Nano-Brick Wall Architectures Account for Super Oxygen Barrier PET Film by Quadlayer Assembly of Polyelectrolytes and α-ZrP Nanoplatelets
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Substrates
2.3. Synthesis and Exfoliation of α-ZrP
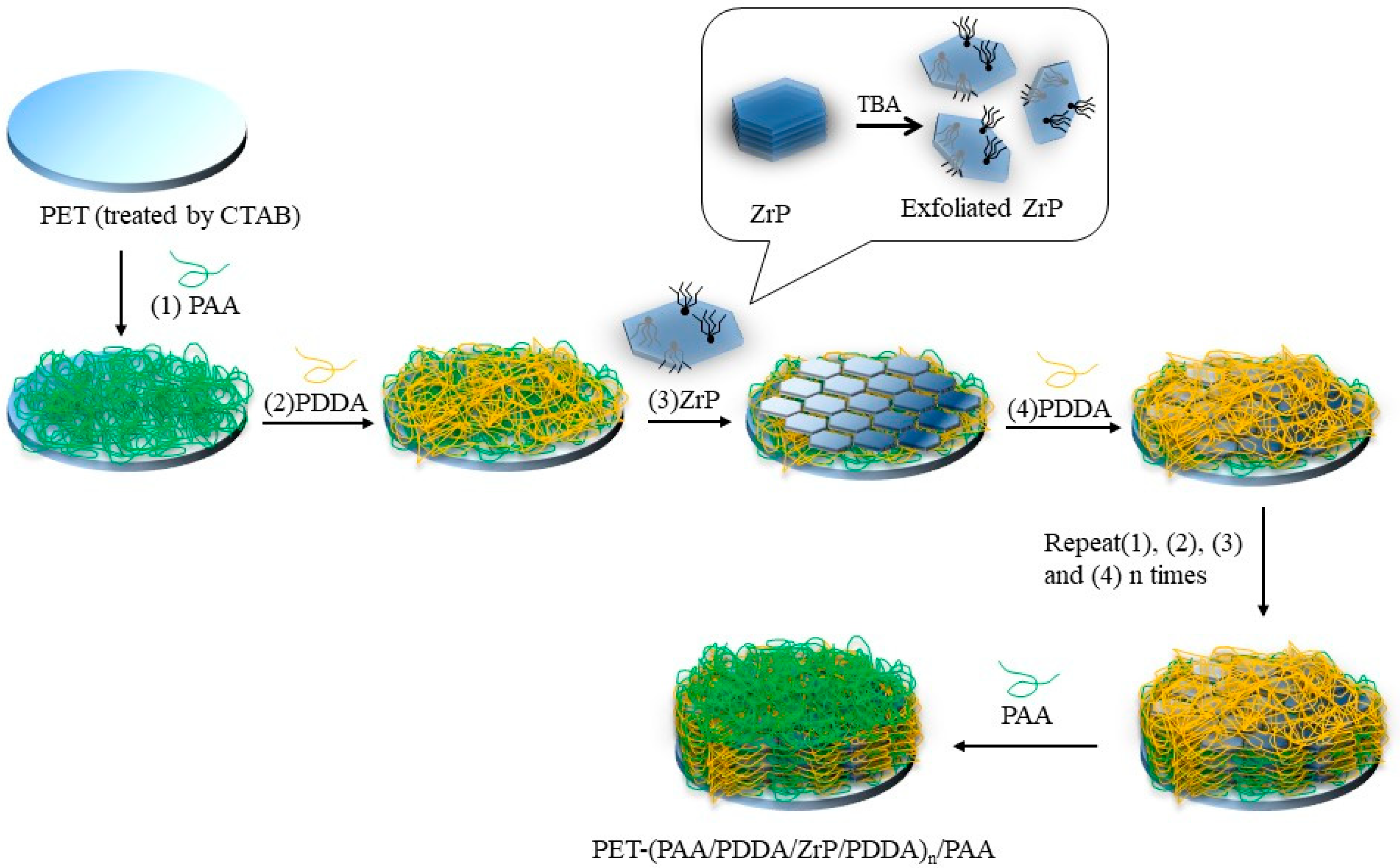
2.4. Layer-by-Layer Deposition
2.5. Film Characterization
2.6. Gas Permeability
2.7. Hazes and Transmittances
3. Results and Discussion
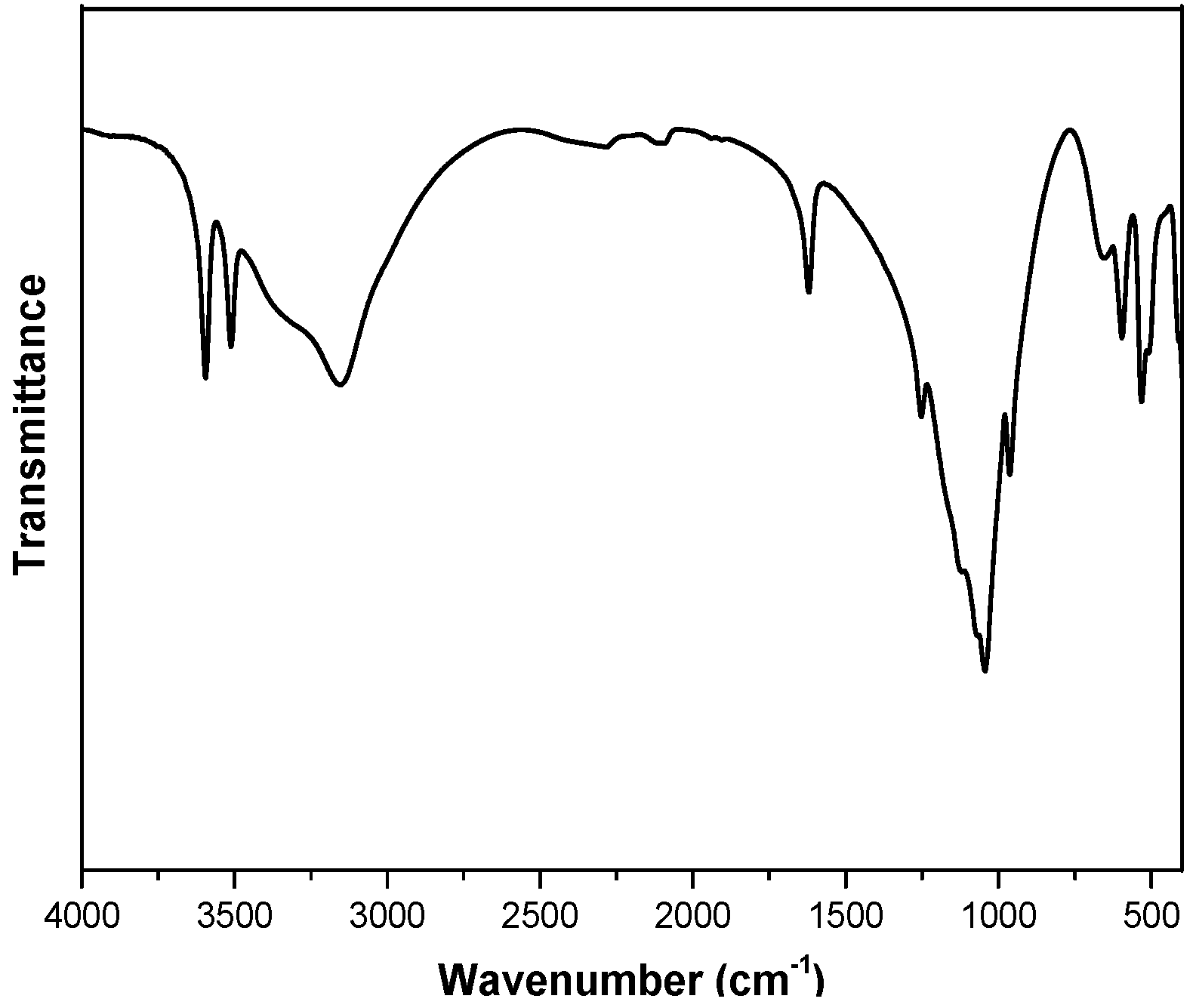
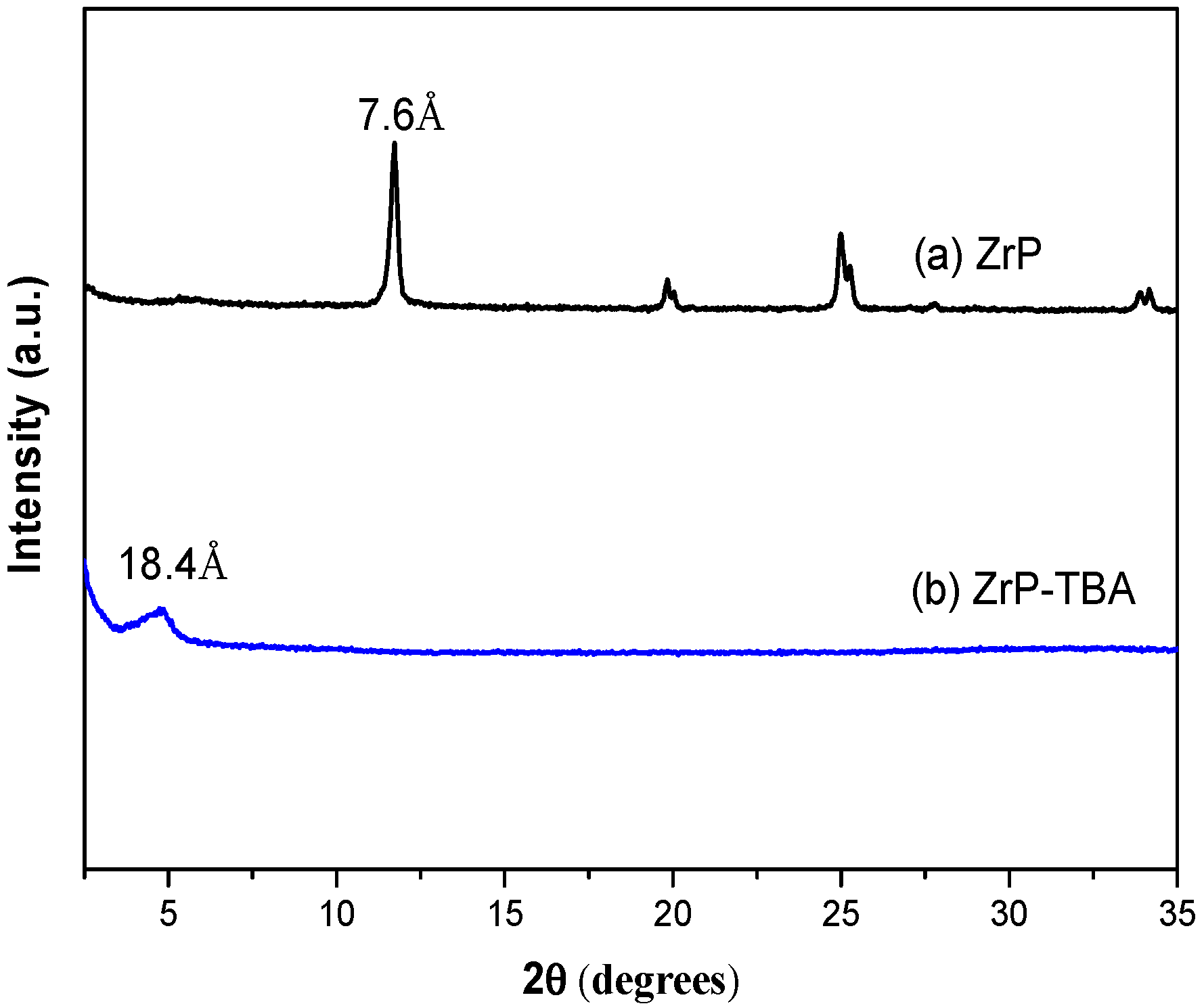
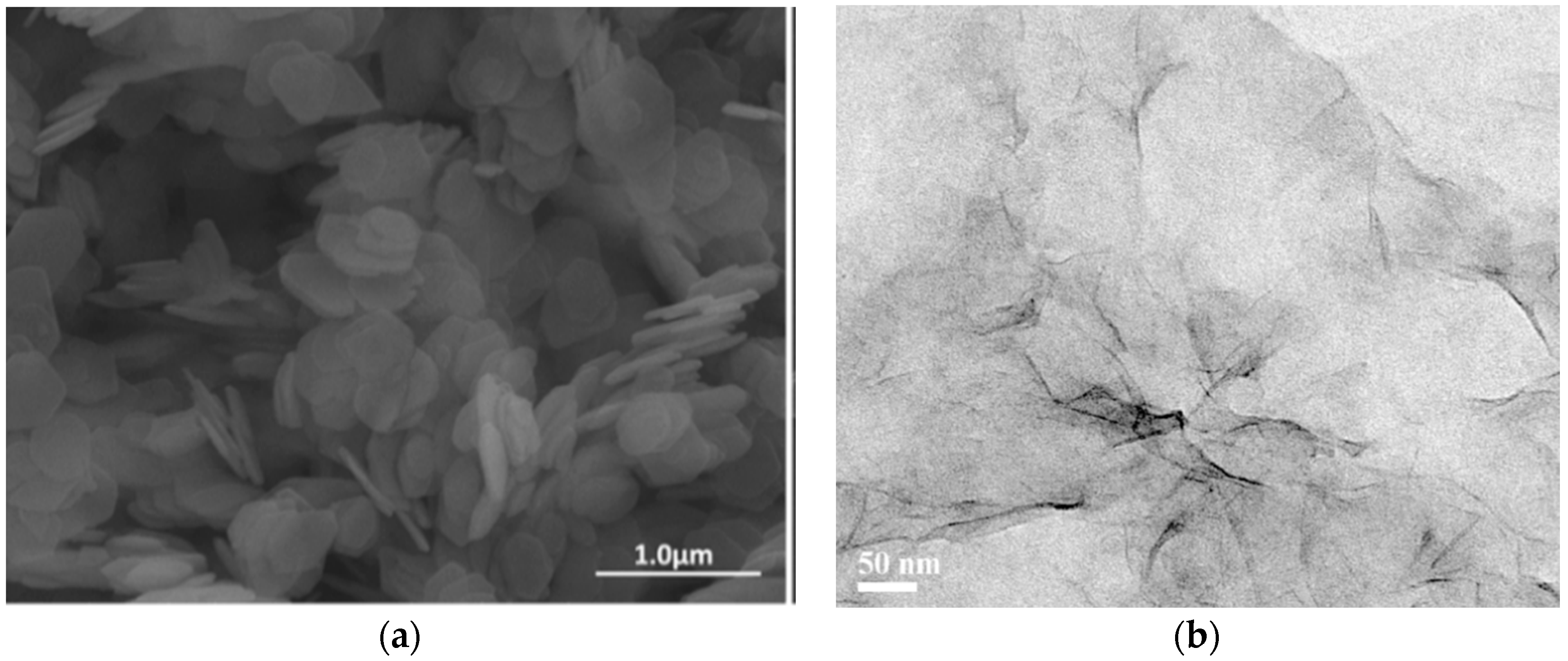
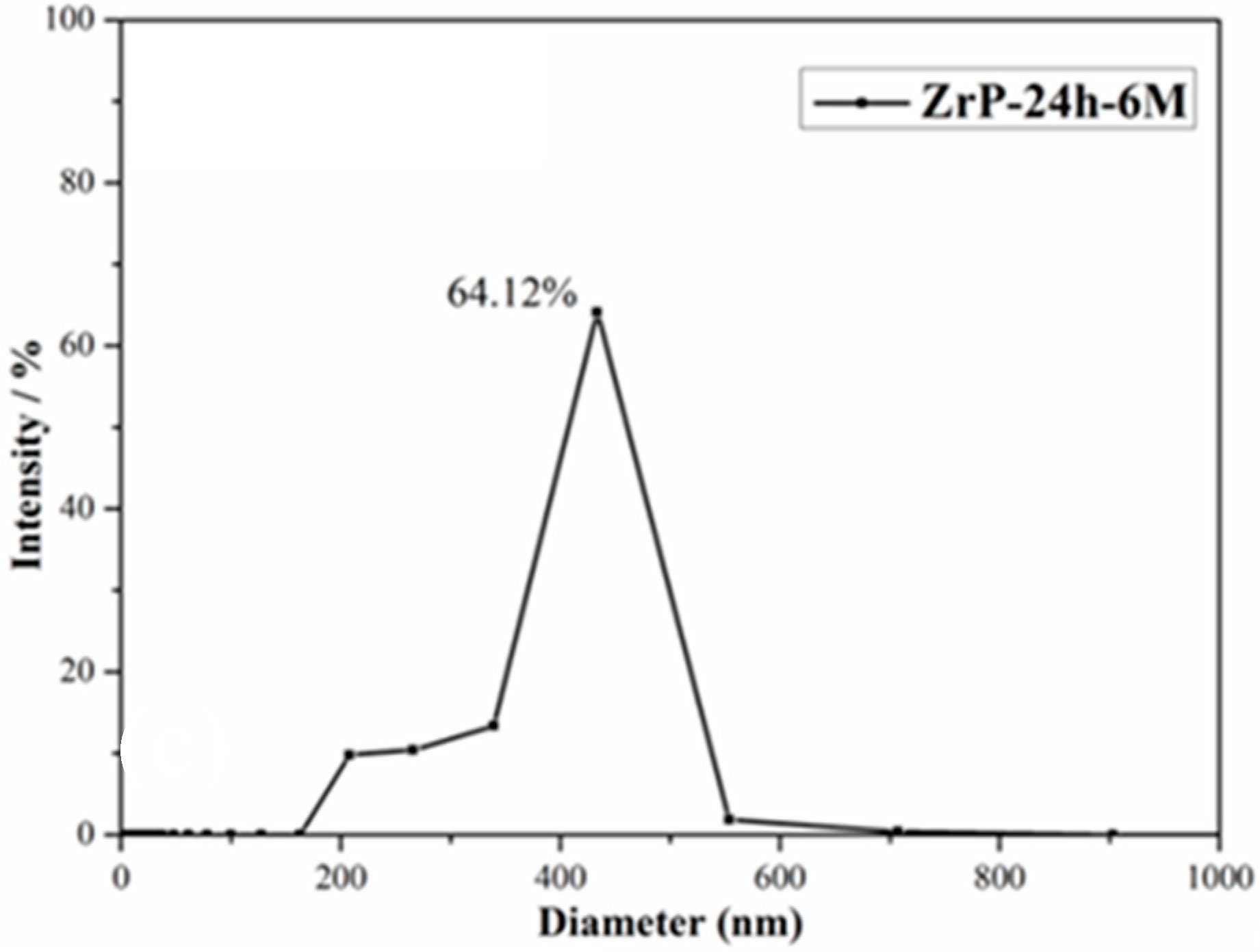
3.1. Exfoliation of α-ZrP
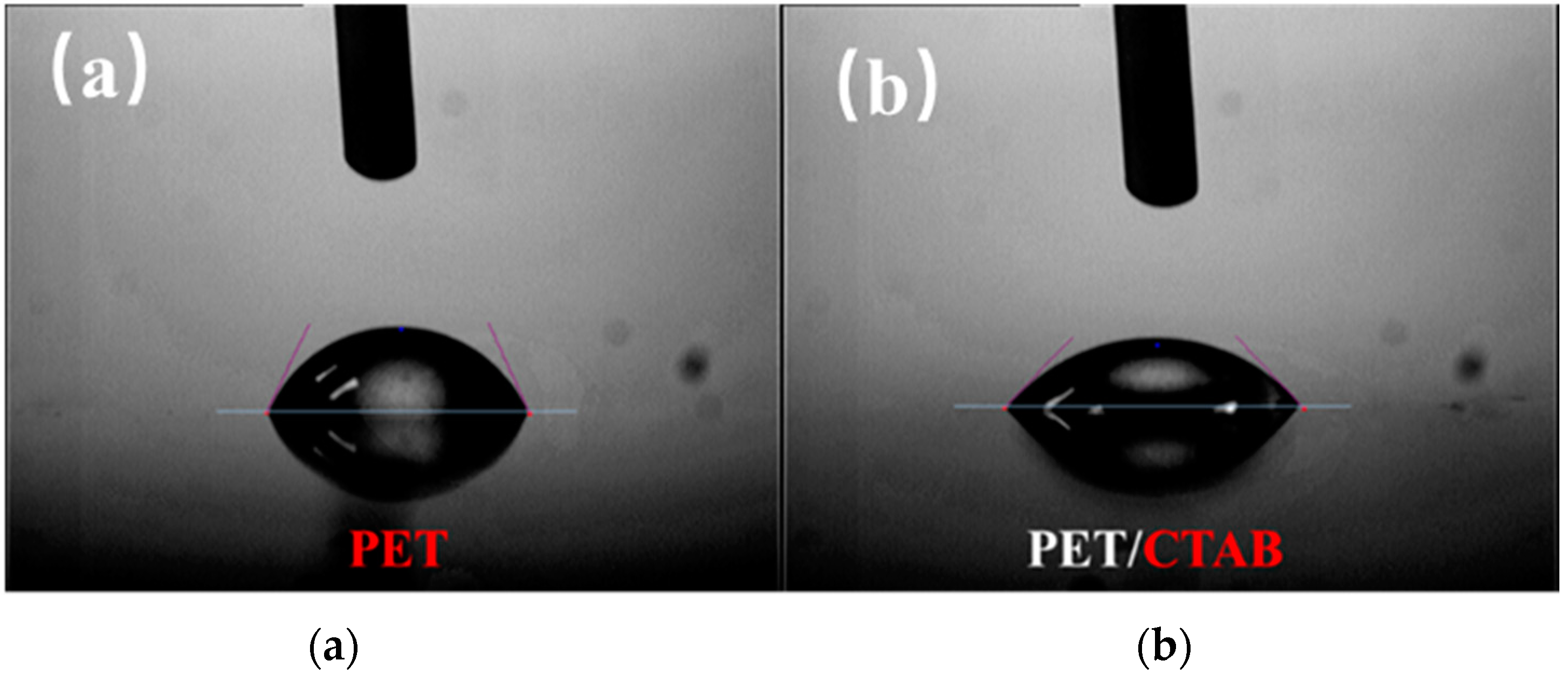
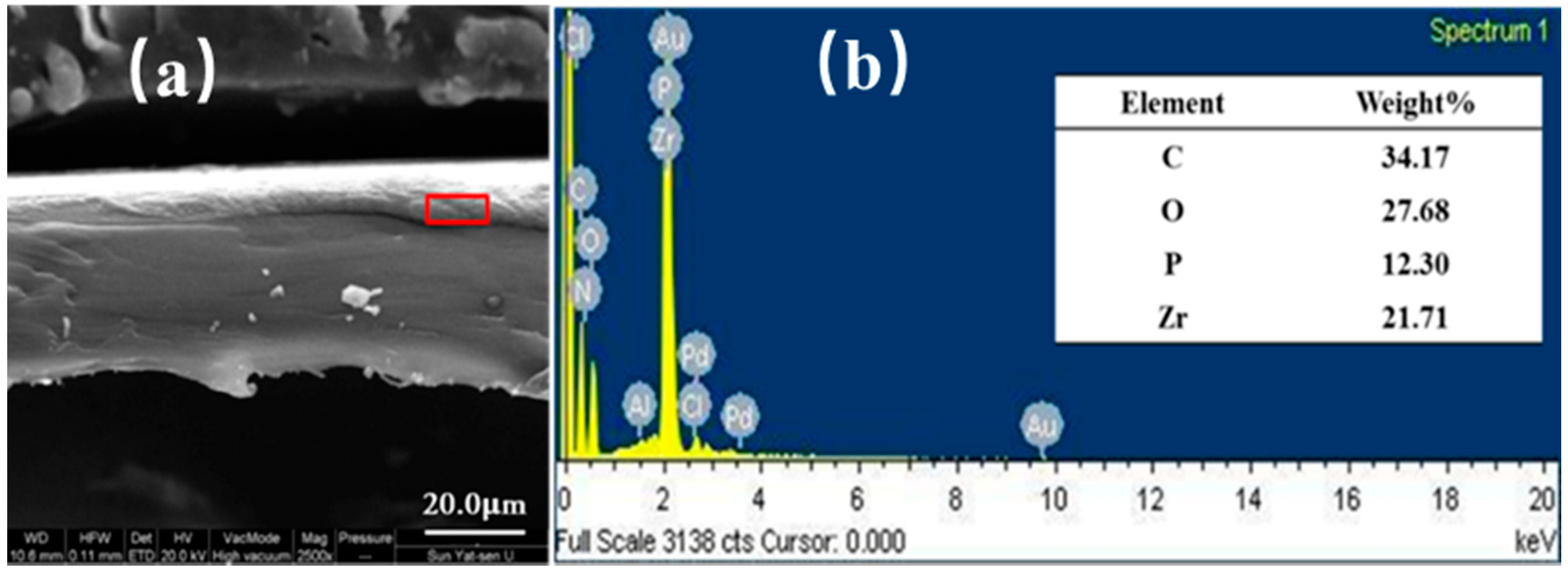
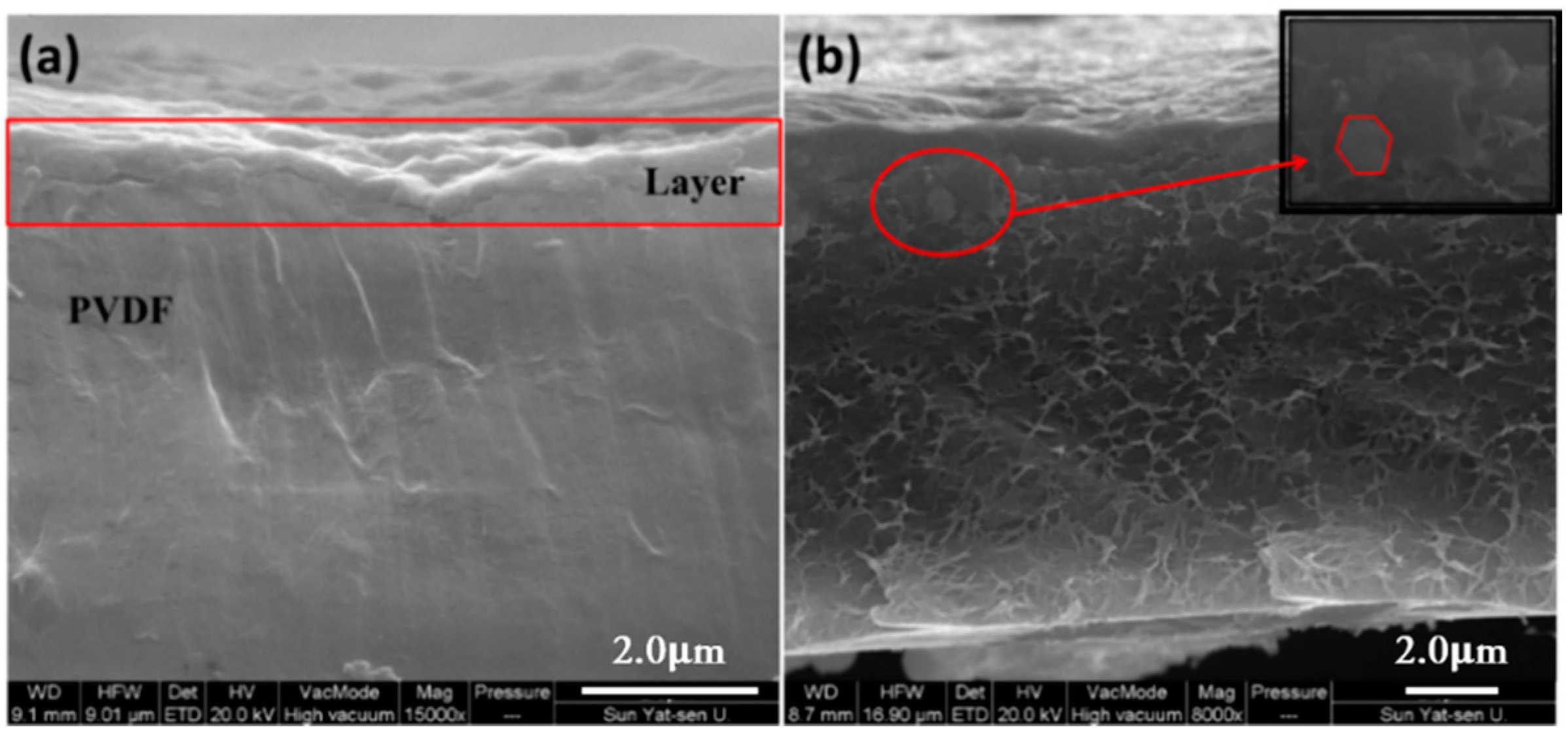
3.2. Characterization of Quadlayer Assembly Film
3.3. Gas Permeability and Transperancy
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Jagadish, R.S.; Raj, B.; Asha, M.R. Blending of low-density polyethylene with improved barrier and aroma-releasing propertis in food packaging. J. Appl. Polym. Sci. 2009, 113, 3732–3741. [Google Scholar] [CrossRef]
- Norrman, K.; Larsen, N.B.; Krebs, F.C. Lifetimes of organic photovoltaics: Combining chemical and physical characterisation techniques to study degradation mechanisms. Sol. Energy Mater. Sol. Cells 2006, 90, 2793–2814. [Google Scholar] [CrossRef]
- Goodyer, C.E.; Bunge, A.L. Comparison of numerical simulations of barrier membranes with impermeable flakes. J. Membr. Sci. 2009, 329, 209–218. [Google Scholar] [CrossRef]
- Lu, C.S.; Mai, Y.W. Permeability modelling of polymer-layered silicate nanocomposites. Compos. Sci. Technol. 2007, 67, 2895–2902. [Google Scholar] [CrossRef]
- Ahn, C.; Kim, S.M.; Jung, J.W.; Park, J.Y.; Kim, T.; Lee, S.E.; Jang, D.C.; Hong, J.W.; Han, S.M.; Jeon, S. Multifunctional Polymer Nanocomposites Reinforced by 3D Continuous Ceramic Nanofillers. ACS Nano 2018, 12, 9126–9133. [Google Scholar] [CrossRef] [PubMed]
- Carosio, F.; Colonna, S.; Fina, A.; Rydzek, G.; Hemmerle, J.; Jierry, L.; Schaaf, P.; Boulmedais, F. Effient gas and water vapor barrier properties of thin poly(lactic acid) packaging films: Fuctionalization with moisture resistant nafion and caly multilayers. Chem. Mater. 2014, 26, 5459–5466. [Google Scholar] [CrossRef]
- Cho, C.Y.; Wallance, K.L.; Hagen, D.A.; Stevents, B.; Regev, O.; Grunlan, J.C. Nanobrick wall multilayer thin films grown faster and stronger using electrophoretic deposition. Nanotechnology 2015, 26, 185703. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.B.; Zhou, A.; Pan, T.; Han, J.B.; Wei, M.; Evans, D.G.; Duan, X. Humidity-triggered self-healing films with excellent oxygen barrier performance. Chem. Commun. 2014, 50, 7136–7138. [Google Scholar] [CrossRef] [PubMed]
- Xiang, F.M.; Tzeng, P.; Sawyer, J.S.; Regev, O.; Grunlan, J.C. Improving the gas barrier prpetiy of clay-polymer multilayer thin films using shorter deposition times. ACS Appl. Mater. Interfaces 2014, 6, 6040–6048. [Google Scholar] [CrossRef] [PubMed]
- Decker, G.; Hong, J.D.; Schmitt, J. Buidup of ultrathin multiplayer films by a self-assembley process: Consecutive adsorption of anionic and cationic bipolar amphiphiles on charged surfaces. Chem. Macromol. Symp. 1991, 46, 321–327. [Google Scholar] [CrossRef]
- Wang, J.J.; Xu, X.Z.; Zhang, J.; Chen, M.T.; Dong, S.Y.; Han, J.B.; Wei, M. Moisture-Permeable, Humidity-Enhanced Gas Barrier Films Based on Organic/Inorganic Multilayers. ACS Appl. Mater. Interfaces 2018, 10, 28130–28138. [Google Scholar] [CrossRef] [PubMed]
- Köklükaya, O.; Carosio, F.; Wågberg, L. Superior Flame-Resistant Cellulose Nanofibril Aerogels Modified with Hybrid Layer-by-Layer Coatings. ACS Appl. Mater. Interfaces 2017, 9, 29082–29092. [Google Scholar] [CrossRef] [PubMed]
- Ming, S.Y.; Chen, G.; He, J.H.; Kuang, Y.D.; Liu, Y.; Tao, R.Q.; Ning, H.L.; Zhu, P.H.; Liu, Y.Y.; Fang, Z.Q. Highly Transparent and Self-Extinguishing Nanofibrillated Cellulose-Monolayer Clay Nanoplatelet Hybrid Films. Langmuir 2017, 33, 8455–8462. [Google Scholar] [CrossRef] [PubMed]
- Priolo, M.A.; Holder, K.M.; Greenlee, S.M.; Stevens, B.E.; Grunlan, J.C. Precisely Tuning the Clay Spacing in Nanobrick Wall Gas Barrier Thin Films. Chem. Mater. 2013, 25, 1649–1655. [Google Scholar] [CrossRef]
- Priolo, M.A.; Holder, K.M.; Gamboa, D.; Grunlan, J.C. Influence of clay concentration on the gas barrier of clay-polymer nanobrick wall thin film assemblies. Langmuir 2011, 27, 12106–12114. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Malek, F.A.; Grunlan, J.C. Influence of Deposition Time on Layer-by-Layer Growth of Clay-Based Thin Films. Ind. Eng. Chem. Res. 2010, 49, 8501–8509. [Google Scholar] [CrossRef]
- Priolo, M.A.; Gamboa, D.; Grunlan, J.C. Transparent Clay-Polymer Nano Brick Wall Assemblies with Tailorable Oxygen Barrier. ACS Appl. Mater. Interfaces 2010, 2, 312–320. [Google Scholar] [CrossRef]
- Priolo, M.A.; Holder, K.M.; Greenlee, S.M.; Grunlan, J.C. Transparency, gas barrier, and moisture resistance of large-aspect-ratio vermiculite nanobrick wall thin films. ACS Appl. Mater. Interfaces 2012, 4, 5529–5533. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Bolling, L.; Priolo, M.A.; Grunlan, J.C. Graphene: Super Gas Barrier and Selectivity of Graphene Oxide-Polymer Multilayer Thin Films. Adv. Mater. 2013, 25, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Bandi, S.; Mehta, S.; Schiraldi, D.A. The mechanism of color generation in poly(ethylene terephthalate)/polyamide blends. Polym. Degrad. Stab. 2005, 88, 341–348. [Google Scholar] [CrossRef]
- Prattipati, V.; Hu, Y.S.; Bandi, S.; Schiraldi, D.A.; Hiltner, A.; Baer, E.; Mehta, S. Effect of compatibilization on the oxygen-barrier properties of poly(ethylene terephthalate)/poly(m-xylylene adipamide) blends. J. Appl. Polym. Sci. 2005, 97, 1361–1370. [Google Scholar] [CrossRef]
- Ahmad, A.J.; Hammam, A.B.; Hannah, H.; Gad, M. Improving the Oxygen Barrier Properties of Polyethylene Terephthalate by Graphite Nanoplatelets. J. Appl. Polym. Sci. 2013, 128, 1534–1539. [Google Scholar] [CrossRef]
- Roberts, A.P.; Henry, B.M.; Sutton, A.P.; Grovenor, C.R.M.; Briggs, G.A.D.; Miyamoto, T.; Kano, M.; Tsukahara, Y.; Yanaka, M. Gas permeation in silicon-oxide/polymer (SiOx/PET) barrier films: role of the oxide lattice, nano-defects and macro-defects. J. Membr. Sci. 2002, 208, 75–88. [Google Scholar] [CrossRef]
- Priolo, M.A.; Holder, K.M.; Guin, T.; Grunlan, J.C. Recent Advances in Gas Barrier Thin Films via Layer-by-Layer Assembly of Polymers and Platelets. Macromol. Rapid Commun. 2015, 36, 866–879. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Song, Y.; Floto, M.E.; Grunlan, J.C. Combined High Stretchability and Gas Barrier in Hydrogen-Bonded Multilayer Nanobrick Wall Thin Films. ACS Appl. Mater. Interfaces 2017, 9, 7903–7907. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, P.; Kaushik, A.K.; Arruda, E.M.; Waas, A.M.; Shim, B.S.; Xu, J.D.; Nandivada, H.; Pumplin, B.G.; Lahann, J.; Ramamoorthy, A.; et al. Ultrastrong and stiff layered polymer nanocomposities. Science 2007, 318, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Schulz, J.; Mannen, S.; Delhom, C.; Condon, B.; Change, S.; Zammarano, M.; Grunlan, J.C. Flame retardant behavior of polyelectrolyte-clay thin film assemblies on cotton fabric. ACS Nano 2010, 6, 3325–3337. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.S.; Rawson, I.; Grunlan, J.C. Layer-layer assembly thin film oxygen barrier. Thin Solid Films 2008, 516, 4819–4825. [Google Scholar] [CrossRef]
- Priolo, M.A.; Gamboa, D.; Holder, K.M.; Grunlan, J.C. Super gas barrier of transparent polymer-clay multilayer ultrathin films. Nano Lett. 2010, 10, 4970–4974. [Google Scholar] [CrossRef] [PubMed]
- Boo, W.J.; Sun, L.Y.; Sun, D.Z.; Clearfild, A.; Sue, H.J. Preparation of exfoliated epoxy/α-zirconium phosphate nanocomposites containing high aspect ratio nanoplatelets. Chem. Mater. 2007, 19, 1749–1754. [Google Scholar]
- Wong, M.H.; Ishige, R.; White, K.L.; Li, P.; Kin, D.; Krishnamoorti, R.; Gunther, R.; Higuchi, T.; Jinnai, H.; Takahara, A.; et al. Molecular traces of alternative social organization in a termite genome. Nat. Commun. 2014, 5, 1–12. [Google Scholar]
- Pan, Y.; Pan, H.F.; Yuan, B.H.; Hong, N.N.; Zhan, J.; Wang, B.B.; Song, L.; Hu, Y. Construction of organic–inorganic hybrid nano-coatings containing α-zirconium phosphate with high efficiency for reducing fire hazards of flexible polyurethane foam. Mater. Chem. Phys. 2015, 163, 107–115. [Google Scholar] [CrossRef]
- Huang, T.C.; Lai, G.H.; Li, C.E.; Tsai, M.H.; Wan, P.Y.; Chung, Y.H.; Lin, M.H. Advanced anti-corrosion coatings prepared from [small alpha]-zirconium phosphate/polyurethane nanocomposites. RSC Adv. 2017, 7, 9908–9913. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, C.; Liu, L.; Hong, J.; Dong, M.; Deng, X. Synergistic flame retardant properties of a layered double hydroxide in combination with zirconium phosphonate in polypropylene. RSC Adv. 2016, 6, 91720–91727. [Google Scholar] [CrossRef]
- Lu, H.; Wilkie, C.A.; Ding, M.; Song, L. Thermal properties and flammability performance of poly (vinyl alcohol)/α-zirconium phosphate nanocomposites. Polym. Degrad. Stab. 2011, 96, 885–891. [Google Scholar] [CrossRef]
- Pan, J.J.; Wang, J.S.; Xiao, M.; Hickner, M.; Meng, Y.Z. Layered zirconium phosphate sulfophenylphosphonates reinforced sulfonated poly (fluorenyl ether ketone) hybrid membranes with high proton conductivity and low vanadium ion permeability. J. Membr. Sci. 2013, 443, 19–27. [Google Scholar] [CrossRef]
- Wang, D.Y.; Liu, X.Q.; Wang, J.S.; Wang, Y.Z.; Stec, A.A.; Hull, T.R. Preparation and characterisation of a novel fire retardant PET/α-zirconium phosphate nanocomposite. Polym. Degrad. Stab. 2009, 94, 544–549. [Google Scholar] [CrossRef] [Green Version]
- Clara, S.; Donatella, D.; Sossio, C. Food packaging based on polymer nanomaterials. J. Progr. Polym. Sci. 2011, 36, 1766–1782. [Google Scholar]
- Mihindukulasuriya, S.D.F.; Lim, L.T. Nanotechnology development in food packaging: A review. Trends Food Sci. Technol. 2014, 40, 149–167. [Google Scholar] [CrossRef]
- Zhang, L.S.; Ling, L.; Xiao, M.; Han, D.M.; Wang, S.J.; Meng, Y.Z. Effectively supperessing vanadium permeation in vanadium redox flow battery application with modified nafion membrane with nacre-like nanoarchitectures. J. Power Sources 2017, 352, 111–117. [Google Scholar] [CrossRef]
- Lu, M.Z.; Huang, S.; Chen, S.; Ju, Q.; Xiao, M.; Peng, X.H.; Wang, S.J.; Meng, Y.Z. Transparent and super-gas-barrier PET film with surface coated by a polyelectrolyte and borax. Polym. J. 2018, 50, 239–250. [Google Scholar] [CrossRef]
- Sun, L.Y.; Boo, W.J.; Sue, H.J.; Clearfield, A. Preparation of alpha-zirconium phosphate nanoplatelets with wide variations in aspect ratios. New J. Chem. 2007, 31, 39–43. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, X.; Shinde, A.; Cheng, Z. Synthesis and Exfoliation of Discotic Zirconium Phosphates to Obtain Colloidal Liquid Crystals. J. Vis. Exp. JoVE 2016, 111. [Google Scholar] [CrossRef] [PubMed]
- Li, G.F.; Luo, W.H.; Xiao, M.; Wang, S.J.; Meng, Y.Z. Biodegradable poly(propylene carbonate)layered double hydroxide composite films with enhanced gas barrier and mechanical properties. Chin. J. Polym. Sci. 2016, 34, 13–22. [Google Scholar] [CrossRef]
- Koleva, V.; Stefov, V.; Cahil, A.; Najdoski, M.; Šoptrajanov, B.; Engelen, B.; Lutz, H.D. Infrared and Raman studies of manganese dihydrogen phosphate dihydrate, Mn(H2PO4)2·2H2O. I: Region of the vibrations of the phosphate ions and external modes of the water molecules. J. Mol. Struct. 2009, 917, 117–124. [Google Scholar] [CrossRef]
- Horsley, S.E.; Nowell, D.V.; Stewart, D.T. The infrared and Raman spectra of α-zirconium phosphate. Spectrochim. Acta Part A Mol. Spectrosc. 1974, 30, 535–541. [Google Scholar] [CrossRef]
- Barraclough, C.G.; Bradley, D.C.; Lewis, J.; Thomas, I.M. 510. The infrared spectra of some metal alkoxides, trialkylsilyloxides, and related silanols. J. Chem. Soc. 1961, 2601–2605. [Google Scholar] [CrossRef]
- Alberti, G. Syntheses, crystalline structure, and ion-exchange properties of insoluble acid salts of tetravalent metals and their salt forms. Acc. Chem. Res. 1978, 11, 163–170. [Google Scholar] [CrossRef]
- Clearfield, A. Group IV Phosphates as Catalysts and Catalyst Supports. J. Mol. Catal. 1984, 27, 251–262. [Google Scholar] [CrossRef]
- Clearfield, A. Inorganic Ion Exchangers with Layered Structures. Annu. Rev. Mater. Sci. 1984, 14, 205–229. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Huang, R.C.; Ding, F.C.; Brittain, A.D.; Liu, J.J.; Zhang, M.; Xiao, M.; Meng, Y.Z.; Sun, L.Y. Sulfonic acid-functionalized α-zirconium phosphate single-layer nanosheets as a strong solid acid for heterogeneous catalysis applications. ACS Appl. Mater. Interfaces 2014, 6, 7417–7425. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.Y.; Boo, W.J.; Browning, R.L.; Sue, H.-J.; Clearfield, A. Effect of crystallinity on the intercalation of monoamine in α-zirconium phosphate layer structure. Chem. Mater. 2005, 17, 5606–5609. [Google Scholar] [CrossRef]
- Xie, Y.H.; Zhang, M.; Gao, X.Z.; Shao, Y.; Liu, H.; Jin, J.H.; Yang, W.G.; Zhang, H.X. Development and antimicrobial application of plantaricin BM-1 incorporating a PVDC film on fresh pork meat during cold storage. J. Appl. Microbiol. 2018, 125, 1108–1116. [Google Scholar] [CrossRef] [PubMed]

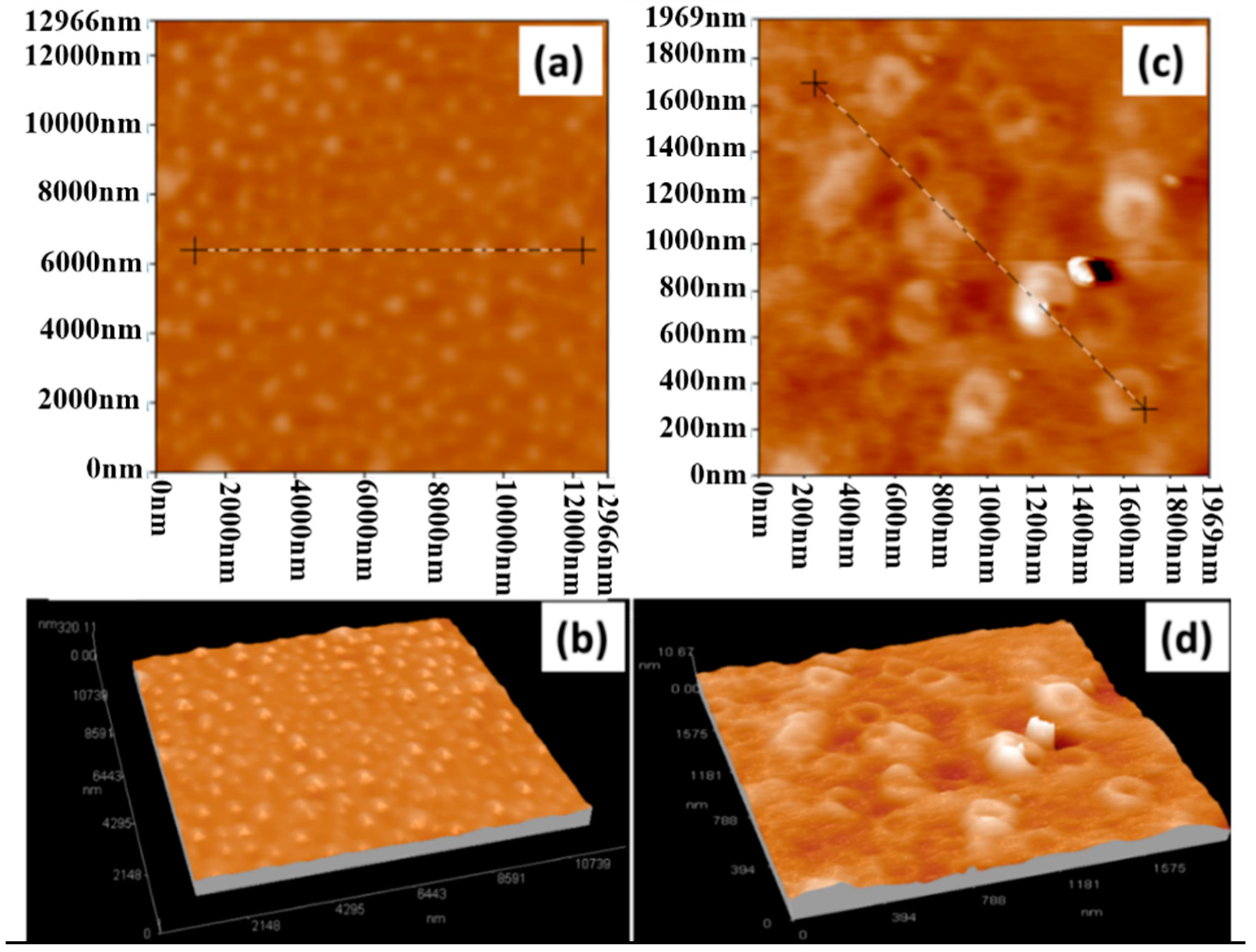
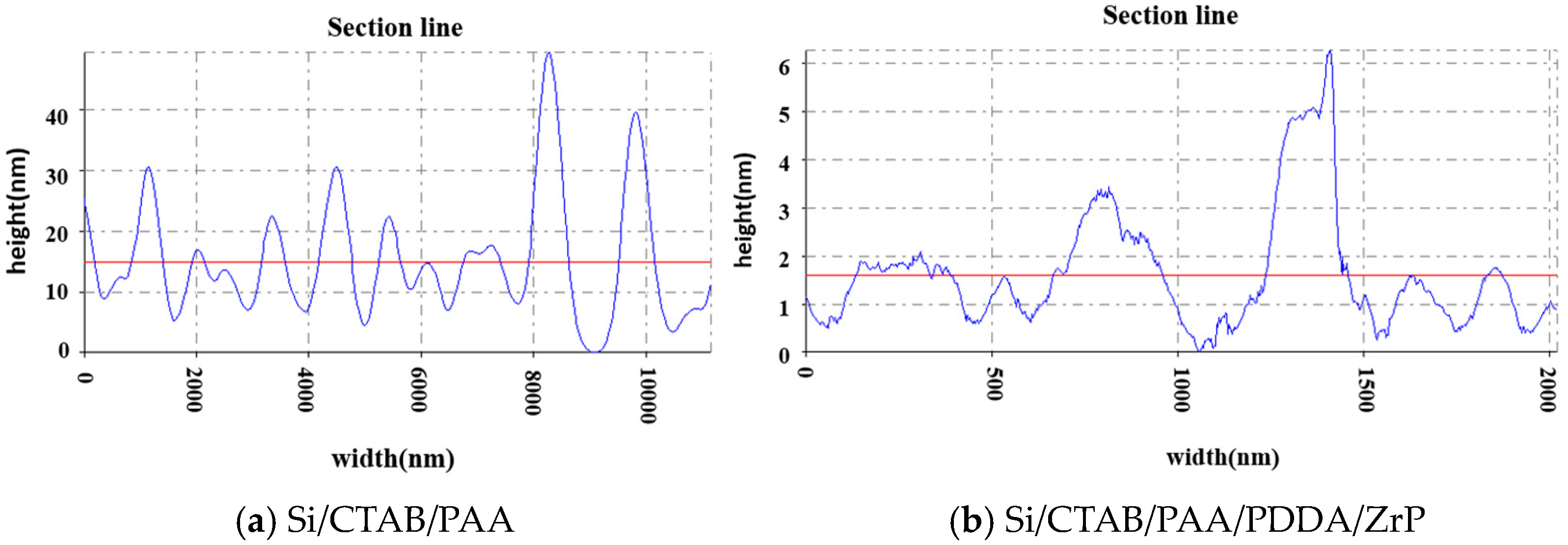
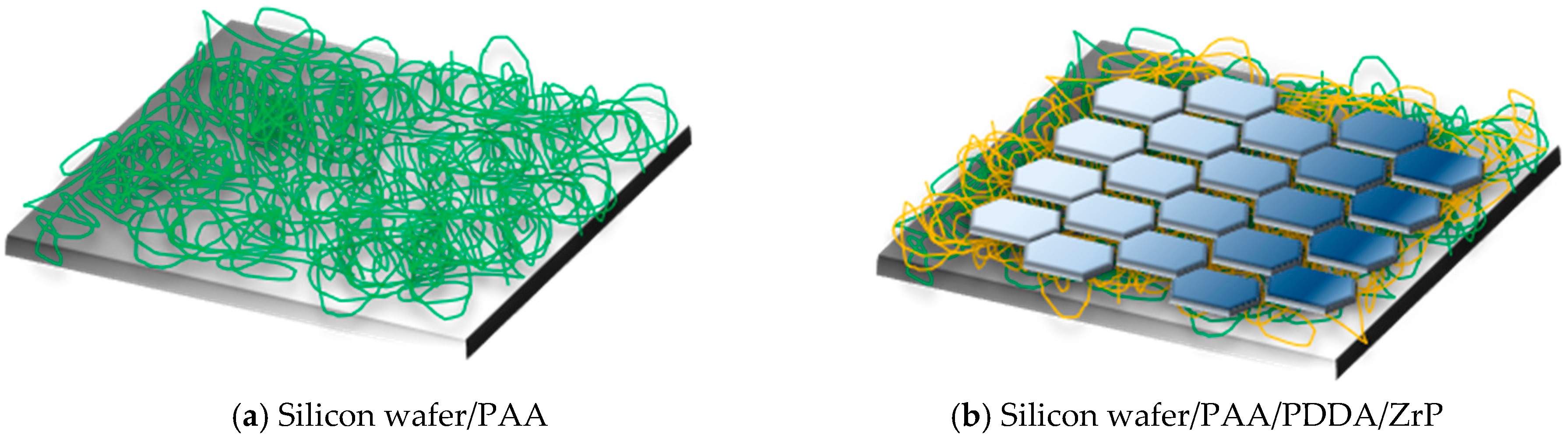
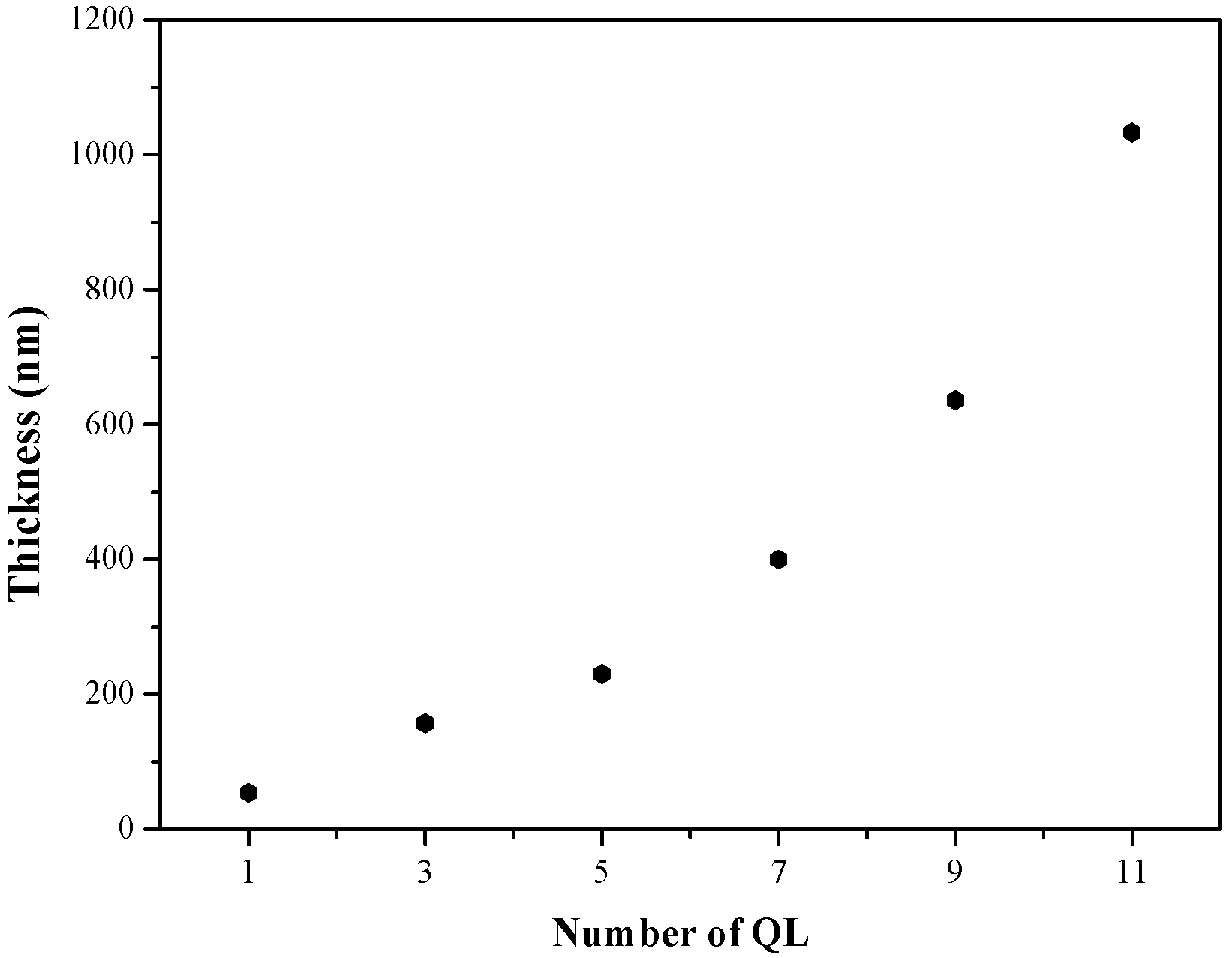
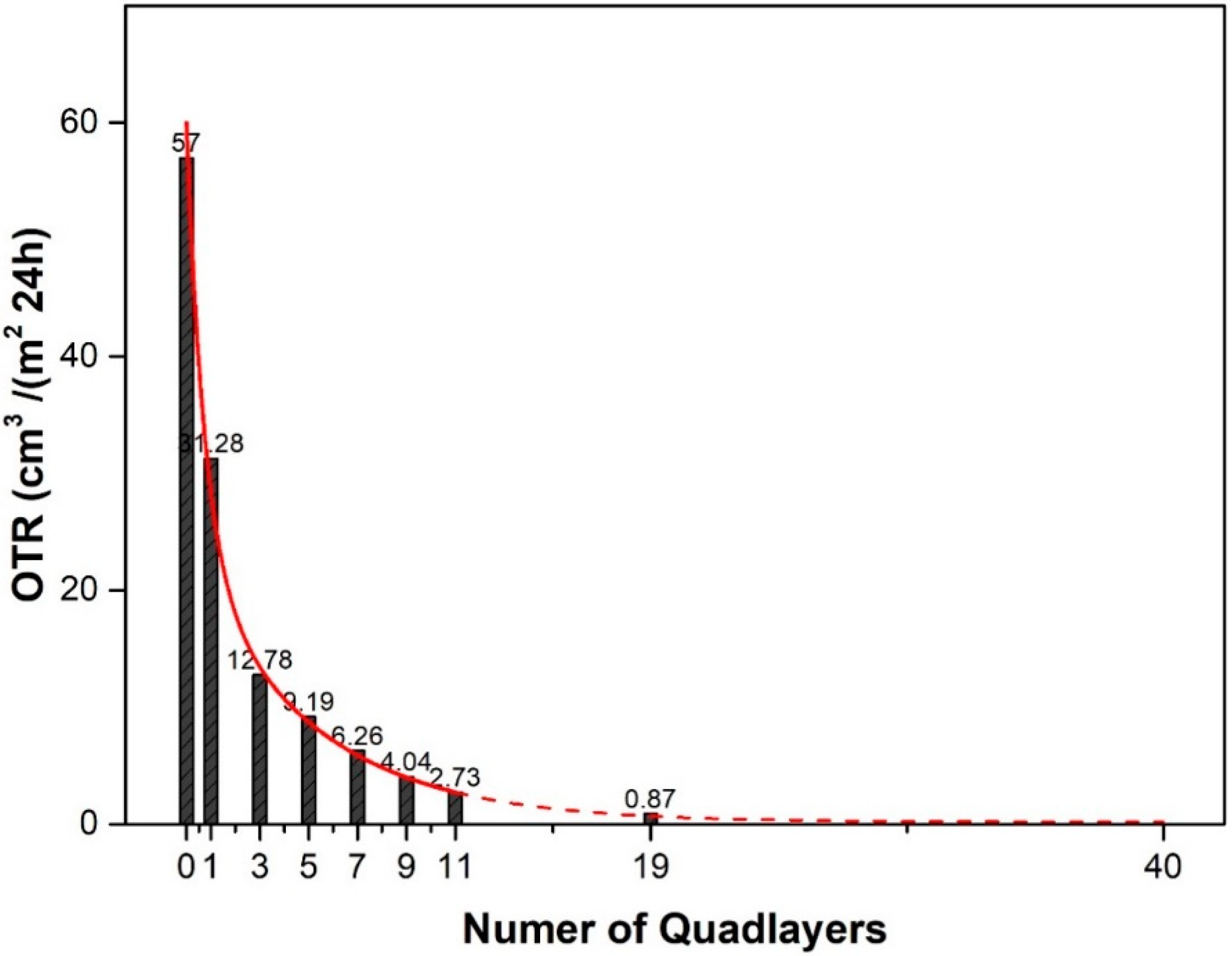


| Sample | OTR (cm3/(m2·24 h)) | OP (cm3·mm/(m2·24 h)) | WVTR (g/(m2·24 h)) | WVP (g mm/(m2·24 h)) |
|---|---|---|---|---|
| PET | 57.0 | 1.43 | 9.97 | 0.25 |
| PET-(QL)1 | 31.3 | 0.78 | 9.30 | 0.23 |
| PET/(QL)3 | 12.8 | 0.32 | 9.22 | 0.23 |
| PET-(QL)5 | 9.19 | 0.23 | 9.15 | 0.23 |
| PET-(QL)7 | 6.26 | 0.16 | 9.10 | 0.23 |
| PET-(QL)9 | 4.04 | 0.10 | 9.08 | 0.22 |
| PET-(QL)11 | 2.73 | 0.07 | 9.06 | 0.23 |
| … | … | … | … | |
| PET-(QL)19 | 0.87 | 0.02 | 8.50 | 0.20 |
| PET-(QL)20 | undetectable | - | 8.45 | 0.19 |
| Sample | Transmittance (%) | Haze (%) |
|---|---|---|
| PET | 89.5 | 2.0 |
| PET/(QL)19/PAA | 88.8 | 2.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, D.; Luo, Y.; Ju, Q.; Xiao, X.; Xiao, M.; Xiao, N.; Chen, S.; Peng, X.; Wang, S.; Meng, Y. Nano-Brick Wall Architectures Account for Super Oxygen Barrier PET Film by Quadlayer Assembly of Polyelectrolytes and α-ZrP Nanoplatelets. Polymers 2018, 10, 1082. https://doi.org/10.3390/polym10101082
Han D, Luo Y, Ju Q, Xiao X, Xiao M, Xiao N, Chen S, Peng X, Wang S, Meng Y. Nano-Brick Wall Architectures Account for Super Oxygen Barrier PET Film by Quadlayer Assembly of Polyelectrolytes and α-ZrP Nanoplatelets. Polymers. 2018; 10(10):1082. https://doi.org/10.3390/polym10101082
Chicago/Turabian StyleHan, Dongmei, Yiqing Luo, Qing Ju, Xujing Xiao, Min Xiao, Naiyu Xiao, Shou Chen, Xiaohua Peng, Shuanjin Wang, and Yuezhong Meng. 2018. "Nano-Brick Wall Architectures Account for Super Oxygen Barrier PET Film by Quadlayer Assembly of Polyelectrolytes and α-ZrP Nanoplatelets" Polymers 10, no. 10: 1082. https://doi.org/10.3390/polym10101082
APA StyleHan, D., Luo, Y., Ju, Q., Xiao, X., Xiao, M., Xiao, N., Chen, S., Peng, X., Wang, S., & Meng, Y. (2018). Nano-Brick Wall Architectures Account for Super Oxygen Barrier PET Film by Quadlayer Assembly of Polyelectrolytes and α-ZrP Nanoplatelets. Polymers, 10(10), 1082. https://doi.org/10.3390/polym10101082






