Droplet-Assisted Microfluidic Fabrication and Characterization of Multifunctional Polysaccharide Microgels Formed by Multicomponent Reactions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials for Microgel Synthesis
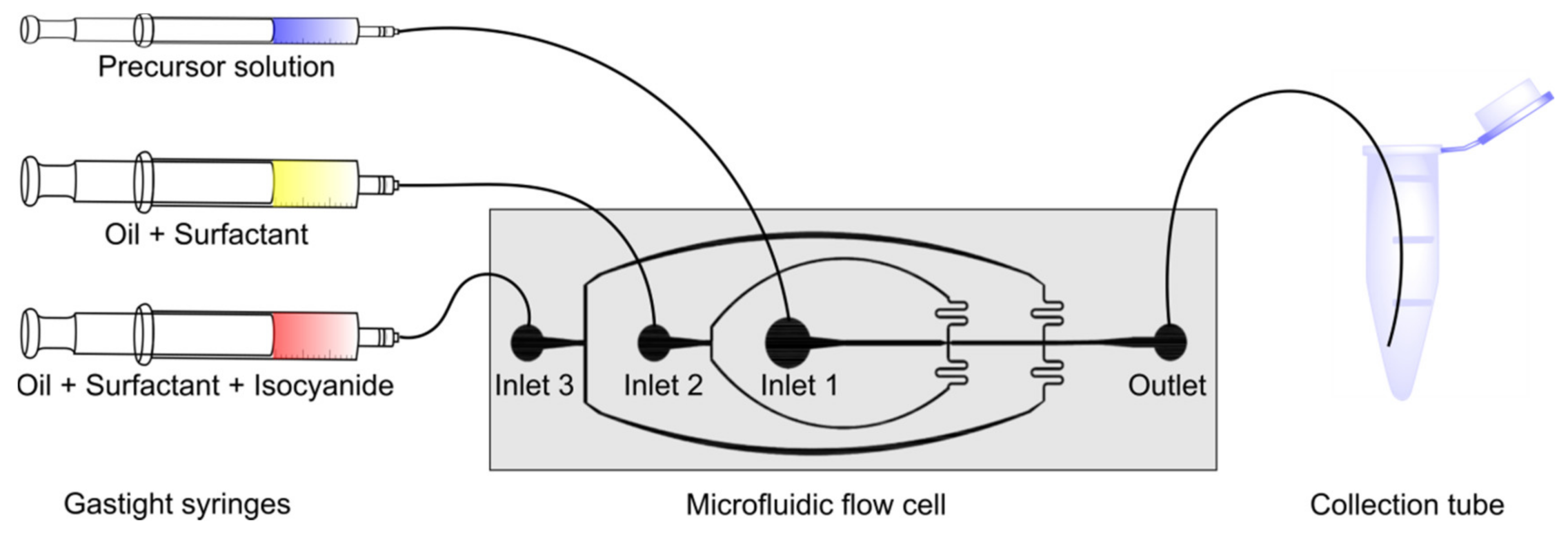
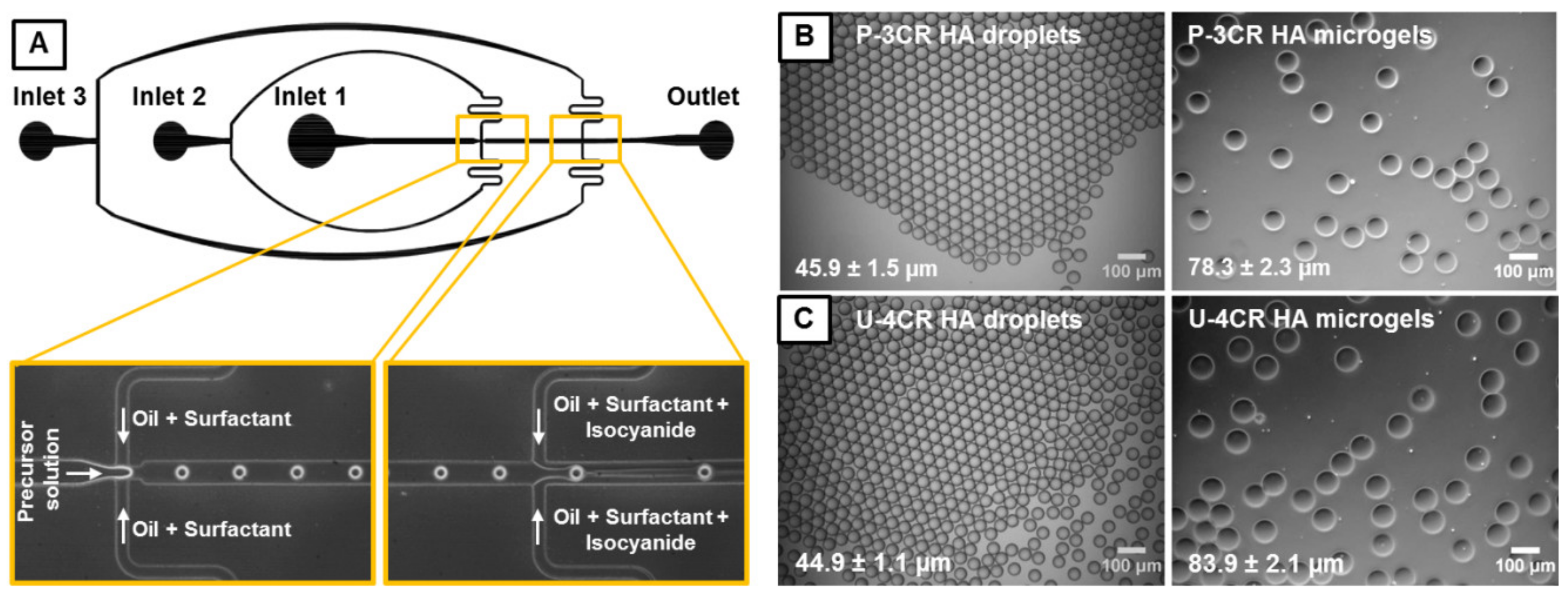
2.2. Microfluidic Device Fabrication and General Microfluidic Experimental Setup
2.3. Microfluidic Fabrication of P-3CR and U-4CR Microgels
2.3.1. General Procedure of Microgel Fabrication
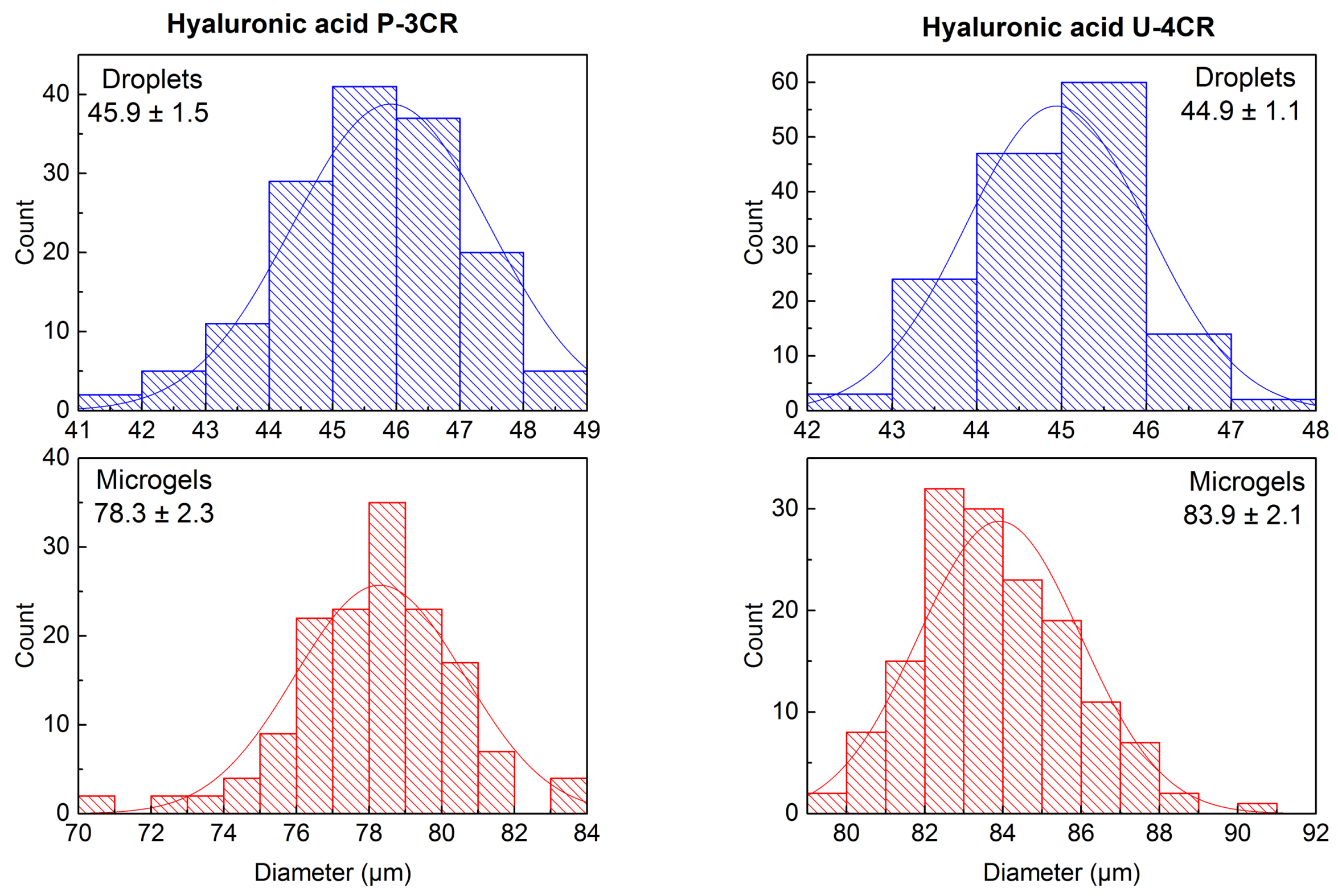
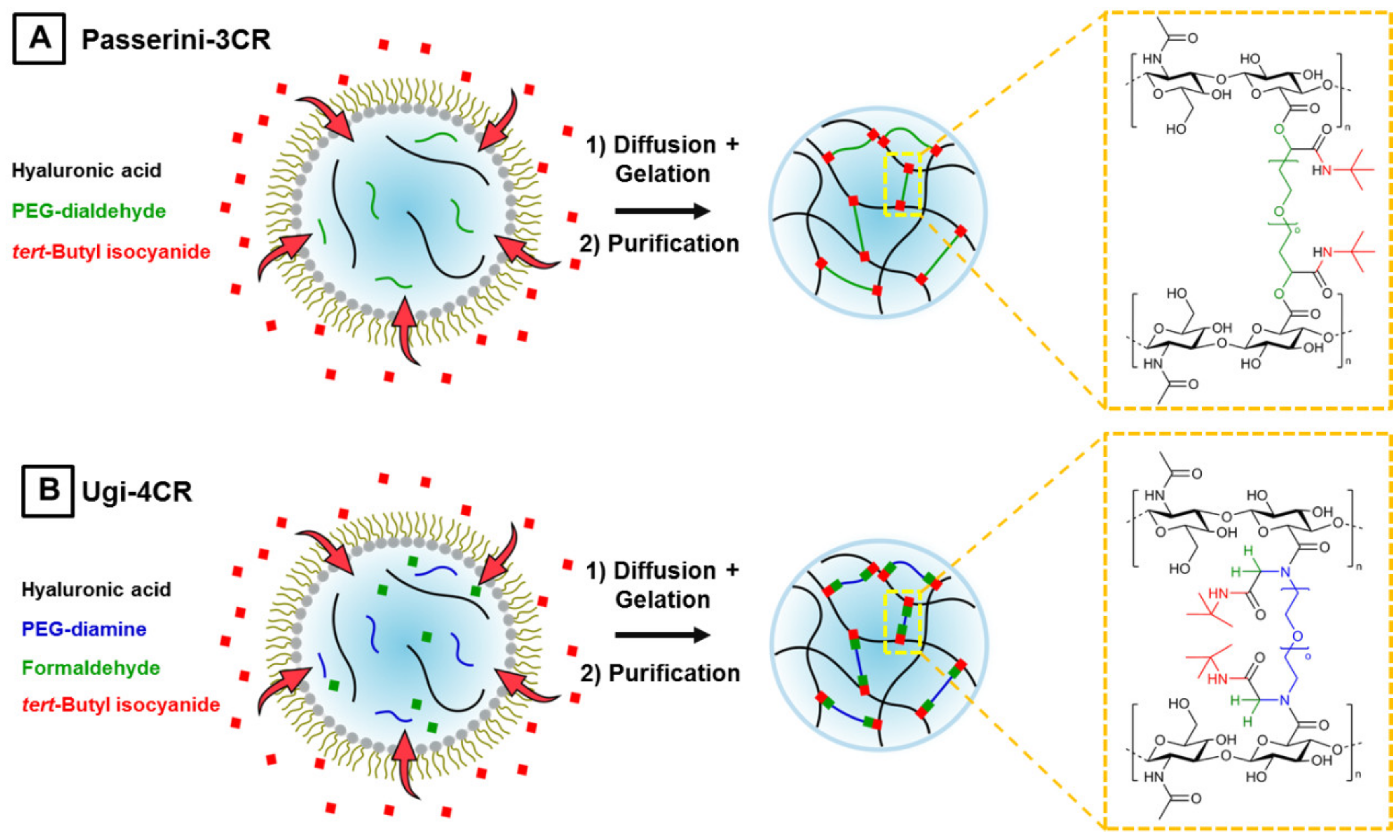
2.3.2. Hyaluronic Acid-Based P-3CR Microgels
2.3.3. Hyaluronic Acid-Based U-4CR Microgels
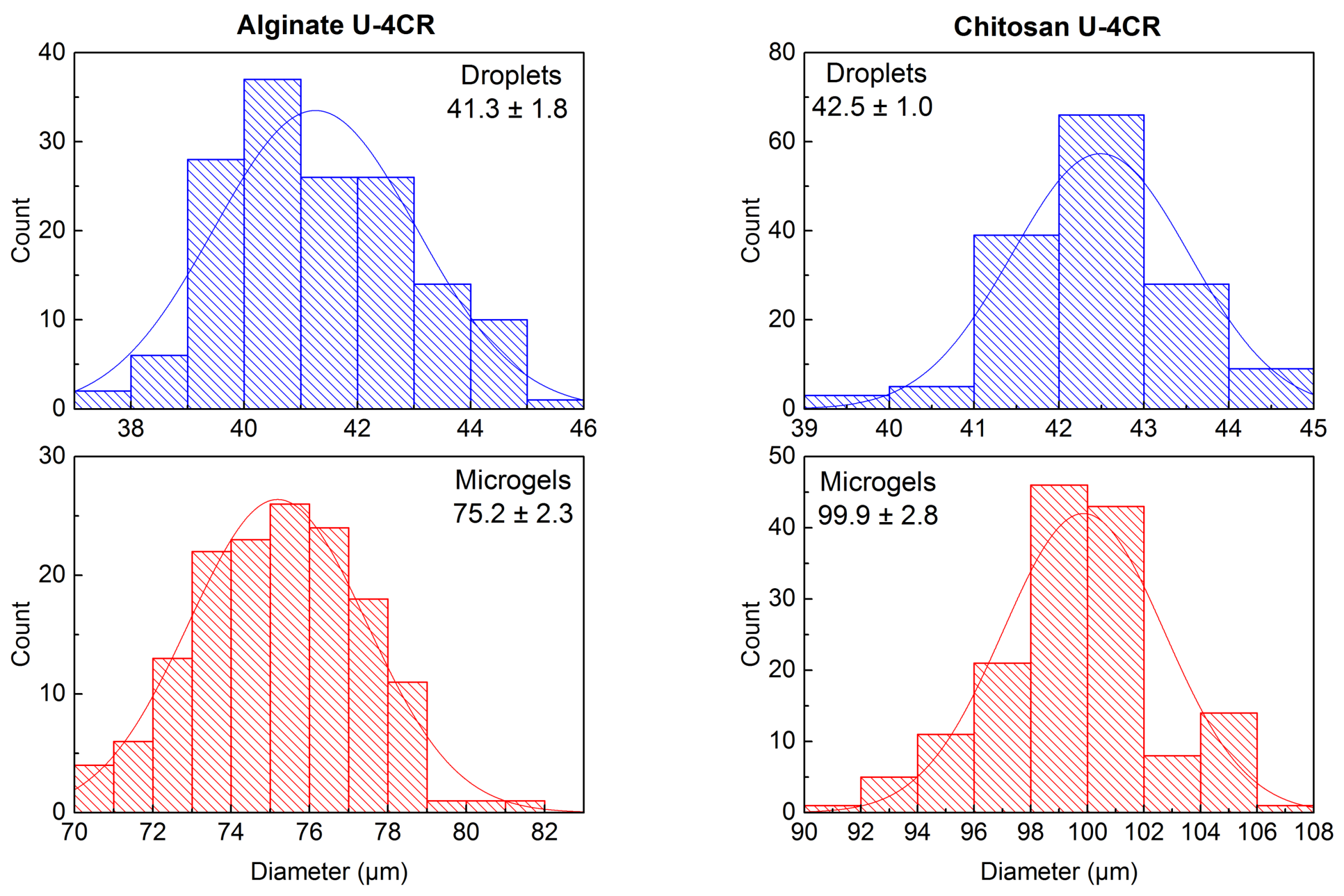
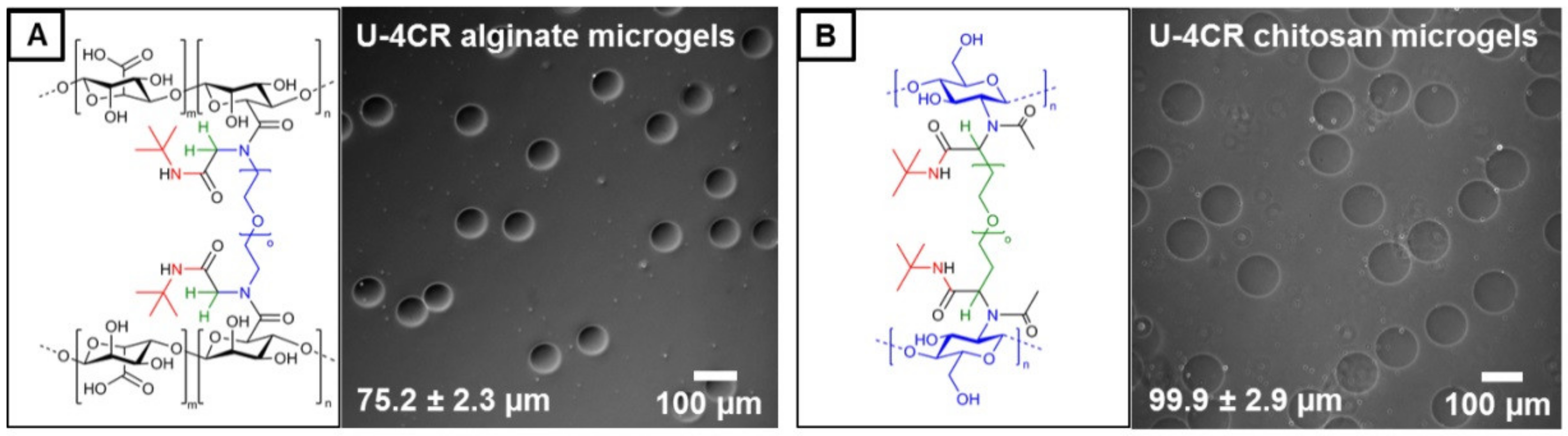
2.3.4. Alginate-Based U-4CR Microgels
2.3.5. Chitosan-Based U-4CR Microgels
2.3.6. Chitosan-Based, Biotin-Functionalized U-4CR Microgels
2.4. Immobilization and Activity Assay of Horseradish Peroxidase (HRP)
2.5. Colloidal Probe Atomic Force Microscopy (CP-AFM)
2.6. Confocal Brillouin Microscopy
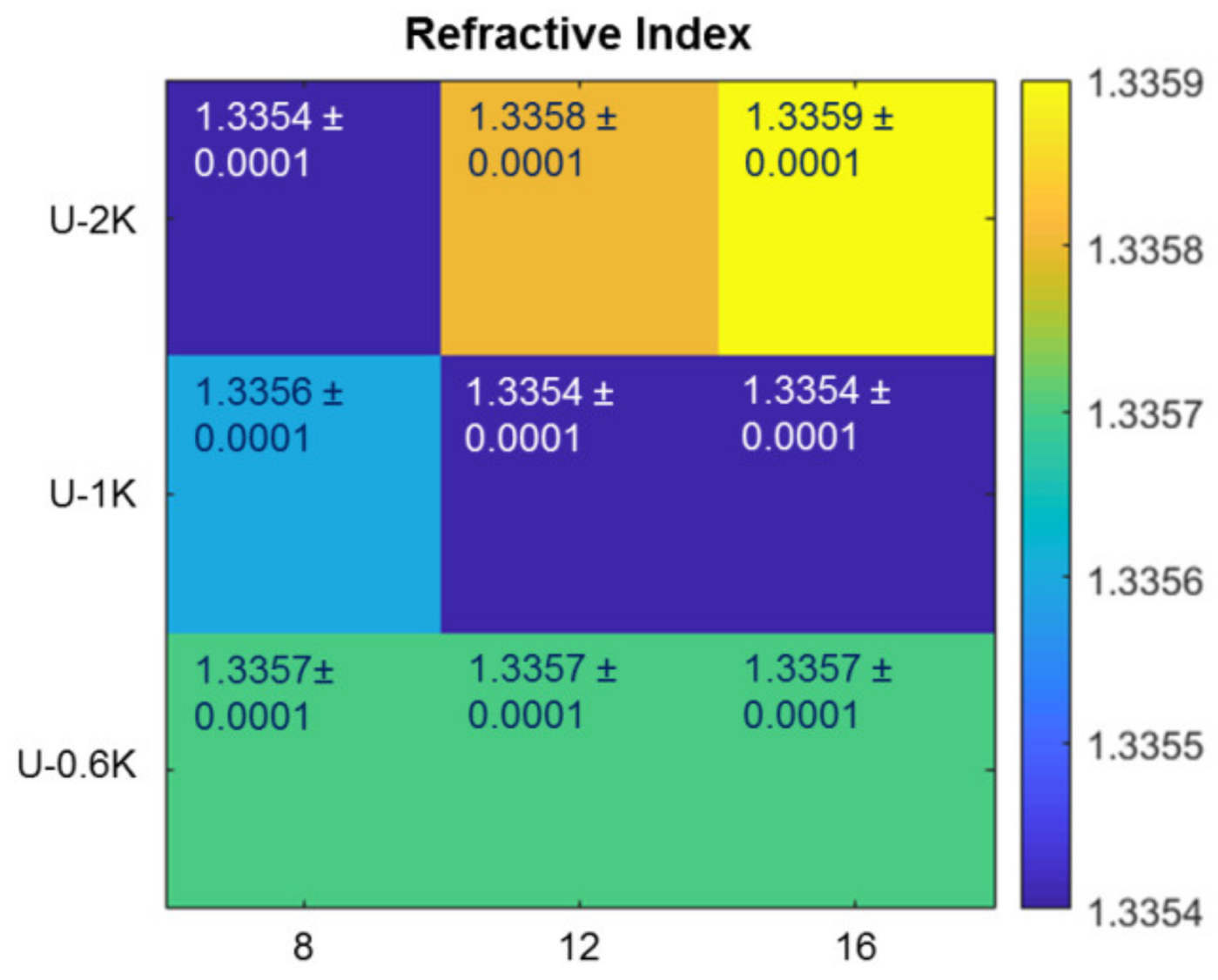
2.7. Refractive Index Measurement
2.8. Fluorescence Correlation Spectroscopy (FCS)
3. Results and Discussion
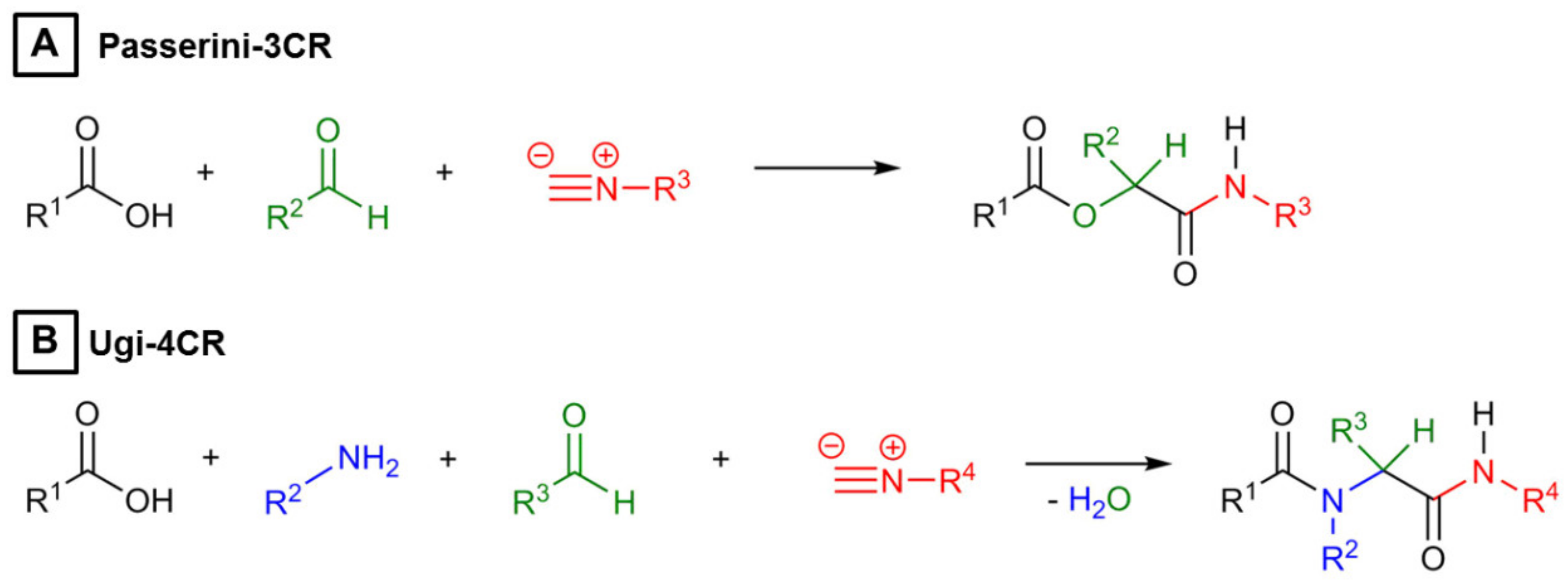
3.1. Microgel Preparation by Multicomponent Reactions via Droplet Microfluidics
3.2. In-Depth Analysis of Hyaluronic Acid-Based U-4CR Microgels
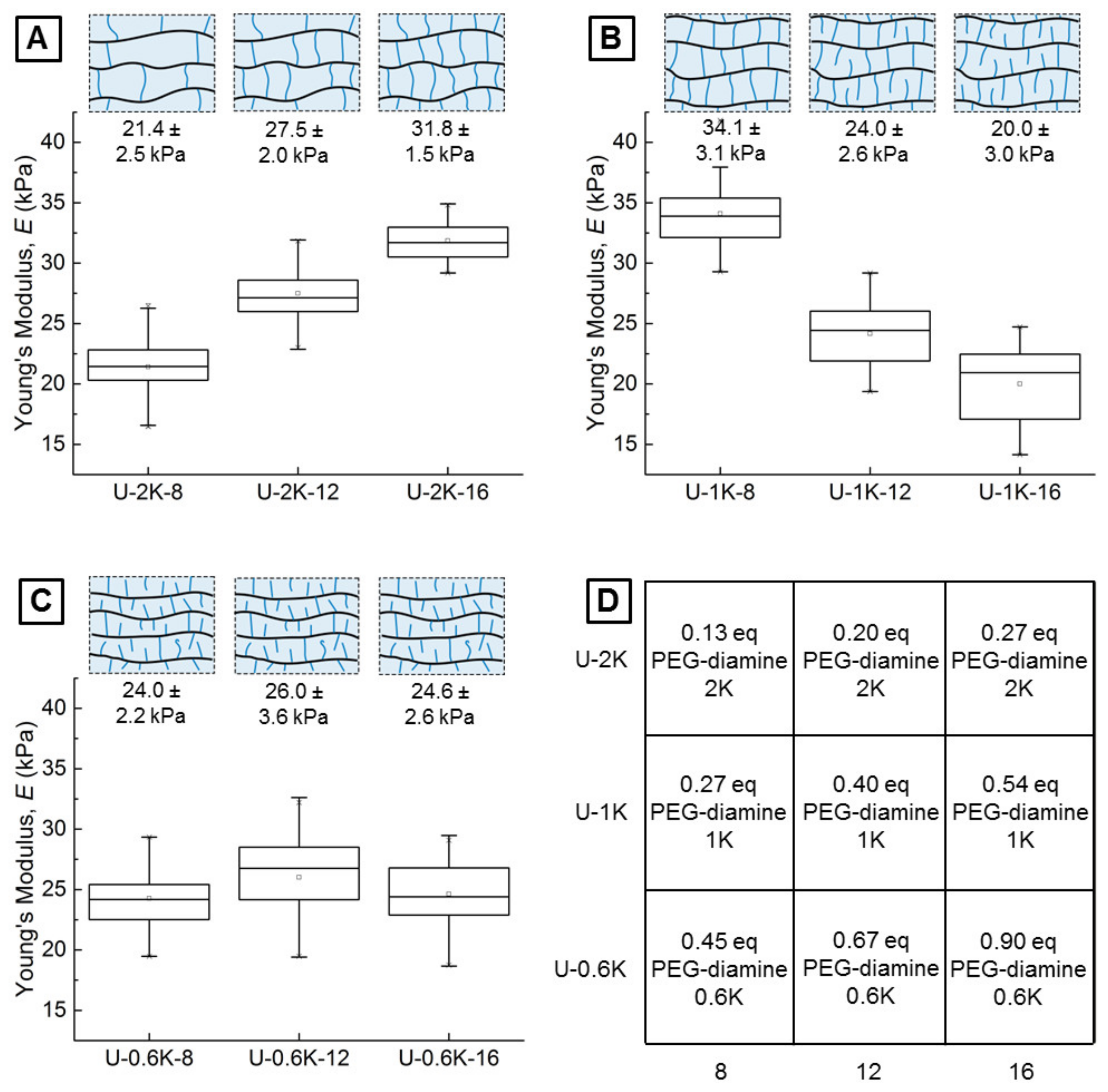
3.2.1. Screening Crosslinker Concentration and Molecular Weight in a Parameter Matrix
3.2.2. Young’s Moduli of Hyaluronic Acid-Based U-4CR Microgels Determined by CP-AFM
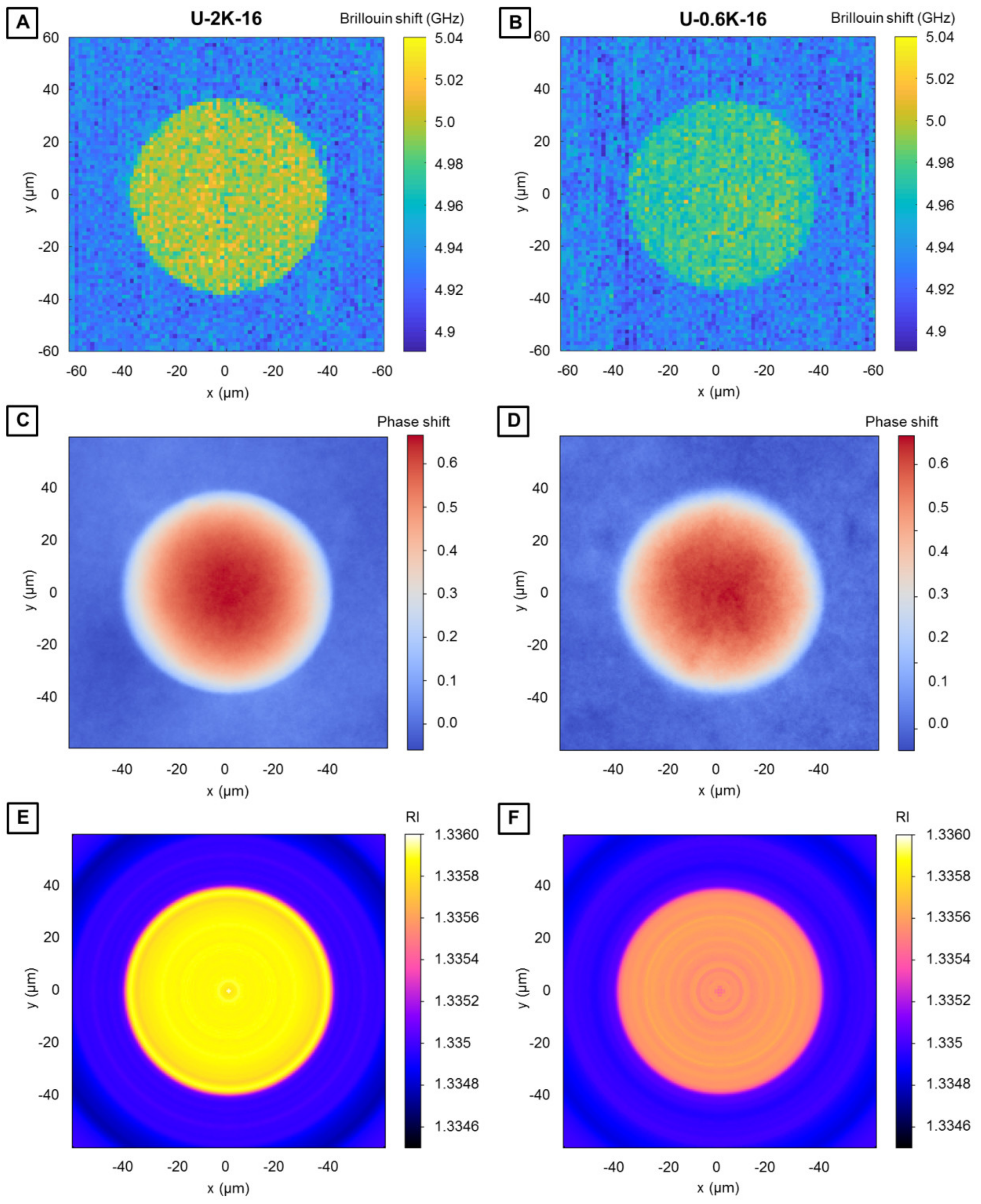
3.2.3. Analysis of Hyaluronic Acid-Based U-4CR Microgels by Optical Techniques
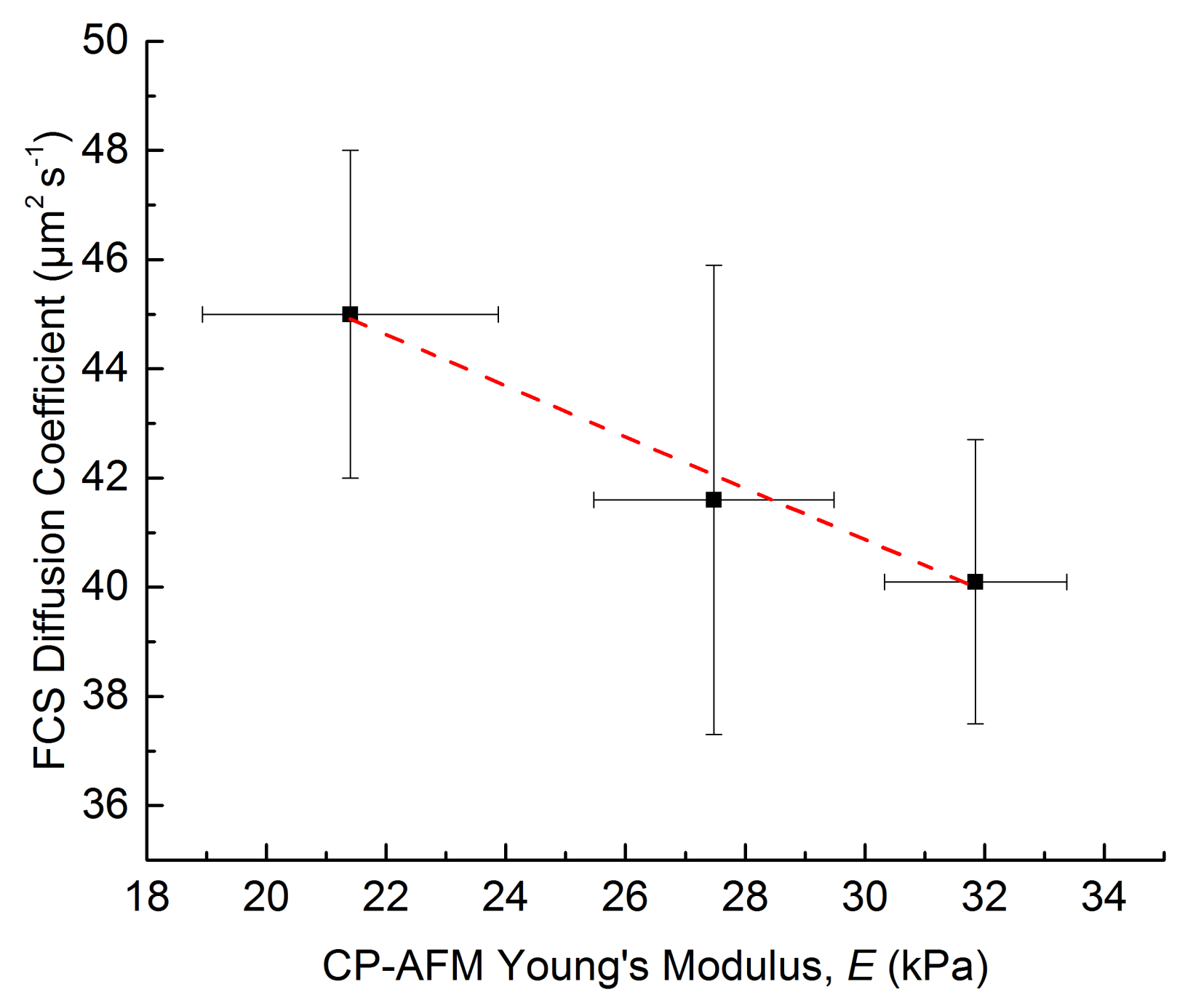
3.2.4. Diffusion Coefficients Determined by FCS and Conclusions on Microgel Network Structure
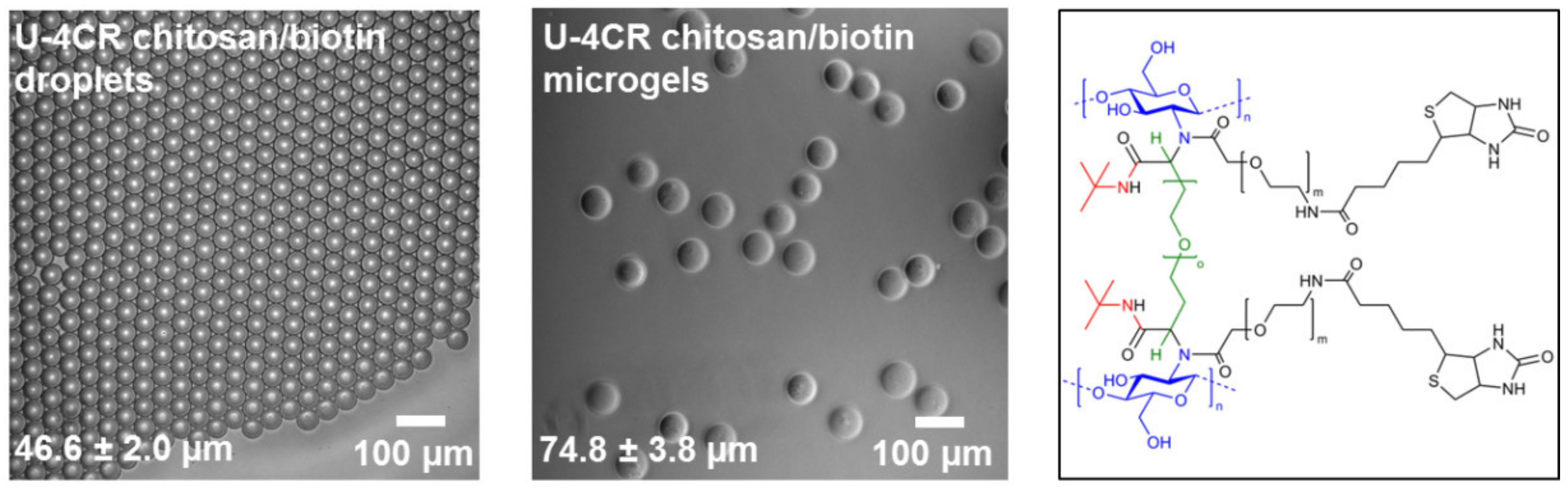
3.3. Introduction of Functionality in Chitosan-Based U-4CR Microgels
3.3.1. Biotin-Functionalized, Chitosan-Based U-4CR Microgels
3.3.2. Proof of Biotin Availability in Chitosan-Based Microgels by Streptavidin Conjugation
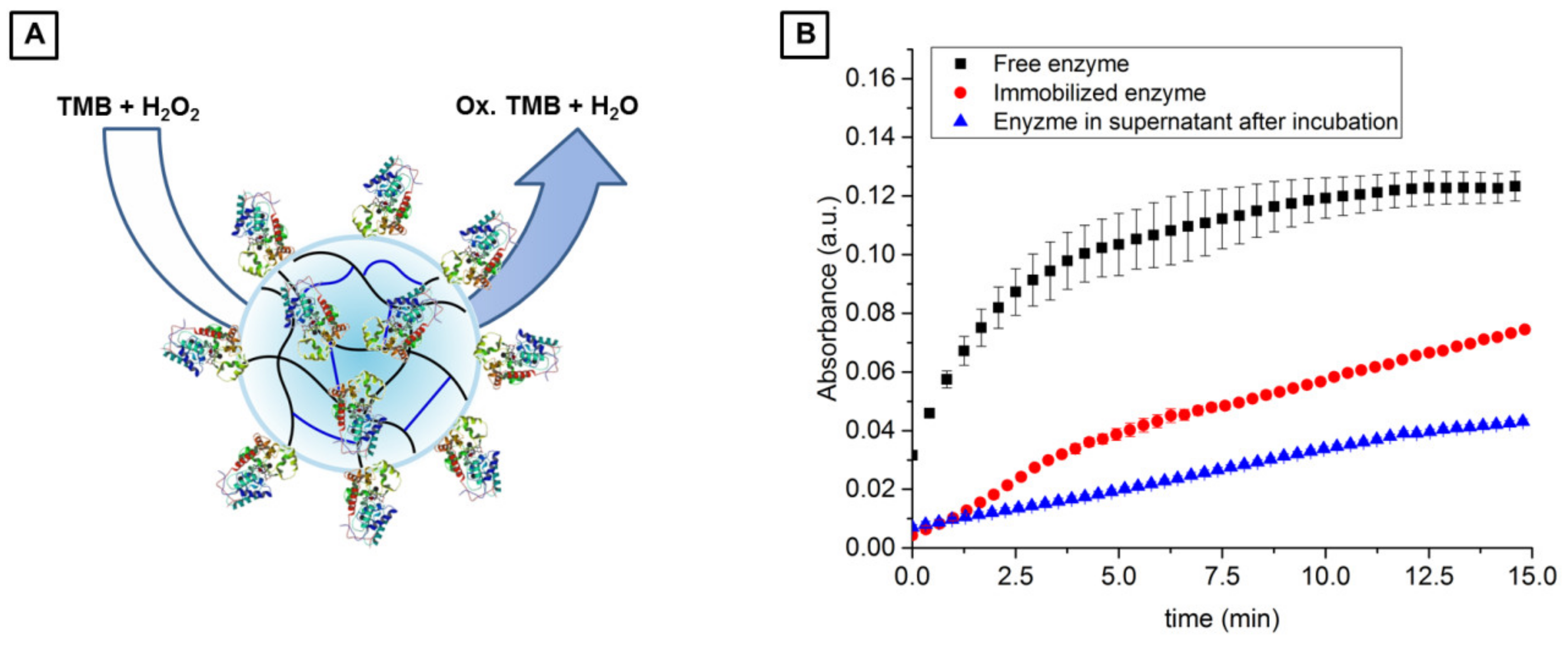
3.3.3. Application of Biotin-Functionalized, Chitosan-Based U-4CR Microgels for Enzyme Immobilization
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
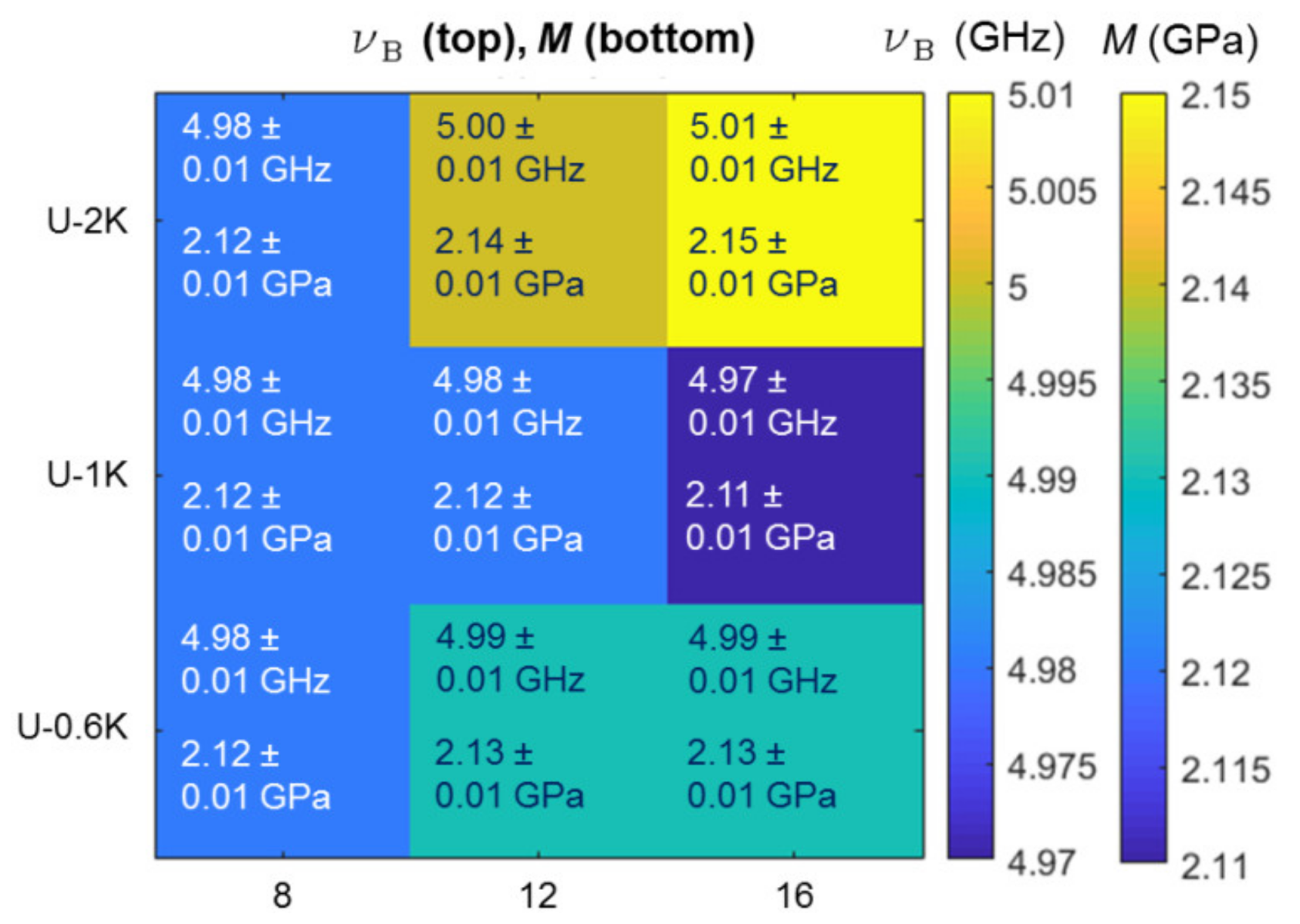
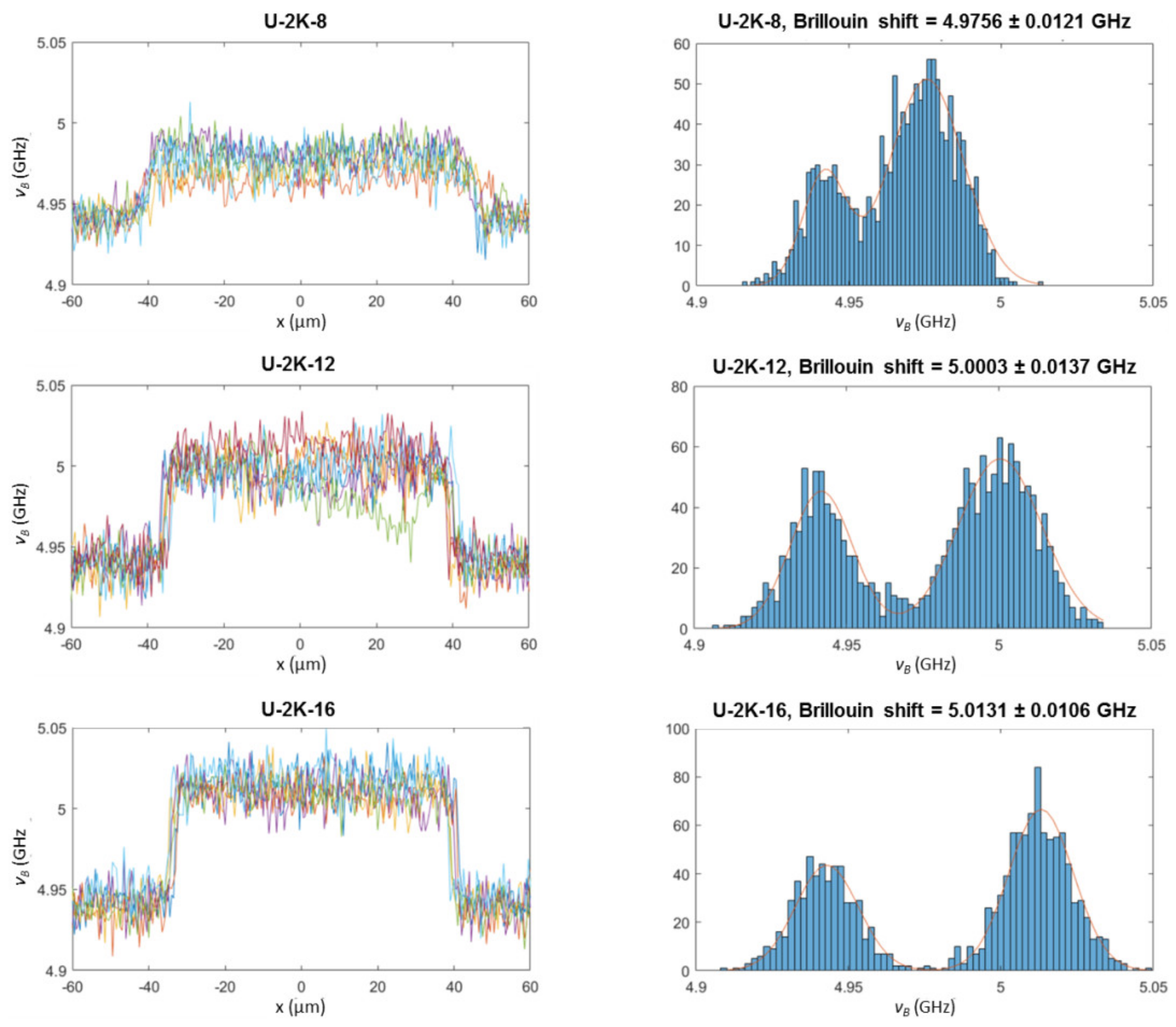
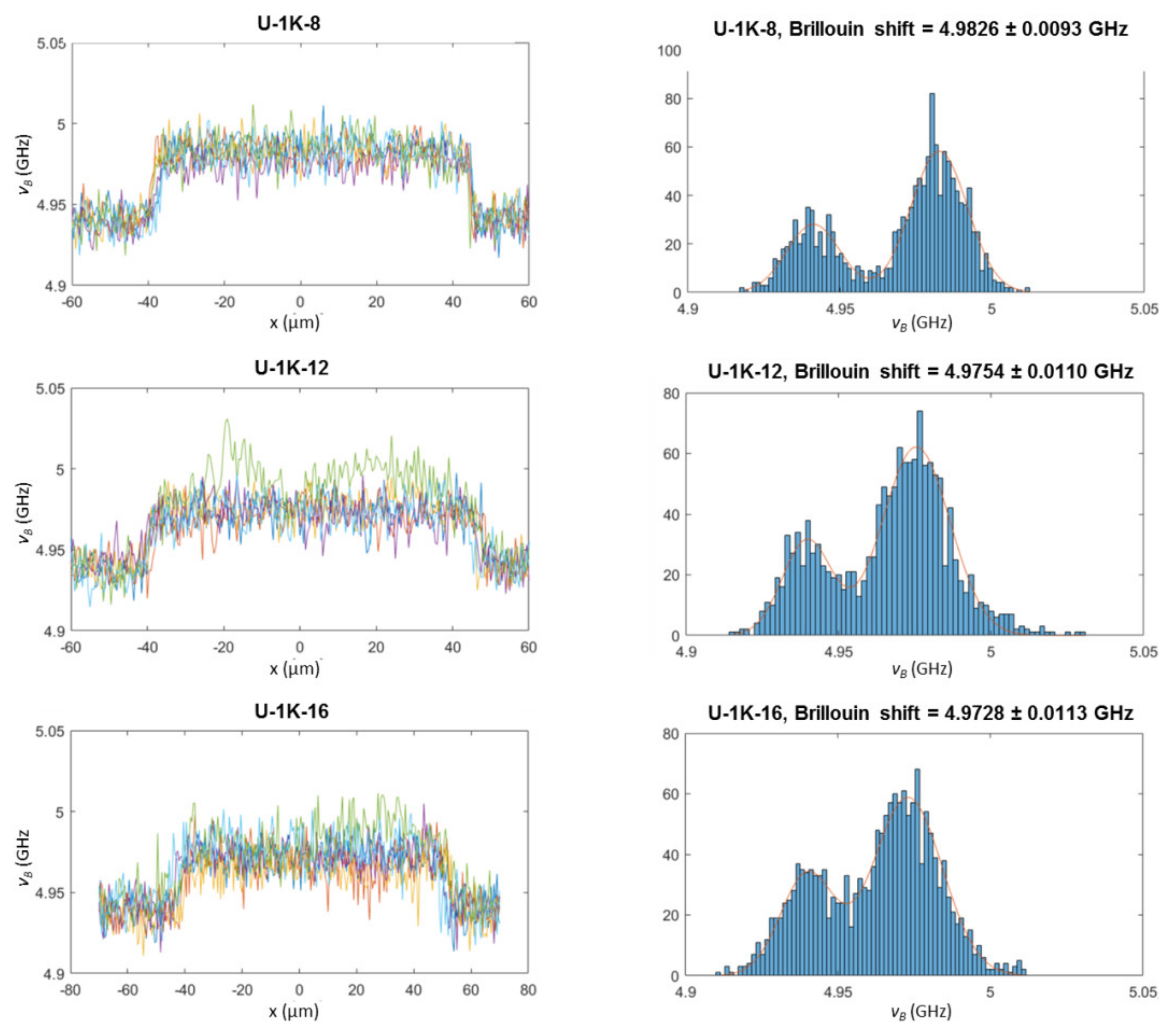
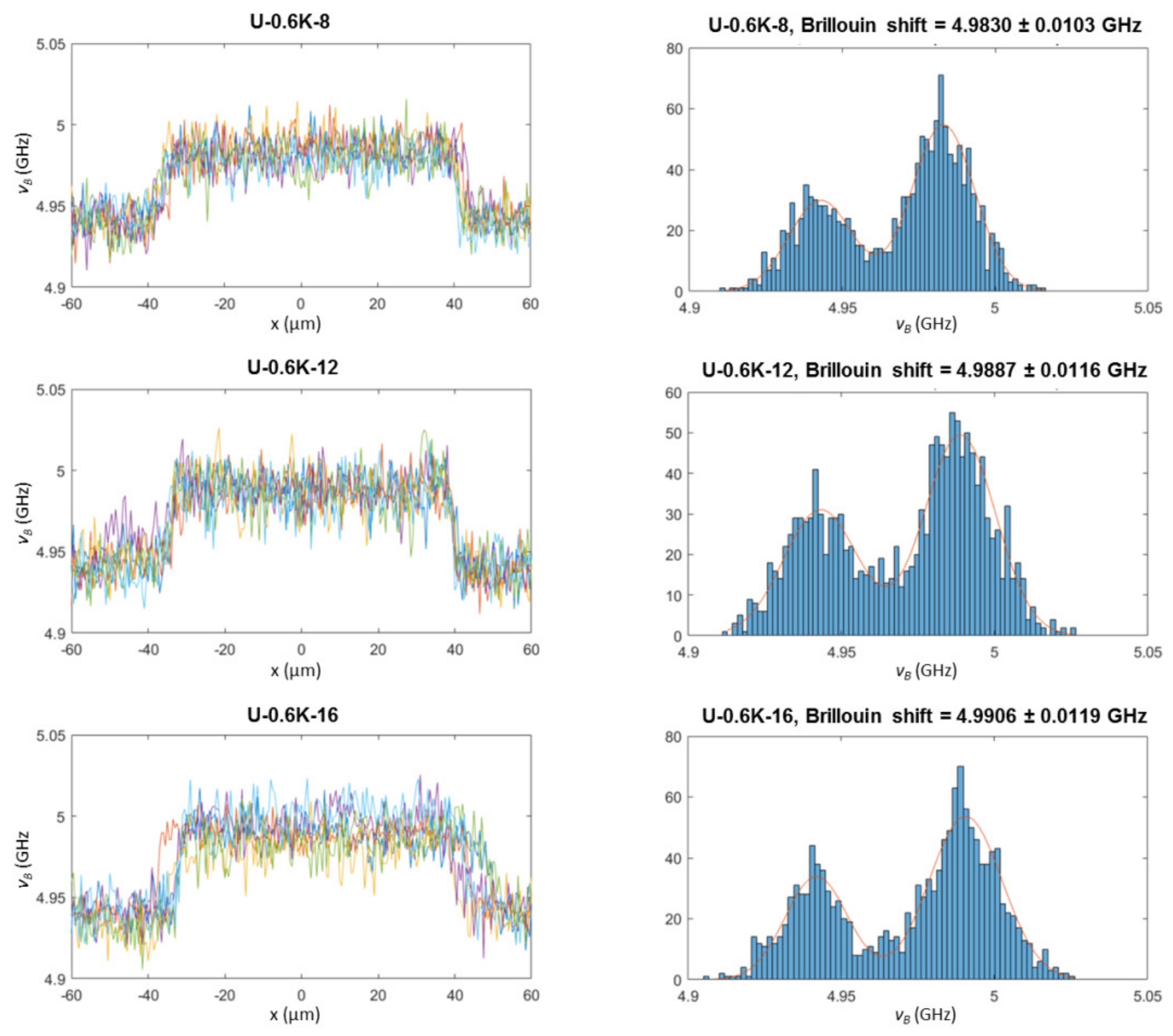
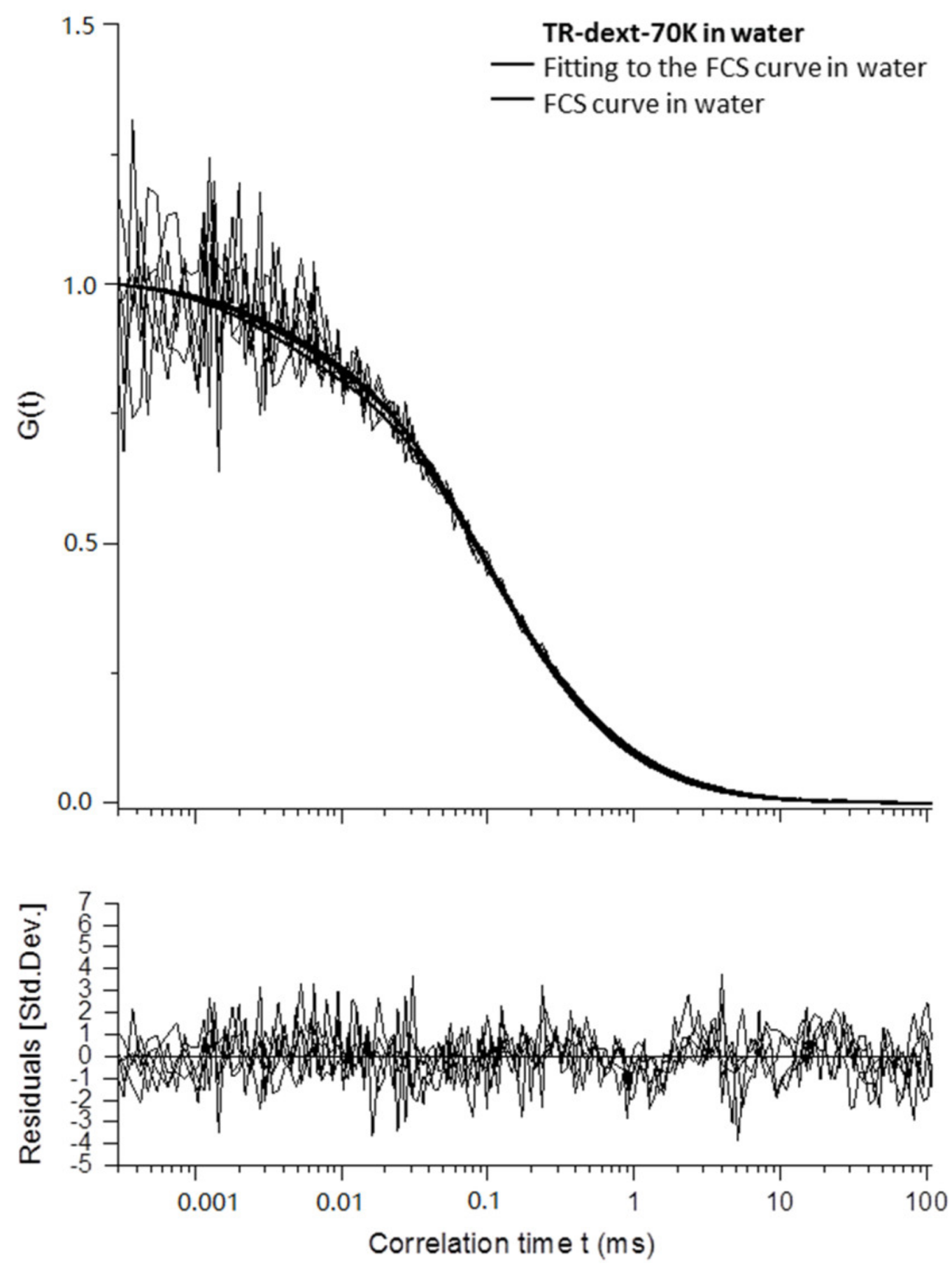
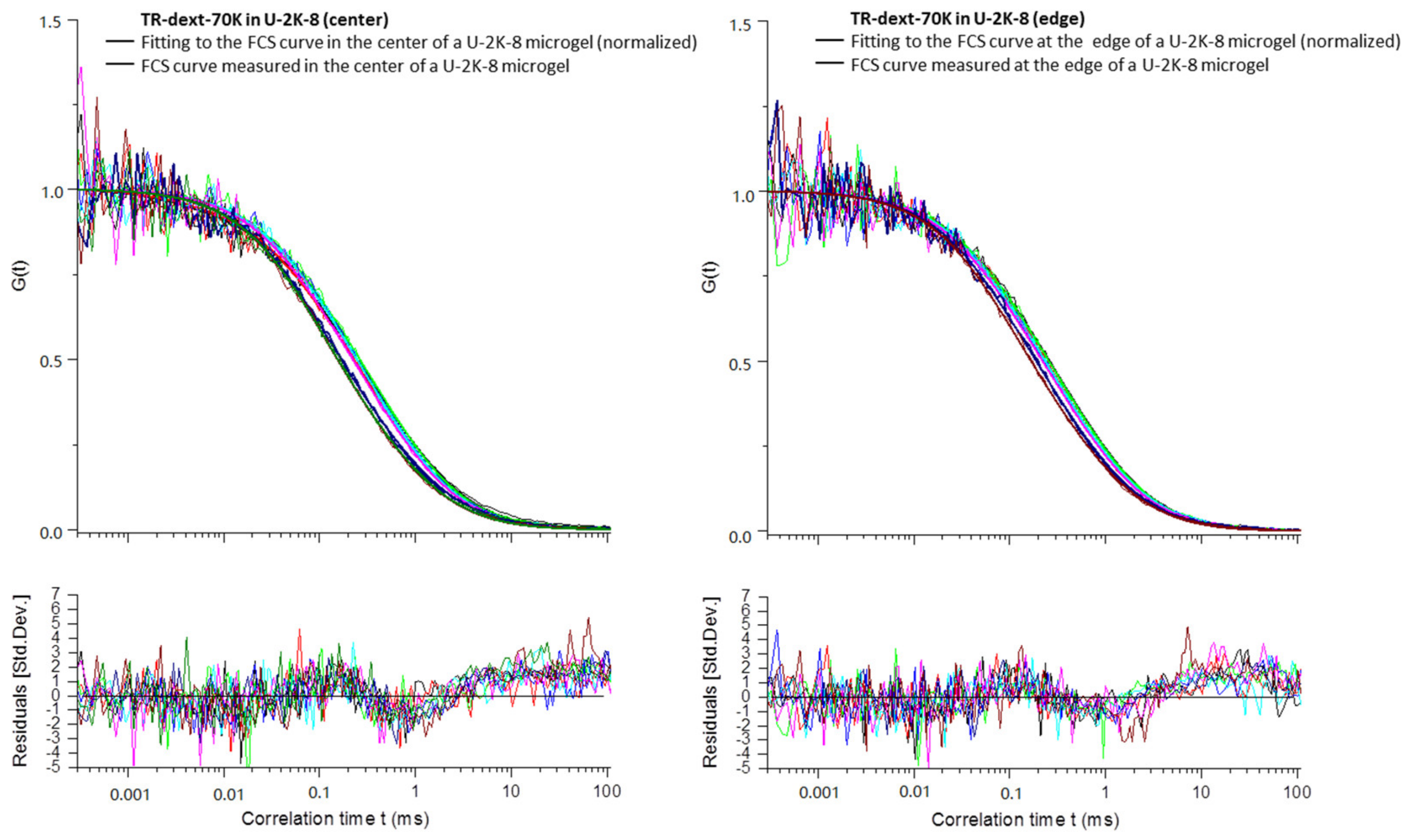
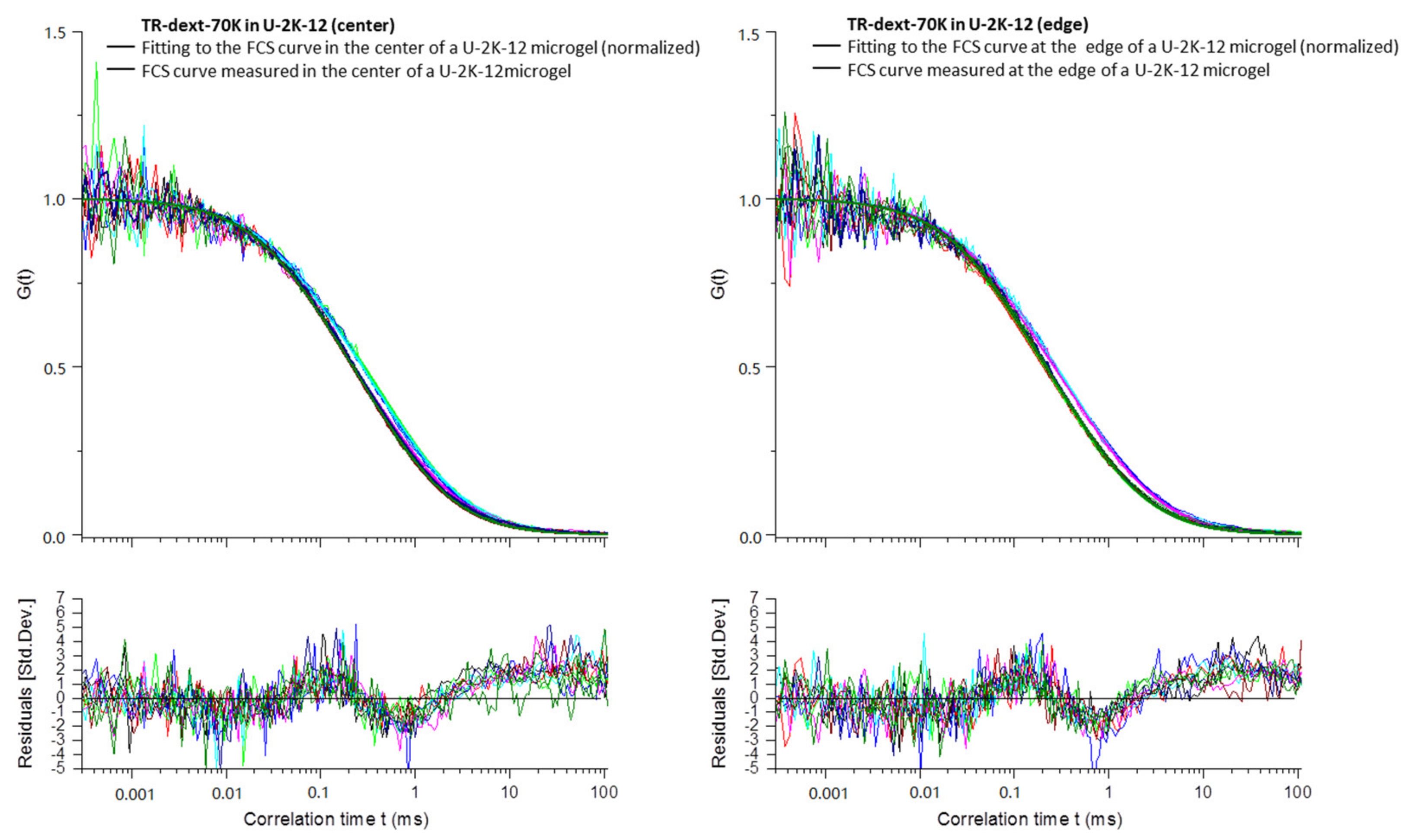
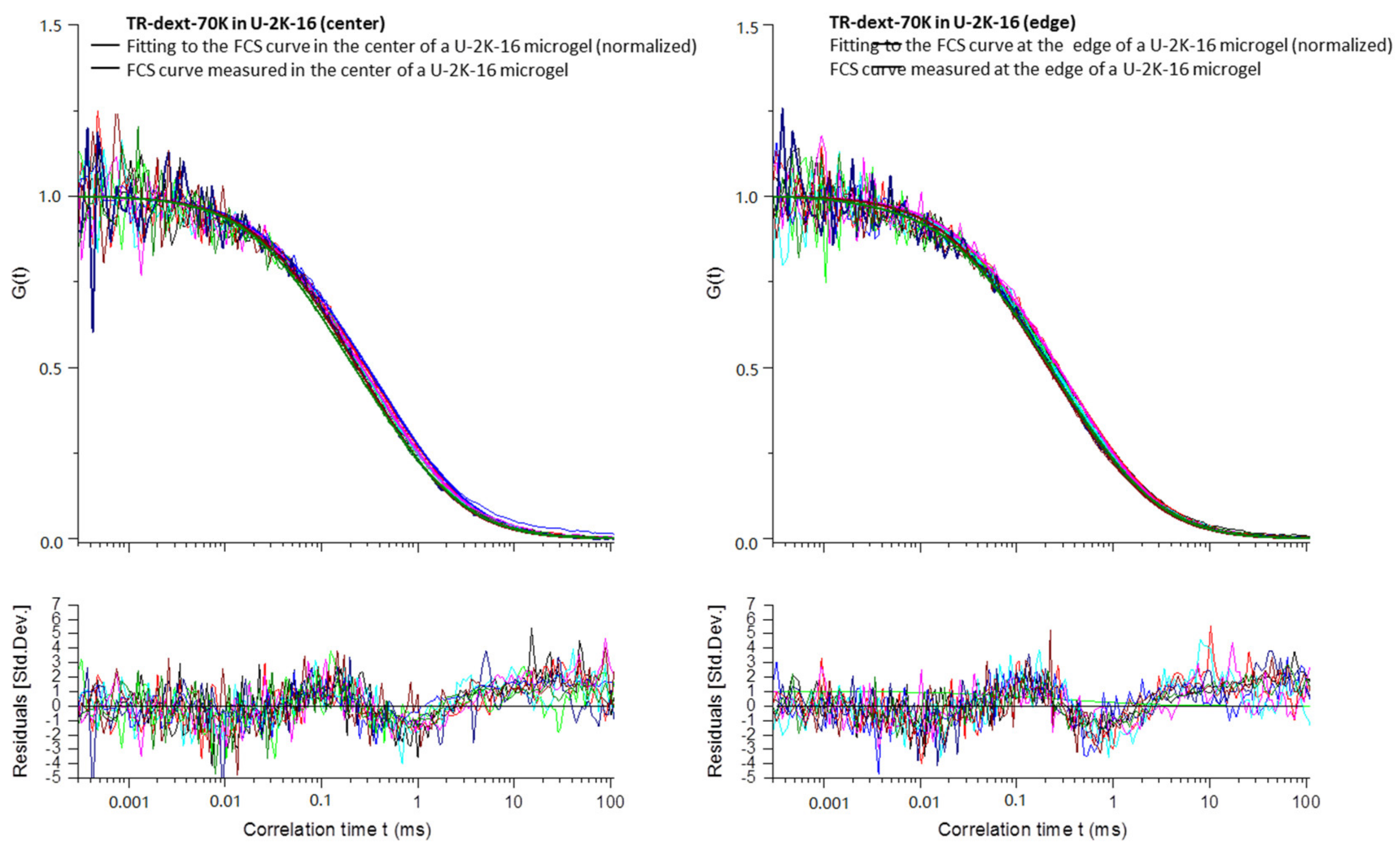
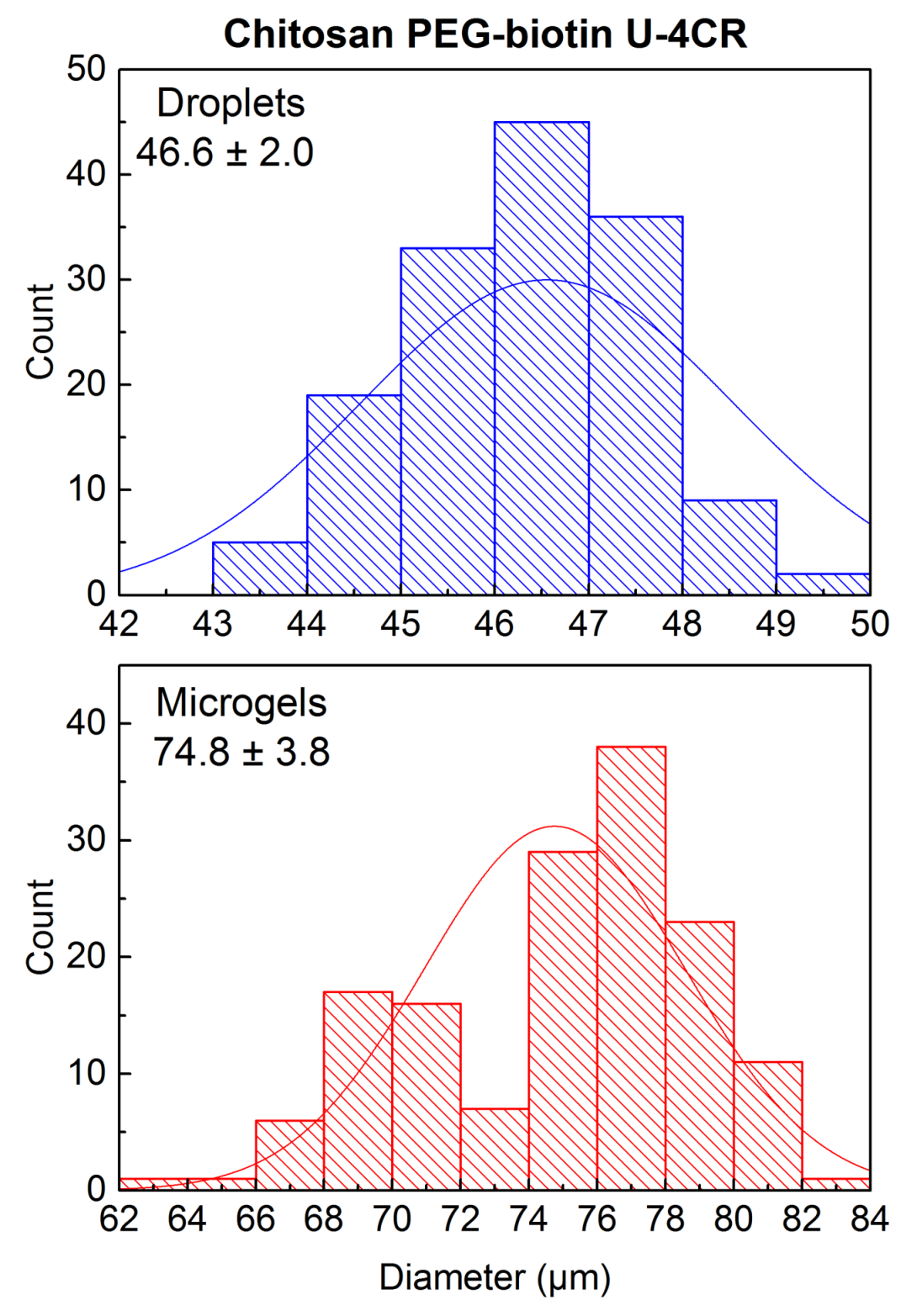
Appendix B
Calculation of Error Propagation of Size Increase during Microdroplet-to-Microgel Transition
References
- Alemán, J.; Chadwick, A.V.; He, J.; Hess, M.; Horie, K.; Jones, R.G.; Kratochvíl, P.; Meisel, I.; Mita, I.; Moad, G. Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials (iupac recommendations 2007). Pure Appl. Chem. 2007, 79, 1801–1829. [Google Scholar] [CrossRef]
- Walta, S.; Pergushov, D.V.; Oppermann, A.; Steinschulte, A.A.; Geisel, K.; Sigolaeva, L.V.; Plamper, F.A.; Wöll, D.; Richtering, W. Microgels enable capacious uptake and controlled release of architecturally complex macromolecular species. Polymer 2017, 119, 50–58. [Google Scholar] [CrossRef]
- Jiang, W.; Li, M.; Chen, Z.; Leong, K.W. Cell-laden microfluidic microgels for tissue regeneration. Lab Chip 2016, 16, 4482–4506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiele, J.; Ma, Y.; Foschepoth, D.; Hansen, M.M.; Steffen, C.; Heus, H.; Huck, W. DNA-functionalized hydrogels for confined membrane-free in vitro transcription/translation. Lab Chip 2014, 14, 2651–2656. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-H.; Lee, K.-Y.; Byun, J.-Y.; Kim, B.-G.; Kim, D.-M. On-bead expression of recombinant proteins in an agarose gel matrix coated on a glass slide. Lab Chip 2012, 12, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Krzek, M.; van Beek, H.L.; Permentier, H.P.; Bischoff, R.; Fraaije, M.W. Covalent immobilization of a flavoprotein monooxygenase via its flavin cofactor. Enzym. Microb. Technol. 2016, 82, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Kim, J.-C. Novel ph-sensitive microgels prepared using salt bridge. Int. J. Pharm. 2010, 388, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Neubauer, M.P.; Thiele, J.; Fery, A.; Huck, W. Artificial microniches for probing mesenchymal stem cell fate in 3d. Biomater. Sci. 2014, 2, 1661–1671. [Google Scholar] [CrossRef] [Green Version]
- Bray, L.J.; Binner, M.; Holzheu, A.; Friedrichs, J.; Freudenberg, U.; Hutmacher, D.W.; Werner, C. Multi-parametric hydrogels support 3d in vitro bioengineered microenvironment models of tumour angiogenesis. Biomaterials 2015, 53, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Crescenzi, V.; Cornelio, L.; Di Meo, C.; Nardecchia, S.; Lamanna, R. Novel hydrogels via click chemistry: Synthesis and potential biomedical applications. Biomacromolecules 2007, 8, 1844–1850. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Angeles, G.; Němcová, M.; Příkopová, E.; Šmejkalová, D.; Pravda, M.; Kučera, L.; Velebný, V. Reductive alkylation of hyaluronic acid for the synthesis of biocompatible hydrogels by click chemistry. Carbohydr. Polym. 2012, 90, 1704–1711. [Google Scholar] [CrossRef] [PubMed]
- Nimmo, C.M.; Owen, S.C.; Shoichet, M.S. Diels−alder click cross-linked hyaluronic acid hydrogels for tissue engineering. Biomacromolecules 2011, 12, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Gao, X.; Dong, H.; He, H.; Cao, X. High-throughput generation of hyaluronic acid microgels via microfluidics-assisted enzymatic crosslinking and/or diels–alder click chemistry for cell encapsulation and delivery. Appl. Mater. Today 2017, 9, 49–59. [Google Scholar] [CrossRef]
- Yu, Y.; Deng, C.; Meng, F.; Shi, Q.; Feijen, J.; Zhong, Z. Novel injectable biodegradable glycol chitosan-based hydrogels crosslinked by michael-type addition reaction with oligo (acryloyl carbonate)-b-poly (ethylene glycol)-b-oligo (acryloyl carbonate) copolymers. J. Biomed. Mater. Res. Part A 2011, 99, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Young, J.L.; Engler, A.J. Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials 2011, 32, 1002–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, T.; Baldwin, A.; Yamaguchi, N.; Kiick, K.L. Production of heparin-functionalized hydrogels for the development of responsive and controlled growth factor delivery systems. J. Control. Release 2007, 122, 287–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cumpstey, I. Chemical modification of polysaccharides. ISRN Organ. Chem. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Kirschning, A.; Dibbert, N.; Dräger, G. Chemical functionalization of polysaccharides—Towards biocompatible hydrogels for biomedical applications. Chem. A Eur. J. 2018, 24, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Vasco, A.V.; Pérez, C.S.; Morales, F.E.; Garay, H.E.; Vasilev, D.; Gavin, J.A.; Wessjohann, L.A.; Rivera, D.G. Macrocyclization of peptide side chains by the ugi reaction: Achieving peptide folding and exocyclic n-functionalization in one shot. J. Organ. Chem. 2015, 80, 6697–6707. [Google Scholar] [CrossRef] [PubMed]
- Sehlinger, A.; Meier, M.A. Passerini and ugi multicomponent reactions in polymer science. In Multi-Component and Sequential Reactions in Polymer Synthesis; Springer: Cham, Switzerland, 2014; pp. 61–86. [Google Scholar]
- Crescenzi, V.; Francescangeli, A.; Capitani, D.; Mannina, L.; Renier, D.; Bellini, D. Hyaluronan networking via ugi’s condensation using lysine as cross-linker diamine. Carbohydr. Polym. 2003, 53, 311–316. [Google Scholar] [CrossRef]
- Crescenzi, V.; Francescangeli, A.; Segre, A.L.; Capitani, D.; Mannina, L.; Renier, D.; Bellini, D. Nmr structural study of hydrogels based on partially deacetylated hyaluronan. Macromol. Biosci. 2002, 2, 272–279. [Google Scholar] [CrossRef]
- Bu, H.; Kjøniksen, A.-L.; Knudsen, K.D.; Nyström, B. Rheological and structural properties of aqueous alginate during gelation via the ugi multicomponent condensation reaction. Biomacromolecules 2004, 5, 1470–1479. [Google Scholar] [CrossRef] [PubMed]
- Bu, H.; Kjøniksen, A.-L.; Nyström, B. Effects of ph on dynamics and rheology during association and gelation via the ugi reaction of aqueous alginate. Eur. Polym. J. 2005, 41, 1708–1717. [Google Scholar] [CrossRef]
- de Nooy, A.E.; Masci, G.; Crescenzi, V. Versatile synthesis of polysaccharide hydrogels using the passerini and ugi multicomponent condensations. Macromolecules 1999, 32, 1318–1320. [Google Scholar] [CrossRef]
- Maleki, A.; Kjøniksen, A.-L.; Nyström, B. Characterization of the chemical degradation of hyaluronic acid during chemical gelation in the presence of different cross-linker agents. Carbohydr. Res. 2007, 342, 2776–2792. [Google Scholar] [CrossRef] [PubMed]
- de Nooy, A.E.; Capitani, D.; Masci, G.; Crescenzi, V. Ionic polysaccharide hydrogels via the passerini and ugi multicomponent condensations: Synthesis, behavior and solid-state nmr characterization. Biomacromolecules 2000, 1, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Shulepov, I.D.; Kozhikhova, K.V.; Panfilova, Y.S.; Ivantsova, M.N.; Mironov, M.A. One-pot synthesis of cross-linked sub-micron microgels from pure cellulose via the ugi reaction and their application as emulsifiers. Cellulose 2016, 23, 2549–2559. [Google Scholar] [CrossRef]
- Mironov, M.A.; Shulepov, I.D.; Ponomarev, V.S.; Bakulev, V.A. Synthesis of polyampholyte microgels from colloidal salts of pectinic acid and their application as ph-responsive emulsifiers. Colloid Polym. Sci. 2013, 291, 1683–1691. [Google Scholar] [CrossRef]
- Heida, T.; Neubauer, J.W.; Seuss, M.; Hauck, N.; Thiele, J.; Fery, A. Mechanically defined microgels by droplet microfluidics. Macromol. Chem. Phys. 2017, 218, 1600418. [Google Scholar] [CrossRef]
- Holtze, C.; Rowat, A.; Agresti, J.; Hutchison, J.; Angile, F.; Schmitz, C.; Köster, S.; Duan, H.; Humphry, K.; Scanga, R. Biocompatible surfactants for water-in-fluorocarbon emulsions. Lab Chip 2008, 8, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- Frey, A.; Meckelein, B.; Externest, D.; Schmidt, M.A. A stable and highly sensitive 3,3′,5,5′-tetramethylbenzidine-based substrate reagent for enzyme-linked immunosorbent assays. J. Immunol. Methods 2000, 233, 47–56. [Google Scholar] [CrossRef]
- Hutter, J.L.; Bechhoefer, J. Calibration of atomic-force microscope tips. Rev. Sci. Instrum. 1993, 64, 1868–1873. [Google Scholar] [CrossRef]
- Seuss, M.; Schmolke, W.; Drechsler, A.; Fery, A.; Seiffert, S. Core–shell microgels with switchable elasticity at constant interfacial interaction. ACS Appl. Mater. Interfaces 2016, 8, 16317–16327. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.L.; Kendall, K.; Roberts, A.D. Surface energy and the contact of elastic solids. Proc. R. Soc. Lond. A 1971, 324, 301–313. [Google Scholar] [CrossRef]
- Schlüßler, R.; Möllmert, S.; Abuhattum, S.; Cojoc, G.; Müller, P.; Kim, K.; Möckel, C.; Zimmermann, C.; Czarske, J.; Guck, J. Mechanical mapping of spinal cord growth and repair in living zebrafish larvae by brillouin imaging. Biophys. J. 2018, 115, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Scarcelli, G.; Yun, S.H. Confocal brillouin microscopy for three-dimensional mechanical imaging. Nat. Photonics 2007, 2, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Scarcelli, G.; Polacheck, W.J.; Nia, H.T.; Patel, K.; Grodzinsky, A.J.; Kamm, R.D.; Yun, S.H. Noncontact three-dimensional mapping of intracellular hydromechanical properties by brillouin microscopy. Nat. Methods 2015, 12, 1132–1134. [Google Scholar] [CrossRef] [PubMed]
- Schürmann, M.; Cojoc, G.; Girardo, S.; Ulbricht, E.; Guck, J.; Müller, P. Three-dimensional correlative single-cell imaging utilizing fluorescence and refractive index tomography. J. Biophotonics 2018, 11, e201700145. [Google Scholar] [CrossRef] [PubMed]
- Schürmann, M.; Scholze, J.; Müller, P.; Guck, J.; Chan, C.J. Cell nuclei have lower refractive index and mass density than cytoplasm. J. Biophotonics 2016, 9, 1068–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, P.; Schürmann, M.; Guck, J. Odtbrain: A python library for full-view, dense diffraction tomography. BMC Bioinform. 2015, 16, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, C.M.; Košovan, P.; Richtering, W.; Wöll, D. Polymers in focus: Fluorescence correlation spectroscopy. Colloid Polym. Sci. 2014, 292, 2399–2411. [Google Scholar] [CrossRef]
- Lehmann, S.; Seiffert, S.; Richtering, W. Spatially resolved tracer diffusion in complex responsive hydrogels. J. Am. Chem. Soc. 2012, 134, 15963–15969. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Loman, A.; Pacheco, V.; Koberling, F.; Willbold, D.; Richtering, W.; Enderlein, J. Precise measurement of diffusion by multi-color dual-focus fluorescence correlation spectroscopy. EPL (Europhys. Lett.) 2008, 83, 46001. [Google Scholar] [CrossRef]
- Widengren, J.; Rigler, R.; Mets, Ü. Triplet-state monitoring by fluorescence correlation spectroscopy. J. Fluoresc. 1994, 4, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Abate, A.R.; Thiele, J.; Weitz, D.A. One-step formation of multiple emulsions in microfluidics. Lab Chip 2011, 11, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Ducker, W.A.; Senden, T.J.; Pashley, R.M. Direct measurement of colloidal forces using an atomic force microscope. Nature 1991, 353, 239–241. [Google Scholar] [CrossRef]
- Jeon, O.; Song, S.J.; Lee, K.-J.; Park, M.H.; Lee, S.-H.; Hahn, S.K.; Kim, S.; Kim, B.-S. Mechanical properties and degradation behaviors of hyaluronic acid hydrogels cross-linked at various cross-linking densities. Carbohydr. Polym. 2007, 70, 251–257. [Google Scholar] [CrossRef]
- Yazici, I.; Okay, O. Spatial inhomogeneity in poly (acrylic acid) hydrogels. Polymer 2005, 46, 2595–2602. [Google Scholar] [CrossRef]
- Girardo, S.; Traeber, N.; Wagner, K.; Cojoc, G.; Herold, C.; Goswami, R.; Schluessler, R.; Abuhattum, S.; Taubenberger, A.; Reichel, F. Standardized microgel beads as elastic cell mechanical probes. J. Mater. Chem. B 2018. [Google Scholar] [CrossRef]
- Zhang, Z.; Nadezhina, E.; Wilkinson, K.J. Quantifying diffusion in a biofilm of streptococcus mutans. Antimicro. Agents Chemother. 2011, 55, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Green, N.M. [5] avidin and streptavidin. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1990; Volume 184, pp. 51–67. [Google Scholar]
- Veitch, N.C. Horseradish peroxidase: A modern view of a classic enzyme. Phytochemistry 2004, 65, 249–259. [Google Scholar] [CrossRef] [PubMed]

| Sample | PEG-Diamine (MW: 2000 g mol−1) | Formaldehyde 37% |
|---|---|---|
| U-2K-8 | 8 mg; 0.13 eq | 0.9 µL; 0.4 eq |
| U-2K-12 | 12 mg; 0.20 eq | 1.4 µL; 0.6 eq |
| U-2K-16 | 16 mg; 0.27 eq | 1.8 µL; 0.81 eq |
| Sample | PEG-Diamine (MW: 1000 g mol−1) | Formaldehyde 37% |
|---|---|---|
| U-1K-8 | 8 mg; 0.27 eq | 1.8 µL; 0.81 eq |
| U-1K-12 | 12 mg; 0.40 eq | 2.7 µL; 1.21 eq |
| U-1K-16 | 16 mg; 0.54 eq | 3.6 µL; 1.61 eq |
| Sample | PEG-Diamine (MW: 600 g mol−1) | Formaldehyde 37% |
|---|---|---|
| U-600-8 | 8 mg; 0.45 eq | 3.0 µL; 1,34 eq |
| U-600-12 | 12 mg; 0.67 eq | 4.5 µL; 2.02 eq |
| U-600-16 | 16 mg; 0.90 eq | 6.0 µL; 2.69 eq |
| Crosslinker Length | 8 mg | Crosslinker Amount 12 mg | 16 mg |
|---|---|---|---|
| 2000 g mol−1 | U-2K-8 | U-2K-12 | U-2K-16 |
| 1000 g mol−1 | U-1K-8 | U-1K-12 | U-1K-16 |
| 600 g mol−1 | U-0.6K-8 | U-0.6K-12 | U-0.6K-16 |
| Sample | Droplet Diameter | Microgel Diameter | Size Increase a |
|---|---|---|---|
| U-2K-8 | 44.5 ± 1.2 µm | 89.2 ± 4.2 µm | 2.0 ± 0.1 |
| U-2K-12 | 42.6 ± 1.2 µm | 72.9 ± 2.1 µm | 1.7 ± 0.1 |
| U-2K-16 | 40.6 ± 1.3 µm | 74.7 ± 1.8 µm | 1.8 ± 0.1 |
| U-1K-8 | 44.7 ± 2.7 µm | 79.7 ± 2.7 µm | 1.8 ± 0.1 |
| U-1K-12 | 44.9 ± 1.1 µm | 83.9 ± 2.1 µm | 1.9 ± 0.1 |
| U-1K-16 | 46.3 ± 1.1 µm | 90.8 ± 1.9 µm | 2.0 ± 0.1 |
| U-0.6K-8 | 47.5 ± 1.9 µm | 78.7 ± 4.1 µm | 1.7 ± 0.1 |
| U-0.6K-12 | 45.7 ± 1.0 µm | 75.7 ± 2.5 µm | 1.7 ± 0.1 |
| U-0.6K-16 | 44.0 ± 1.0 µm | 75.2 ± 4.1 µm | 1.7 ± 0.1 |
| Exp.a | Type | D1 (µm2 s−1) | D2 (µm2 s−1) | D1/D1sol | D2/D2sol | t1 (ms) | t2 (ms) |
|---|---|---|---|---|---|---|---|
| Mean | U-2K-8 | 45.0 ± 3.0 | 363 c | 0.85 | 1.00 | 0.66 ± 0.04 | 0.082 c |
| Mean | U-2K-12 | 41.6 ± 4.3 | 363 c | 0.78 | 1.00 | 0.73 ± 0.08 | 0.082 c |
| Mean | U-2K-16 | 40.1 ± 2.6 | 363 c | 0.76 | 1.00 | 0.75 ± 0.05 | 0.082 c |
| Mean | solution | 53.0 ± 12.0 | 366 ± 24 | - | - | 0.58 ± 0.16 | 0.082 c |
| Lit.b | solution | 37.0 ± 6.6 | 412 ± 18 | - | - | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hauck, N.; Seixas, N.; Centeno, S.P.; Schlüßler, R.; Cojoc, G.; Müller, P.; Guck, J.; Wöll, D.; Wessjohann, L.A.; Thiele, J. Droplet-Assisted Microfluidic Fabrication and Characterization of Multifunctional Polysaccharide Microgels Formed by Multicomponent Reactions. Polymers 2018, 10, 1055. https://doi.org/10.3390/polym10101055
Hauck N, Seixas N, Centeno SP, Schlüßler R, Cojoc G, Müller P, Guck J, Wöll D, Wessjohann LA, Thiele J. Droplet-Assisted Microfluidic Fabrication and Characterization of Multifunctional Polysaccharide Microgels Formed by Multicomponent Reactions. Polymers. 2018; 10(10):1055. https://doi.org/10.3390/polym10101055
Chicago/Turabian StyleHauck, Nicolas, Nalin Seixas, Silvia P. Centeno, Raimund Schlüßler, Gheorghe Cojoc, Paul Müller, Jochen Guck, Dominik Wöll, Ludger A. Wessjohann, and Julian Thiele. 2018. "Droplet-Assisted Microfluidic Fabrication and Characterization of Multifunctional Polysaccharide Microgels Formed by Multicomponent Reactions" Polymers 10, no. 10: 1055. https://doi.org/10.3390/polym10101055
APA StyleHauck, N., Seixas, N., Centeno, S. P., Schlüßler, R., Cojoc, G., Müller, P., Guck, J., Wöll, D., Wessjohann, L. A., & Thiele, J. (2018). Droplet-Assisted Microfluidic Fabrication and Characterization of Multifunctional Polysaccharide Microgels Formed by Multicomponent Reactions. Polymers, 10(10), 1055. https://doi.org/10.3390/polym10101055







