The Influence of B, N and Si Doping on the CH3 Adsorption on the Diamond Surface Based on DFT Calculations
Abstract
1. Introduction
2. Methodology
3. Results and Discussions
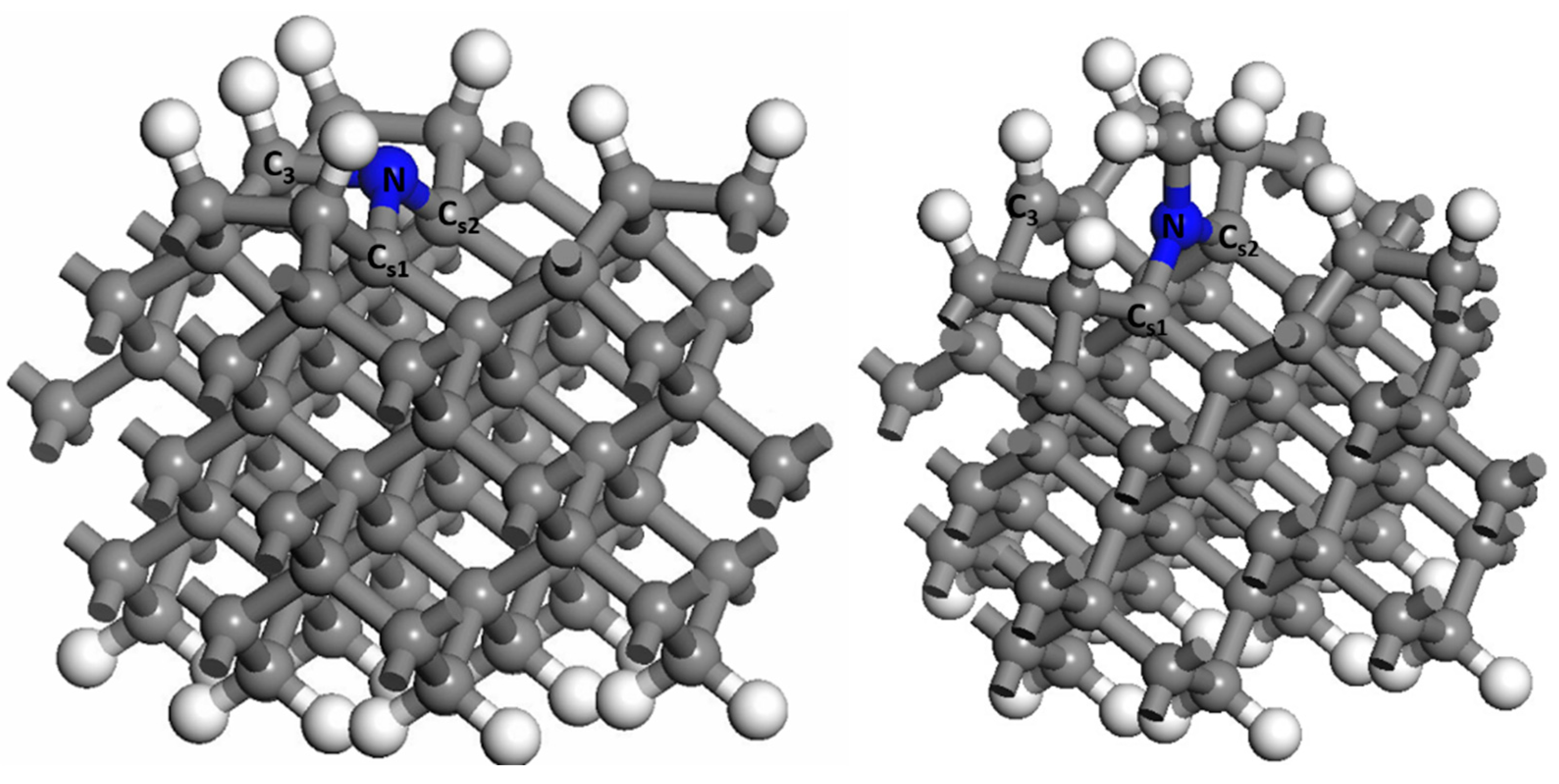
3.1. The Adsorption of CH3 on Different Doped Atoms
3.2. The Effects of Doped Radicals on the Adsorption of CH3
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Najar, K.A.; Sheikh, N.A.; Shah, M.A. Enhancement in Tribological and Mechanical Properties of Cemented Tungsten Carbide Substrates using CVD-diamond Coatings. Tribol. Ind. 2017, 39, 20–30. [Google Scholar] [CrossRef]
- Radhika, R.; Rao, M.S.R. Growth and tribological properties of diamond films on silicon and tungsten carbide substrates. Appl. Phys. A Mater. Sci. Process. 2016, 122, 937. [Google Scholar] [CrossRef]
- Nadolinny, V.; Komarovskikh, A.; Palyanov, Y. Incorporation of Large Impurity Atoms into the Diamond Crystal Lattice: EPR of Split-Vacancy Defects in Diamond. Crystals 2017, 7, 237. [Google Scholar] [CrossRef]
- Ashkinazi, E.E.; Khmelnitskii, R.A.; Sedov, V.S.; Khomich, A.A.; Khomich, A.V.; Ralchenko, V.G. Morphology of Diamond Layers Grown on Different Facets of Single Crystal Diamond Substrates by a Microwave Plasma CVD in CH4-H-2-N-2 Gas Mixtures. Crystals 2017, 7, 12. [Google Scholar] [CrossRef]
- Wang, Q.J.; Wu, G.; Liu, S.; Gan, Z.Y.; Yang, B.; Pan, J.H. Simulation-Based Development of a New Cylindrical-Cavity Microwave-Plasma Reactor for Diamond-Film Synthesis. Crystals 2019, 9, 11. [Google Scholar] [CrossRef]
- Halliwell, S.C.; May, P.W.; Fox, N.A.; Othman, M.Z. Investigations of the co-doping of boron and lithium into CVD diamond thin films. Diam. Relat. Mat. 2017, 76, 115–122. [Google Scholar] [CrossRef]
- Vanpoucke, D.E.P.; Nicley, S.S.; Raymakers, J.; Maes, W.; Haenen, K. Can europium atoms form luminescent centres in diamond: A combined theoretical–experimental study. Diam. Relat. Mater. 2019, 94, 233–241. [Google Scholar] [CrossRef]
- Chou, J.P.; Retzker, A.; Gali, A. Nitrogen-Terminated Diamond (111) Surface for Room-Temperature Quantum Sensing and Simulation. Nano Lett. 2017, 17, 2294–2298. [Google Scholar] [CrossRef]
- Bernardi, E.; Nelz, R.; Sonusen, S.; Neu, E. Nanoscale Sensing Using Point Defects in Single-Crystal Diamond: Recent Progress on Nitrogen Vacancy Center-Based Sensors. Crystals 2017, 7, 21. [Google Scholar]
- Zou, Y.; Larsson, K. Effect of Boron Doping on the CVD Growth Rate of Diamond. J. Phys. Chem. C 2016, 120, 10658–10666. [Google Scholar] [CrossRef]
- Srikanth, V.V.; Sampath Kumar, P.; Kumar, V.B. A Brief Review on the In Situ Synthesis of Boron-Doped Diamond Thin Films. Int. J. Electrochem. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Kuntumalla, M.K.; Elfimchev, S.; Chandran, M.; Hoffman, A. Raman scattering of nitrogen incorporated diamond thin films grown by hot filament chemical vapor deposition. Thin Solid Films 2018, 653, 284–292. [Google Scholar] [CrossRef]
- Rakha, S.A.; Xintai, Z.; Zhu, D.; Guojun, Y. Effects of N2 addition on nanocrystalline diamond films by HFCVD in Ar/CH4 gas mixture. Curr. Appl. Phys. 2010, 10, 171–175. [Google Scholar] [CrossRef]
- Ullah, M.; Rana, A.M.; Ahmed, E.; Malik, A.S.; Shah, Z.A.; Ahmad, N.; Mehtab, U.; Raza, R. Tribological performance of polycrystalline tantalum-carbide-incorporated diamond films on silicon substrates. Physica B 2018, 537, 277–282. [Google Scholar] [CrossRef]
- Liu, X.J.; Lu, P.F.; Wang, H.C.; Ren, Y.; Tan, X.; Sun, S.Y.; Jia, H.L. Morphology and structure of Ti-doped diamond films prepared by microwave plasma chemical vapor deposition. Appl. Surf. Sci. 2018, 442, 529–536. [Google Scholar] [CrossRef]
- Din, S.H.; Shah, M.A.; Sheikh, N.A.; Najar, K.A.; Ramasubramanian, K.; Balaji, S.; Ramachandra Rao, M.S. Influence of boron doping on mechanical and tribological properties in multilayer CVD-diamond coating systems. Bull. Mat. Sci. 2016, 39, 1753–1761. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.F.; Tang, T.; Xie, N.; Sun, F.H. The optimization of the doping level of boron, silicon and nitrogen doped diamond film on Co-cemented tungsten carbide inserts. Physica B 2018, 550, 280–293. [Google Scholar] [CrossRef]
- Wang, L.; Shen, B.; Sun, F.H.; Zhang, Z.M. Effect of pressure on the growth of boron and nitrogen doped HFCVD diamond films on WC-Co substrate. Surf. Interface Anal. 2015, 47, 572–586. [Google Scholar] [CrossRef]
- Wang, L.; Lei, X.L.; Shen, B.; Sun, F.H.; Zhang, Z.M. Tribological properties and cutting performance of boron and silicon doped diamond films on Co-cemented tungsten carbide inserts. ScienceDirect 2013, 33, 54–62. [Google Scholar] [CrossRef]
- May, P.W.; Harvey, J.N.; Allan, N.L.; Richley, J.C.; Mankelevich, Y.A. Simulations of chemical vapor deposition diamond film growth using a kinetic Monte Carlo model. J. Appl. Phys. 2010, 108, 014905. [Google Scholar] [CrossRef]
- Richley, J.C.; Harvey, J.N.; Ashfold, M.N.R. CH2 group migration between H-terminated 2 × 1 reconstructed {100} and {111} surfaces of diamond. J. Phys. Chem. C 2012, 116, 7810–7816. [Google Scholar] [CrossRef]
- Brenner, D.W. Empirical potential for hydrocarbons for use in simulating the chemical vapor deposition of diamond films. Phys. Rev. B 1990, 42, 9458–9471. [Google Scholar] [CrossRef] [PubMed]
- Larsson, K.; Carlsson, J.O. Surface migration during diamond growth studied by molecular orbital calculations. Phys. Rev. B 1999, 59, 8315–8322. [Google Scholar] [CrossRef]
- James, M.C.; Croot, A.; May, P.W.; Allan, N.L. Negative electron affinity from aluminium on the diamond (100) surface: A theoretical study. J. Phys. Condes. Matter 2018, 30, 9. [Google Scholar] [CrossRef] [PubMed]
- Croot, A.; Othman, M.Z.; Conejeros, S.; Fox, N.A.; Allan, N.L. A theoretical study of substitutional boron-nitrogen clusters in diamond. J. Phys. Condes. Matter 2018, 30, 10. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.B.; Chen, W.B.; Cui, Y.; Jiao, Z.W.; Qu, M.; Cao, L.G. Interactions between hydrogenated diamond surface and adsorbates with different concentration of NO2 molecules: A first-principles study. Surf. Interface Anal. 2018, 50, 962–968. [Google Scholar] [CrossRef]
- Liu, X.J.; Qiao, H.M.; Kang, C.J.; Ren, Y.; Tan, X.; Sun, S.Y. Adsorption and migration behavior of Si atoms on the hydrogen-terminated diamond (001) surface: A first principles study. Appl. Surf. Sci. 2017, 420, 542–549. [Google Scholar] [CrossRef]
- Tang, L.; Yue, R.F.; Wang, Y. N-type B-S co-doping and S doping in diamond from first principles. Carbon 2018, 130, 458–465. [Google Scholar] [CrossRef]
- Cheesman, A.; Harvey, J.N.; Ashfold, M.N. Computational studies of elementary steps relating to boron doping during diamond chemical vapour deposition. Phys. Chem. Chem. Phys. 2005, 7, 1121–1126. [Google Scholar] [CrossRef]
- Richley, J.C.; Harvey, J.N.; Ashfold, M.N.R. Boron Incorporation at a Diamond Surface: A QM/MM Study of Insertion and Migration Pathways during Chemical Vapor Deposition. J. Phys. Chem. C 2012, 116, 18300–18307. [Google Scholar] [CrossRef]
- James, M.C.; May, P.W.; Allan, N.L. Ab initio study of negative electron affinity from light metals on the oxygen-terminated diamond (1 1 1) surface. J. Phys. Condens. Matter 2019, 31, 295002. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Kristallogr. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Van Regemorter, T.; Larsson, K. Effect of coadsorbed dopants on diamond initial growth processes: CH3 adsorption. J. Phys. Chem. A 2008, 112, 5429–5435. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.N.; Larsson, K. Theoretical Study of the Energetic Stability and Geometry of Terminated and B-Doped Diamond (111) Surfaces. J. Phys. Chem. C 2014, 118, 1944–1957. [Google Scholar] [CrossRef] [PubMed]
- Van Regemorter, T.; Larsson, K. Effect of substitutional N on the diamond CVD growth process: A theoretical approach. Diam. Relat. Mat. 2008, 17, 1076–1079. [Google Scholar] [CrossRef]


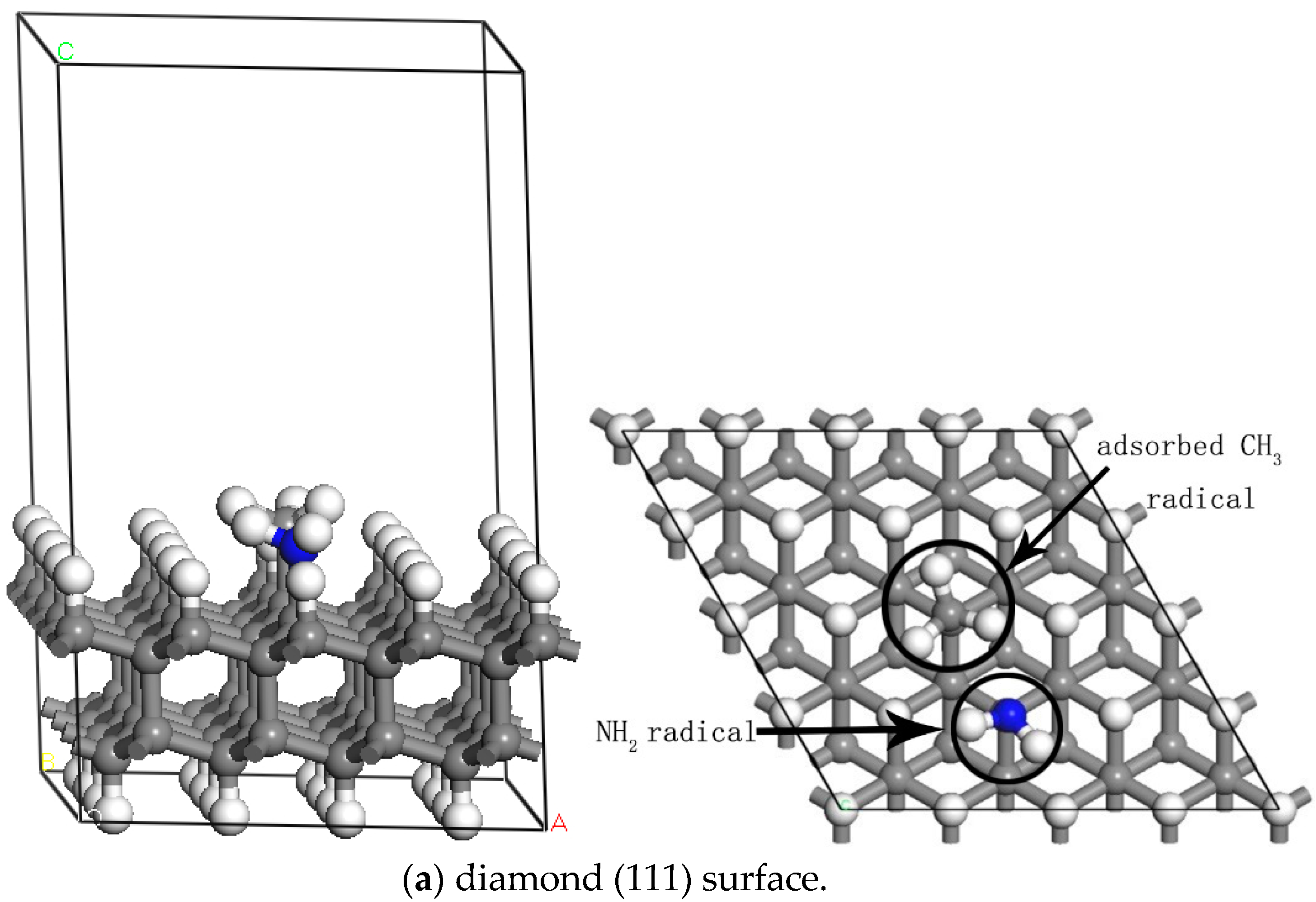
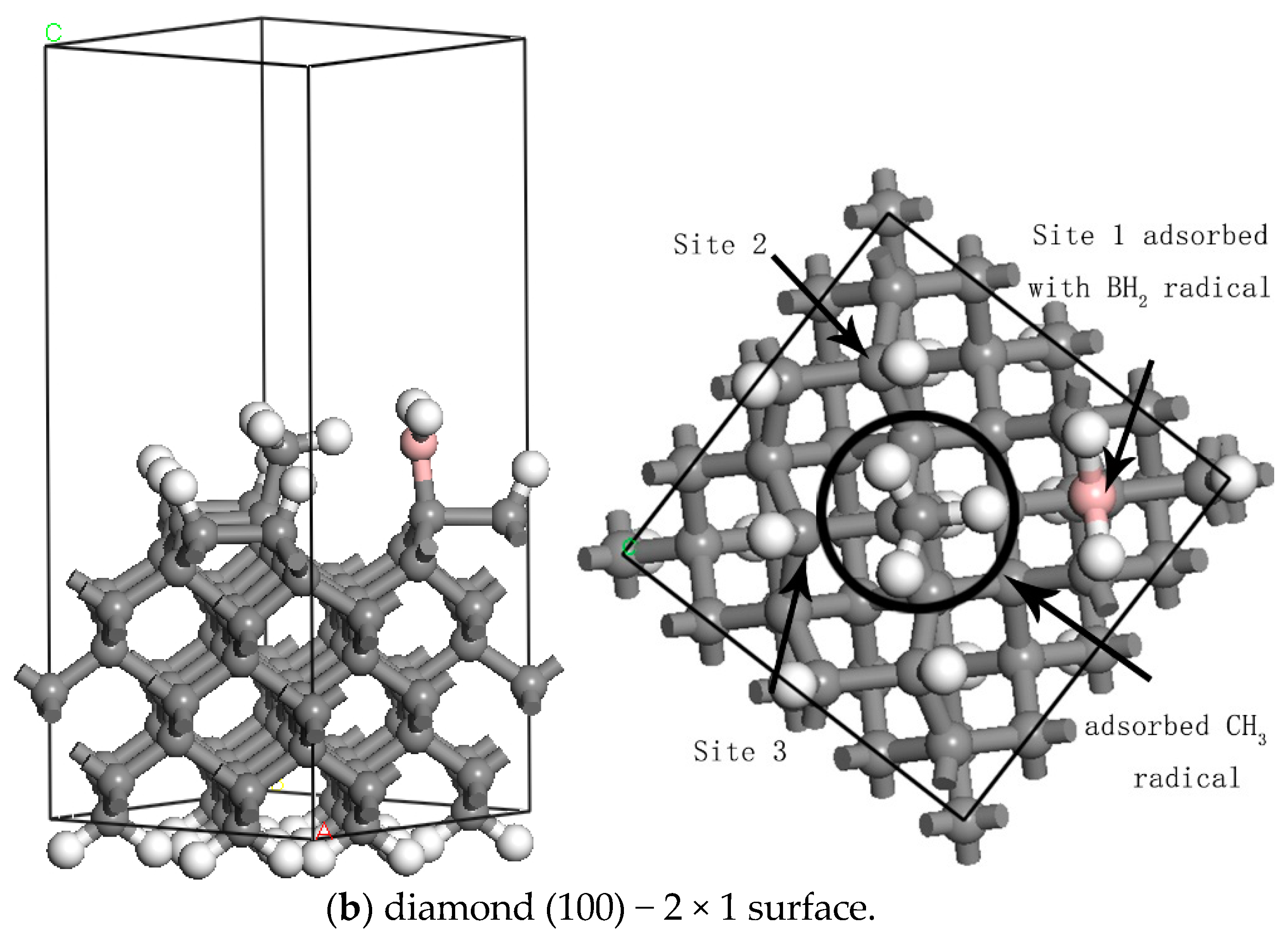
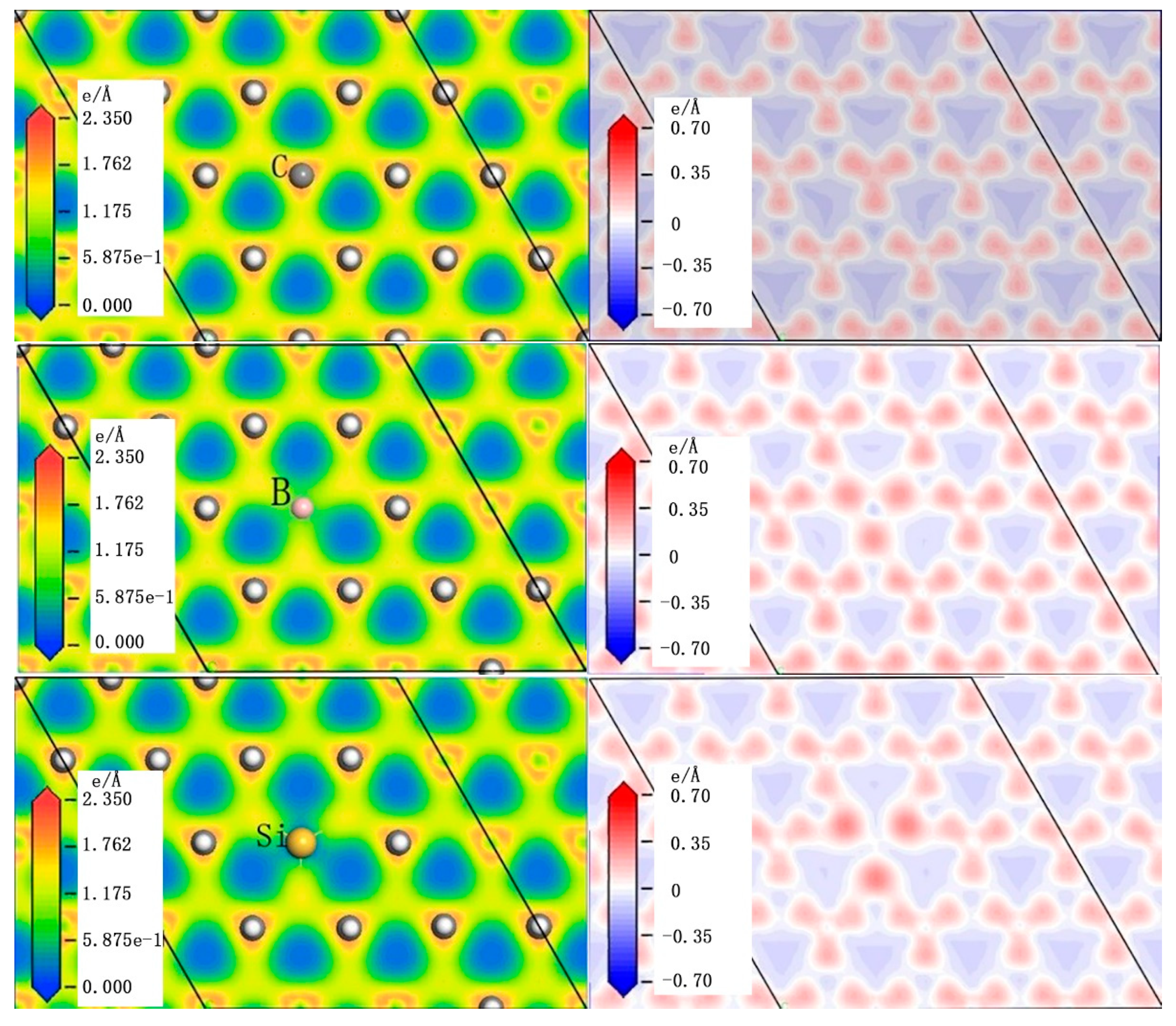

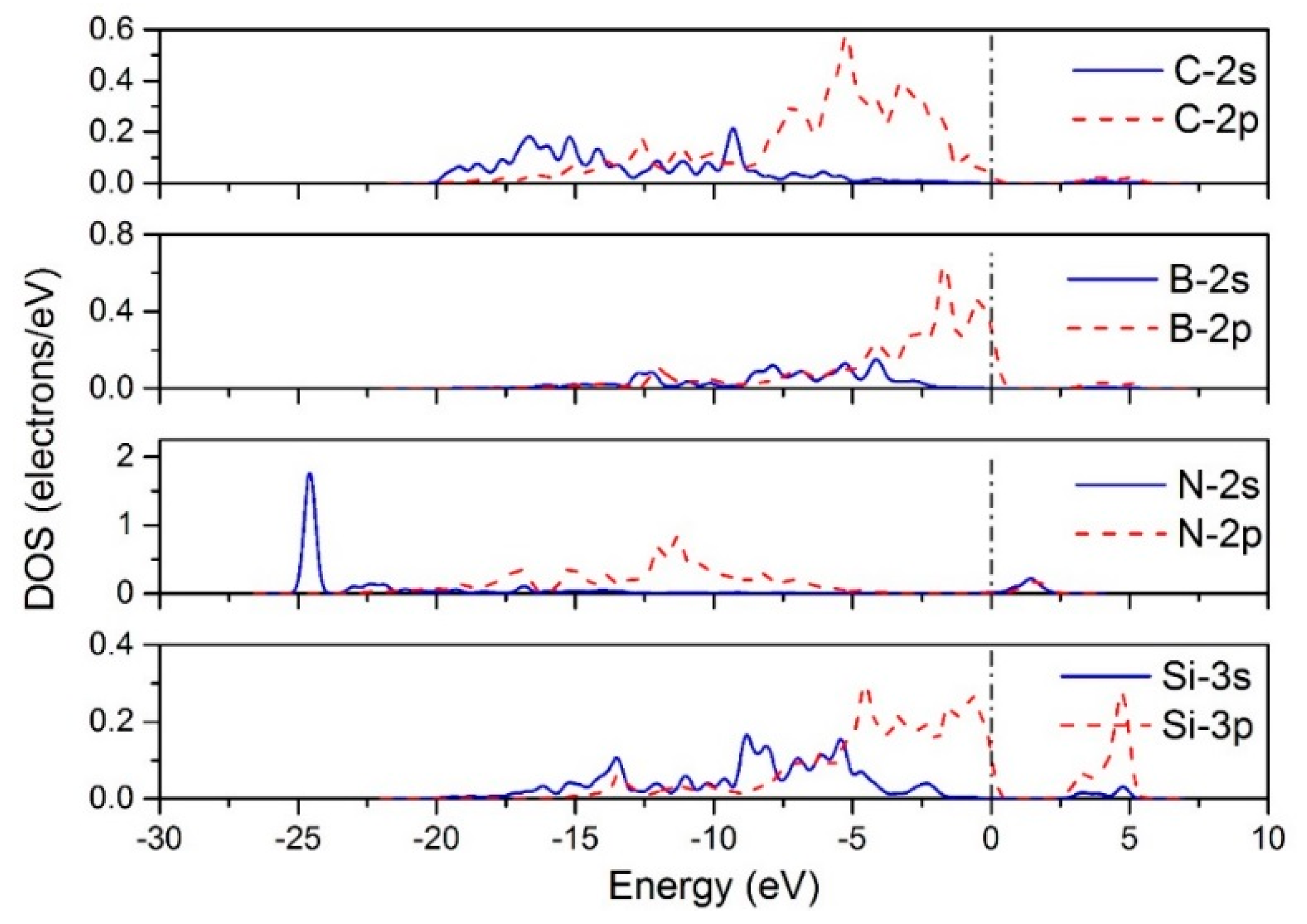

| (111) | (100) | |
|---|---|---|
| C | −312.82 | −349.40 |
| B | −130.45 | −234.33 |
| Si | −360.90 | −333.94 |
| N | 0.07 | −37.44 |
| (111) | (100) | |||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| BH2 | −320.94 | −363.34 | −345.23 | −264.33 |
| SiH3 | −271.25 | −346.77 | −297.71 | −297.11 |
| NH2 | −283.65 | −319.14 | −317.38 | −346.77 |
| CH3 | −267.87 | −289.54 | −299.67 | −327.63 |
| H | −314.69 | −349.40 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Liu, J.; Tang, T. The Influence of B, N and Si Doping on the CH3 Adsorption on the Diamond Surface Based on DFT Calculations. Crystals 2019, 9, 427. https://doi.org/10.3390/cryst9080427
Wang L, Liu J, Tang T. The Influence of B, N and Si Doping on the CH3 Adsorption on the Diamond Surface Based on DFT Calculations. Crystals. 2019; 9(8):427. https://doi.org/10.3390/cryst9080427
Chicago/Turabian StyleWang, Liang, Jiangshan Liu, and Tang Tang. 2019. "The Influence of B, N and Si Doping on the CH3 Adsorption on the Diamond Surface Based on DFT Calculations" Crystals 9, no. 8: 427. https://doi.org/10.3390/cryst9080427
APA StyleWang, L., Liu, J., & Tang, T. (2019). The Influence of B, N and Si Doping on the CH3 Adsorption on the Diamond Surface Based on DFT Calculations. Crystals, 9(8), 427. https://doi.org/10.3390/cryst9080427





