Quartz Microcrystal-Hybridized Organosilicone Encapsulant with Enhanced Optical and Thermal Performances
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
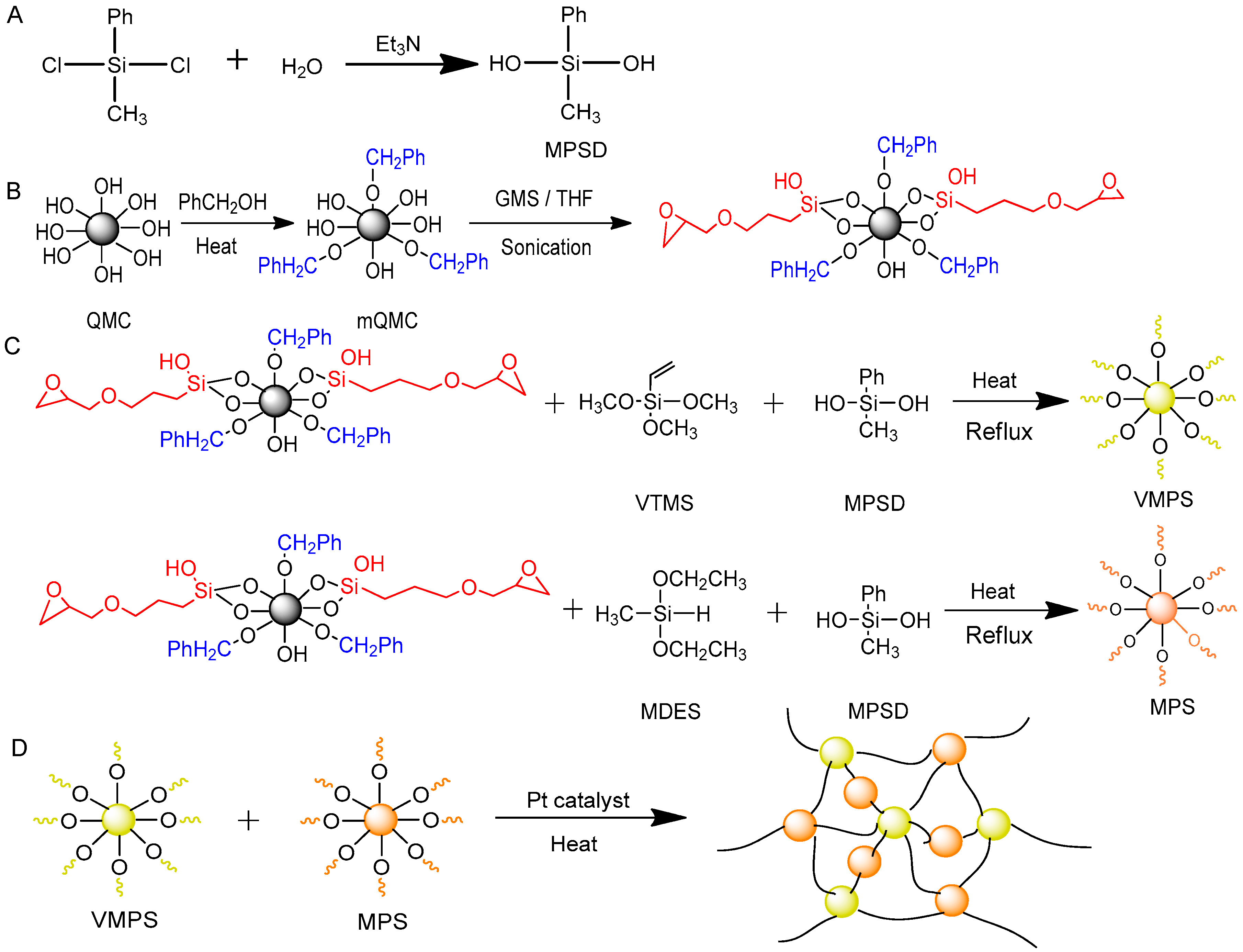
2.2. Synthesis of MPSD
2.3. Modification of Quartz Microcrystal
2.4. Synthesis of Vinylmethylphenylsiloxane Resin (VMPS)
2.5. Synthesis of Methylphenylsiloxane Resin (MPS)
2.6. Fabrication of Quartz−Organosilicone Composite
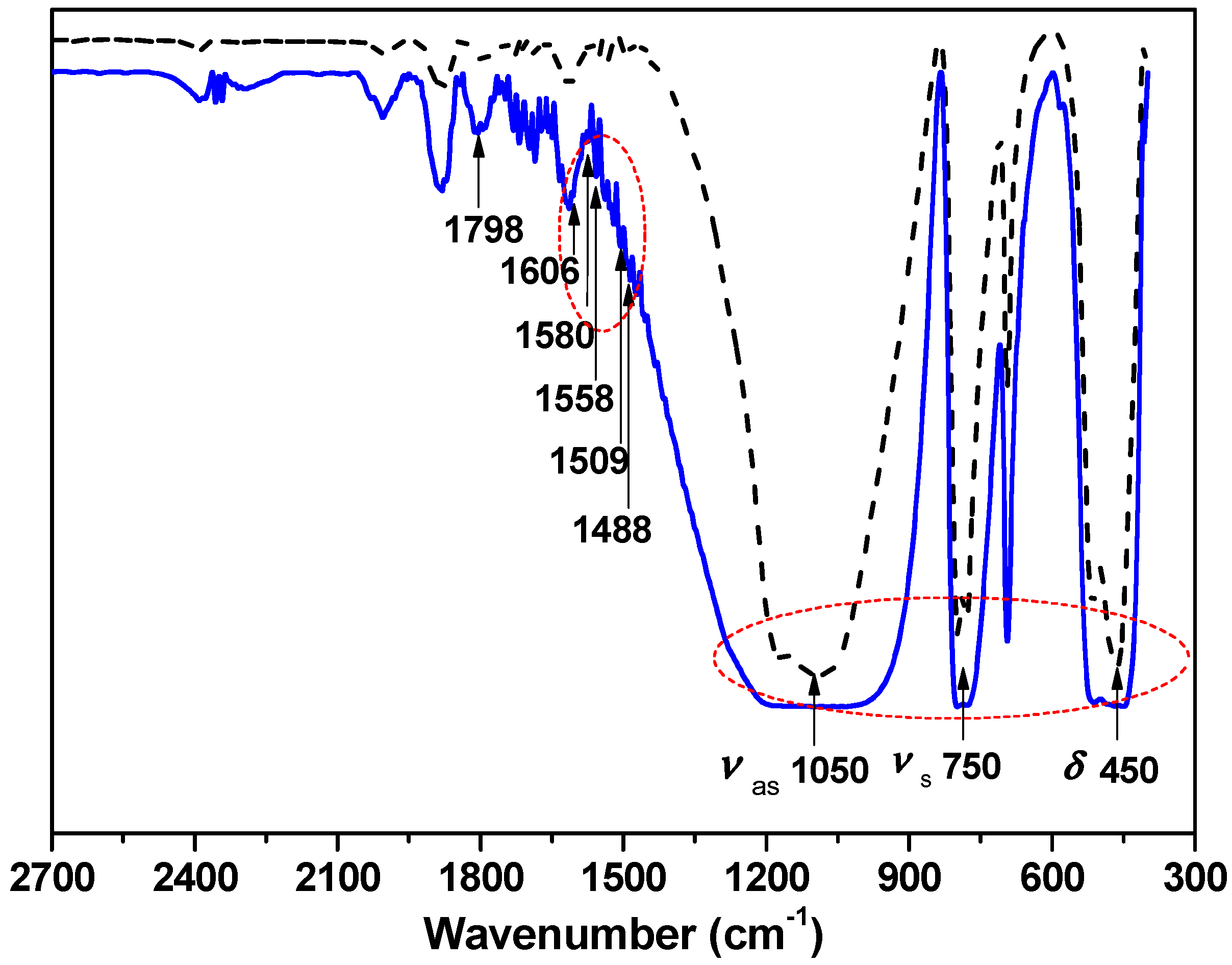
2.7. Chemical Characterizations
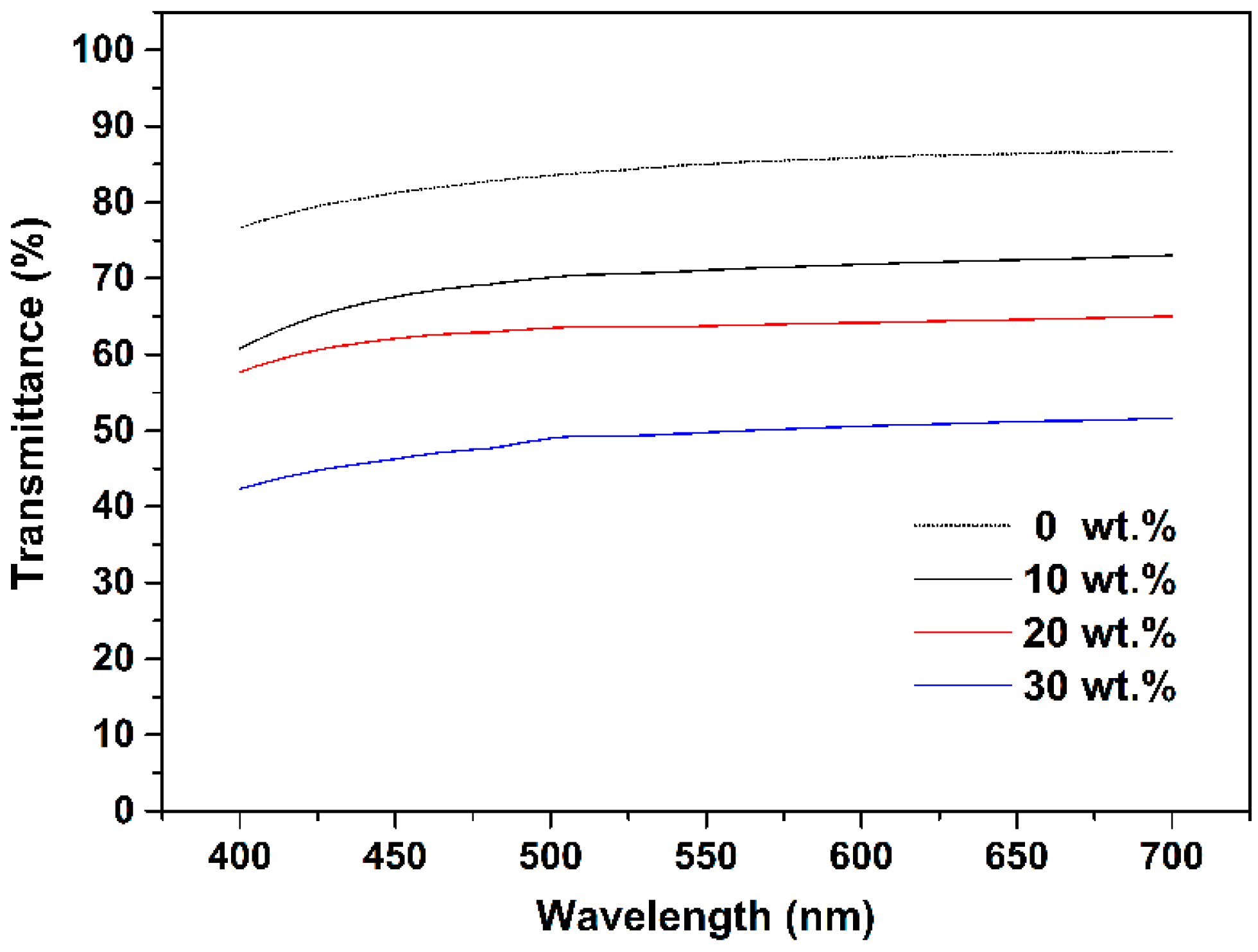
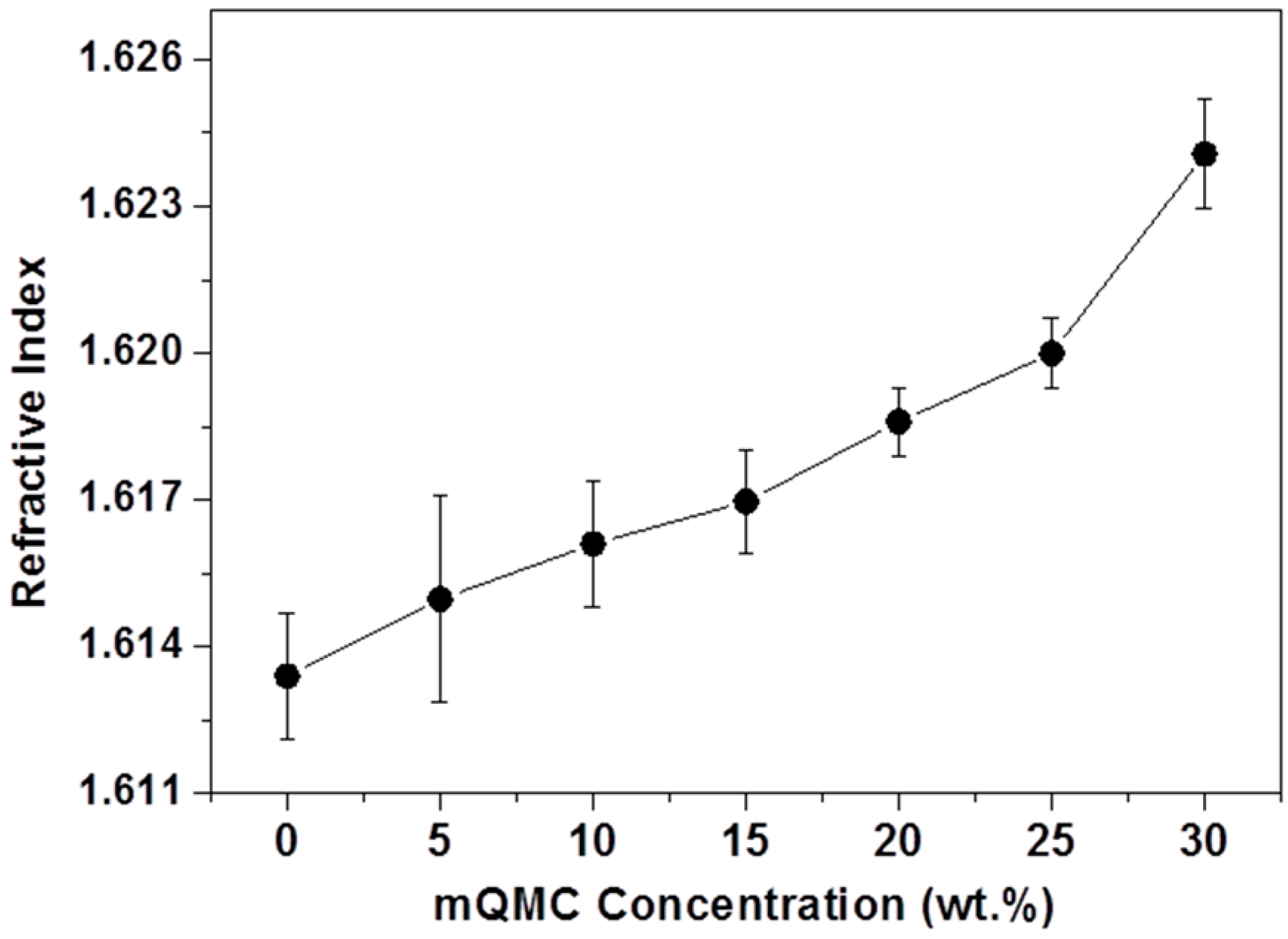
2.8. Optical Measurements
2.9. Thermal Analysis
2.10. Mechanical Test
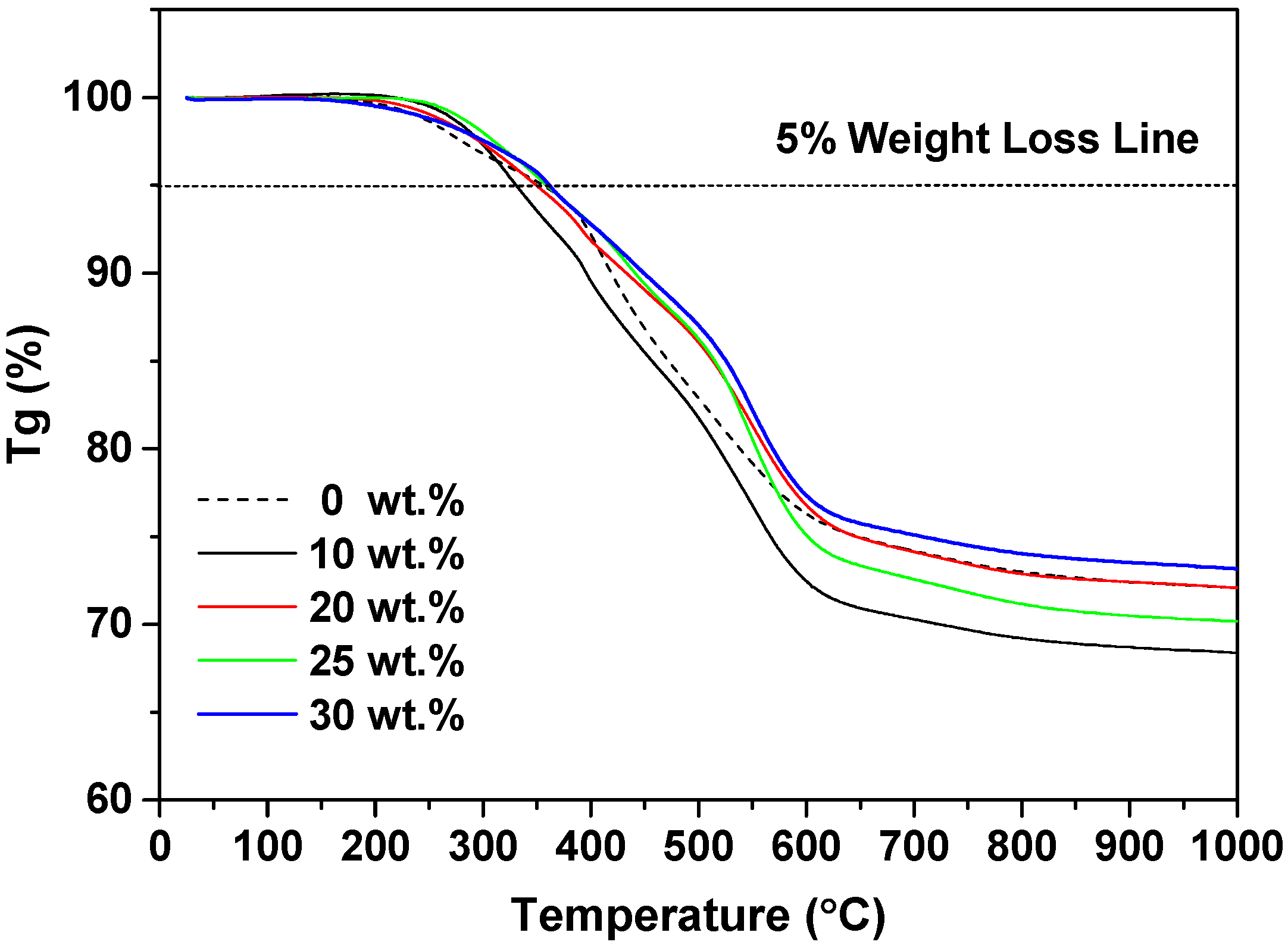
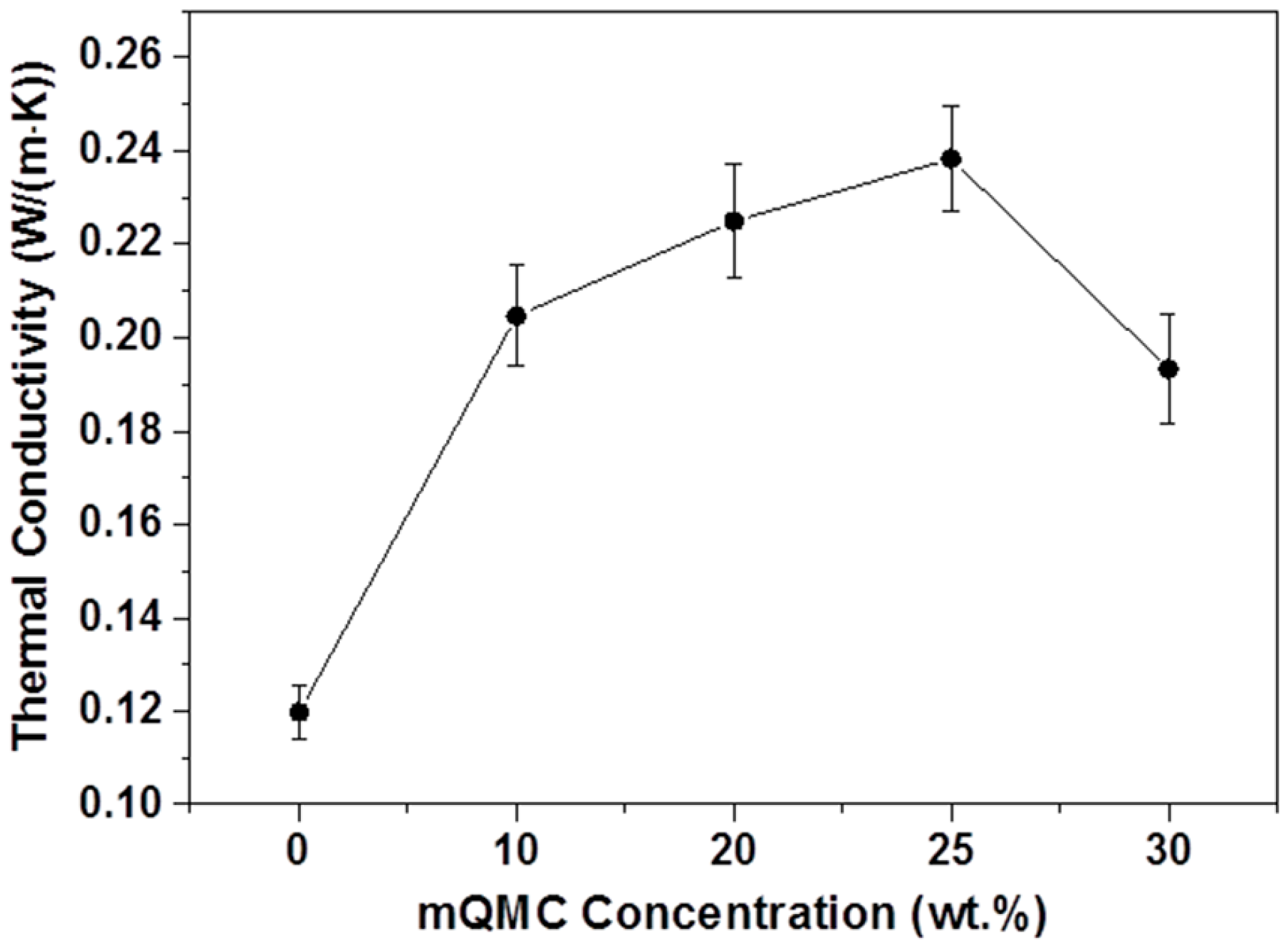
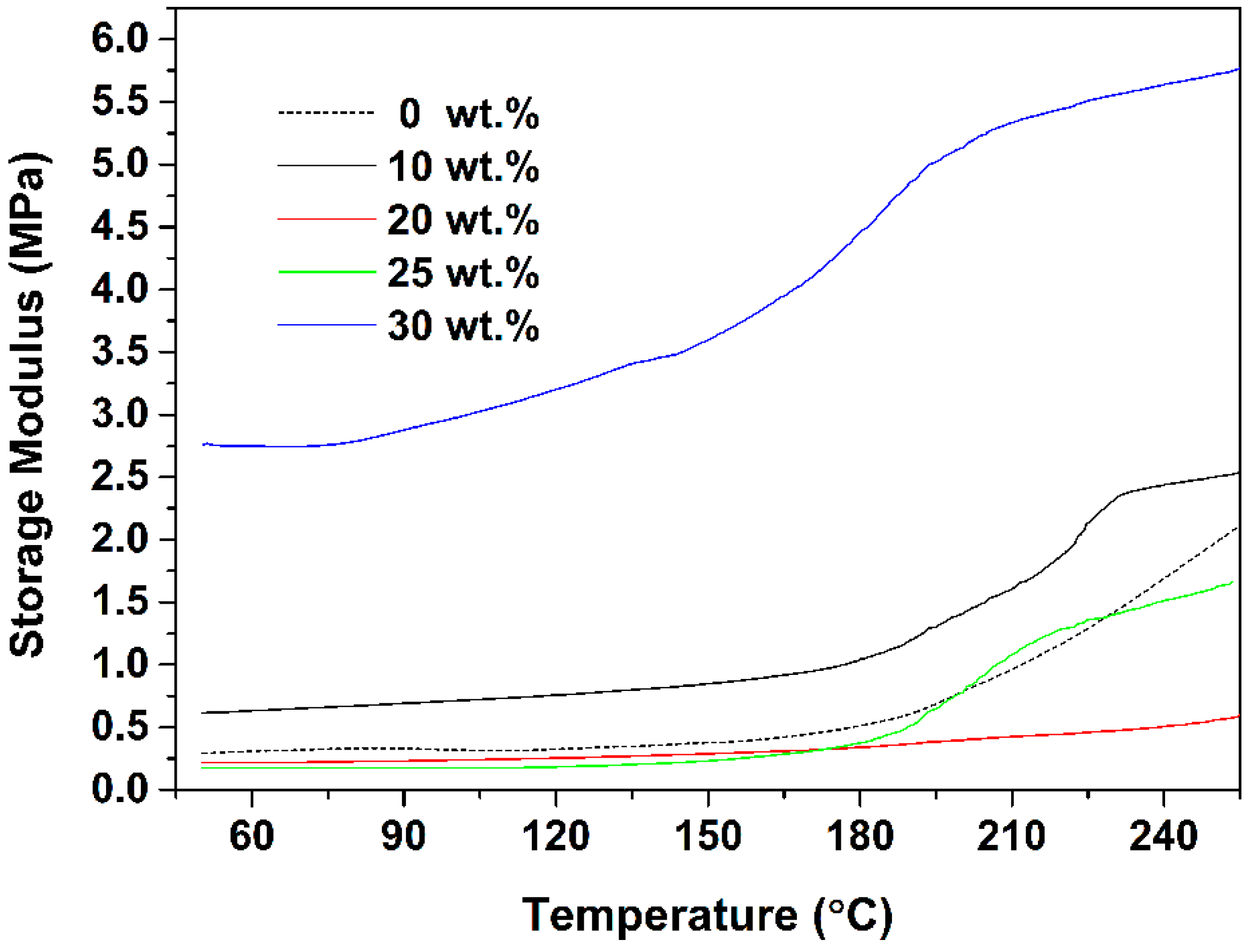
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De Mello, J.; Anthony, J.; Lee, S. Organic electronics: Recent developments. Chemphyschem 2015, 16, 1099–1100. [Google Scholar] [CrossRef] [PubMed]
- Thejo Kalyani, N.; Dhoble, S.J. Novel materials for fabrication and encapsulation of OLEDs. Renew. Sustain. Energy Rev. 2015, 44, 319–347. [Google Scholar] [CrossRef]
- Tao, P.; Li, Y.; Siegel, R.W.; Schadler, L.S. Transparent dispensible high-refractive index ZrO2/epoxy nanocomposites for LED encapsulation. J. Appl. Polym. Sci. 2013, 130, 3785–3793. [Google Scholar] [CrossRef]
- Li, T.; Zhang, J.; Wang, H.; Hu, Z.; Yu, Y. High-performance light-emitting diodes encapsulated with silica-filled epoxy materials. ACS Appl. Mater. Interfaces 2013, 5, 8968–8981. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Kwak, S.-Y.; Jin, J.; Kim, J.-S.; Choi, Y.; Paik, K.-W.; Bae, B.-S. Thermally resistant UV-curable epoxy–siloxane hybrid materials for light emitting diode (LED) encapsulation. J. Mater. Chem. 2012, 22, 8874–8880. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Z.; Shen, G.; Wen, R.; Lv, J.; Huo, J.; Yu, Y. Effect of chain flexibility of epoxy encapsulants on the performance and reliability of light-emitting diodes. Ind. Eng. Chem. Res. 2016, 55, 7635–7645. [Google Scholar] [CrossRef]
- Chen, J.; Huang, X.; Zhu, Y.; Jiang, P. Cellulose nanofiber supported 3D interconnected BN nanosheets for epoxy nanocomposites with ultrahigh thermal management capability. Adv. Funct. Mater. 2017, 27, 1604754. [Google Scholar] [CrossRef]
- Kim, J.-S.; Yang, S.; Bae, B.-S. Thermally stable transparent sol-gel based siloxane hybrid material with high refractive index for light emitting diode (LED) encapsulation. Chem. Mater. 2010, 22, 3549–3555. [Google Scholar] [CrossRef]
- Lee, S.; Hong, J.Y.; Jang, J. Multifunctional graphene sheets embedded in silicone encapsulant for superior performance of light-emitting diodes. ACS Nano 2013, 7, 5784–5790. [Google Scholar] [CrossRef] [PubMed]
- Varenik, M.; Nadiv, R.; Levy, I.; Vasilyev, G.; Regev, O. Breaking through the solid/liquid processability barrier: Thermal conductivity and rheology in hybrid graphene–graphite polymer composites. ACS Appl. Mater. Interfaces 2017, 9, 7556–7564. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, M.D.; Kazem, N.; Powell-Palm, M.J.; Huang, X.; Sun, W.; Malen, J.A.; Majidi, C. High thermal conductivity in soft elastomers with elongated liquid metal inclusions. Proc. Natl. Acad. Sci. USA 2017, 114, 2143–2148. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.-Y.; Kim, H.-Y.; Lim, Y.-W.; Kim, Y.-H.; Bae, B.-S. Optically recoverable, deep ultraviolet (UV) stable and transparent sol–gel fluoro siloxane hybrid material for a UV LED encapsulant. RSC Adv. 2016, 6, 26826–26834. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, G.Y.; Liang, X.; Zhang, W.S.; Zhou, L.; He, B.F.; Song, P.; Yuan, X.; Zhang, C.H.; Zhang, L.Y.; et al. Thermally stable transparent sol–gel based active siloxane-oligomer materials with tunable high refractive index and dual reactive groups. RSC Adv. 2016, 6, 70825–70831. [Google Scholar] [CrossRef]
- Kleine, T.S.; Nguyen, N.A.; Anderson, L.E.; Namnabat, S.; LaVilla, E.A.; Showghi, S.A.; Dirlam, P.T.; Arrington, C.B.; Manchester, M.S.; Schwiegerling, J.; et al. High refractive index copolymers with improved thermomechanical properties via the inverse vulcanization of sulfur and 1,3,5-triisopropenylbenzene. ACS Macro Lett. 2016, 5, 1152–1156. [Google Scholar] [CrossRef]
- Sun, Z.; Huang, H.; Li, L.; Liu, L.; Chen, Y. Polythioamides of high refractive index by direct polymerization of aliphatic primary diamines in the presence of elemental sulfur. Macromolecules 2017, 50, 8505–8511. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, H.; Yeo, H.; Goh, M.; Ku, B.-C.; Hahn, J.R.; You, N.-H. Preparation of UV-curable acryl resin for high refractive index based on 1,5-bis(2-acryloylenethyl)-3,4-ethylenedithiothiophene. Eur. Polym. J. 2016, 75, 303–309. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Imai, T.; Fu, M.-C.; Ando, S.; Higashihara, T.; Ueda, M. Poly(phenylene thioether)s with fluorene-based cardo structure toward high transparency, high refractive index, and low birefringence. Macromolecules 2016, 49, 5849–5856. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Han, W.-G.; Chiang, S.-J.; Su, W.-C.; Liu, Y.-L. Multi-functional branched polysiloxanes polymers for high refractive index and flame retardant LED encapsulants. RSC Adv. 2016, 6, 4377–4381. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Z.Y.; Moon, K.-S.; Yoo, S.; Choi, J.; Wong, P. High refractive index and transparency nanocomposites as encapsulant for high brightness LED packing. Electron. Compon. Technol. Conf. 2013, 4, 553–556. [Google Scholar]
- Macdonald, E.K.; Lacey, J.C.; Ogura, I.; Shaver, M.P. Aromatic polyphosphonates as high refractive index polymers. Eur. Polym. J. 2017, 87, 14–23. [Google Scholar] [CrossRef]
- Chung, P.T.; Chiou, S.H.; Tseng, C.Y.; Chiang, A.S. Preparation and evaluation of a zirconia/oligosiloxane nanocomposite for LED encapsulation. ACS Appl. Mater. Interfaces 2016, 8, 9986–9993. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Lim, Y.-W.; Lee, D.; Kim, Y.H.; Bae, B.-S. A highly adhesive siloxane LED encapsulant optimized for high thermal stability and optical efficiency. J. Mater. Chem. C 2016, 4, 10791–10796. [Google Scholar] [CrossRef]
- Ireni, N.G.; Karuppaiah, M.; Narayan, R.; Raju, K.V.S.N.; Basak, P. TiO2 /poly(thiourethane-urethane)-urea nanocomposites: Anticorrosion materials with NIR-reflectivity and high refractive index. Polymer 2017, 119, 142–151. [Google Scholar] [CrossRef]
- Wang, F.; Zeng, X.; Yao, Y.; Sun, R.; Xu, J.; Wong, C.P. Silver nanoparticle-deposited boron nitride nanosheets as fillers for polymeric composites with high thermal conductivity. Sci. Rep. 2016, 6, 19394. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zeng, X.; Yao, Y.; Gong, Z.; Wang, F.; Sun, R.; Xu, J.; Wong, C.P. Ice-templated assembly strategy to construct 3D boron nitride nanosheet networks in polymer composites for thermal conductivity improvement. Small 2015, 11, 6205–6213. [Google Scholar]
- Zhu, J.; Park, H.; Chen, J.-Y.; Gu, X.; Zhang, H.; Karthikeyan, S.; Wendel, N.; Campbell, S.A.; Dawber, M.; Du, X.; et al. Revealing the origins of 3D anisotropic thermal conductivities of black phosphorus. Adv. Electron. Mater. 2016, 2, 1600040. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, Z.; Mahoney, C.; Yan, J.; Ferebee, R.; Luo, D.; Matyjaszewski, K.; Bockstaller, M.R. Transparent and high refractive index thermoplastic polymer glasses using evaporative ligand exchange of hybrid particle fillers. ACS Appl. Mater. Interfaces 2017, 9, 7515–7522. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zeng, X.; Pan, G.; Sun, J.; Hu, J.; Huang, Y.; Sun, R.; Xu, J.B.; Wong, C.P. Interfacial engineering of silicon carbide nanowire/cellulose microcrystal paper toward high thermal conductivity. ACS Appl. Mater. Interfaces 2016, 8, 31248–31255. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, L.; Zhang, H.; Bai, Y.; Niu, Y.; Wang, H. Enhanced high thermal conductivity and low permittivity of polyimide based composites by core-shell Ag@SiO2 nanoparticle fillers. Appl. Phys. Lett. 2012, 101, 012903. [Google Scholar] [CrossRef]
- Peters, J.E.; Papavassiliou, D.V.; Grady, B.P. Unique thermal conductivity behavior of single-walled carbon nanotube–polystyrene composites. Macromolecules 2008, 41, 7274–7277. [Google Scholar] [CrossRef]
- James, A.C.; John, C.C. Procedures for preparation of silanols. J. Organomet. Chem. 1994, 480, 23–26. [Google Scholar]
- Kim, Y.H.; Bae, J.Y.; Jin, J.; Bae, B.S. Sol-gel derived transparent zirconium-phenyl siloxane hybrid for robust high refractive index LED encapsulant. ACS Appl. Mater. Interfaces 2014, 6, 3115–3121. [Google Scholar] [CrossRef] [PubMed]
- Chen, S. Molecular composites comprising TiO2 and their optical properties. Macromolecules 2008, 41, 4838–4844. [Google Scholar]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Feng, Y.; Wang, X.; Li, E.; Wang, Y.; Shui, L.; Li, H.; Li, N.; Zhou, G. Quartz Microcrystal-Hybridized Organosilicone Encapsulant with Enhanced Optical and Thermal Performances. Polymers 2018, 10, 84. https://doi.org/10.3390/polym10010084
Chen X, Feng Y, Wang X, Li E, Wang Y, Shui L, Li H, Li N, Zhou G. Quartz Microcrystal-Hybridized Organosilicone Encapsulant with Enhanced Optical and Thermal Performances. Polymers. 2018; 10(1):84. https://doi.org/10.3390/polym10010084
Chicago/Turabian StyleChen, Xin, Yancong Feng, Xiao Wang, En Li, Yao Wang, Lingling Shui, Hao Li, Nan Li, and Guofu Zhou. 2018. "Quartz Microcrystal-Hybridized Organosilicone Encapsulant with Enhanced Optical and Thermal Performances" Polymers 10, no. 1: 84. https://doi.org/10.3390/polym10010084
APA StyleChen, X., Feng, Y., Wang, X., Li, E., Wang, Y., Shui, L., Li, H., Li, N., & Zhou, G. (2018). Quartz Microcrystal-Hybridized Organosilicone Encapsulant with Enhanced Optical and Thermal Performances. Polymers, 10(1), 84. https://doi.org/10.3390/polym10010084






