Titanium(III, IV)-Containing Catalytic Systems for Production of Ultrahigh Molecular Weight Polyethylene Nascent Reactor Powders, Suitable for Solventless Processing—Impact of Oxidation States of Transition Metal
Abstract
:1. Introduction
2. Materials and Methods
Polymer Evaluation Methods
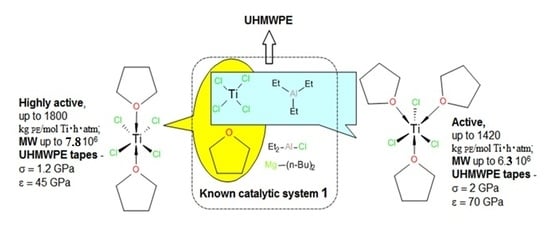
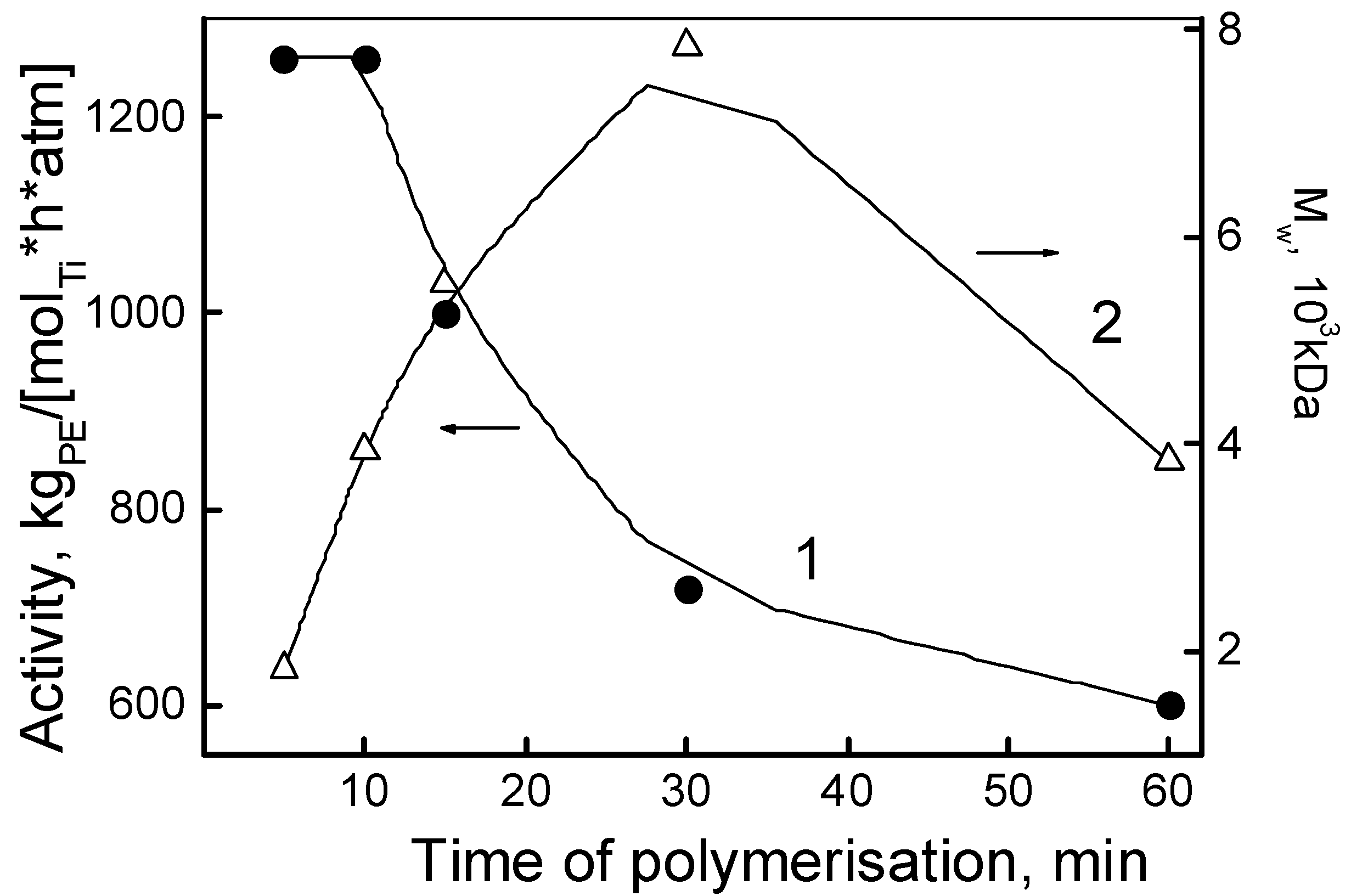
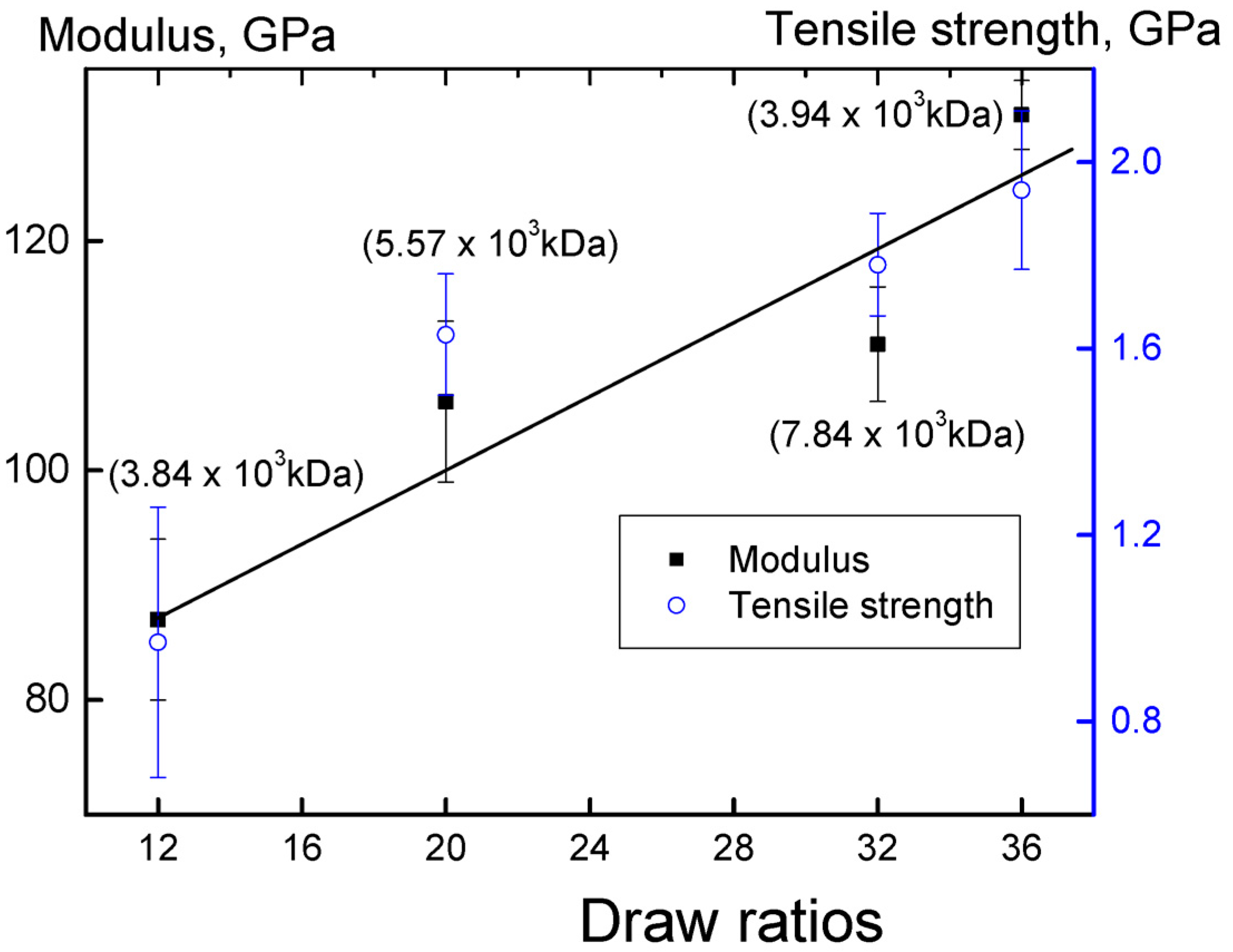
3. Results and Discussion
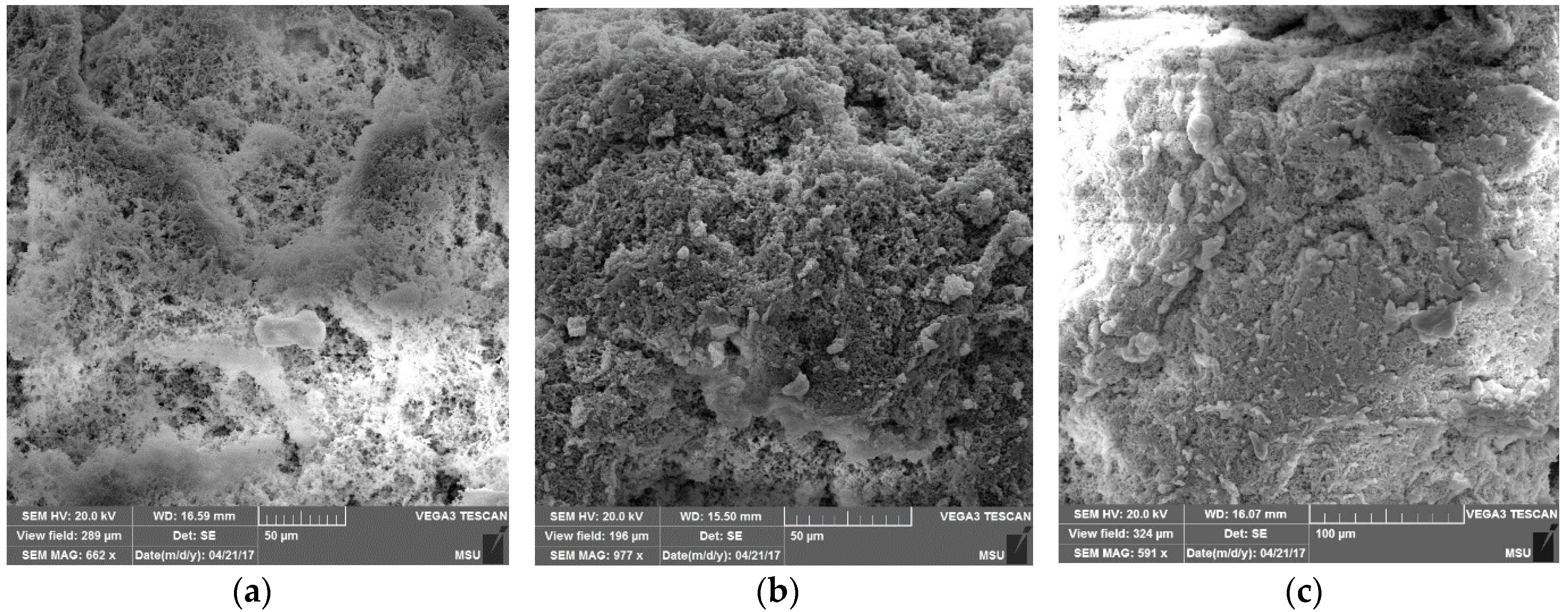
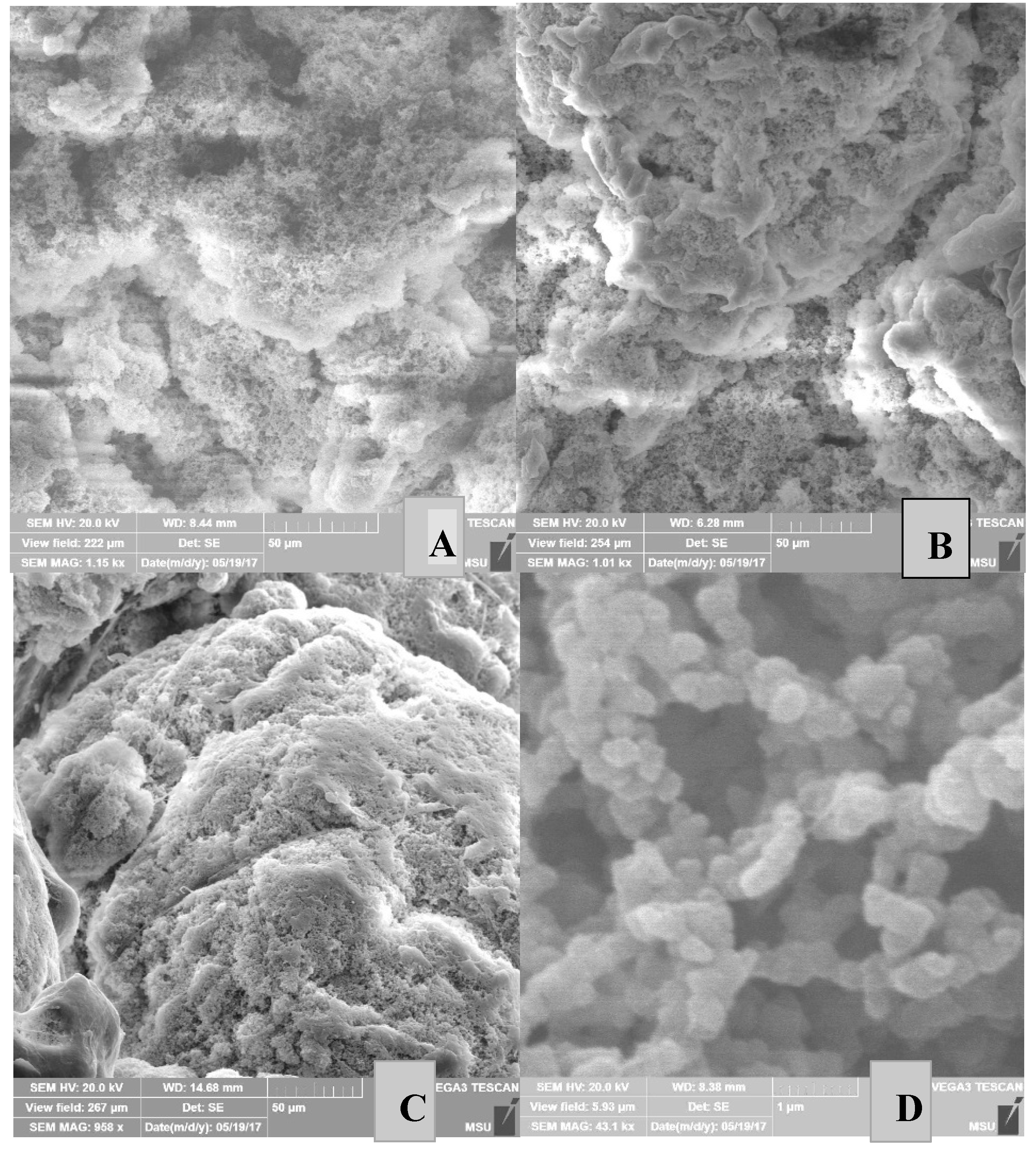
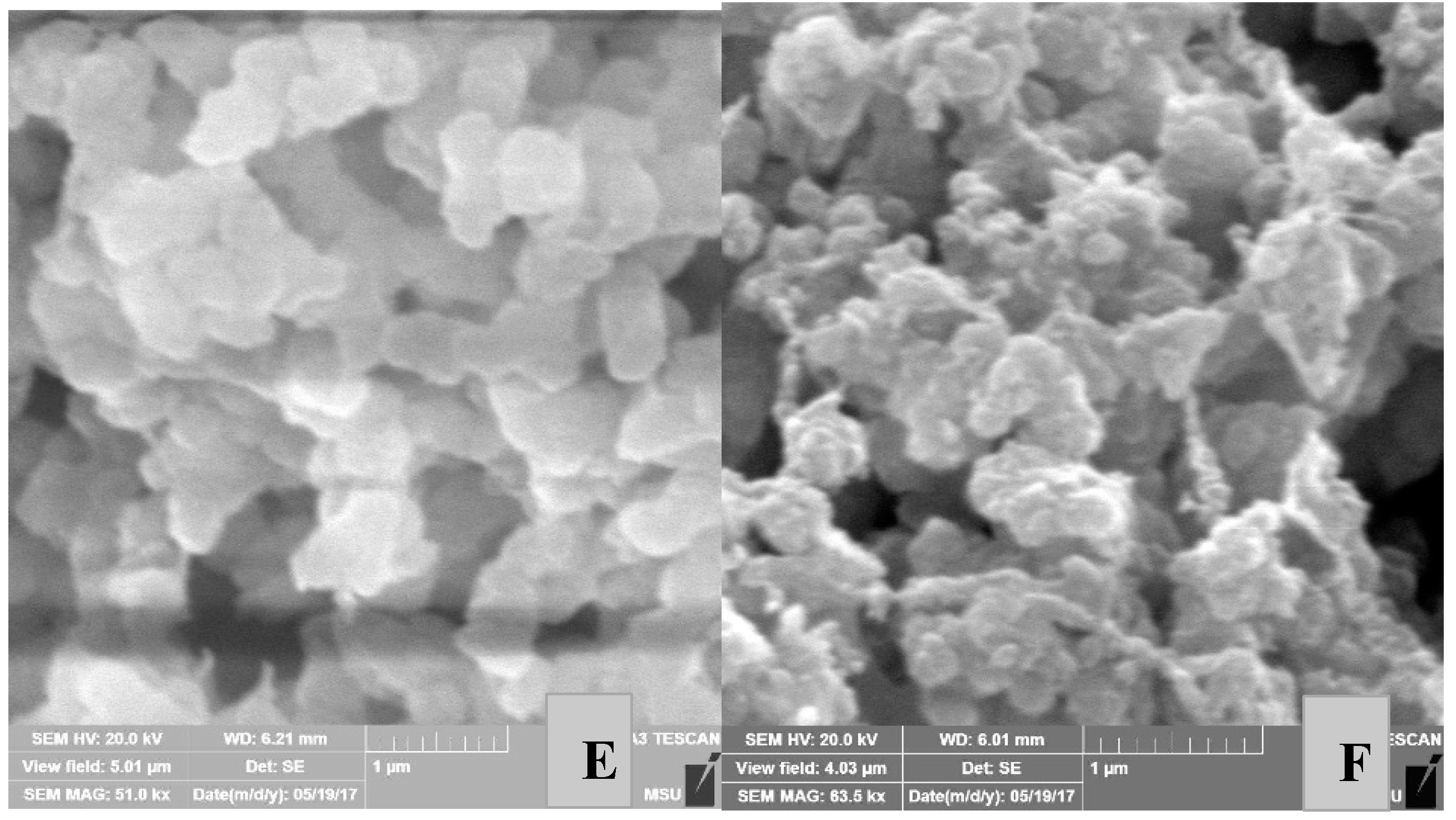
Properties of Reactor Powders
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kurtz, S.M. The UHMWPE Handbook: Ultra High Molecular Weight Polyethylene in Total Joint Replacement, 1st ed.; Elsevier Academic Press: New York, NY, USA, 2004; ISBN 9780080481463. [Google Scholar]
- Smith, P.; Chanzy, H.D.; Rotzinger, B.P. Drawing of virgin ultrahigh molecular weight polyethylene: An alternative route to high strength fibres. Polym. Commun. 1985, 26, 258. [Google Scholar]
- Smith, P.; Chanzy, H.D.; Rotzinger, B.P. Drawing of virgin ultrahigh molecular weight polyethylene: An alternative route to high strength/high modulus materials. J. Mater. Sci. 1987, 22, 523–531. [Google Scholar] [CrossRef]
- Rastogi, S.; Yao, Y.; Ronca, S.; Bos, J.; van der Eem, J. Unprecedented High-Modulus High-Strength Tapes and Films of Ultrahigh Molecular Weight Polyethylene via Solvent-Free Route. Macromolecules 2011, 44, 5558–5568. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Jiang, S.; Rastogi, S. 13C Solid State NMR Characterization of Structure and Orientation Development in the Narrow and Broad Molar Mass Disentangled UHMWPE. Macromolecules 2014, 47, 1371–1382. [Google Scholar] [CrossRef]
- Romano, D.; Ronca, S.; Rastogi, S. A Hemi-metallocene Chromium Catalyst with Trimethylaluminum-Free Methylaluminoxane for the Synthesis of Disentangled Ultra-High Molecular Weight Polyethylene. Macromol. Rapid Commun. 2015, 36, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Romano, D.; Andablo-Reyes, E.A.; Ronca, S.; Rastogi, S. Effect of a Cocatalyst Modifier in the Synthesis of Ultrahigh Molecular Weight Polyethylene having Reduced Number of Entanglements. J. Polym. Sci. A 2013, 51, 1630–1635. [Google Scholar] [CrossRef]
- Ozerin, A.N.; Ivanchev, S.S.; Chvalun, S.N.; Aulov, V.A.; Ivancheva, N.I.; Bakeev, N.F. Properties of Oriented Film Tapes Prepared via Solid State Processing of a Nascent Ultrahigh Molecular Weight Polyethylene Reactor Powder Synthesized with a Postmetallocene Catalyst. Polym. Sci. Ser. A 2012, 54, 950–954. [Google Scholar] [CrossRef]
- Won, H.Y.; Song, J.K.; Joo, Y.L.; Lee, H.K.; Park, N.C. Ultra-High Molecular Weight Polyethylene Subject Tub Method. Korean Patent KP98-15409, 29 April 1998. [Google Scholar]
- Joo, Y.K.; Zhou, H.; Lee, S.G.; Lee, H.K.; Song, J.K. Solid-State Compaction and Drawing of Nascent Reactor Powders of Ultra-High-Molecular-Weight Polyethylene. J. Appl. Polym. Sci. 2005, 98, 718–730. [Google Scholar] [CrossRef]
- Joo, Y.L.; Han, O.H.; Lee, H.K.; Song, J.K. Characterization of ultra high molecular weight polyethyelene nascent reactor powders by X-ray diffraction and solid state NMR. Polymer 2000, 41, 1355–1368. [Google Scholar] [CrossRef]
- Czaja, K.; Białek, M. Vanadium-based Ziegler-Natta catalyst supported on MgCl2(THF)2 for ethylene polymerization. Macromol. Rapid Commun. 1996, 17, 253–260. [Google Scholar] [CrossRef]
- Czaja, K.; Białek, M.; Ochedzan, W. Organometallic VCl4-based Catalyst supported on MgCl2(THF)2 for Ethylene Polymerization. Polimery 1997, 42, 714–716. [Google Scholar]
- Białek, M.; Czaja, K. Synthesis and characterization of ethylene-1-hexene copolymers prepared by using MgCl2(THF)2-supported Ziegler-Natta catalysts. Polimery 2000, 45, 293–296. [Google Scholar]
- Białek, M.; Czaja, K. The effect of the comonomer on the copolymerization of ethylene with long chain α-olefins using Ziegler–Natta catalysts supported on MgCl2(THF)2. Polymer 2000, 41, 7899–7904. [Google Scholar] [CrossRef]
- Kissin, Y.V.; Mink, R.I.; Brandolini, A.J.; Nowlin, T.E. AlR2Cl/MgR2 combinations as universal cocatalysts for Ziegler–Natta, metallocene, and post-metallocene catalysts. J. Polym. Sci. A 2009, 47, 3271–3285. [Google Scholar] [CrossRef]
- Kim, I.; Kim, J.H.; Choi, H.K.; Chung, M.C.; Woo, S.I. Comonomer enhancement effect of 1-hexene in ethylene copolymerization catalyzed over MgCl2/THF/TiCl4 catalysts. J. Appl. Polym. Sci. 1993, 48, 721–730. [Google Scholar] [CrossRef]
- Czala, K.; Bialek, M. Microstructure of ethylene-1-hexene and ethylene-1-octene copolymers obtained over Ziegler–Natta catalysts supported on MgCl2(THF)2. Polymer 2001, 42, 2289–2298. [Google Scholar] [CrossRef]
- Sobota, P. Metal-assembled compounds: Precursors of polymerization catalysts and new materials. Coord. Chem. Rev. 2004, 248, 1047–1060. [Google Scholar] [CrossRef]
- Seenivasan, K.; Sommazzi, A.; Bonino, F.; Bordiga, S.; Groppo, E. Spectroscopic Investigation of Heterogeneous Ziegler–Natta Catalysts: Ti and Mg Chloride Tetrahydrofuranates, Their Interaction Compound, and the Role of the Activator. Chem. Eur. J. 2011, 17, 8648–8656. [Google Scholar] [CrossRef] [PubMed]
- Grau, E.; Lesage, A.; Norsic, S.; Copéret, C.; Monteil, V.; Sautet, P. Tetrahydrofuran in TiCl4/THF/MgCl2: A Non-Innocent Ligand for Supported Ziegler–Natta Polymerization Catalysts. ACS Catal. 2013, 3, 52–56. [Google Scholar] [CrossRef]
- Belsky, V.K.; Bulychev, B.M.; Streltsova, N.R. Crystaline-structure of homometallic and heterometallic crown-compounds of [TiClx15K5(MU-2 = 0)TiCl5], [TiCl2x15K5][AlCl4] and [Mgx15K5x(CH3CN)2][TiCl6]. Russ. J. Inorg. Chem. 1992, 37, 1531. [Google Scholar]
- Sobota, P.; Utko, J.; Janas, Z. MgCl2—One of the factors controlling the mechanism of the reaction between grignard reagents and titanium or zirconium tetrachloride. II. J. Organomet. Chem. 1986, 316, 19–23. [Google Scholar] [CrossRef]
- Belsky, V.K.; Bulychev, B.M. Structural-chemical aspects of complexation in metal halide–macrocyclic polyether systems. Russ. Chem. Rev. 1999, 68, 119–135. [Google Scholar] [CrossRef]
- Busico, V. Metal-catalysed olefin polymerization into the new millennium: A perspective outlook. Dalton Trans. 2009, 41, 8794–8802. [Google Scholar] [CrossRef] [PubMed]
- Bryliakov, K.P.; Talsi, E.P. Frontiers of mechanistic studies of coordination polymerization and oligomerization of α-olefins. Coord. Chem. Rev. 2012, 256, 2994–3007. [Google Scholar] [CrossRef]
- Valente, A.; Mortreux, A.; Visseaux, M.; Zinck, P. Coordinative Chain Transfer Polymerization. Chem. Rev. 2013, 113, 3836–3857. [Google Scholar] [CrossRef] [PubMed]
- Sloan, T.E. Tetrahydrofuran Complexes of Selected Early Transition Metals. Inorg. Synth. 1982, 21, 135–136. [Google Scholar]
- Jones, N.A.; Liddle, S.T.; Wilson, C.; Arnold, P.L. Titanium(III) Alkoxy-N-heterocyclic Carbenes and a Safe, Low-Cost Route to TiCl3(THF)3. Organometallics 2007, 26, 755–757. [Google Scholar] [CrossRef]
- Bravaya, N.M.; Saratovskikh, S.L.; Mukhina, E.V.; Smirnova, O.V.; Gagieva, S.C.; Tuskaev, V.A.; Bulychev, B.M. Influence of polymerization conditions on catalytic properties of the TiCl2{η2-1-[C(H)=N(2,3,5,6-tetrafluorophenyl)]-2-O-3,5-di-But-C6H2}2/MAO system in ethylene homopolymerization and copolymerization with α-olefins. Russ. Chem. Bull. 2013, 62, 2500–2508. [Google Scholar] [CrossRef]
- Forte, G.; Ronca, S. Synthesis of Disentangled Ultra-High Molecular Weight Polyethylene: Influence of Reaction Medium on Material Properties. Int. J. Polym. Sci. 2017, 7431419. [Google Scholar] [CrossRef]
- Mehta, A.; Tembe, G.; Parikh, P.; Mehta, G. Titanasilsesquioxane-Alkylaluminum Catalyst System for Ethylene Polymerization. Mod. Res. Catal. 2012, 1, 29–42. [Google Scholar] [CrossRef]
- Dessy, R.E.; King, R.B.; Waldrop, M. Organometallic Electrochemistry. V. The Transition Series. J. Am. Chem. Soc. 1966, 88, 5112–5117. [Google Scholar] [CrossRef]
- Wailes, P.C.; Coutts, R.S.P.; Weigold, H. Organometallic Chemistry of Titanium, Zirconium and Hafnium; Academic Press: New York, NY, USA, 1974; p. 302. [Google Scholar] [CrossRef]
- Samuel, E. Reduction of zirconocene dihalides with magnesium studied by ESR. Evidence of zirconium(III) hydride formation by hydrogen transfer from the cyclopentadienyl ring. Inorg. Chem. 1983, 22, 2967–2970. [Google Scholar] [CrossRef]
- Basso, N.R.; Greco, P.P.; Carone, C.L.P.; Livotto, P.R.; Simplıcio, L.M.T.; da Rocha, Z.N.; Galland, G.B.; dos Santos, J.H.Z. Reactivity of zirconium and titanium alkoxides bidentade complexes on ethylene polymerization. J. Mol. Catal. A 2007, 267, 129–136. [Google Scholar] [CrossRef]
- Tuskaev, V.A.; Gagieva, S.C.; Kurmaev, D.A.; Kolosov, N.A.; Fedyanin, I.V.; Bulychev, B.M. Titanium (IV) complexes stabilized by 2,6-bis-(α,α’-diphenyl(hydroxy)methyl)-pyridine: Catalytic activity in olefin polymerization and impact of lithium and magnesium chlorides. Inorg. Chim. Acta 2015, 425, 275–281. [Google Scholar] [CrossRef]
- Tuskaev, V.A.; Gagieva, S.C.; Maleev, V.I.; Borissova, A.O.; Solov’ev, M.V.; Starikova, Z.A.; Bulychev, B.M. Titanium (IV) and zirconium (IV) chloride complexes on the base of chiral tetraaryl-1,3-dioxolane-4,5-dimetanol ligands in the polymerization of ethylene: The promoting role of lithium and magnesium chloride. Polymer 2013, 54, 4455–4462. [Google Scholar] [CrossRef]
- Garkhail, S.K. Easily Processable Ultra High Molecular Weight Polyethylene with Narrow Molecular Weight Distribution; Technische Universiteit Eindhoven: Eindhoven, The Netherlands, 2005. [Google Scholar] [CrossRef]
- Jones, R.L.; Armoush, M. Catalysts for UHMWPE. Macromol. Symp. 2009, 283–284, 88–95. [Google Scholar] [CrossRef]
- Jones, R.L., Jr.; Armoush, M.Z.; Harjati, T.; Elder, M.; Hummel, A.A.; Sullivan, J. Catalysts for UHMWPE and UHMWPE-copolymers. Inorg. Chim. Acta 2010, 364, 275–281. [Google Scholar] [CrossRef]
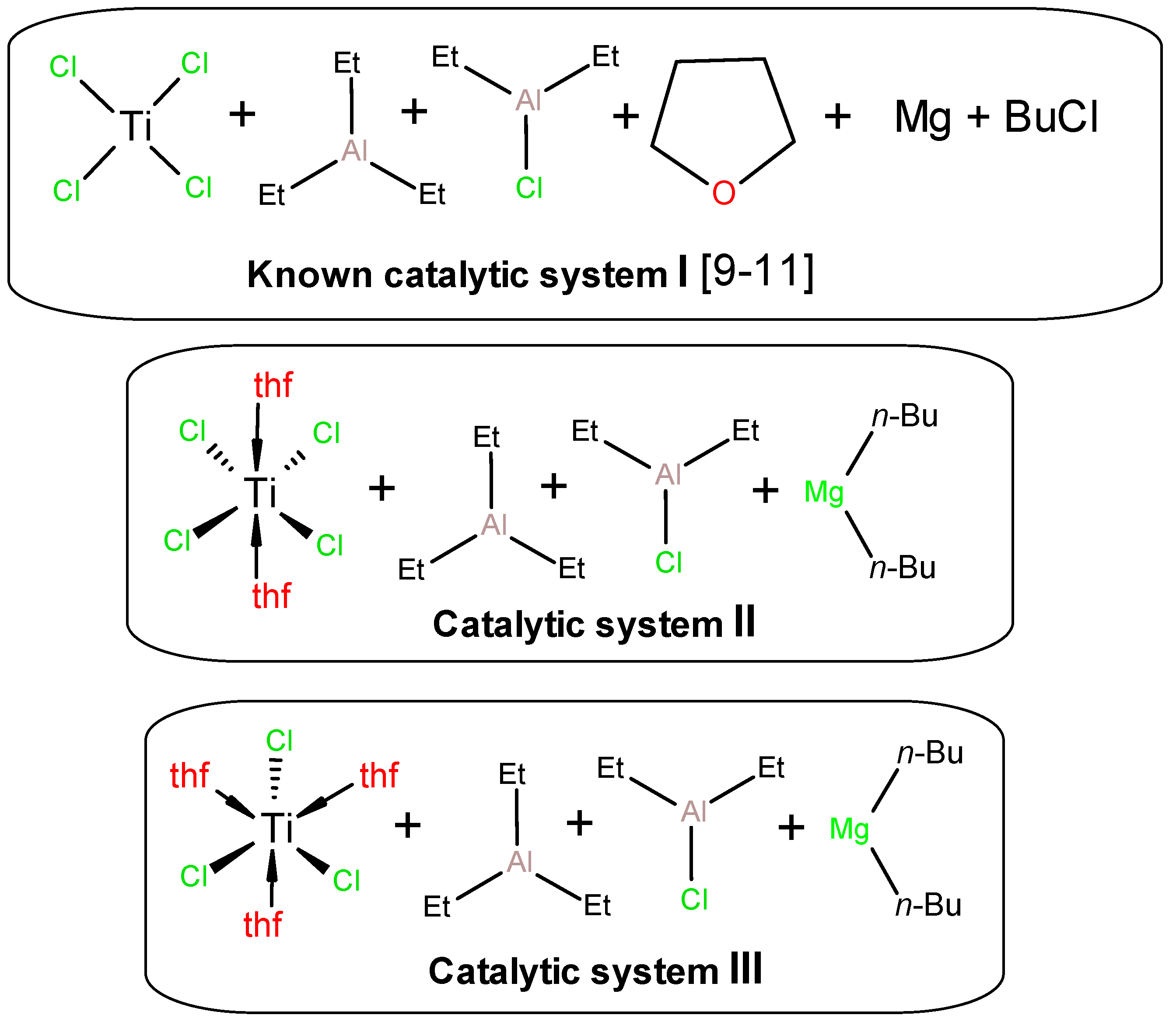
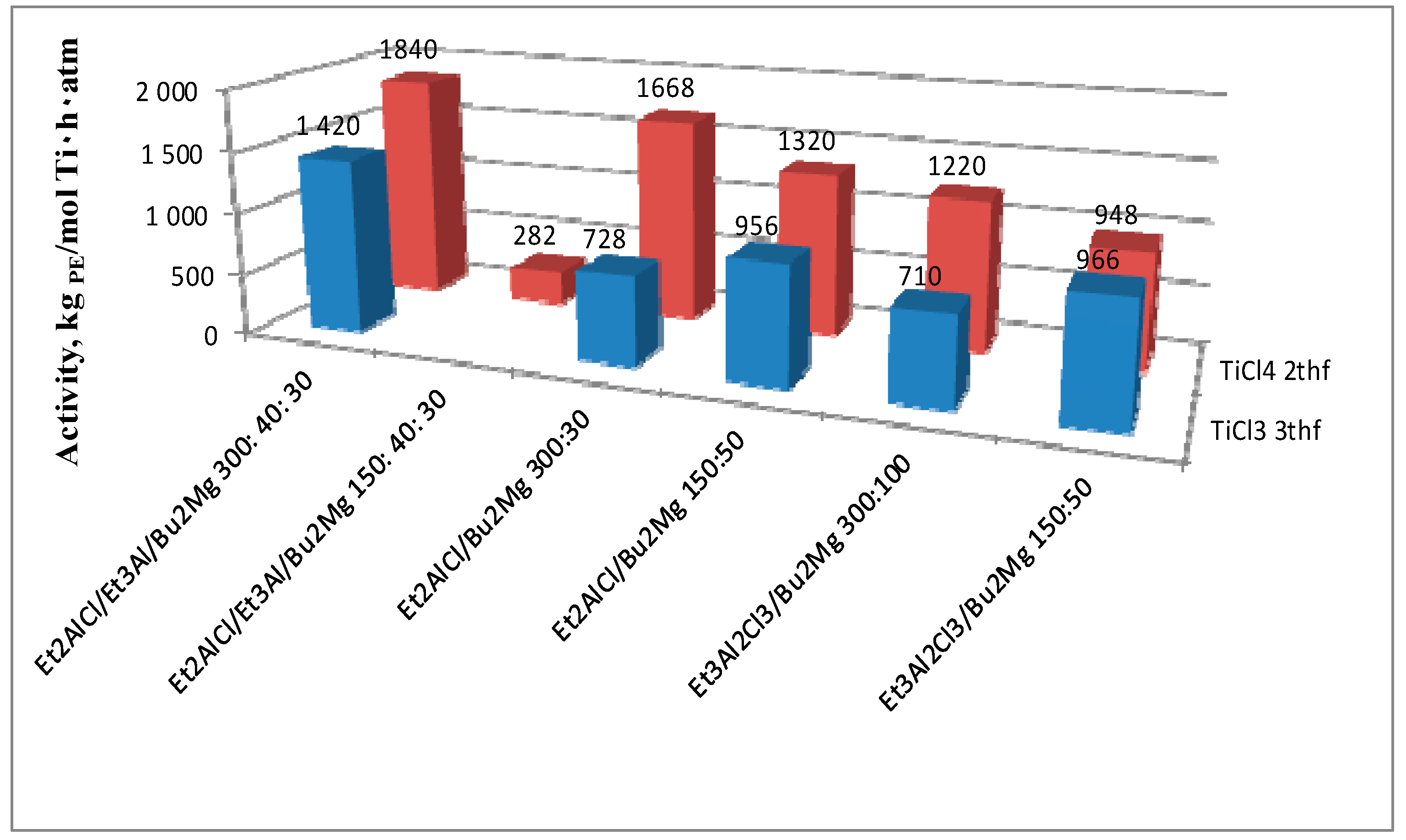
| Entry | Catal. system | (Ti), mol | Activators, (Et2AlCl):(Et3Al):(Bu2Mg) | t, min | mpolym, g | A b | Tm c | Degree of crystal., c % | Bulk density, g/cm3 | MW 106 Da |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | I | 2 × 10−5 | 300:40:30 | 5 | 2.10 | 1260 | 144 | 77 | 0.06 | 1.8 |
| 2 | I | 2 × 10−5 | 300:40:30 | 10 | 4.20 | 1260 | 144 | 76 | 0.07 | 3.8 |
| 3 | I | 2 × 10−5 | 300:40:30 | 15 | 5.00 | 1000 | 146 | 76 | 0.06 | 5.6 |
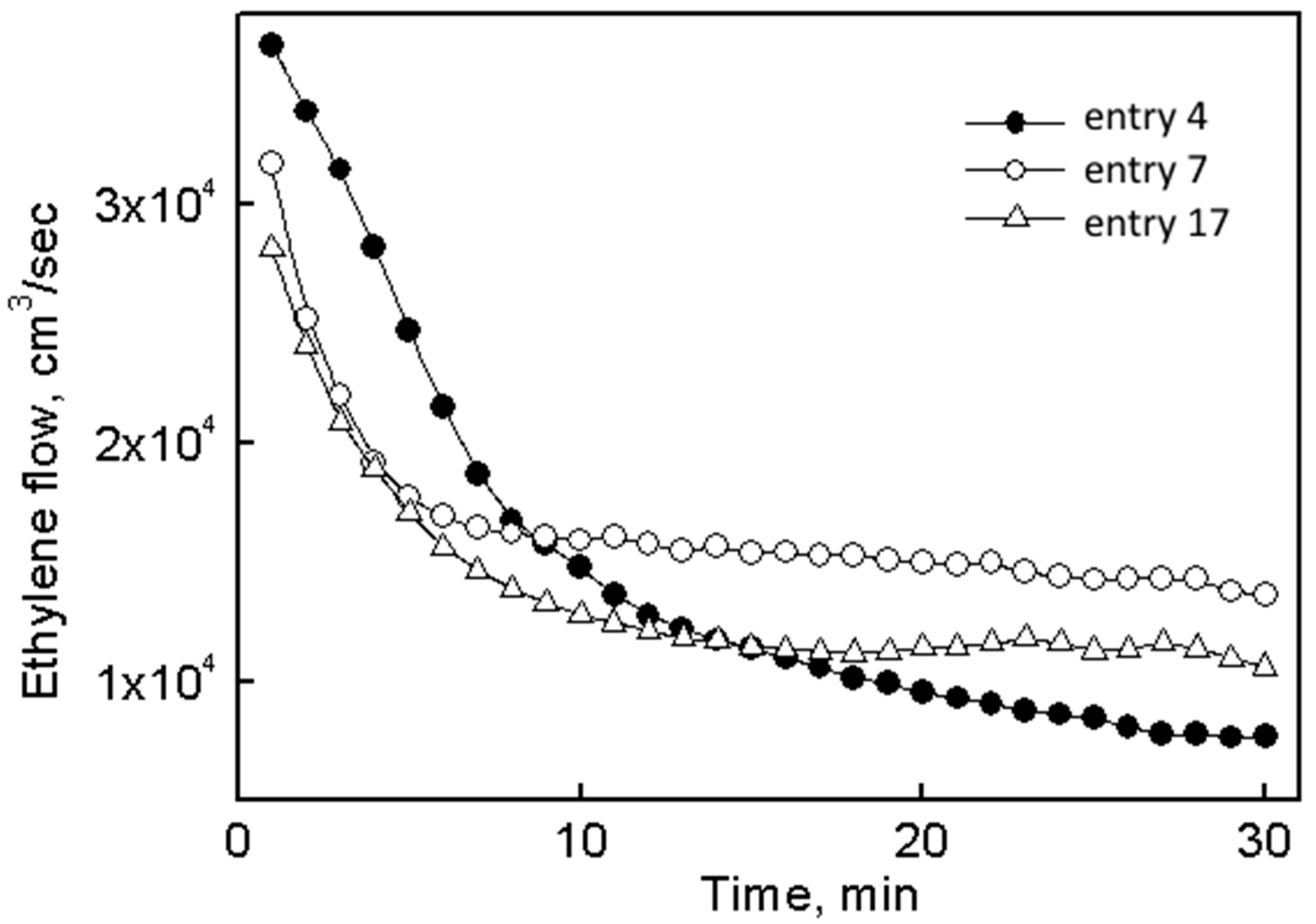
| 4 | I | 2 × 10−5 | 300:40:30 | 30 | 7.20 | 720 | 144 | 71 | 0.07 | 7.8 |
| 5 | I | 2 × 10−5 | 300:40:30 | 60 | 12.00 | 600 | 144 | 74 | 0.05 | 3.9 |
| 6 | II | 1 × 10−5 | 300:40:30 | 30 | 4.10 | 820 | 146 | 77 | 0.05 | 3.3 |
| 7 | II | 1 × 10−5 | 300:40:30 | 30 | 9.20 | 1840 | 141 | 63 | 0.07 | 7.2 |
| 8 | II f | 1 × 10−5 | 300:40:30 | 30 | 1.50 | 300 | 148 | 78 | 0.07 | 6.2 |
| 9 | II | 1 × 10−5 | 150:40:30 | 30 | 1.41 | 282 | 142 | 61 | 0.09 | 2.9 |
| 10 | II | 1 × 10−5 | 300:0:30 | 30 | 8.34 | 1668 | 143 | 66 | 0.07 | 3.9 |
| 11 | II | 1 × 10−5 | 150:0:50 | 30 | 6.60 | 1320 | 142 | 69 | 0.06 | 5.7 |
| 12 | II g | 1 × 10−5 | 150:0:50 | 30 | 4.74 | 948 | 144 | 69 | 0.09 | n.d |
| 13 | II d | 1 × 10−5 | 300:0:30 | 30 | 1.15 | 282 | 137 | 70 | 0.31 | 1.1 |
| 14 | II e | 1 × 10−5 | 300:0:30 | 30 | 0.34 | 68 | 140 | 45 | 0.45 | n.d |
| 15 | Ti–Zr | 1 × 10−5 + 1 × 10−5 | 300:40:30 | 30 | 3.00 | 600 | 142 | 58 | 0.06 | n.d |
| 16 | Ti–Zr | 1 × 10−5 + 1 × 10−5 | 600:80:60 | 30 | 5.96 | 1192 | 142 | 0.05 | 3.7 | |
| 17 | III | 1 × 10−5 | 300:40:30 | 30 | 6.97 | 1420 | 144 | 77 | 0.06 | 2.2 |
| 18 | III g | 1 × 10−5 | 150:0:50 | 30 | 4.83 | 966 | 142 | 67 | 0.06 | 6.3 |
| 19 | III | 1 × 10−5 | 300:0:30 | 30 | 3.64 | 728 | 144 | 69 | 0.07 | 6.3 |
| 20 | III | 1 × 10−5 | 300:0:100 | 30 | 2.70 | 540 | 149 | 78 | 0.09 | 3.8 |
| 21 | III | 1 × 10−5 | 150:0:50 | 30 | 4.78 | 956 | 143 | 67 | 0.06 | 2.5 |
| 22 | I d | 2 × 10−5 | 300:0:30 | 30 | 1.95 | 286 | 139 | 58 | 0.26 | 0.8 |
| 23 | I e | 2 × 10−5 | 300:0:30 | 30 | 0.40 | 68 | 140 | 45 | 0.38 | 0.7 |
| Entry a | Catalytic system | MW (106) | Elongation, % | Drawing ratio | Breaking strength, GPa | Elastic modulus, GPa |
|---|---|---|---|---|---|---|
| 1 | I | 1.83 | 2.4 | 1.2 | 60 | |
| 2 | I | 3.84 | 2.0 | 36 | 2.1 | 125 |
| 3 | I | 5.57 | 2.1 | 20 | 1.6 | 106 |
| 4 | I | 7.84 | 2.2 | 32 | 1.9 | 100 |
| 5 | I | 3.94 | 2.1 | 12 | 1.2 | 60 |
| 6 | II | 3.33 | 3.0 | 12 | 1.2 | 45 |
| 16 | Ti–Zr | 3.70 | 3.2 | 20 | 1.0 | 44 |
| 17 | III | 2.15 | 2.7 | 20 | 1.8 | 78 |
| 18 | III | 6.28 | 2.7 | 20 | 1.8 | 78 |
| 19 | III | 6.28 | 2.3 | 20 | 1.6 | 81 |
| 20 | III | 3.79 | 2.9 | 24 | 2.0 | 70 |
| 21 | III | 2.47 | 2.5 | 20 | 1.8 | 88 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuskaev, V.A.; Gagieva, S.C.; Kurmaev, D.A.; Kolosov, N.A.; Mikhaylik, E.S.; Golubev, E.K.; Sizov, A.I.; Zubkevich, S.V.; Vasil’ev, V.G.; Nikiforova, G.G.; et al. Titanium(III, IV)-Containing Catalytic Systems for Production of Ultrahigh Molecular Weight Polyethylene Nascent Reactor Powders, Suitable for Solventless Processing—Impact of Oxidation States of Transition Metal. Polymers 2018, 10, 2. https://doi.org/10.3390/polym10010002
Tuskaev VA, Gagieva SC, Kurmaev DA, Kolosov NA, Mikhaylik ES, Golubev EK, Sizov AI, Zubkevich SV, Vasil’ev VG, Nikiforova GG, et al. Titanium(III, IV)-Containing Catalytic Systems for Production of Ultrahigh Molecular Weight Polyethylene Nascent Reactor Powders, Suitable for Solventless Processing—Impact of Oxidation States of Transition Metal. Polymers. 2018; 10(1):2. https://doi.org/10.3390/polym10010002
Chicago/Turabian StyleTuskaev, Vladislav A., Svetlana C. Gagieva, Dmitrii A. Kurmaev, Nikolay A. Kolosov, Elena S. Mikhaylik, Evgenii K. Golubev, Alexander I. Sizov, Sergey V. Zubkevich, Viktor G. Vasil’ev, Galina G. Nikiforova, and et al. 2018. "Titanium(III, IV)-Containing Catalytic Systems for Production of Ultrahigh Molecular Weight Polyethylene Nascent Reactor Powders, Suitable for Solventless Processing—Impact of Oxidation States of Transition Metal" Polymers 10, no. 1: 2. https://doi.org/10.3390/polym10010002
APA StyleTuskaev, V. A., Gagieva, S. C., Kurmaev, D. A., Kolosov, N. A., Mikhaylik, E. S., Golubev, E. K., Sizov, A. I., Zubkevich, S. V., Vasil’ev, V. G., Nikiforova, G. G., Buzin, M. I., Serenko, O. A., & Bulychev, B. M. (2018). Titanium(III, IV)-Containing Catalytic Systems for Production of Ultrahigh Molecular Weight Polyethylene Nascent Reactor Powders, Suitable for Solventless Processing—Impact of Oxidation States of Transition Metal. Polymers, 10(1), 2. https://doi.org/10.3390/polym10010002