Long-Term Stable Solid-State Dye-Sensitized Solar Cells Assembled with Solid-State Polymerized Hole-Transporting Material
Abstract
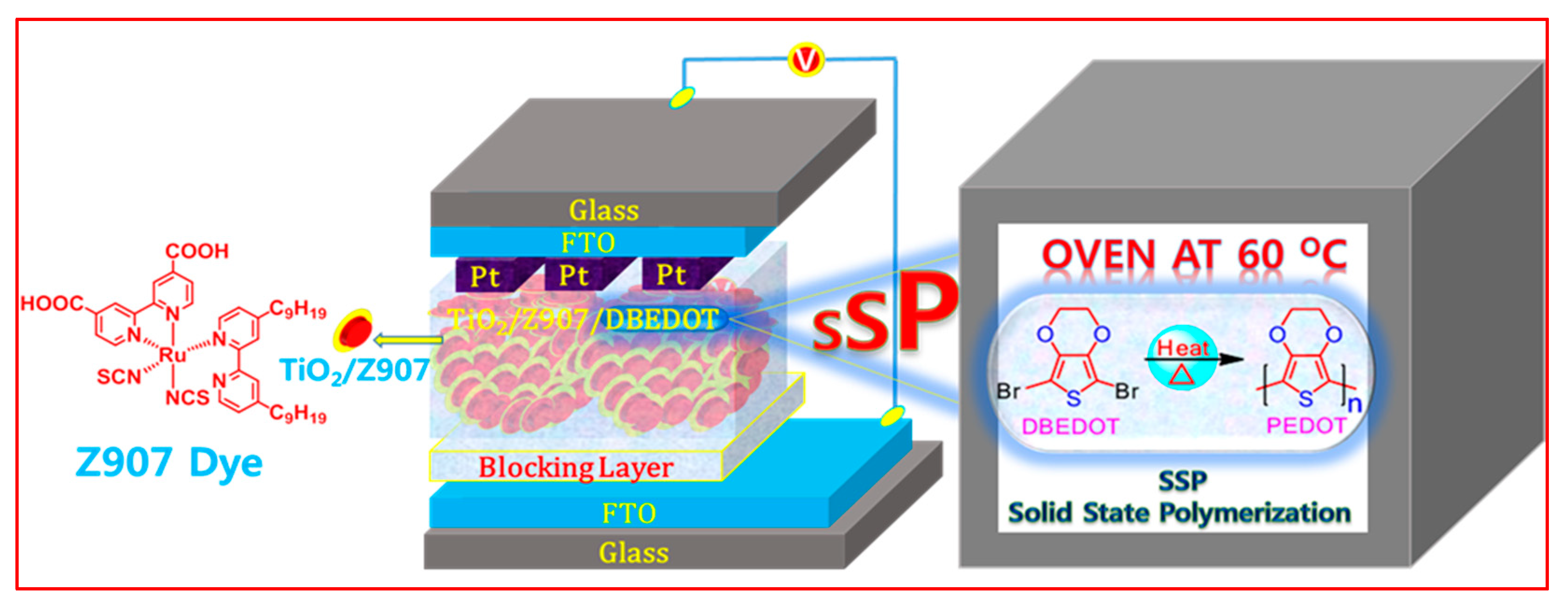
:1. Introduction
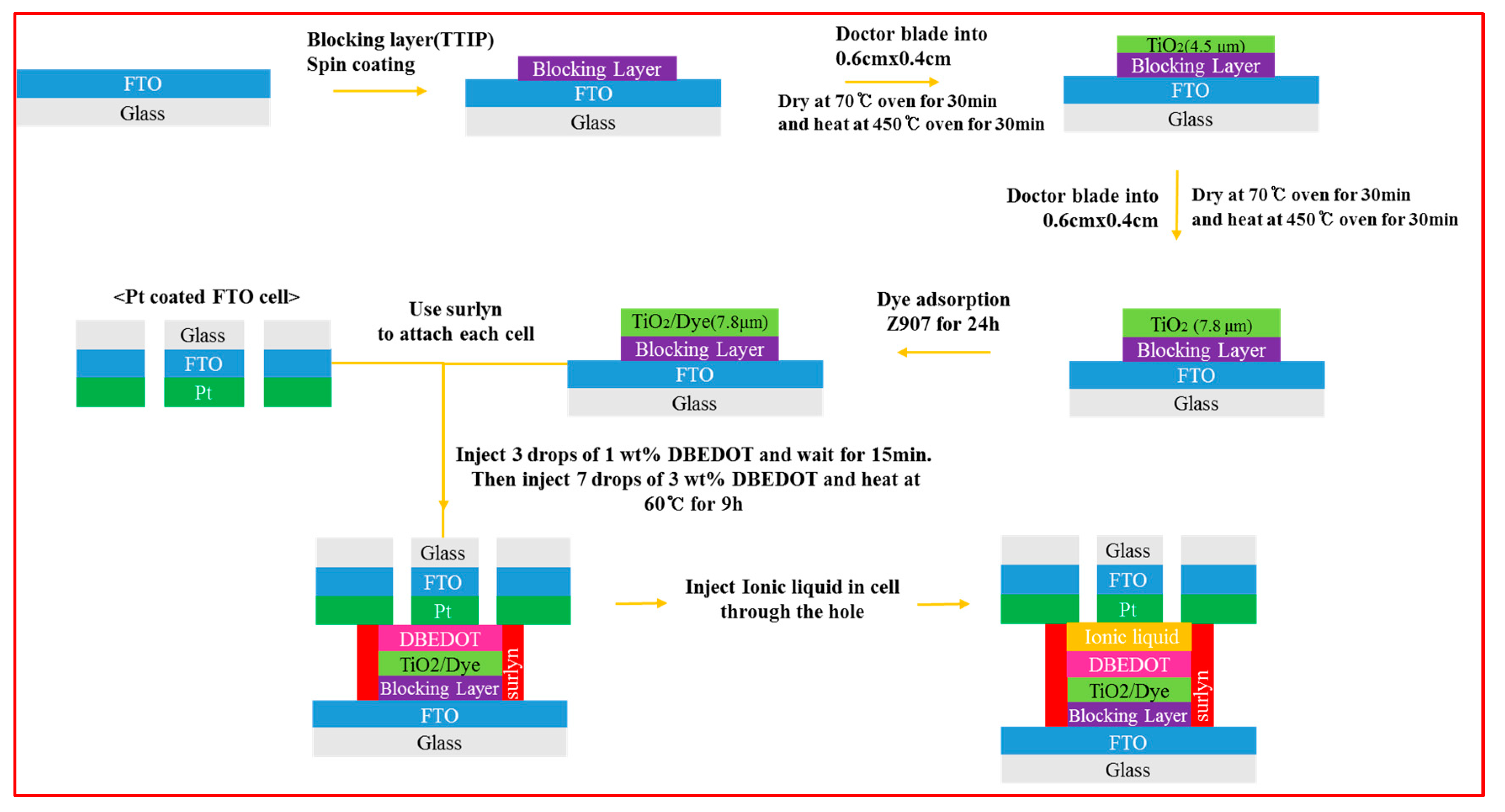
2. Materials and Methods
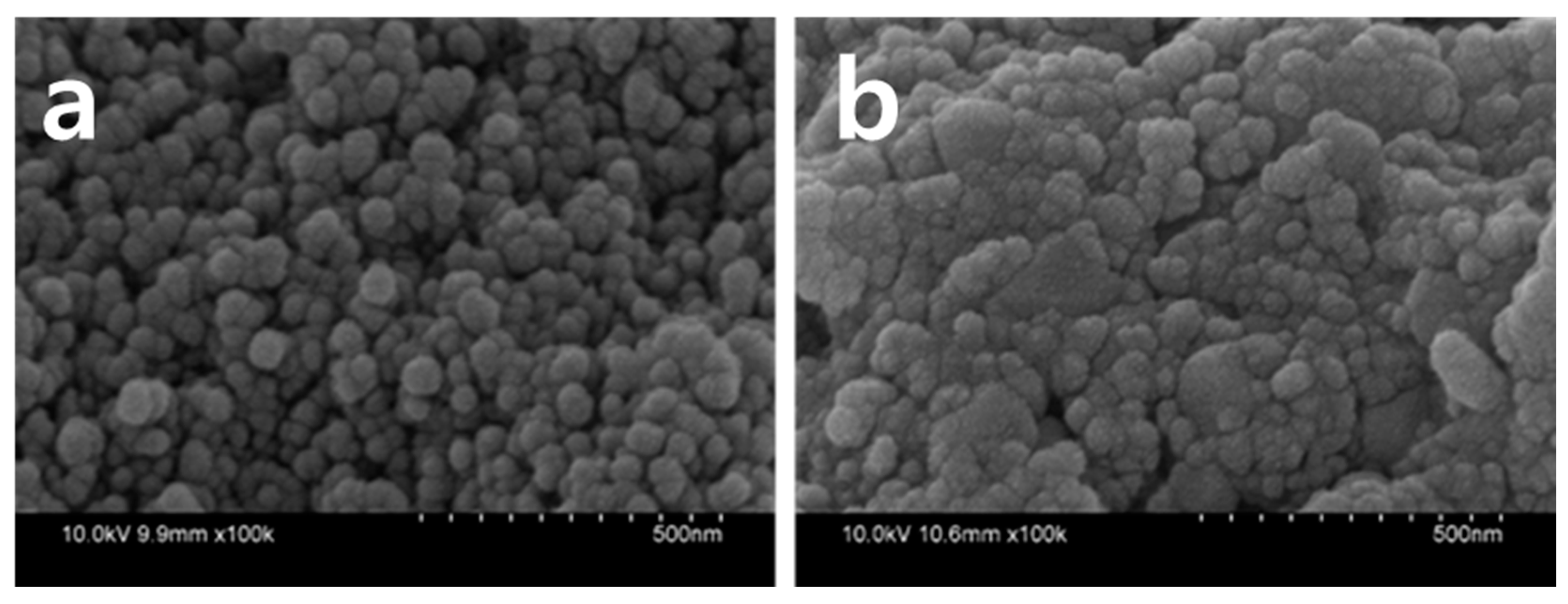
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Thomas, S.W.; Joly, G.D.; Swager, T.M. Chemical sensors base on amplifying fluorescent conjugated polymers. Chem. Rev. 2007, 107, 1339–1386. [Google Scholar] [CrossRef] [PubMed]
- Beaujuge, P.M.; Reynolds, J.R. Color control in π-conjugated organic polymers for use in electrochromic devices. Chem. Rev. 2010, 110, 268–320. [Google Scholar] [CrossRef] [PubMed]
- Burroughes, J.H.; Bradley, D.D.C.; Brown, A.R.; Marks, R.N.; Mackay, K.; Friend, R.H.; Burns, P.L.; Holmes, A.B. Light-emitting diodes based on conjugated polymers. Nature 1990, 347, 539–541. [Google Scholar] [CrossRef]
- Zaumseil, J.; Sirringhaus, H. Electron and ambipolar transport in organic field-effect transistors. Chem. Rev. 2007, 107, 1296–1323. [Google Scholar] [CrossRef] [PubMed]
- Senadeera, R.; Fukuri, N.; Saito, Y.; Takayuki, K.; Wada, Y.; Yanagida, S. Volatile solvent-free solid-state polymer-sensitized TiO2 solar cells with poly(3,4-ethylenedioxythiophene) as a hole-transporting medium. Chem. Commun. 2005, 17, 2259–2261. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Torres, P.; Tscharner, R.; Wyrsch, N.; Keppner, H. Photovoltaic technology: the case for thin-film solar cells. Science 1999, 285, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Levermore, P.A.; Chen, L.; Wang, X.; Das, R.; Bradley, D.D.C. Highly conductive poly(3,4-ethylenedioxythiophene) films by vapor phase polymerization for application in efficient organic light-emitting diodes. Adv. Mater. 2007, 19, 2379–2385. [Google Scholar] [CrossRef]
- Kim, Y.H.; Sachse, C.; Machala, M.L.; May, C.; Mller-Meskamp, L.; Leo, K. Highly conductive PEDOT:PSS electrode with optimized solvent and thermal post-treatment for ITO-free organic solar cells. Adv. Funct. Mater. 2011, 21, 1076–1081. [Google Scholar] [CrossRef]
- Charvet, R.; Acharya, S.; Hill, J.P.; Akada, M.; Liao, M.; Seki, S.; Honsho, Y.; Saeki, A.; Ariga, K. Block-copolymer-nanowires with nanosized domain segregation and high charge mobilities as stacked p/n heterojunction arrays for repeatable photocurrent switching. Am. Chem. Soc. 2009, 131, 18030–18031. [Google Scholar] [CrossRef]
- Thuy, C.T.T.; Jung, J.H.; Thogiti, S.; Jung, W.S.; Ahn, K.S.; Kim, J.H. Graphene coated alumina-modified polypyrrole composite films as an efficient Pt-free counter electrode for dye-sensitized solar cells. Electrochim. Acta. 2016, 205, 170–177. [Google Scholar] [CrossRef]
- Thuy, C.T.T.; Park, J.Y.; Lee, S.W.; Suresh, T.; Kim, J.H. Surfactant effect in polypyrrole and polypyrrole with multi wall carbon nanotube counter electrodes: improved power conversion efficiency of dye-sensitized solar cell. J. Nanosci. Nanotechnol. 2016, 16, 5263–5267. [Google Scholar] [CrossRef] [PubMed]
- Rumbau, V.; Pomposo, A.; Eleta, A.; Rodriguez, J.; Grande, H.; Mecerreyes, D.; Ochoteco, E. First enzymatic synthesis of water-soluble conducting poly(3,4-ethylenedioxythiophene). Biomacromolecules 2007, 2, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Heinze, J.; Frontana-Uribe, B.A.; Ludwigs, S. Electrochemistry of conducting polymers--persistent models and new concepts. Chem. Rev. 2010, 110, 4724–4771. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, R.H.; Herland, A.; Hamedi, M.; Wigenius, J.A.; Åslund, A.; Liu, X.; Fahlman, M.; Inganas, O.; Konradsson, P. Iron-catalyzed polymerization of alkoxysulganate-functionalized 3,4-ethylenedioxythiophene gives water-soluble poly(3,4-Ethylenedioxythiophene) of high conductivity. Chem. Mater. 2009, 21, 1815–1821. [Google Scholar] [CrossRef]
- Lee, S.; Panie, D.C.; Gleason, K.K. Heavily doped poly(3,4-ethylenedioxythiophene) thin films with high carrier mobility deposited using oxidative CVD: conductivity stability and carrier transport. Adv. Funct. Mater. 2014, 24, 7187–7196. [Google Scholar] [CrossRef]
- Kim, J.; You, J.; Kim, B.; Park, T.; Kim, E. Solution processable and patternable poly(3,4-alkylenedioxythiophene)s for large-area electrochromic films. Adv. Mater. 2011, 23, 4168–4173. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Perepichka, D.F.; Bendikov, M.; Wudl, R.; Pan, G.Z.; Yu, W.; Dong, W.; Brown, W. Solid-state synthesis of a conducting polythiophene via an unprecedented heterocyclic coupling reaction. J. Am. Chem. Soc. 2003, 125, 15151–15162. [Google Scholar] [CrossRef]
- Kim, B.; Koh, J.K.; Kim, J.; Chi, W.S.; Kim, J.H.; Kim, E. Room temperature solid-state synthesis of a conductive polymer for applications in stable I2-free dye-sensitized solar cells. ChemSusChem 2012, 11, 2173–2180. [Google Scholar] [CrossRef]
- Koh, J.K.; Kim, J.; Kim, B.; Kim, J.H.; Kim, E. Highly efficient, iodine-free dye-sensitized solar cells with solid-state synthesis of conducting polymers. Adv. Mater. 2011, 23, 1641–1646. [Google Scholar] [CrossRef]
- Kim, J.; Koh, J.K.; Kim, B.; Ahn, S.H.; Ahn, H.; Ryu, D.Y.; Kim, J.H.; Kim, E. Enhanced performance of I2-free solid-state dye-sensitized solar cells with conductive polymer up to 6.8%. Adv. Funct. Mater. 2011, 21, 4633–4639. [Google Scholar] [CrossRef]
- Kim, D.W.; Cheruku, R.; Thogiti, S.; Koyyada, G.; Ho, P.; Kim, J.H. The effect of difference molar ratios of Dibromo-EDOT as hole transporting material for solid state dye-sensitized solar cells. J. Inorg. Organomet. Polym. Mater. 2017, 28, 2871–2874. [Google Scholar] [CrossRef]
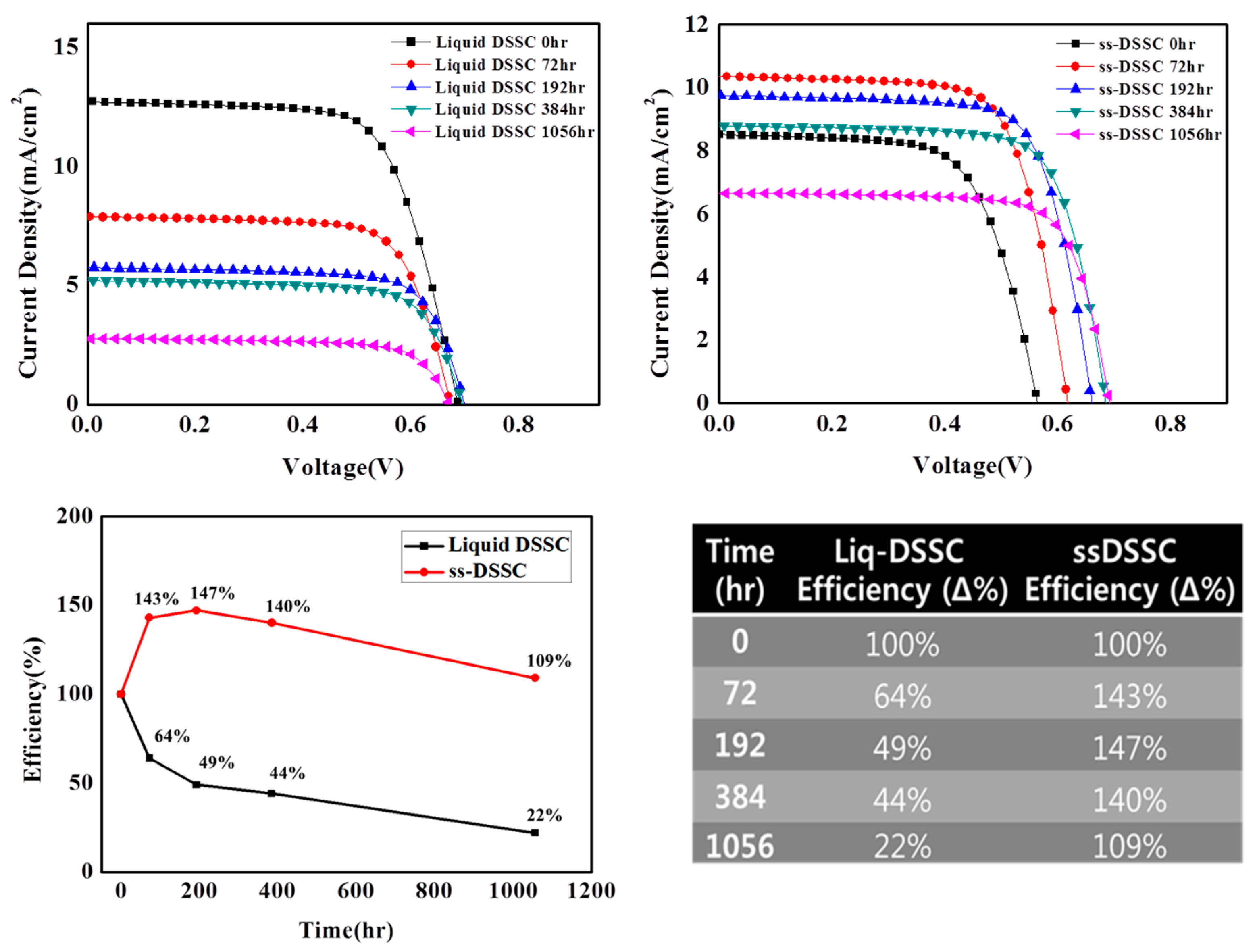
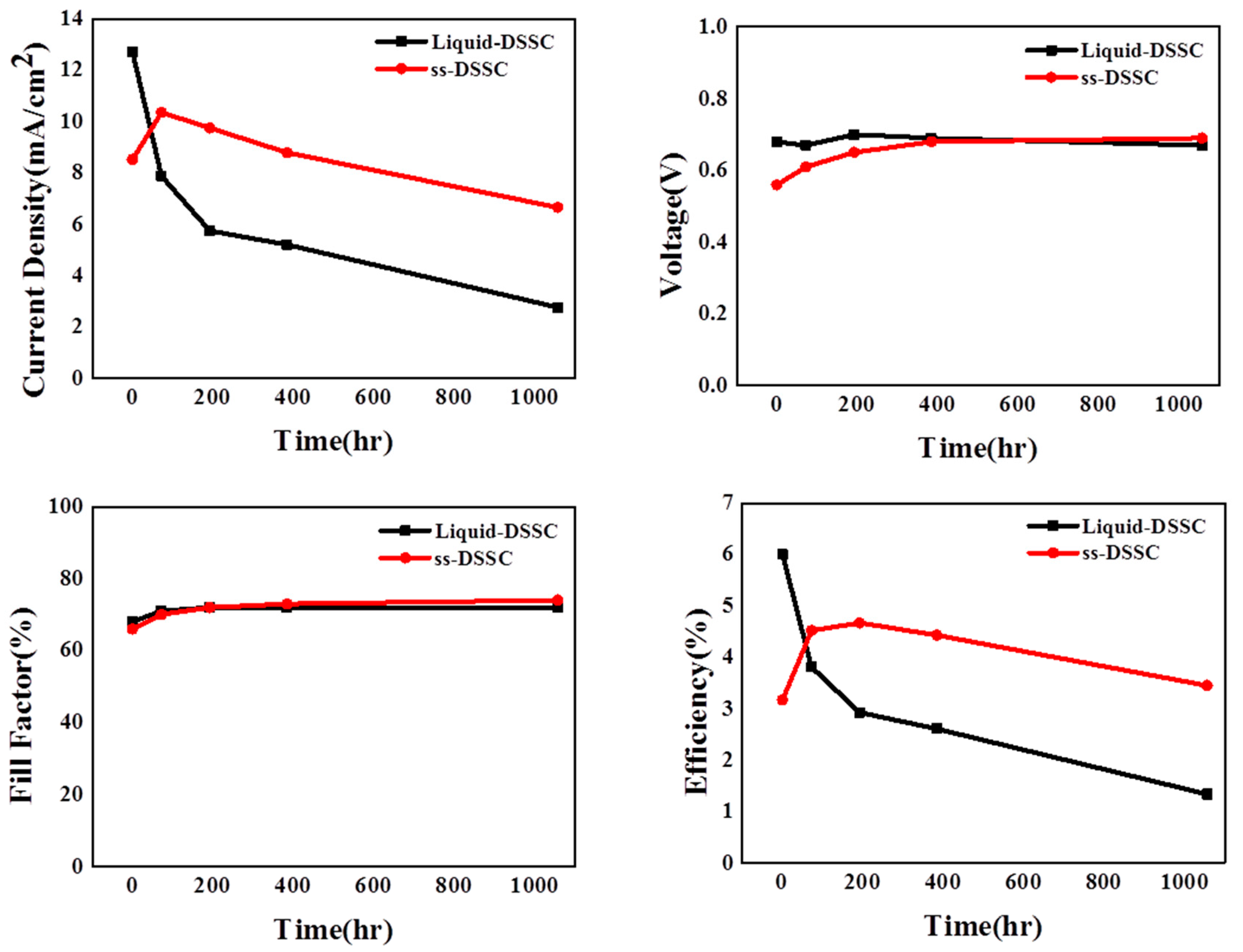
| Sample | Jsc | Voc | FF | η |
|---|---|---|---|---|
| Liquid DSSC 0 h | 12.70 ± 0.1 | 0.68 ± 0.002 | 0.68 ± 0.02 | 5.97 ± 0.27 |
| ss-DSSC 0 h | 8.54 ± 0.36 | 0.56 ± 0.005 | 0.66 ± 0.01 | 3.19 ± 0.20 |
| Liquid DSSC 72 h | 7.83 ± 0.12 | 0.68 ± 0.011 | 0.70 ± 0.01 | 3.73 ± 0.02 |
| ss-DSSC 72 h | 10.38 ± 0.44 | 0.61 ± 0.006 | 0.71 ± 0.01 | 4.51 ± 0.24 |
| Liquid DSSC 192 h | 5.75 ± 0.15 | 0.70 ± 0.01 | 0.73 ± 0.02 | 2.94 ± 0.20 |
| ss-DSSC 192 h | 9.78 ± 0.23 | 0.65 ± 0.001 | 0.72 ± 0.002 | 4.61 ± 0.12 |
| Liquid DSSC 384 h | 5.25 ± 0.45 | 0.68 ± 0.02 | 0.69 ± 0.03 | 2.46 ± 0.12 |
| ss-DSSC 384 h | 8.80 ± 0.20 | 0.68 ± 0.004 | 0.73 ± 0.005 | 4.36 ± 0.09 |
| Liquid DSSC 1056 h | 2.80 ± 0.19 | 0.67 ± 0.009 | 0.70 ± 0.02 | 1.30 ± 0.12 |
| ss-DSSC 1056 h | 6.66 ± 0.25 | 0.69 ± 0.004 | 0.75 ± 0.004 | 3.42 ± 0.15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, Y.J.; Thogiti, S.; Lee, K.-y.; Kim, J.H. Long-Term Stable Solid-State Dye-Sensitized Solar Cells Assembled with Solid-State Polymerized Hole-Transporting Material. Crystals 2019, 9, 452. https://doi.org/10.3390/cryst9090452
Jang YJ, Thogiti S, Lee K-y, Kim JH. Long-Term Stable Solid-State Dye-Sensitized Solar Cells Assembled with Solid-State Polymerized Hole-Transporting Material. Crystals. 2019; 9(9):452. https://doi.org/10.3390/cryst9090452
Chicago/Turabian StyleJang, Yu Jeong, Suresh Thogiti, Kang-yong Lee, and Jae Hong Kim. 2019. "Long-Term Stable Solid-State Dye-Sensitized Solar Cells Assembled with Solid-State Polymerized Hole-Transporting Material" Crystals 9, no. 9: 452. https://doi.org/10.3390/cryst9090452
APA StyleJang, Y. J., Thogiti, S., Lee, K.-y., & Kim, J. H. (2019). Long-Term Stable Solid-State Dye-Sensitized Solar Cells Assembled with Solid-State Polymerized Hole-Transporting Material. Crystals, 9(9), 452. https://doi.org/10.3390/cryst9090452





