Abstract
Catalytic systems containing TiCl4 or TiCl3, THF, organomagnesium (n-Bu2Mg) and organoaluminum compounds capable of producing ultrahigh molecular weight polyethylene (UHMWPE) were developed. The resulting polymers were characterized by a molecular weight in the range of (1.8–7.8) × 106 Da and desirable morphology, suitable for modern methods of polymer processing—the solvent-free solid-state processing of superhigh-strength (tensile strength up to 2.1 GPa) and high-modulus (elastic modulus up to 125 GPa) oriented films and film tapes. The impacts of a THF additive, the oxidation state of the titanium atom, and the composition and nature of the nontransition organometallic compounds on the formation of catalytic systems for UHMWPE production were evaluated. The results indicate the suitability of individual titanium chloride tetrahydrofuran complex application for the formation of THF-containing catalytic systems. This approach also results in a significant increase in the system catalytic activity and mechanical properties of UHMWPE. The catalysts based on Ti(III) were inferior to systems containing Ti(IV) in productivity but were markedly superior in the mechanical properties of UHMWPE.
1. Introduction
Ultrahigh molecular weight polyethylene (UHMWPE) is a perspective engineering polymer that is widely used in demanding applications [1]. Because of its high melt viscosity, the polymer cannot be processed via conventional methods (i.e., injection molding, blow molding, extrusion, etc.). By reducing the entanglement density, the UHMWPE nascent reactor powders can be processed in solid-state below their melting temperature, leading to the high-strength and high-modulus oriented materials. This methodology, which was begun by Smith et al. [2,3], received considerable development by Rastogi et al. [4,5]. However, not only metallocenes [6] or nonmetallocene catalysts [4,5,7,8] allow us to obtain UHMWPE with a reduced number of entanglements.
Joo et al. reported that the catalytic system consisting of TiCl4, Bu2Mg, nonstoichiometric amounts of THF, and two types of organoaluminum compounds—Et3Al and Et2AlCl—allows the synthesis of UHMWPE with molecular weights in the range of (1.0–3.8) × 106 under mild conditions (at 10–60 °C and ethylene pressures of 1 to 3 atm, solvent—petroleum ether). Polymer film tapes obtained using the solvent-free solid-phase monolithisation of reactor powders (below melting point) had a tensile strength of 1.7–2.0 GPa [9,10,11].
Despite the fact that Ziegler–Natta (ZN) catalysts, containing THF, are well studied and are widely used in industry, the true role of THF in the formation of the catalytic system remains the subject of discussion. For example, titanium- and vanadium-containing ZN catalysts, which include THF complexes of MgCl2, are highly active in ethylene polymerization [12,13] and its copolymerization with α-olefins [14,15] resulting in ultrahigh molecular weight polymers.
One of the possible functions of THF in titanium–magnesium ZN catalysts is the modification of MgCl2, formed by the reaction (1) as described by Kissin et al. [16].
R2Mg + 2Et2AlCl = 2MgCl2 + 2Et2AlR
Reaction of MgCl2 with a Lewis base, typically alcohols or ethers such as THF, is a generally known method of its activation [17,18,19,20].
Alternatively, THF can serve as a ligand interacting with TiCl4. Such interaction would convert the purely Ziegler type catalytic system to a nonmetallocene type. This possibility is illustrated by Sautet et al. [21], who described the formation of Ti(IV) alkoxo-complexes due to the THF ring opening in the presence of a strong Lewis acid-Ti(IV) species. This information indicates that the multivariance of chemical processes that may occur in the system {TiCl4 + R2Mg + THF + Et3Al + Et2AlCl} will inevitably lead to a certain nonreproducibility of the results and difficulties in the control of the catalytic process.
Undoubtedly, the basic THF molecule will react with all inorganic and organometallic compounds of the system which are sufficiently strong Lewis acids. The key question is which of the formed products will have the greatest impact on the catalytic activity and properties of the polymer. It was previously demonstrated that in the presence of electron-donor organic ligands, such as THF, acetonitrile, or crown-ethers, ionic binuclear complexes of different composition can be formed. The precise composition of such complexes depends on the oxidation state of Group 4 metals and differences in acidity of electron-deficient molecules that are introduced to the system. Thus, the authors [22,23] have shown that titanium (or zirconium) in the formal +4 oxidation state in the presence of magnesium compounds is always included in the anion: [Mg(CH3CN)2(15C5)]2+[TiIVCl6]2−, [Mg(thf)6]2+[ZrIVCI6]2−, [Mg(thf)6]2+[ZrIVCl5(thf)]2− [Mg(thf)6]2+[TiCl5(thf)]−2, [(thf)4Mg(μ-CI)2TiIVCI4] and [Mg2(μ-Cl)3(thf)6]+[TiCl5(thf)]−. At the same time, titanium (III) chloride in the presence of aluminum compounds is always included in the cation, e.g., {[(TiIIICl2)(15-crown-5)]+[AlCl4]−} [24]. The above-mentioned behavior of titanium compounds in different oxidation states, in our view, is of fundamental importance to understanding the mechanism of system I (Figure 1) in terms of catalytically active site formation. Thus, it is expected that the formation of chlorine-containing titanium(IV) (Zr, Hf) anions will withdraw a transition metal atom from the catalytic process. In contrast, formation of a titanium-containing cation will promote the formation of catalytically active centers, corresponding to the most common point of view regarding their nature [25,26,27]. In other words, if the qualitative and quantitative composition of the cocatalyst will be constant, we can assume that the catalytic activity of the system in ethylene polymerization will be determined by the structure of the cationic active site containing Ti(III) with some optimal amount of THF.
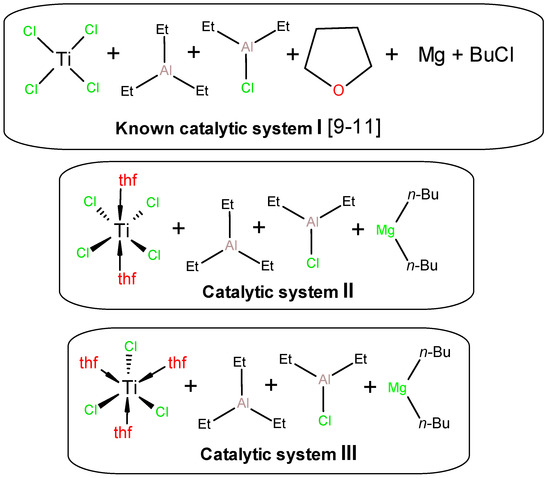
Figure 1.
Compositions of catalytic systems I–III used in the present study. The exact ratio of activators for each experiment is given in Table 1.
The aim of this article is twofold. The first is to develop and optimize a cheap and technologically acceptable catalytic system capable of producing UHMWPE nascent reactor powders which will be processed via a solid-state solventless method. Secondly, we aim to examine the impact of a THF additive, the oxidation state of the titanium atom, and the composition and nature of nontransition organometallic compounds on the productivity of the catalytic systems, the properties of UHMWPE powders, and the specific features of plastic deformation during the orientational drawing of the compacted and monolithized material in the film tapes.
2. Materials and Methods
Toluene, hexane, and THF were distilled from Na/benzophenone prior to use. The water content of these solvents was periodically tested using Karl Fischer coulometry with a Methrom 756 KF apparatus. Argon and ethylene of special-purity grade were dried by purging through Super Clean™ Gas Filters (Zoetermeer, The Netherlands). Diethylaluminumchloride, aluminium sesquichloride, di-n-butylmagnesium, triethyl aluminium (Sigma-Aldrich, St. Louis, MO, USA) were used as 1.0 M solution in heptane. Tetrahydrofuran complexes of Group 4 transition metal chlorides TiCl4·2THF, TiCl3·3THF, ZrCl4·2THF, and HfCl4·2THF were synthesized according to known methods [28,29]. All manipulations with air-sensitive materials (precatalysts, organoaluminum compounds, Bu2Mg) were performed with rigorous exclusion of oxygen and moisture in oven-dried Schlenk glassware on a dual manifold Schlenk line, interfaced to a high-vacuum line.
The polymerization of ethylene was carried out in a jacketed 100 or 300 mL stainless steel reactor (Parr Instrument Co., Moline, IL, USA) equipped with a mechanical stirrer, a temperature controller, and inlets for loading components of catalytic systems and ethylene. The reactor was kept under vacuum for 1 h at 50 °C before each experiment, after which it was filled with argon and cooled to the required temperature. An appropriate solvent (petroleum ether, b.p. 70–80 °C, or toluene) and the necessary amount of a cocatalyst and additives (for example, Et3Al (0.8 mL of 1 M solution in toluene, 8 × 10−4 mol), Bu2Mg (0.6 mL of 1 M solution in toluene, 6 × 10−4 mol), THF (0.008 mL, an aliquot in toluene), Et2AlCl (6 mL of 1 M solution in toluene, 6 × 10−3 mol) were loaded into the argon-purged reactor and stirred vigorously. Depending on the system, precatalysts were activated with Et2AlCl, Et3Al2Cl3, Et3Al, iBu3Al, methylaluminoxane (MAO), and Bu2Mg in various combinations; the composition of the activator mixtures for each experiment is indicated in Table 1. The reactor was heated to a specified temperature and the reaction mixture was saturated with ethylene to achieve a total ethylene and solvent vapor pressure of 0.7 atm. Polymerization was initiated by the addition of precatalyst to the reaction mixture. The pressure of ethylene was maintained constant during polymerization; the temperature was thermostatically controlled as indicated. After a desired period of time, the reactor was vented. Polymerization was stopped by the addition of 10% HCl solution in ethanol to the reactor. The polymer was filtered out, washed several times with a water–ethanol mixture, immersed in acidified ethanol for 12 h to remove inorganic impurities, and then dried under vacuum at 50–60 °C until a constant weight was achieved.

Table 1.
Ethylene polymerization results using catalytic systems I–III a.
Polymer Evaluation Methods
DSC was performed using a differential scanning calorimeter DSC-822e (Mettler-Toledo, Greifensee, Switzerland) at a heating rate of 10 °C/min in air. TGA and DTA measurements were done using a “Derivatograph-C” (MOM, Budapest, Hungary) at a heating rate 10 °C/min in air. The viscosity average molecular weight of the synthesized UHMWPE samples was calculated with the Mark–Houwink equation: MW = 5.37 × 104 [η]1.37 [1], where MW is the viscosity average molecular weight (g/mol); [η] is the intrinsic viscosity in decalin at 135 °C (dL/g); [η] = (2ηsp − 2lnηr)1/2/0.056 (ηsp is the specific viscosity decalin at 135 °C; ηr is the relative viscosity in decalin at 135 °C; ηr = ηsp + 1).
The mechanical characteristics of the oriented materials were evaluated on the oriented tapes obtained by solid-state processing of the UHMWPE nascent reactor powders. Monolithic tapes (100 microns in thickness and 10 mm in width), uniform over the entire length, were formed at a pressure and shear deformation below the polymer melting point (124–126 °C). The tapes were subjected to uniaxial drawing using Spinline Daca equipment (Shanghai, China). The drawing temperature was set 4 °C below the polymer melting point. The mechanical characteristics of the tapes were measured with a Hounsfield H1KS machine (Redhill, UK) at the gauge length of the tested samples (120 mm) with a 2 mm/min initial deformation rate. The reported values are the average of at least eight samples.
Scanning Electron Microscopy investigations on morphologies of nascent reactor powders were carried out with a high resolution Tescan Vega 3 (Carl Zeiss Leo 1530 VP, LaB6, Oberkochen, Germany) operated at 15 kV. As-polymerized particles were carefully deposited on SEM stubs, and the samples were coated with gold using a sputtering technique.
3. Results and Discussion
When testing UHMWPE synthesis using the catalytic system {TiCl4 + R2Mg + THF + Et3Al + Et2AlCl} (I), proposed by Joo et al. [9,10,11], we used published data, but, for technical reasons, have introduced some modifications. As such, we used commercial di-n-butylmagnesium, reduced the amounts of reactants and the solvent volume proportionally to the reactor volume, and increased the TiCl4 concentration to 4 times the original concentration to overcome the constraints when adding the reagent. The components used for the catalytic system formation are presented in Figure 1.
Figure 2 shows the kinetic dependences of the activity of the catalytic system I and the MW of the resulting PE. From the 15 min mark onwards, a monotonic decrease in activity was observed, and at 60 min it reduced twofold (Figure 2; Table 1, entries 1–5). Simultaneously, within the time interval of 5 to 30 min, the molecular weight of the polymer increased from 1.83 × 103 to 7.84 × 103 kDa (Figure 2, curve 2), and then decreased to 3.5 × 103 kDa at 60 min. Therefore, during that same interval of time (5–30 min) the catalytically active sites are characterized by homogeneity providing a living or “pseudo-living” polymerization regime, but only for a given time interval. Based on this observation, all subsequent polymerization experiments were conducted for 30 min.
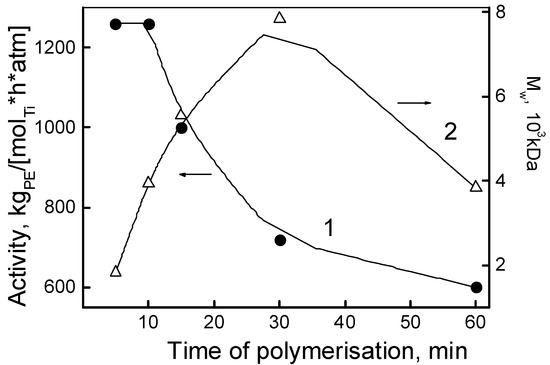
Figure 2.
Catalytic activity (1) of the system I and molecular weight of the obtained ultrahigh molecular weight polyethylene (UHMWPE) samples (2) versus the polymerization time.
In order to elucidate the impact of the titanium oxidation state and the amounts of THF and nontransition organometallic compounds in the polymerization of ethylene, we used different catalytic systems, the composition of which is given in Table 1. Their properties were compared with the catalytic system I described by Joo et al. [9,10,11]. It is not possible to use other known catalysts for comparison with the considered catalytic systems, since commercial supported ZN catalysts do not give PE with the desired morphology (i.e., such polymers cannot be processed by a solventless method). On the other hand, single-site catalysts (metallocenes or phenoxyimine complexes of titanium) are MAO-activated catalysts but MAO has not shown sufficient efficiency on our systems.
Application of system II allowed an approximately twofold reduction of the concentration of titanium compared with system I (entries 6–12). Moreover, application of activators in a ratio Et2AlCl/Et3Al/Bu2Mg (300:40:30) increased the activity of the catalytic system by ~2.5 times (entry 7 vs. entry 4); its resistance to deactivation was also slightly improved (Figure 3, curves 1 and 2).
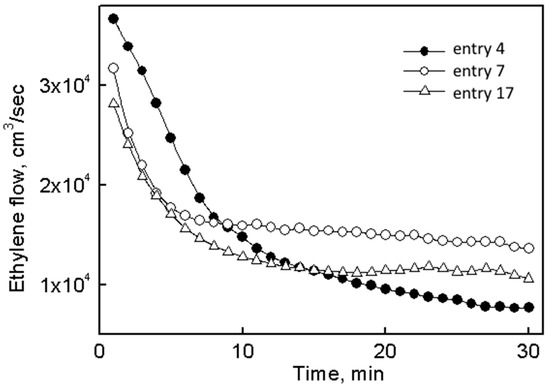
Figure 3.
Kinetic dependence of catalytic systems based on catalytic systems I (entry 4), II (entry 7) and III (entry 17).
Generally, both activity and stability of nonmetallocene systems increase when the aliphatic solvent is replaced by toluene (e.g., [30,31]). The systems evaluated in this work display similar behavior (e.g., entries 6 and 7); this may be due to the limited solubility of tetrahydrofuran complexes of group 4 metal chlorides in petroleum ether. For this reason, all further experiments were carried out in toluene.
The introduction of an additional amount of THF into the system II, significantly exceeding complex stoichiometry, led to a sharp decrease in its activity. Thus, in entry 8, conducted under the same quantities of cocatalysts but with large amounts of THF, the system activity decreased almost threefold. This can be explained, for example, by the formation of inactive or much less active heterometallic magnesium complexes with anions [TiCl6−n·THFn]−x, similar to that previously described [23,24].
A twofold decrease in the concentration of Et2AlCl results in a considerable decrease in activity (entry 9 vs. 7), whereas the complete elimination of Et3Al from the catalytic system II has practically no influence on its activity (entry 10 vs. 7). TiCl4·2THF, activated by the mixture of Et2AlCl and Bu2Mg in a ratio of 150:50, showed good activity (1320 kg PE/mol Ti·h·atm, entry 11). It is interesting that the replacement of Et2AlCl for Et3Al2Cl3, which usually leads to increased activity of nonmetallocene systems (for example, [32]), in our case led to its decrease by one-third (entry 11 vs. 12, Table 1).
The activation of catalytic systems I (entries 21–22, Table 1) and II (entries 13–14) by methylaluminoxane (MAO) or triisobutylaluminium (TIBA) in the Ti/Al molar ratio 1:300 is accompanied by a sharp drop in the activity and molecular weight of the obtained polymers. The morphology of the reactor powders also undergoes negative changes, which, in particular, is manifested in a marked increase in the bulk density.
Attempts to form catalytic systems similar to II but using ZrCl4·2THF or HfCl4·2THF or equimolar mixtures of ZrCl4·2THF and TiCl4 THF were either unsuccessful or had no effect on the heterometallic system activity (entries 15–16). This is not surprising given the results of all early works on Ziegler catalysis, starting with metallocene and nonmetallocene systems containing groups 4 and 5 metals, as well as a significantly higher reduction potential M4+/M3+ for zirconium and hafnium compounds (for metallocene Cp2MCl2: −E12 = 1.4 (Ti), 1.6–2.3 (Zr) and 2.7 (Hf) [33,34,35] and titanium and zirconium nonmetallocene compounds with a pyrone ligand: −E1\2 = 0.48 (Ti); 1.3 (Zr)) [36].
Thus, the results discussed above clearly indicate the involvement of Ti(III) in the formation of catalytic systems initially containing TiCl4 or TiCl4·2THF. As can be seen from Table 1, the properties of the catalytic systems III formed from TiCl3·3THF, with composition shown in Figure 1, behave directly opposite to systems based on TiCl4 2THF. For example, the presence of Et3Al in the system with TiCl3·3THF increases its activity (entry 17 vs. 19), and the use of aluminum sesquichloride instead of Et2AlCl does not lead to its fall (entry 18 vs. 20).
Catalytic system III was fairly active without the Et3Al cocatalyst (entries 18–20), but, in most cases, it was inferior to system II (Figure 4). In some cases, the ZN catalysts formed in situ (systems I and II, entries 1–16, in our opinion) showed superior results to systems obtained from single components [37,38]. As seen from Table 1, the presence of Et3Al in mixture (ceteris paribus) led to a significant increase in activity of the catalyst based on TiCl3·3THF (entry 17 vs. 19), which suggests that its role is not limited to the reduction of Ti(IV) to Ti(III), but is necessary for the effective alkylation of the transition metal.
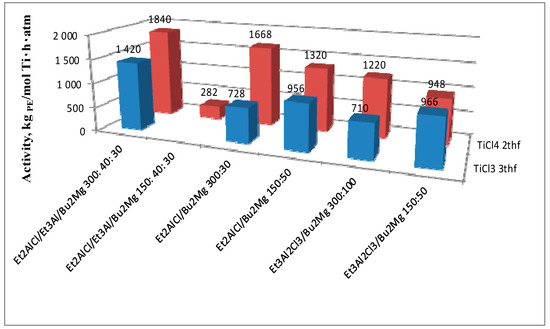
Figure 4.
Activities of catalytic systems with TiCl3 and TiCl4 tetrahydrofuran complexes versus the composition of the activation mixture.
Properties of Reactor Powders
All obtained polymers lack branching at the main chain, which is evident from the absence of an IR absorption band at 1378 cm−1, characteristic of the symmetric deformational vibrations of CH3 groups. The bulk density of the obtained UHMWPE reactor powders varies in the range from 0.05 to 0.09 g/cm3. The melting point and degree of crystallinity are typical for UHMWPE [1,39] and are in the range of 141–149 °C and 58–78% respectively (Table 1). Explicit differences in the characteristics of reactor powders of polymers obtained using catalytic systems of different compositions were not found.
Figure 5 shows the surface morphology of nascent PE powders obtained from the catalytic system I at different polymerization times. As is evident from Figure 5, the PE powder has an irregular surface, which makes it favorable for solvent-free processing. With an increase in the polymerization time, the porous structure of the particles changes, namely, the pore size decreases, and their upper layer is densified. Similar phenomena are observed for nascent PE powders obtained from the catalytic systems II and III (Figure 6).
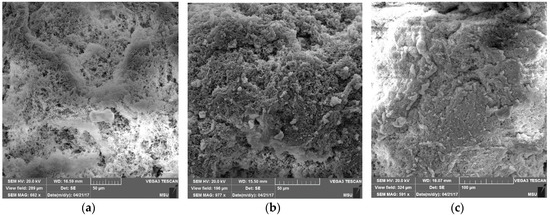
Figure 5.
SEM images of the surface morphology of PE powders obtained from the catalytic system I for 10 min (a), 15 min (b), and 30 min (c).
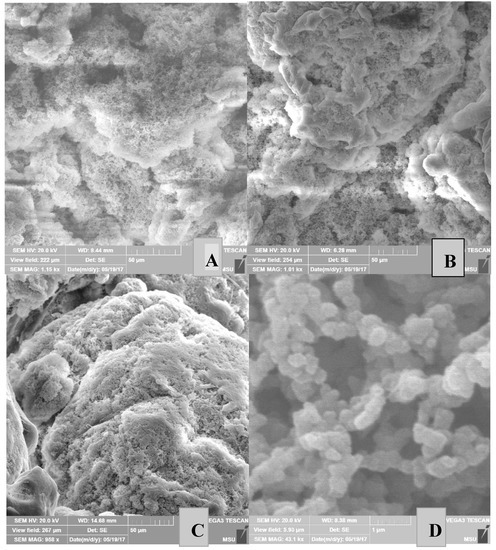
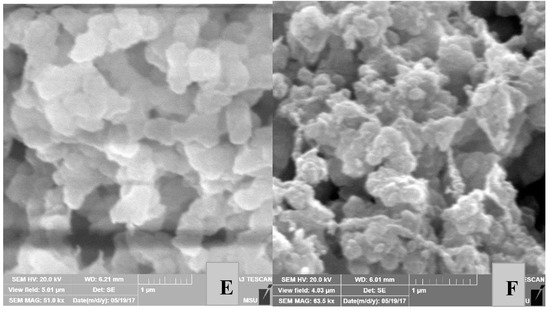
Figure 6.
SEM images of the surface morphology of PE powders obtained from catalytic systems III in entries 17 (A), 19 (B), 21 (C), 17 (D, increase 1 μm), 19 (E, increase 1 μm), and 21 (F, increase 1 μm).
The processing of reactor powders of UHMWPE obtained from catalytic systems I–III into high-modulus oriented films was carried out by preparing monolithic samples under pressure and shear deformation at an elevated temperature below the polymer melting point with subsequent uniaxial stretching by the method described by Ozerin et al. [8].
Table 2 summarizes the set mechanical characteristics of UHMWPE films. It should be noted that these films are characterized by a single-step character of the rupture, which indicates the uniformity of the obtained samples.

Table 2.
Mechanical properties of monolithic UHMWPE tapes.
The best values of the draw ratio as well as the elastic modulus and tensile strength for catalytic system I were achieved on the sample obtained after 10 min of polymerization (entry 2). Figure 7 shows the dependence of the elastic modulus and tensile strength of oriented films obtained by solvent-free processing of the reactor powder synthesized in catalytic system I on the drawing ratio.
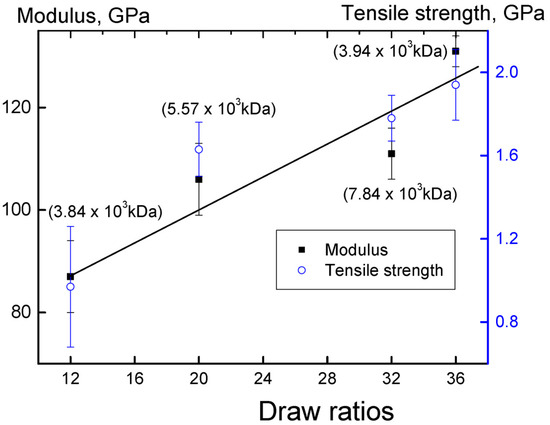
Figure 7.
The dependence of the elastic modulus and tensile strength of oriented films on the drawing ratio. Reactor powders obtained from the catalytic system I at different polymerization times were used (entries 2–5). Polymer molecular weights are indicated in parentheses.
With an increase in the degree of stretching, the strength properties of the oriented films increase linearly, and there is no apparent relationship between the molar masses of the polymer and its mechanical characteristics.
It is known that a solid-phase method can be used to process UHMWPE powders with a porous, “fluffy” particle surface [39]. It is probable that the appearance of fused zones on their surface (Figure 5) with an increase in the polymerization time reduces the quality of the resulting films and prevents the achievement of a high—more than 20-fold—drawing rate.
For correct comparison of the mechanical properties of UHMWPE films obtained from different catalytic systems, we selected samples with the same drawing rate equal to 20 (entries 3, 16–19, 21). As seen from the Table 2 (entries 6 and 16), the samples obtained from catalytic system II and from a mixture of TiCl4 2THF + ZrCl4 2THF did not show good mechanical properties, probably due to a long polymerization time. Films from reactor powders synthesized using the TiCl3 2THF are characterized by similar mechanical properties (within the error limits of the corresponding values), which confirms the high quality of the UHMWPE reactor powder of this type. They are inferior in elastic modulus to sample 3, obtained from catalytic system I, but superior to the samples obtained from system II and the mixed system {TiCl4 2THF + ZrCl4 2THF}.
Uniaxially drawn tapes from UHMWPE powders obtained from a system with Ti(III) significantly outperform the analogues obtained with the participation of Ti(IV) in terms of mechanical properties.
As shown by the experiments, polymers obtained from catalytic system I and from catalytic system III with the participation of TiCl3·2THF possess the highest drawing ratios. For the samples obtained in entries 2 and 21, the maximum values of the elastic modulus and tensile strength are achieved at the optimum value of elongation at break (Table 2, Figure 7).
4. Conclusions
The results indicate the expediency of individual titanium chloride tetrahydrofuran complex application for the formation of THF-containing catalyst systems. Aside from the obvious technological advantages, this approach allows a significant increase in the catalytic activity of systems without any reduction in the mechanical properties of UHMWPE. The introduction of an additional amount of THF into system II led to a sharp decrease in its activity.
The catalytic systems containing Ti(III) are, in most cases, inferior in productivity to systems with Ti(IV). This conclusion is consistent with the results reported by Jones et al. [40,41]. A comparison of the productivity of various catalysts for UHMWPE synthesis, including classic Ziegler–Natta (ZN), current commercial generation ZN, metallocene single site, and nonmetallocene catalysts, allowed them to reveal the following activity trend: TiCl3 < Mg/Si–TiCl3 < metallocenes ≈ nonmetallocenes.
Oriented film tapes from UHMWPE powders obtained from a Ti(III)-containing system noticeably outperform the physicomechanical properties of analogs obtained from a system with TiCl4·2THF. This fact confirms the correctness of the assumption that the Ti(IV) atoms are reduced to Ti(III) in systems I and II, and the fact of increased activity in system III in the presence of Et3Al indicates that its role is not limited to the reduction reaction alone and that it is apparently necessary for more effective alkylation of the transition metal.
The obtained data on the mechanical characteristics of UHMWPE oriented film tapes with an equal degree of drawing allow us to conclude that the nature of the catalytic system used in the synthesis of the reactor powder affects the strength properties of the filaments. In this case, both the activity of the catalytic system and the MW of the reactor powder are not among the main factors determining the mechanical properties of the film tapes. Perhaps the nature of the catalytic system, influencing the morphology of the reactor powder, indirectly predetermines the topology of the fibrillar structure, which is formed during the solid-phase forming of films and their subsequent uniaxial drawing. Its study, depending on the nature of the catalyst, requires additional research and is beyond the scope of this paper.
Acknowledgments
Financial support from the Russian Science Foundation (Project No. 16-13-10502) is gratefully acknowledged.
Author Contributions
Boris M. Bulychev, Vladislav A. Tuskaev and Svetlana C. Gagieva conceived and designed the experiments; Vladislav A. Tuskaev, Svetlana C. Gagieva, Dmitrii A. Kurmaev, Nikolay A. Kolosov, Elena S. Mikhaylik, Evgenii K. Golubev, Alexander I. Sizov, Sergey V. Zubkevich, Viktor G. Vasil’ev, Galina G. Nikiforova and Mikhail I. Buzin performed the experiments; Boris M. Bulychev, Olga A. Serenko, Vladislav A. Tuskaev, and Mikhail I. Buzin analyzed the data; Boris M. Bulychev, Vladislav A. Tuskaev and Olga A. Serenko wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kurtz, S.M. The UHMWPE Handbook: Ultra High Molecular Weight Polyethylene in Total Joint Replacement, 1st ed.; Elsevier Academic Press: New York, NY, USA, 2004; ISBN 9780080481463. [Google Scholar]
- Smith, P.; Chanzy, H.D.; Rotzinger, B.P. Drawing of virgin ultrahigh molecular weight polyethylene: An alternative route to high strength fibres. Polym. Commun. 1985, 26, 258. [Google Scholar]
- Smith, P.; Chanzy, H.D.; Rotzinger, B.P. Drawing of virgin ultrahigh molecular weight polyethylene: An alternative route to high strength/high modulus materials. J. Mater. Sci. 1987, 22, 523–531. [Google Scholar] [CrossRef]
- Rastogi, S.; Yao, Y.; Ronca, S.; Bos, J.; van der Eem, J. Unprecedented High-Modulus High-Strength Tapes and Films of Ultrahigh Molecular Weight Polyethylene via Solvent-Free Route. Macromolecules 2011, 44, 5558–5568. [Google Scholar] [CrossRef]
- Yao, Y.; Jiang, S.; Rastogi, S. 13C Solid State NMR Characterization of Structure and Orientation Development in the Narrow and Broad Molar Mass Disentangled UHMWPE. Macromolecules 2014, 47, 1371–1382. [Google Scholar] [CrossRef]
- Romano, D.; Ronca, S.; Rastogi, S. A Hemi-metallocene Chromium Catalyst with Trimethylaluminum-Free Methylaluminoxane for the Synthesis of Disentangled Ultra-High Molecular Weight Polyethylene. Macromol. Rapid Commun. 2015, 36, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Romano, D.; Andablo-Reyes, E.A.; Ronca, S.; Rastogi, S. Effect of a Cocatalyst Modifier in the Synthesis of Ultrahigh Molecular Weight Polyethylene having Reduced Number of Entanglements. J. Polym. Sci. A 2013, 51, 1630–1635. [Google Scholar] [CrossRef]
- Ozerin, A.N.; Ivanchev, S.S.; Chvalun, S.N.; Aulov, V.A.; Ivancheva, N.I.; Bakeev, N.F. Properties of Oriented Film Tapes Prepared via Solid State Processing of a Nascent Ultrahigh Molecular Weight Polyethylene Reactor Powder Synthesized with a Postmetallocene Catalyst. Polym. Sci. Ser. A 2012, 54, 950–954. [Google Scholar] [CrossRef]
- Won, H.Y.; Song, J.K.; Joo, Y.L.; Lee, H.K.; Park, N.C. Ultra-High Molecular Weight Polyethylene Subject Tub Method. Korean Patent KP98-15409, 29 April 1998. [Google Scholar]
- Joo, Y.K.; Zhou, H.; Lee, S.G.; Lee, H.K.; Song, J.K. Solid-State Compaction and Drawing of Nascent Reactor Powders of Ultra-High-Molecular-Weight Polyethylene. J. Appl. Polym. Sci. 2005, 98, 718–730. [Google Scholar] [CrossRef]
- Joo, Y.L.; Han, O.H.; Lee, H.K.; Song, J.K. Characterization of ultra high molecular weight polyethyelene nascent reactor powders by X-ray diffraction and solid state NMR. Polymer 2000, 41, 1355–1368. [Google Scholar] [CrossRef]
- Czaja, K.; Białek, M. Vanadium-based Ziegler-Natta catalyst supported on MgCl2(THF)2 for ethylene polymerization. Macromol. Rapid Commun. 1996, 17, 253–260. [Google Scholar] [CrossRef]
- Czaja, K.; Białek, M.; Ochedzan, W. Organometallic VCl4-based Catalyst supported on MgCl2(THF)2 for Ethylene Polymerization. Polimery 1997, 42, 714–716. [Google Scholar]
- Białek, M.; Czaja, K. Synthesis and characterization of ethylene-1-hexene copolymers prepared by using MgCl2(THF)2-supported Ziegler-Natta catalysts. Polimery 2000, 45, 293–296. [Google Scholar]
- Białek, M.; Czaja, K. The effect of the comonomer on the copolymerization of ethylene with long chain α-olefins using Ziegler–Natta catalysts supported on MgCl2(THF)2. Polymer 2000, 41, 7899–7904. [Google Scholar] [CrossRef]
- Kissin, Y.V.; Mink, R.I.; Brandolini, A.J.; Nowlin, T.E. AlR2Cl/MgR2 combinations as universal cocatalysts for Ziegler–Natta, metallocene, and post-metallocene catalysts. J. Polym. Sci. A 2009, 47, 3271–3285. [Google Scholar] [CrossRef]
- Kim, I.; Kim, J.H.; Choi, H.K.; Chung, M.C.; Woo, S.I. Comonomer enhancement effect of 1-hexene in ethylene copolymerization catalyzed over MgCl2/THF/TiCl4 catalysts. J. Appl. Polym. Sci. 1993, 48, 721–730. [Google Scholar] [CrossRef]
- Czala, K.; Bialek, M. Microstructure of ethylene-1-hexene and ethylene-1-octene copolymers obtained over Ziegler–Natta catalysts supported on MgCl2(THF)2. Polymer 2001, 42, 2289–2298. [Google Scholar] [CrossRef]
- Sobota, P. Metal-assembled compounds: Precursors of polymerization catalysts and new materials. Coord. Chem. Rev. 2004, 248, 1047–1060. [Google Scholar] [CrossRef]
- Seenivasan, K.; Sommazzi, A.; Bonino, F.; Bordiga, S.; Groppo, E. Spectroscopic Investigation of Heterogeneous Ziegler–Natta Catalysts: Ti and Mg Chloride Tetrahydrofuranates, Their Interaction Compound, and the Role of the Activator. Chem. Eur. J. 2011, 17, 8648–8656. [Google Scholar] [CrossRef] [PubMed]
- Grau, E.; Lesage, A.; Norsic, S.; Copéret, C.; Monteil, V.; Sautet, P. Tetrahydrofuran in TiCl4/THF/MgCl2: A Non-Innocent Ligand for Supported Ziegler–Natta Polymerization Catalysts. ACS Catal. 2013, 3, 52–56. [Google Scholar] [CrossRef]
- Belsky, V.K.; Bulychev, B.M.; Streltsova, N.R. Crystaline-structure of homometallic and heterometallic crown-compounds of [TiClx15K5(MU-2 = 0)TiCl5], [TiCl2x15K5][AlCl4] and [Mgx15K5x(CH3CN)2][TiCl6]. Russ. J. Inorg. Chem. 1992, 37, 1531. [Google Scholar]
- Sobota, P.; Utko, J.; Janas, Z. MgCl2—One of the factors controlling the mechanism of the reaction between grignard reagents and titanium or zirconium tetrachloride. II. J. Organomet. Chem. 1986, 316, 19–23. [Google Scholar] [CrossRef]
- Belsky, V.K.; Bulychev, B.M. Structural-chemical aspects of complexation in metal halide–macrocyclic polyether systems. Russ. Chem. Rev. 1999, 68, 119–135. [Google Scholar] [CrossRef]
- Busico, V. Metal-catalysed olefin polymerization into the new millennium: A perspective outlook. Dalton Trans. 2009, 41, 8794–8802. [Google Scholar] [CrossRef] [PubMed]
- Bryliakov, K.P.; Talsi, E.P. Frontiers of mechanistic studies of coordination polymerization and oligomerization of α-olefins. Coord. Chem. Rev. 2012, 256, 2994–3007. [Google Scholar] [CrossRef]
- Valente, A.; Mortreux, A.; Visseaux, M.; Zinck, P. Coordinative Chain Transfer Polymerization. Chem. Rev. 2013, 113, 3836–3857. [Google Scholar] [CrossRef] [PubMed]
- Sloan, T.E. Tetrahydrofuran Complexes of Selected Early Transition Metals. Inorg. Synth. 1982, 21, 135–136. [Google Scholar]
- Jones, N.A.; Liddle, S.T.; Wilson, C.; Arnold, P.L. Titanium(III) Alkoxy-N-heterocyclic Carbenes and a Safe, Low-Cost Route to TiCl3(THF)3. Organometallics 2007, 26, 755–757. [Google Scholar] [CrossRef]
- Bravaya, N.M.; Saratovskikh, S.L.; Mukhina, E.V.; Smirnova, O.V.; Gagieva, S.C.; Tuskaev, V.A.; Bulychev, B.M. Influence of polymerization conditions on catalytic properties of the TiCl2{η2-1-[C(H)=N(2,3,5,6-tetrafluorophenyl)]-2-O-3,5-di-But-C6H2}2/MAO system in ethylene homopolymerization and copolymerization with α-olefins. Russ. Chem. Bull. 2013, 62, 2500–2508. [Google Scholar] [CrossRef]
- Forte, G.; Ronca, S. Synthesis of Disentangled Ultra-High Molecular Weight Polyethylene: Influence of Reaction Medium on Material Properties. Int. J. Polym. Sci. 2017, 7431419. [Google Scholar] [CrossRef]
- Mehta, A.; Tembe, G.; Parikh, P.; Mehta, G. Titanasilsesquioxane-Alkylaluminum Catalyst System for Ethylene Polymerization. Mod. Res. Catal. 2012, 1, 29–42. [Google Scholar] [CrossRef]
- Dessy, R.E.; King, R.B.; Waldrop, M. Organometallic Electrochemistry. V. The Transition Series. J. Am. Chem. Soc. 1966, 88, 5112–5117. [Google Scholar] [CrossRef]
- Wailes, P.C.; Coutts, R.S.P.; Weigold, H. Organometallic Chemistry of Titanium, Zirconium and Hafnium; Academic Press: New York, NY, USA, 1974; p. 302. [Google Scholar] [CrossRef]
- Samuel, E. Reduction of zirconocene dihalides with magnesium studied by ESR. Evidence of zirconium(III) hydride formation by hydrogen transfer from the cyclopentadienyl ring. Inorg. Chem. 1983, 22, 2967–2970. [Google Scholar] [CrossRef]
- Basso, N.R.; Greco, P.P.; Carone, C.L.P.; Livotto, P.R.; Simplıcio, L.M.T.; da Rocha, Z.N.; Galland, G.B.; dos Santos, J.H.Z. Reactivity of zirconium and titanium alkoxides bidentade complexes on ethylene polymerization. J. Mol. Catal. A 2007, 267, 129–136. [Google Scholar] [CrossRef]
- Tuskaev, V.A.; Gagieva, S.C.; Kurmaev, D.A.; Kolosov, N.A.; Fedyanin, I.V.; Bulychev, B.M. Titanium (IV) complexes stabilized by 2,6-bis-(α,α’-diphenyl(hydroxy)methyl)-pyridine: Catalytic activity in olefin polymerization and impact of lithium and magnesium chlorides. Inorg. Chim. Acta 2015, 425, 275–281. [Google Scholar] [CrossRef]
- Tuskaev, V.A.; Gagieva, S.C.; Maleev, V.I.; Borissova, A.O.; Solov’ev, M.V.; Starikova, Z.A.; Bulychev, B.M. Titanium (IV) and zirconium (IV) chloride complexes on the base of chiral tetraaryl-1,3-dioxolane-4,5-dimetanol ligands in the polymerization of ethylene: The promoting role of lithium and magnesium chloride. Polymer 2013, 54, 4455–4462. [Google Scholar] [CrossRef]
- Garkhail, S.K. Easily Processable Ultra High Molecular Weight Polyethylene with Narrow Molecular Weight Distribution; Technische Universiteit Eindhoven: Eindhoven, The Netherlands, 2005. [Google Scholar] [CrossRef]
- Jones, R.L.; Armoush, M. Catalysts for UHMWPE. Macromol. Symp. 2009, 283–284, 88–95. [Google Scholar] [CrossRef]
- Jones, R.L., Jr.; Armoush, M.Z.; Harjati, T.; Elder, M.; Hummel, A.A.; Sullivan, J. Catalysts for UHMWPE and UHMWPE-copolymers. Inorg. Chim. Acta 2010, 364, 275–281. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).