The Structural Dimorphism of Lanthanum Oxide Fluoride Selenide La2OF2Se
Abstract
:1. Introduction
2. Materials and Methods
3. Results
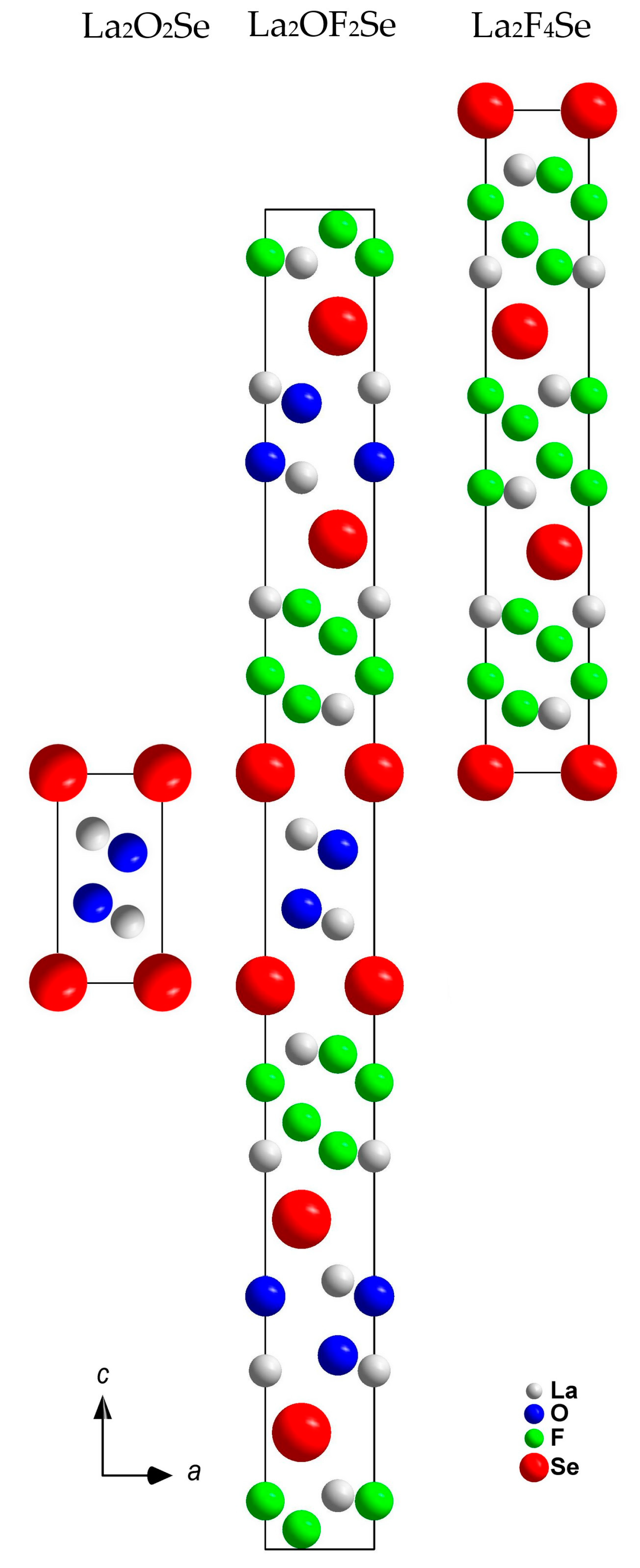
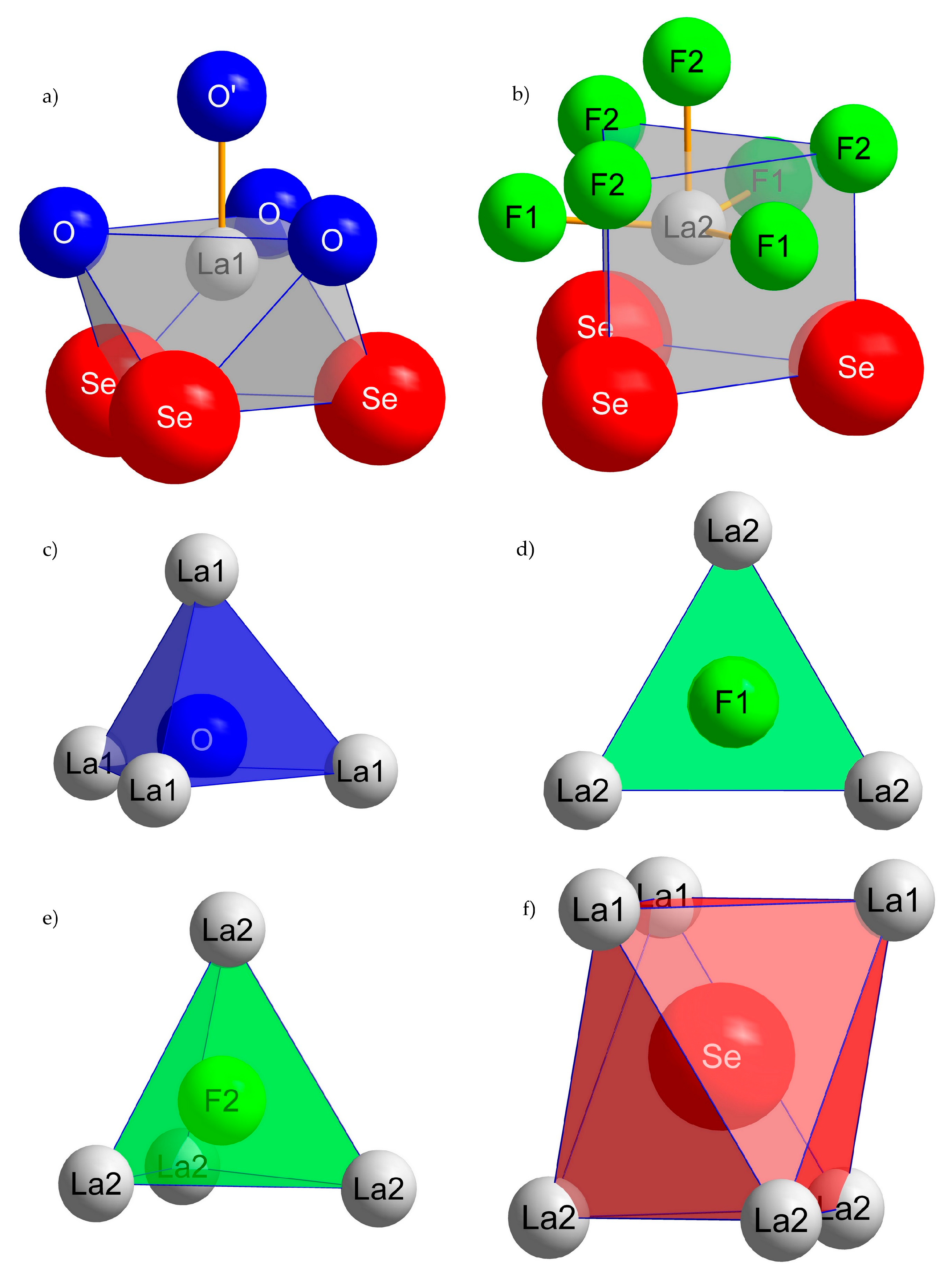
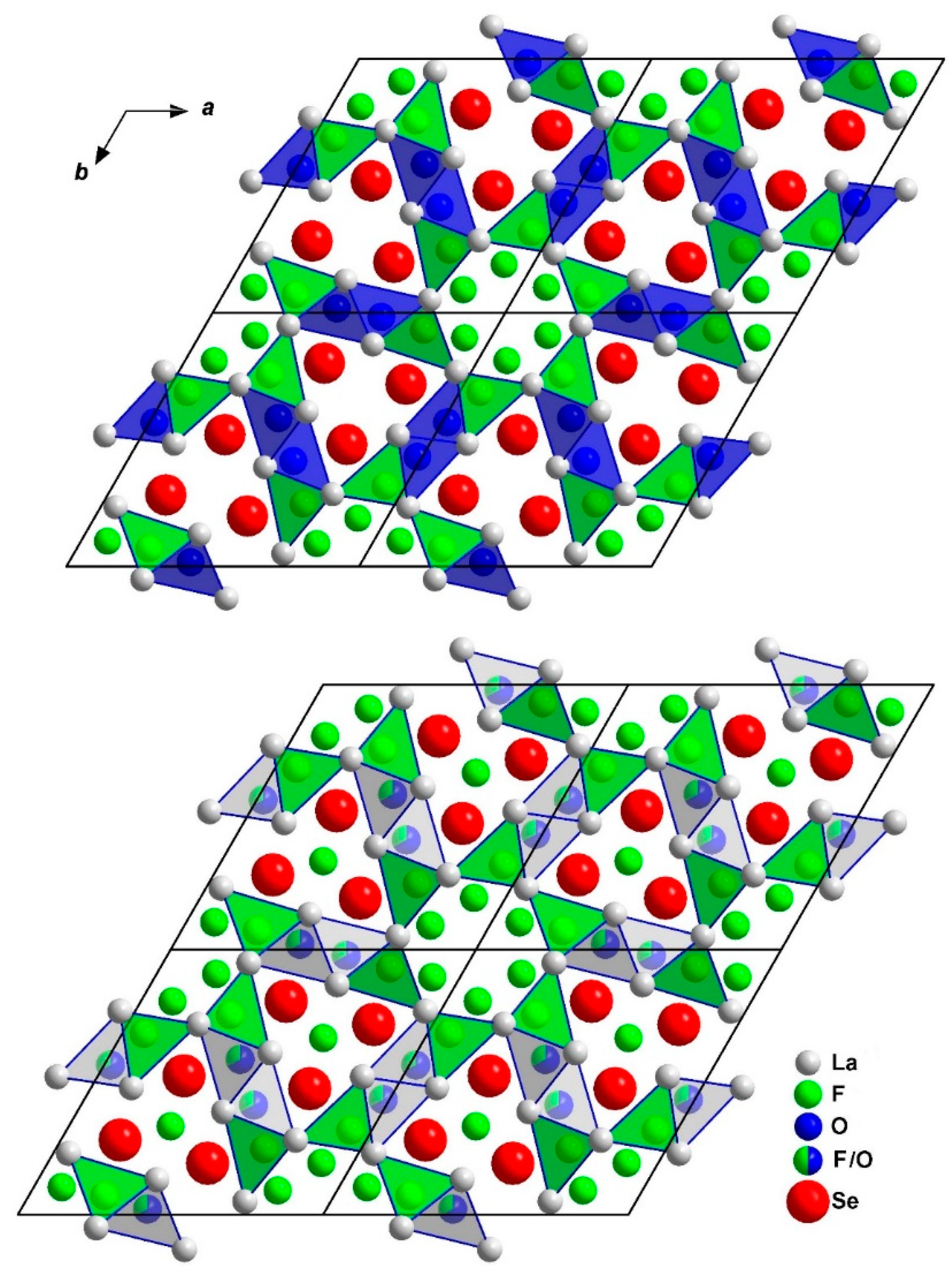
3.1. Structure Description of A-Type La2OF2Se
3.2. Structure Description of B-Type La2OF2Se
3.3. Single-Crystal Data of A- and B-Type La2OF2Se
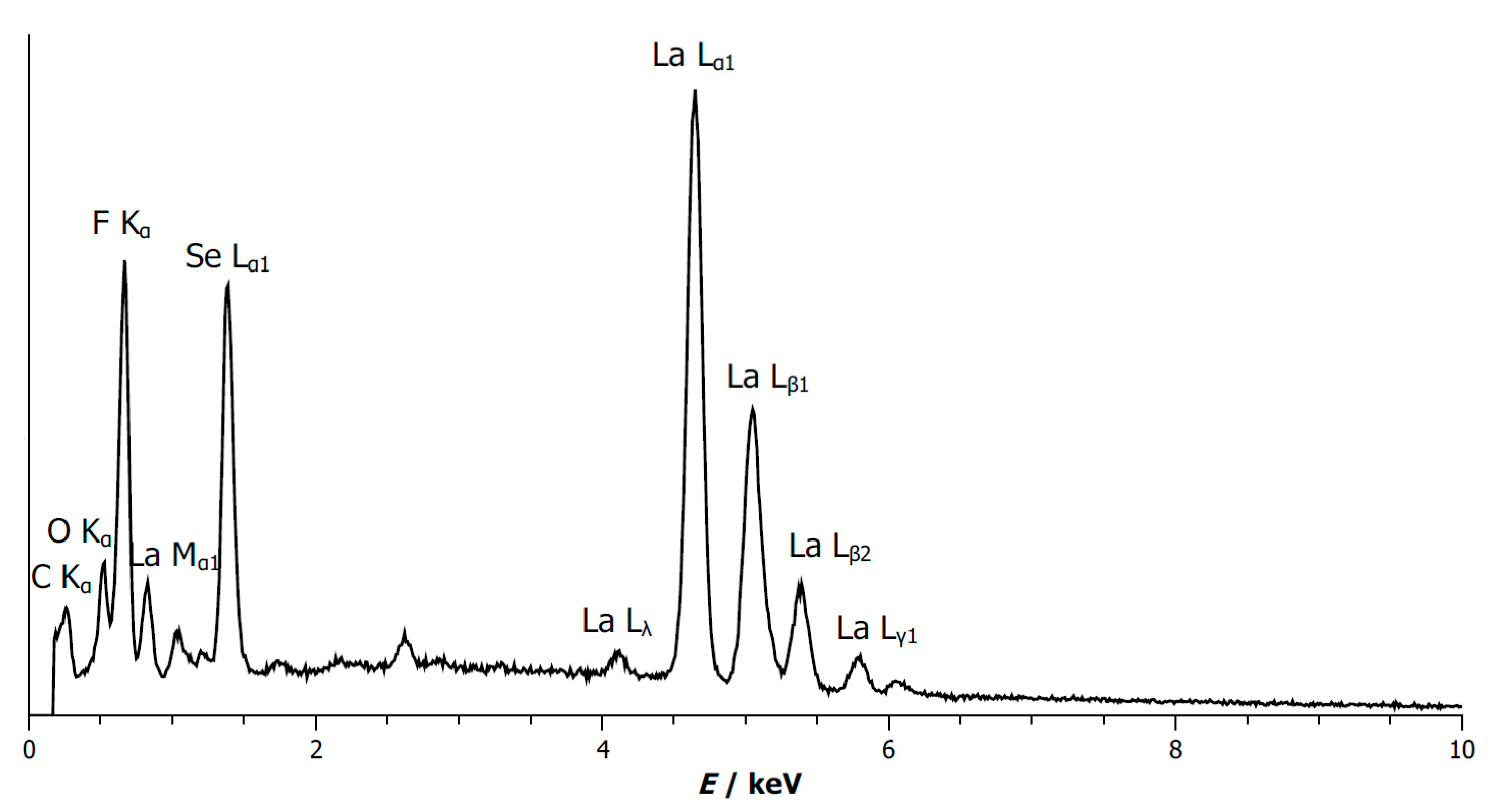
3.4. Energy-Dispersive X-Ray Spectrum of B-Type La2OF2Se
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Folchnandt, M.; Schleid, T. Single Crystals of C-La2Se3, C-Pr2Se3, and C-Gd2Se3 with Cation-Deficient Th3P4-Type Structure. Z. Anorg. Allg. Chem. 2001, 627, 1411–1413. [Google Scholar] [CrossRef]
- Schneck, C.; Höss, P.; Schleid, T. C-type Nd2Se3. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, i20. [Google Scholar] [CrossRef]
- Folchnandt, M.; Schneck, C.; Schleid, T. Über Sesquiselenide der Lanthanoide: Einkristalle von Ce2Se3 im C-, Gd2Se3 im U- und Lu2Se3 im Z-Typ. Z. Anorg. Allg. Chem. 2004, 630, 149–155. [Google Scholar] [CrossRef]
- Prokof’ev, A.V.; Shelykh, A.I.; Golubkov, A.V.; Sharenkova, N.V. Preparation and optical properties of Ln2Se3 rare-earth selenides. Inorg. Mater. 1994, 30, 326–327. [Google Scholar]
- Grundmeier, T.; Urland, W. Zur Polymorphie von Sm2Se3. Z. Anorg. Allg. Chem. 1995, 621, 1977–1979. [Google Scholar] [CrossRef]
- Julien Pouzol, M.; Guittard, M. Les phases cubiques de type Th3P4 dans les systèmes Cu2Se - L2Se3 et Ag2Se - L2Se3. Bull. Soc. Chim. Fr. 1968, 35, 2293–2295. [Google Scholar]
- Julien-Pouzol, M.; Guittard, M. Etude cristallochimique des combinaisons ternaires cuivre-terre rare soufre ou selenium, situees le long des binaires Cu2X-L2X3. Ann. Chim. France 1972, 7, 253–264. [Google Scholar]
- Golabi, S.M.; Flahaut, J.; Domange, L. Compounds of the Th3P4 type formed between rare-earth selenides and alkaline-earth selenides. Compt. Rend. Hebd. Acad. Sci. 1964, 259, 820–822. [Google Scholar]
- Dudnik, E.M.; Lashkarev, G.V.; Paderno, Y.B.; Obolonchik, V.A. Thermal expansion of the chalcogenides of rare-earth metals. Inorg. Mater. 1966, 2, 833–836. [Google Scholar]
- Loriers, J.; Suchet, J.; Weill, G.; Collin, G. Structure, susceptibilité magnétique et conductibilité thermique de quelques séléniures ternaires d’argent et de terres rares. Compt. Rend. Hebd. Acad. Sci. 1965, 35, 2219–2222. [Google Scholar]
- Miller, J.F.; Matson, L.K.; Himes, R.C. Studies on the selenides and tellurides of selected rare-earth metals. In Proceedings of the Conference on Rare Earths Research, Glenwood Springs, CO, USA, 15–16 February 1961; pp. 233–248. [Google Scholar]
- Pribyl’skii, N.Y.; Gamidov, R.S. The Cu2Se - Pr2Se3 system. Russ. J. Inorg. Chem. 1984, 11, 1707–1708. [Google Scholar]
- Efendiev, G.K.; Karaev, Z.S.; Nasibov, I.O. About the interaction of selenides A(III)2B(VI)3 of neodymium and gallium. Azerb. Khim. Zh. 1964, 4, 111–114. [Google Scholar]
- Pribyl’skaya, N.Y.; Eliseev, A.A.; Pribyl’skii, N.Y.; Gamidov, R.S. Homogeneity regions of lanthanide selenides with a Th3P4 structure. Russ. J. Inorg. Chem. 1984, 3, 451–452. [Google Scholar]
- Holtzberg, F.; Okaya, Y.; Stemple, N.R. Th3P4 type rare earth compounds. In Proceedings of the Annual Meeting of American Crystallographic Association, Gatlinburg, TN, USA, 27 June–2 July 1965; p. 46. [Google Scholar]
- Guittard, M.; Benacerraf, A.; Flahaut, J. Les seleniures L2Se3 et L3Se4 des elements des terres rares. Ann. Chim. France 1964, 9, 25–34. [Google Scholar]
- Flahaut, J.; Laruelle, P.; Pardo, M.P.; Guittard, M. Les sulfures, seleniures et tellurures L2X3 de terres rares, d’yttrium et de scandium orthorhombiques du type Sc2S3. Bull. Soc. Chim. Fr. 1965, 32, 1399–1404. [Google Scholar]
- Holtzberg, F.; McGuire, T.R.; Methfessel, S.; Suits, J.C. Ferromagnetism in Rare-Earth Group VA and VIA Compounds with Th3P4 Structure. J. Appl. Phys. 1964, 35, 1033–1038. [Google Scholar] [CrossRef]
- Pribyl’skii, N.Y.; Gamidov, R.S.; Vasil’eva, I.G. Phase equilibria in the Cu-Gd-Se system. Izv. Sibirsk. Otdel. Akad. Nauk SSSR Ser. Khim. Nauk 1983, 1983, 51–54. [Google Scholar]
- Eatough, N.L.; Webb, A.W.; Hall, H.T. High-pressure Th3P4-type polymorphs of rare earth sesquichalcogenides. Inorg. Chem. 1969, 8, 2069–2071. [Google Scholar] [CrossRef]
- Grundmeier, T.; Urland, W. Zur Kristallstruktur von Tb2Se3. Z. Anorg. Allg. Chem. 1997, 623, 1744–1746. [Google Scholar] [CrossRef]
- Range, K.J.; Leeb, R. Die Kristallstruktur von Dy2Se3. Z. Naturforsch. 1976, 31 b, 685–686. [Google Scholar] [CrossRef]
- Guittard, M.; Flahaut, J.; Domange, L. Seleniures d’yttrium, de gadolinium, de dysprosium et d’erbium. Compt. Rend. Hebd. Acad. Sci. 1963, 256, 427–429. [Google Scholar]
- Slovyanskikh, V.K.; Kuznetsov, N.T.; Gracheva, N.V. Lanthanide selenides Ln Se1.4+-x of the yttrium subgroup. Russ. J. Inorg. Chem. 1982, 27, 745–746. [Google Scholar]
- Kuznetsov, N.T.; Slovyanskikh, V.K.; Gracheva, N.V. The compounds Dy2Se3 and USe3. Russ. J. Inorg. Chem. 1980, 25, 1130–1132. [Google Scholar]
- Eliseev, A.A.; Sadovskaya, O.S.; Tam, N.V. X-ray investigation of europium selenides. Inorg. Mater. 1975, 11, 361–364. [Google Scholar]
- Urland, W.; Person, H. Zur Kristallstruktur von Ho2Se3/On the Crystal Structure of Ho2Se3. Z. Naturforsch. 1998, 53 b, 900–902. [Google Scholar] [CrossRef]
- Range, K.J.; Eglmeier, C. Crystal data for rare earth sesquiselenides Ln2Se3 (Ln ≡ Ho, Er, Tm, Yb, Lu) and structure refinement of Er2Se3. J. Less-Common Met. 1991, 171, L27–L30. [Google Scholar] [CrossRef]
- Fang, C.M.; Meetsma, A.; Wiegers, G.A. Crystal structure of erbium sesquiselenide, Er2Se3. J. Alloy. Compd. 1995, 218, 224–227. [Google Scholar] [CrossRef]
- Assoud, A.; Kleinke, H. Ytterbium sesquiselenide Yb2Se3. Acta Crystallogr. Sect. E Struct. Rep. Online 2003, 59, i103–i104. [Google Scholar] [CrossRef]
- Flahaut, J.; Domange, L.; Guittard, M.; Pardo, M.P. Etude cristallographique des seleniures et des tellures des terres rares orthorhombiques, de type U2S3. Bull. Soc. Chim. Fr. 1965, 32, 326–327. [Google Scholar]
- Filatkina, V.S.; Kustova, G.N.; Batsanov, S.S. X-ray diffraction and IR spectroscopic investigation of selenido- and telluridofluorides of neodymium. Russ. Chem. Bull. 1972, 21, 2107–2110. [Google Scholar] [CrossRef]
- Dung, N.H. Stucture cristalline de la variete alpha du fluoroseleniure de lanthane LaSeF. Bull. Soc. Fr. Mineral. Cristallogr. 1973, 96, 41–43. [Google Scholar]
- Dung, N.H. Etude structurale du tetrafluoroseleniure de cerium Ce2SeF4. Bull. Soc. Fr. Mineral. Cristallogr. 1973, 96, 44–47. [Google Scholar]
- Zimmermann, D.D.; Schleid, T. Synthesis and crystal structures of the dimorphic holmium(III) fluoride selenide HoFSe. Z. Kristallogr. Suppl. 2014, 34, 139. [Google Scholar]
- Zimmermann, D.D. Synthese, Charakterisierung Sowie Erweiterung von Ternären und Quaternären Seltenerdmetall-Verbindungen mit Fluoridhaltigen Gittern Unter Chalkogenidischen Einflüssen. Ph.D. Thesis, University Stuttgart, Stuttgart, Germany, 2016. [Google Scholar]
- Dung, N.H.; Dagron, C.; Laruelle, P. Etude structurale des polytypes à deux anions LSeF (L = Y, Ho, Er). I. Structure cristalline du polytype orthorhombique à six couches du fluoroséléniure d’erbium ErSeF 6 O. Acta Crystallogr. Sect B Struct. Sci. 1975, 31, 514–518. [Google Scholar] [CrossRef]
- Dung, N.H.; Laruelle, P. Etude stucturale des polytypes à deux anions LSeF (L = Y, Ho, Er). IV. Structure cristalline du polytype monoclinique à huit couches du fluoroséléniure d’ytrrium ‘YSeF’ 8 M. Acta Crystallogr. Sect. B Struct. Sci. 1977, 33, 3360–3363. [Google Scholar] [CrossRef]
- Dung, N.H.; Dagron, C.; Laruelle, P. Etude structurale des polytypes à deux anions LSeF (L = Y, Ho, Er). II. Structure cristalline du fluoroséléniure d’yttrium YSeF, polytype monoclinique à quatre couches 4 M. Acta Crystallogr. Sect. B Struct. Sci. 1975, 31, 519–521. [Google Scholar] [CrossRef]
- Dung, N.H. Structure cristalline du fluoroséléniure d’yttrium orthorhombique YSeF 1 O. Acta Crystallogr. Sect B Struct. Sci. 1973, 29, 2095–2097. [Google Scholar] [CrossRef]
- Weber, F.A.; Schurz, C.M.; Frunder, S.; Kuhn, C.F.; Schleid, T. The Short Series of the Oxygen-Poor Lanthanide Oxide Selenides M10OSe14 with M = La–Nd. Crystals 2012, 2, 1136–1145. [Google Scholar] [CrossRef]
- Weber, F.A.; Schleid, T. Vier Oxidselenide des Praseodyms: Pr10OSe14, Pr2OSe2, Pr2O2Se und Pr4O4Se3. Z. Anorg. Allg. Chem. 2001, 627, 1383–1388. [Google Scholar] [CrossRef]
- Tougait, O.; Ibers, J.A. Gd2OSe2. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2000, 56, 623–624. [Google Scholar] [CrossRef]
- Eick, H.A. The crystal structure and lattice parameters of some rare earth mono-seleno oxides. Acta Crystallogr. 1960, 13, 161. [Google Scholar] [CrossRef]
- Киряков, А.С.; Тарасенко, М.С. Синтез твердых растворов состава: Y1,98RE0,02O2Se (RE = Ce-Nd, Sm)-И ИЗУЧЕНИЕ ИХ ФОТОФИЗИЧЕСКИХ СВОЙСТВ. МНСК-2018: Химия. 2018, p. 80. Available online: https://issc.nsu.ru/upload/Химия_МНСК–2017.pdf (accessed on 19 August 2019).
- Zimmermann, D.D.; Schleid, T. Ho3OFSe3 and Ho3OF3Se2: Two surprising new structure types for rare-earth metall(III) oxide fluoride selenides. Z. Kristallogr. Suppl. 2014, 34, 138. [Google Scholar]
- Zimmermann, D.D.; Schleid, T. Ce6O4F4Se3: A New Variant for Lanthanoid Oxide Fluoride Selenides. Z. Anorg. Allg. Chem. 2014, 2351. [Google Scholar] [CrossRef]
- Zimmermann, D.D.; Grossholz, H.; Wolf, S.; Janka, O.; Müller, A.C.; Schleid, T. Two Hexagonal Series of Lanthanoid(III) Oxide Fluoride Selenides: M6O2F8Se3 (M = La–Nd) and M2OF2Se (M = Nd, Sm, Gd–Ho). Z. Anorg. Allg. Chem. 2015, 641, 1926–1933. [Google Scholar] [CrossRef]
- Grossholz, H.; Zimmermann, D.D.; Janka, O.; Schleid, T. Oxidfluoridsulfide der Lanthanoide vom Formeltyp M6O2F8S3 (M=La–Nd, Sm, Gd)/Oxide Fluoride Sulfides of the Lanthanoids with the Formula M6O2F8S3 (M = La–Nd, Sm, Gd). Z. Naturforsch. 2013, 68b, 751–760. [Google Scholar] [CrossRef]
- Grossholz, H.; Janka, O.; Schleid, T. Oxide Fluoride Sulfides of the Lanthanoids with the Formula M3OF5S (M = Nd, Sm, Gd–Ho). Z. Naturforsch. 2011, 66b, 213–220. [Google Scholar] [CrossRef]
- Grossholz, H.; Schleid, T. Dy3OF5S: Das erste Oxidfluoridsulfid eines Lanthanoids. Z. Anorg. Allg. Chem. 2002, 628, 1012–1016. [Google Scholar] [CrossRef]
- Müller, A.C.; Janka, O.; Zimmermann, D.D.; Schleid, T. A-YFS and Y3OF5S: oxygen-free and oxygen-containing yttrium fluoride sulfides. Z. Anorg. Allg. Chem. 2014, 640, 2352. [Google Scholar]
- Pauwels, D.; Demourgues, A.; Laronze, H.; Gravereau, P.; Guillen, F.; Isnard, O.; Tressaud, A. Structural features of new rare earth-based mixed anions (O, S, F) compounds: relationships between optical absorption and rare earth environment. Solid State Sci. 2002, 4, 1471–1479. [Google Scholar] [CrossRef]
- Strobel, S.; Müller, A.C.; Schleid, T. The Crystal Structures of Er3OFS3 and Er3OF3S2: Two Erbium Oxide Fluoride Sulfides with Condensed Tetrahedral [ZEr4] Units (Z = O and F). Z. Anorg. Allg. Chem. 2009, 635, 1940–1946. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Program Suite for the Solution and Refinement of Crystal Structures; University Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Wolf, S. Darstellung und Charakterisierung Ternärer und Quaternärer Selten-Erd-Metall-Fluoride und -Oxidfluoride mit Elementen der 16. und 17. Gruppe. Staatsexamensarbeit; University Stuttgart: Stuttgart, Germany, 2012. [Google Scholar]
- Weber, F.A. Präparative Studien in den Mehrstoffsystemen Selten-Erd-Metall–Selen bzw. Tellur und Sauerstoff. Ph.D. Thesis, University Stuttgart, Stuttgart, Germany, 1999. [Google Scholar]
- Buyer, C.; Wolf, S.; Schleid, T. NaCe18O9F19Se9: A Sodium-Containing Compound in the Ce2OF2Se—Ce6O2F8Se3 System. Z. Kristallogr. Suppl. 2019, 39, 95. [Google Scholar]
- Fischer, R.X.; Tillmanns, E. The equivalent isotropic displacement factor. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1988, 44, 775–776. [Google Scholar] [CrossRef] [Green Version]
- Hoppe, R. Madelung Constants. Angew. Chem. Int. Ed. Engl. 1966, 5, 95–106. [Google Scholar] [CrossRef]
- Hoppe, R. The Coordination Number—An “Inorganic Chameleon”. Angew. Chem. Int. Ed. Engl. 1970, 9, 25–34. [Google Scholar] [CrossRef]
- Hübenthal, R.; Hoppe, R. MAPLE; Program for the Calculation of the Madelung Part of Lattice Energy; University of Gießen: Gießen, Germany, 1993. [Google Scholar]


| Compound | A-La2OF2Se | B-La2OF2Se |
|---|---|---|
| crystal system | trigonal | hexagonal |
| space group | Rm (no. 166) | P63/m (no. 172) |
| lattice parameters, | ||
| a/pm | 418.13(3) | 1396.82(9) |
| c/pm | 4478.2(4) | 401.08(3) |
| c/a | 10.710 | 0.287 |
| number of formula units (Z) | 6 | 6 |
| unit-cell volume (Vuc in nm3) | 0.67804(9) | 0.67771(8) |
| molar volume (Vm in cm3/mol) | 68.053 | 68.020 |
| calculated density (Dx in g/cm3) | 6.036 | 6.039 |
| diffractometer | IPDS (Stoe and Cie) | κ-CCD (Bruker-Nonius) |
| wavelength (λ in pm) | Mo-Kα (λ = 71.07 pm) | Mo-Kα (λ = 71.07 pm) |
| index range (±hmax, ±kmax, ±lmax) | 6/6/67 | 19/19/5 |
| number of e− per unit cell (F(000)) | 1044 | 1044 |
| absorption coefficient (μ in mm−1) | 26.59 | 26.60 |
| number of collected vs. unique reflections | 3237/383 | 13042/749 |
| data-set residuals (Rint/Rσ) | 0.070/0.027 | 0.097/0.052 |
| structure residuals (R1/wR2) | 0.026/0.088 | 0.052/0.120 |
| goodness of fit (GooF) | 1.072 | 1.028 |
| residual electron density (max./min. in e− 10−6 pm−3) | 3.12/−2.67 | 3.57/−1.98 |
| CSD number | 1919109 | 1919110 |
| Atom | Site | x/a | y/b | z/c | Ueq/pm2 |
|---|---|---|---|---|---|
| La1 | 6c | 0 | 0 | 0.133109(14) | 206(3) |
| La2 | 6c | 0 | 0 | 0.293363(14) | 111(2) |
| O | 6c | 0 | 0 | 0.18868(19) | 248(18) |
| F1 | 6c | 0 | 0 | 0.03591(17) | 178(14) |
| F2 | 6c | 0 | 0 | 0.34832(17) | 171(13) |
| Se | 6c | 0 | 0 | 0.42052(2) | 138(3) |
| Atom | Site | x/a | y/b | z/c | Ueq/pm2 |
|---|---|---|---|---|---|
| La1 | 6h | 0.12574(8) | 0.51625(8) | 231(3) | |
| La2 | 6h | 0.23351(8) | 0.28450(8) | 239(3) | |
| O | 6h | 0.4323(7) | 0.4156(8) | 114(19) | |
| F1 | 6h | 0.1895(9) | 0.0901(9) | 335(24) | |
| F2 | 6h | 0.0696(8) | 0.3151(8) | 263(21) | |
| Se | 6h | 0.48066(14) | 0.19870(14) | 232(4) |
| Distance | (d/pm) | Angle | (∢/°) | ||
|---|---|---|---|---|---|
| La1–O | (3×) | 246.9 | La1–O–La1 | (3×) | 102.1 |
| La1–O’ | (1×) | 248.9 | La1–O–La1′ | (3×) | 115.8 |
| La1–Se | (3×) | 317.1 | La2–F1–La2 | (3×) | 119.4 |
| La2–F1 | (3×) | 242.1 | La2–F2–La2 | (3×) | 103.4 |
| La2–F2 | (1×) | 245.2 | La2–F2–La2′ | (3×) | 115.1 |
| La2–F2′ | (3×) | 266.5 | La1–Se–La1 | (3×) | 82.5 |
| La2–Se | (3×) | 320.9 | La1–Se–La2 | (6×) | 98.1 |
| O–La1 | (3×) | 246.9 | La1–Se–La2′ | (3×) | 179.2 |
| O–La1′ | (1×) | 248.9 | La2–Se–La2 | (3×) | 81.3 |
| F1–La2 | (3×) | 242.1 | |||
| F2–La2 | (1×) | 245.2 | |||
| F2–La2′ | (3×) | 266.5 | |||
| Se–La1 | (3×) | 317.1 | |||
| Se–La2 | (3×) | 320.9 |
| Distance | (d/pm) | Angle | (∢/°) | ||
|---|---|---|---|---|---|
| La1–O | (1×) | 243.0 | La1–O–La1 | (2×) | 105.4 |
| La1–O’ | (2×) | 243.5 | La1–O–La1′ | (1×) | 110.9 |
| La1–F2 | (1×) | 251.1 | La1–O–La2 | (2×) | 109.8 |
| La1–Se | (2×) | 318.0 | La1′–O–La2 | (1×) | 115.3 |
| La1–Se’ | (2×) | 318.3 | La2–F1–La2′ | (2×) | 100.4 |
| La2–O | (1×) | 244.5 | La2–F1–La2 | (1×) | 110.9 |
| La2–F1 | (1×) | 246.6 | La1–F2–La2 | (2×) | 105.9 |
| La2–F1′ | (2×) | 260.9 | La1–F2–La2′ | (1×) | 112.7 |
| La2–F2 | (2×) | 249.8 | La2–F2–La2 | (1×) | 106.8 |
| La2–F2′ | (1×) | 253.5 | La2–F2–La2′ | (2×) | 112.5 |
| La2–Se | (2×) | 318.3 | La1–Se–La1 | (2×) | 78.1 |
| O–La1 | (1×) | 243.0 | La1–Se–La1′ | (2×) | 89.8 |
| O–La1′ | (2×) | 243.5 | La1–Se–La2 | (2×) | 82.5 |
| O–La2 | (1×) | 244.4 | La1′–Se–La2 | (2×) | 80.6 |
| F1–La2 | (1×) | 246.6 | La2–Se–La2 | (2×) | 78.1 |
| F1–La2′ | (2×) | 260.9 | |||
| F2–La1 | (1×) | 251.1 | |||
| F2–La2 | (2×) | 249.8 | |||
| F2–La2′ | (1×) | 253.5 | |||
| Se–La1 | (2×) | 318.0 | |||
| Se–La1′ | (2×) | 318.3 |
| A-La2OF2Se: | |||||
| F1 | F2 | O | Se | C.N. | |
| La1 | 7 | ||||
| La2 | 10 | ||||
| C.N. | 3 | 4 | 4 | 6 | |
| B-La2OF2Se: | |||||
| F1 | F2 | O | Se | C.N. | |
| La1 | 8 | ||||
| La2 | 9 | ||||
| C.N. | 3 | 4 | 4 | 6 |
| Element | Measured Content [wt-%] | Theoretical Content [wt-%] |
|---|---|---|
| La | 68.4(5) | 67.63 |
| O | 4.15 | 3.90 |
| F | 9.66(14) | 9.25 |
| Se | 17.79(16) | 19.22 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buyer, C.; Grossholz, H.; Schleid, T. The Structural Dimorphism of Lanthanum Oxide Fluoride Selenide La2OF2Se. Crystals 2019, 9, 435. https://doi.org/10.3390/cryst9090435
Buyer C, Grossholz H, Schleid T. The Structural Dimorphism of Lanthanum Oxide Fluoride Selenide La2OF2Se. Crystals. 2019; 9(9):435. https://doi.org/10.3390/cryst9090435
Chicago/Turabian StyleBuyer, Constantin, Hagen Grossholz, and Thomas Schleid. 2019. "The Structural Dimorphism of Lanthanum Oxide Fluoride Selenide La2OF2Se" Crystals 9, no. 9: 435. https://doi.org/10.3390/cryst9090435
APA StyleBuyer, C., Grossholz, H., & Schleid, T. (2019). The Structural Dimorphism of Lanthanum Oxide Fluoride Selenide La2OF2Se. Crystals, 9(9), 435. https://doi.org/10.3390/cryst9090435





