Absolute Luminescence Efficiency of Europium-Doped Calcium Fluoride (CaF2:Eu) Single Crystals under X-ray Excitation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Absolute and X-ray Luminescence Efficiency
2.2. Spectral Matching
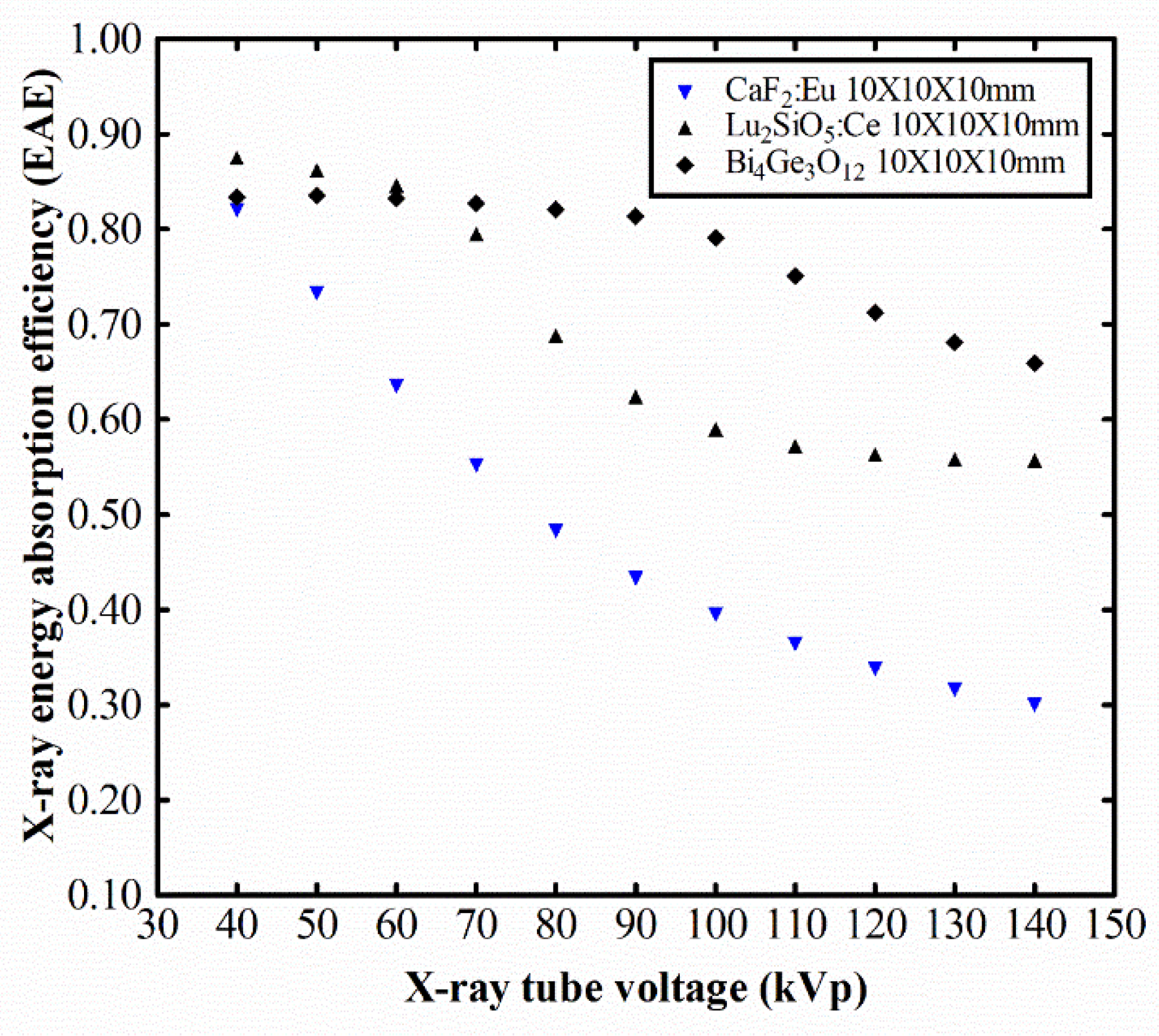
2.3. Energy Absorption Efficiency
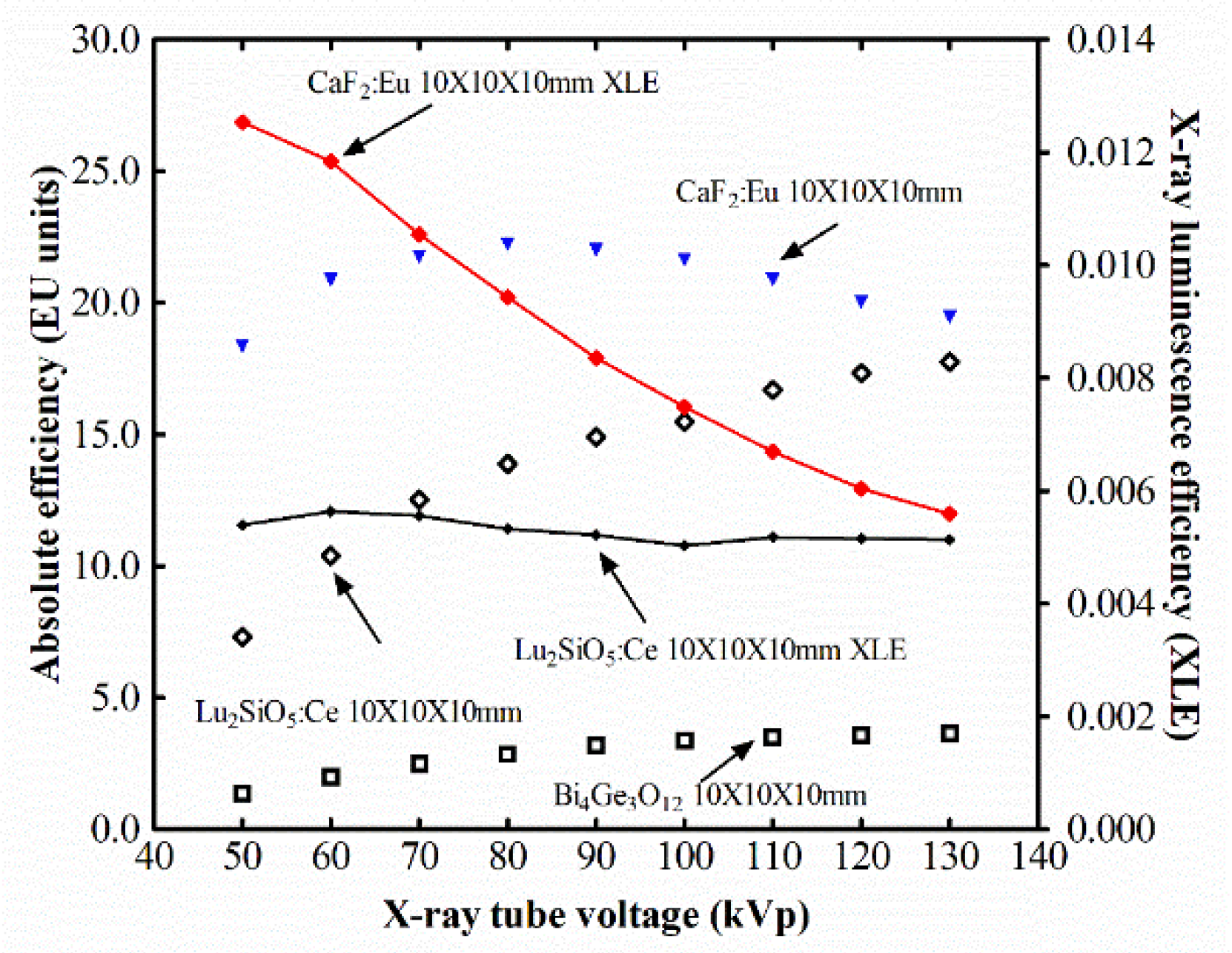
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Salomoni, M.; Pots, R.; Auffray, E.; Lecoq, P. Enhancing Light Extraction of Inorganic Scintillators Using Photonic Crystals. Crystals 2018, 8, 78. [Google Scholar] [CrossRef]
- Maddalena, F.; Tjahjana, L.; Xie, A.; Arramel, A.; Zeng, S.; Wang, H.; Coquet, P.; Drozdowski, W.; Dujardin, C.; Dang, C.; et al. Inorganic, Organic, and Perovskite Halides with Nanotechnology for High–Light Yield X- and γ-ray Scintillators. Crystals 2019, 9, 88. [Google Scholar] [CrossRef]
- Drozdowski, W.; Brylew, K.; Chruścińska, A.; Kamada, K.; Yanagida, T.; Yoshikawa, A. Scintillation yield enhancement in LuAG:Pr crystals following thermal annealing. Opt. Mater. 2012, 34, 1975–1978. [Google Scholar] [CrossRef]
- Michail, C.; Karpetas, G.; Kalyvas, N.; Valais, I.; Kandarakis, I.; Agavanakis, K.; Panayiotakis, G.; Fountos, G. Information Capacity of Positron Emission Tomography Scanners. Crystals 2018, 8, 459. [Google Scholar] [CrossRef]
- Mares, J.; Nikl, M.; Beitlerova, A.; Horodysky, P.; Blazek, K.; Bartos, K.; D’Ambrosio, C. Scintillation Properties of Ce3+-and Pr3+-Doped LuAG, YAG and Mixed LuxY1-xAG Garnet Crystals. IEEE Trans. Nucl. Sci. 2012, 59, 2120–2125. [Google Scholar] [CrossRef]
- Mares, J.; Beitlerova, A.; Nikl, M.; Vedda, A.; D’Ambrosio, C.; Blazek, K.; Nejezchleb, K. Time development of scintillating response in Ce- or Pr-doped crystals. Phys. Stat. Sol. C 2007, 4, 996–999. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, X.; Chen, H.; Shi, Y.; Liu, X.; Xie, T.; Kou, H.; Pan, Y.; Mihokova, E.; Nikl, M.; et al. Suppression of the slow scintillation component of Pr:Lu3Al5012 transparent ceramics by increasing Pr concentration. J. Lumin. 2019, 210, 14–20. [Google Scholar] [CrossRef]
- Nikl, M.; Pejchal, J.; Mihokova, E.; Mares, J.; Ogino, H.; Yoshikawa, A.; Fukuda, T.; Vedda, A.; D’Ambrosio, C. Antisite defect-free Lu3(GaxAl1−x)5O12:PrLu3(GaxAl1−x)5O12:Pr scintillator. Appl. Phys. Lett. 2006, 88, 141916. [Google Scholar] [CrossRef]
- Kamada, K.; Tsutsumi, K.; Usuki, Y.; Ogino, H.; Yanagida, T.; Yoshikawa, A. Crystal Growth and Scintillation Properties of 2-Inch-Diameter Pr:Lu3Al5O12 (Pr:LuAG) Single Crystal. IEEE Trans. Nucl. Sci. 2008, 55, 1488–1491. [Google Scholar] [CrossRef]
- Yoshikawa, A.; Yanagida, T.; Kamada, K.; Yokota, Y.; Pejchal, J.; Usuki, Y.; Yamamoto, S.; Miyake, M.; Kumagai, K.; Yamaji, A.; et al. Positron emission mammography using Pr:LuAG scintillator—Fusion of optical material study and systems engineering. Opt. Mater. 2010, 32, 1294–1297. [Google Scholar] [CrossRef]
- Ogino, H.; Yoshikawa, A.; Nikl, M.; Krasnikov, A.; Kamada, K.; Fukuda, T. Growth and scintillation properties of Pr-doped Lu. J. Cryst. Growth 2006, 287, 335–338. [Google Scholar] [CrossRef]
- Yanagida, T.; Yoshikawa, A.; Yokota, Y.; Kamada, K.; Usuki, Y.; Yamamoto, S.; Miyake, M.; Baba, M.; Kumagai, K.; Sasaki, K.; et al. Development of Pr:LuAG Scintillator Array and Assembly for Positron Emission Mammography. IEEE Trans. Nucl. Sci. 2010, 57, 1492–1495. [Google Scholar] [CrossRef]
- Valais, I.; Kandarakis, I.; Nikolopoulos, D.; Michail, C.; David, S.; Loudos, G.; Cavouras, D.; Panayiotakis, G. Luminescence properties of (Lu,Y)2SiO5:Ce and Gd2SiO5:Ce single crystal scintillators under X-ray excitation for use in medical imaging systems. IEEE Trans. Nucl. Sci. 2007, 54, 11–18. [Google Scholar] [CrossRef]
- Valais, I.; Michail, C.; David, S.; Liaparinos, P.; Fountos, G.; Paschalis, T.; Kandarakis, I.; Panayiotakis, G. Comparative Investigation of Ce3+ doped Scintillators in a wide Range of Photon Energies covering X-ray CT, Nuclear Medicine and Megavoltage Radiation Therapy Portal Imaging applications. IΕΕΕ Trans. Nucl. Sci. 2010, 57, 3–7. [Google Scholar] [CrossRef]
- Michail, C.; David, S.; Liaparinos, P.; Valais, I.; Nikolopoulos, D.; Kalivas, N.; Toutountzis, A.; Cavouras, D.; Kandarakis, I.; Panayiotakis, G. Evaluation of the imaging performance of LSO powder scintillator for use in X-ray mammography. Nucl. Instrum. Meth. Phys. Res. A 2007, 580, 558–561. [Google Scholar] [CrossRef]
- Michail, C.; Valais, I.; Martini, N.; Koukou, V.; Kalyvas, N.; Bakas, A.; Kandarakis, I.; Fountos, G. Determination of the Detective Quantum Efficiency (DQE) of CMOS/CsI Imaging Detectors following the novel IEC 62220-1-1:2015 International Standard. Radiat. Meas. 2016, 94, 8–17. [Google Scholar] [CrossRef]
- Kato, T.; Kataoka, J.; Nakamori, T.; Miura, T.; Matsuda, H.; Sato, K.; Ishikawa, Y.; Kawabata, N.; Ikeda, H.; Sato, G.; et al. Development of a large-area monolithic 4X4 MPPC array for a future PET scanner employing pixelized Ce:LYSO and Pr:LuAG crystals. Nucl. Instrum. Meth. Phys. Res. A 2011, 638, 83–91. [Google Scholar] [CrossRef]
- Hu, C.; Li, J.; Yang, F.; Jiang, B.; Zhang, L.; Zhu, R. LuAG ceramic scintillators for future HEP experiments. Nucl. Instrum. Meth. Phys. Res. A 2019. [Google Scholar] [CrossRef]
- Kastengren, A. Thermal behavior of single-crystal scintillators for high-speed X-ray imaging. J. Synchrotron Rad. 2019, 26, 205–214. [Google Scholar] [CrossRef]
- Grammaticos, P.; Fountos, G. The physician should benefit, not harm the patient. Hell. J. Nucl. Med. 2006, 9, 82–84. [Google Scholar]
- Gundacker, S.; Martinez Turtos, R.; Auffray, E.; Paganoni, M.; Lecoq, P. High-frequency SiPM readout advances measured coincidence time resolution limits in TOF-PET. Phys. Med. Biol. 2019, 64, 055012. [Google Scholar] [CrossRef] [PubMed]
- Grodzicka, M.; Moszynski, M.; Szczesniak, T. Silicon Photomultipliers in Detectors for Nuclear Medicine. In Radiation Detectors for Medical Imaging Devices, Circuits, and Systems; Iniewski, K., Iwanczyk, J., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 119–148. [Google Scholar]
- Gupta, T. Radiation, Ionization, and Detection in Nuclear Medicine; Springer: Heidelberg, Germany, 2013. [Google Scholar]
- Preston, K., Jr.; Taylor, K.; Johnson, S.; Ayers, W. Medical Imaging Techniques, a Comparison; Plenum Press: New York, NY, USA, 1979. [Google Scholar]
- Lecoq, P.; Korzhik, M.; Alexander Gektin, A. Inorganic Scintillators for Detector Systems. Physical Principles and Crystal Engineering; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Michail, C.; Karpetas, G.; Fountos, G.; Kalyvas, N.; Valais, I.; Fountzoula, C.; Zanglis, A.; Kandarakis, I.; Panayiotakis, G. A novel method for the Optimization of Positron Emission Tomography Scanners Imaging Performance. Hell. J. Nucl. Med. 2016, 19, 231–240. [Google Scholar] [PubMed]
- Vaquero, J.; Kinahan, P. Positron Emission Tomography: Current Challenges and Opportunities for Technological Advances in Clinical and Preclinical Imaging Systems. Annu. Rev. Biomed. Eng. 2015, 17, 385–414. [Google Scholar] [CrossRef] [PubMed]
- Valais, I.; Michail, C.; David, S.; Nomicos, C.; Panayiotakis, G.; Kandarakis, I. A Comparative Study of the Luminescence Properties of LYSO:Ce, LSO:Ce, GSO:Ce and BGO Single Crystal Scintillators for Use in Medical X-ray Imaging. Phys. Med. 2008, 24, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Van Eijk, C. Inorganic scintillators in medical imaging. Phys. Med. Biol. 2002, 47, R85–R106. [Google Scholar] [CrossRef] [PubMed]
- Humm, J.; Rosenfeld, A.; Guerra, A. From PET detectors to PET scanners. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 1574–1597. [Google Scholar] [CrossRef]
- Trébossen, R.; Comtat, C.; Brulon, V.; Bailly, P.; Meyer, M. Comparison of two commercial whole body PET systems based on LSO and BGO crystals respectively for brain imaging. Med. Phys. 2009, 36, 1399–1409. [Google Scholar]
- Karpetas, G.; Michail, C.; Fountos, G.; Kalyvas, N.; Valais, I.; Kandarakis, I.; Panayiotakis, G. Detective Quantum Efficiency (DQE) in PET Scanners: A Simulation Study. Appl. Radiat. Isot. 2017, 125, 154–162. [Google Scholar] [CrossRef]
- Hong, B.; Kawano, K. Syntheses of Eu-Activated Alkaline Earth Fluoride MF2 (M=Ca, Sr) Nanoparticles. Jpn. J. Appl. Phys. 2007, 46, 6319–6323. [Google Scholar] [CrossRef]
- Holl, I.; Lorenz, E.; Mageras, G. A Measurement of the Light Yield of Common Inorganic Scintillators. IEEE Trans. Nucl. Sci. 1988, 35, 105–109. [Google Scholar] [CrossRef]
- Knoll, G. Radiation Detection and Measurement; John Wiley and Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- Shimizu, Y.; Minowa, M.; Suganuma, W.; Inoue, Y. Dark matter search experiment with CaF2(Eu) scintillator at Kamioka Observatory. Phys. Lett. B 2006, 633, 195–200. [Google Scholar] [CrossRef]
- Chen, M. Double beta decay: Scintillators. J. Phys. Conf. Ser. 2008, 136, 022035. [Google Scholar] [CrossRef]
- Ely, J.; Aalseth, C.; McIntyre, J. Novel beta-gamma coincidence measurements using phoswich detectors. J. Radioanal. Nucl. Chem. 2005, 263, 245–250. [Google Scholar] [CrossRef]
- Plettner, C.; Pausch, G.; Scherwinski, F.; Herbach, C.; Lentering, R.; Kong, Y.; Römer, K.; Grodzicka, M.; Szcześniak, T.; Iwanowska, J.; et al. CaF2(Eu): An “old” scintillator revisited. J. Inst. 2013, 8, P06010. [Google Scholar] [CrossRef]
- Bernabei, R.; Belli, P.; Montecchia, F.; Incicchitti, A.; Nicolantonio, W.; Prosperi, D.; Bacci, C.; Dai, C.; Ding, L.; Kuang, H.; et al. Improved limits on WIMP-19F elastic scattering and first limit on the 2EC2ν 40Ca decay by using a low radioactive CaF2(Eu) scintillator. Astropart. Phys. 1997, 7, 73–76. [Google Scholar] [CrossRef]
- Sunta, C. A review of thermoluminescence of calcium fluoride, calcium sulphate and calcium carbonate. Radiat. Prot. Dosim. 1984, 8, 25–44. [Google Scholar] [CrossRef]
- Takada, M.; Shibata, T.; Uwamino, Y.; Nakamura, T. A performance study on a phoswich detector consisting of an inner NE213 scintillator and an outer CaF2(Eu) crystal wall. Nucl. Instrum. Meth. A 1996, 379, 293. [Google Scholar] [CrossRef]
- Song, L.; Gao, J.; Song, R. Synthesis and luminescent properties of oleic acid (OA)-modified CaF2:Eu nanocrystals. J. Lumin. 2010, 130, 1179–1182. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; White, D.; Finch, A.; Townsend, P. Factors controlling the thermoluminescence spectra of rare earth doped calcium fluoride. J. Lumin. 2017, 184, 55–63. [Google Scholar] [CrossRef]
- Dubey, V.; Kaur, J.; Agrawal, S. Effect of europium concentration on photoluminescence and thermoluminescence behavior of Y2O3:Eu3+ Phosphor. Res. Chem. Intermed. 2015, 41, 4727–4739. [Google Scholar] [CrossRef]
- Reddy, B.; Colon, T. White light emission characteristics of europium doped fluoride crystals. Proc. SPIE 2013, 8621, 86210. [Google Scholar]
- Straßer, M.; Schrauth, J.; Dembski, S.; Haddad, D.; Ahrens, B.; Schweizer, S.; Christ, B.; Cubukova, A.; Metzger, M.; Walles, H.; et al. Calcium fluoride based multifunctional nanoparticles for multimodal imaging. Beilstein J. Nanotechnol. 2017, 8, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Nakhaei, O.; Shahtahmassebi, N. Study structural and up-conversion luminescence properties of polyvinyl alcohol/CaF:erbium nanofibers for potential medical applications. Nanomed. J. 2015, 2, 160–166. [Google Scholar]
- Zhi, G.; Song, J.; Mei, B.; Zhou, W. Synthesis and characterization of Er3+ doped CaF2 nanoparticles. J. Alloys Compd. 2011, 509, 9133–9137. [Google Scholar] [CrossRef]
- Dong, N.-N.; Pedroni, M.; Piccinelli, F.; Conti, G.; Sbarbati, A.; Ramírez-Hernández, J.; Maestro, L.; Iglesias-de la Cruz, M.; Sanz-Rodriguez, F.; Juarranz, A.; et al. NIR-to-NIR Two-Photon Excited CaF2:Tm3+,Yb3+ Nanoparticles: Multifunctional Nanoprobes for Highly Penetrating Fluorescence Bio-Imaging. ACS Nano 2011, 5, 8665–8671. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Peng, Q.; Li, Y. Upconversion Luminescence of Monodisperse CaF2:Yb3+/Er3+ Nanocrystals. J. Am. Chem. Soc. 2009, 131, 14200–14201. [Google Scholar] [CrossRef]
- Bensalaha, A.; Mortiera, M.; Patriarcheb, G.; Gredinc, P.; Viviena, D. Synthesis and optical characterizations of undoped and rare-earth-doped CaF2 nanoparticles. J. Solid State Chem. 2006, 179, 2636–2644. [Google Scholar] [CrossRef]
- Wang, F.; Fan, X.; Pi, D.; Wang, M. Synthesis and luminescence behavior of Eu3+-doped CaF2 nanoparticles. Solid State Commun. 2005, 133, 775–779. [Google Scholar] [CrossRef]
- Wang, J.; Miao, W.; Li, Y.; Yao, H.; Li, Z. Water-soluble Ln3+-doped calcium fluoride nanocrystals: Controlled synthesis and luminescence properties. Mater. Lett. 2009, 63, 1794–1796. [Google Scholar] [CrossRef]
- Salah, N.; Alharbi, N.; Habib, S.; Lochab, S. Luminescence Properties of CaF2 Nanostructure Activated by Different Elements. J. Nanomater. 2015, 2015, 136402. [Google Scholar] [CrossRef]
- Fan, T.; Lü, J.; Huang, Y.; Li, G. Monodispersing Eu3+ and Li+ codoped CaF2 nanoparticles for efficient luminescence. Micro. Nano Lett. 2018, 13, 393–396. [Google Scholar] [CrossRef]
- Lina, L.; Leitnera, D.; Benattia, C.; Perdikakis, G.; Krausea, S.; Rencsoka, R.; Nasha, S.; Wittmer, W. Investigation of ion induced damage in KBr, YAG:Ce, CaF2:Eu and CsI:Tl irradiated by various-energy protons. J. Inst. 2015, 10, P03024. [Google Scholar] [CrossRef]
- Dujardin, C.; Auffray, E.; Bourret-Courchesne, E.; Dorenbos, P.; Lecoq, P.; Nikl, M.; Vasil’ev, A.; Yoshikawa, A.; Zh, R. Needs, Trends, and Advances in Inorganic Scintillators. IEEE Trans. Nucl. Sci. 2018, 65, 1977–1997. [Google Scholar] [CrossRef]
- Yanagida, T. Inorganic scintillating materials and scintillation detectors. Proc. Jpn. Acad. Ser. B 2018, 94, 75–97. [Google Scholar] [CrossRef] [PubMed]
- Cortelletti, P.; Pedroni, M.; Boschi, F.; Pin, S.; Ghigna, P.; Canton, P.; Vetrone, F.; Speghini, A. Luminescence of Eu3+ Activated CaF2 and SrF2 Nanoparticles: Effect of the Particle Size and Codoping with Alkaline Ions. Cryst. Growth Des. 2018, 18, 686–694. [Google Scholar] [CrossRef]
- Sasidharan, S.; Jayasree, A.; Fazal, S.; Koyakutty, M.; Nair, S.; Menon, D. Ambient temperature synthesis of citrate stabilized and biofunctionalized, fluorescent calcium fluoride nanocrystals for targeted labeling of cancer cells. Biomater. Sci. 2013, 1, 294–305. [Google Scholar] [CrossRef]
- Maushake, P. Calcium Fluoride Crystals. Opt. Photonik 2008, 2, 46–47. [Google Scholar] [CrossRef]
- Belli, P.; Bernabei, R.; Dai, C.; Grianti, F.; He, H.; Incicchitti, A.; Kuang, H.; Ma, J.; Montecchia, F.; Ignesti, G.; et al. New limits on spin-dependent coupled WIMPs and on 2β processes in 40Ca and 46Ca by using low radioactive CaF2(Eu) crystal scintillators. Nucl. Phys. B 1999, 563, 97–106. [Google Scholar] [CrossRef]
- Heath, R.; Hofstadter, R.; Hughes, E. Inorganic scintillators: A review of techniques and applications. Nucl. Instr. Meth. 1979, 162, 431–476. [Google Scholar] [CrossRef]
- Menefee, J.; Sweinehart, C.; O’Dell, E. Calcium fluoride as an X-ray and charged particle detector. IEEE Trans. Nucl. Sci. 1966, 13, 720–724. [Google Scholar] [CrossRef]
- Shcheulin, A.; Semenova, T.; Koryakina, L.; Petrova, M.; Angervaks, A.; Ryskin, A. Additive coloring rate and intensity for pure and doped fluorite crystals. Opt. Spectroscop. 2011, 110, 617–623. [Google Scholar] [CrossRef]
- Nakamura, F.; Kato, T.; Okada, G.; Kawaguchi, N.; Fukuda, K.; Yanagida, T. Scintillation and dosimeter properties of CaF2 translucent ceramic produced by SPS. J. Eur. Ceram. Soc. 2017, 37, 1707–1711. [Google Scholar] [CrossRef]
- Heaton, R.; Lin, C. Electronic energy-band structure of the calcium fluride crystal. Phys. Rev. B 1980, 22, 3629. [Google Scholar] [CrossRef]
- Mikhailik, V.; Kraus, H.; Imber, J.; Wahl, D. Scintillation properties of pure CaF2. Nucl. Instrum. Meth. Phys. Res. A 2006, 566, 522–525. [Google Scholar] [CrossRef]
- Aliaga-Kelly, D.; Nicoll, D. Recent developments in scintillation detectors. Nucl. Instrum. Meth. 1966, 43, 110–115. [Google Scholar] [CrossRef]
- Taulbeel, T.; Rooney, B.; Mengesha, W.; Valentine, J. The Measured Electron Response Nonproportionality of CaF2, BGO, LSO, and GSO. IEEE Nucl. Sci. Symp. Conf. Rec. 1996, 1, 326–330. [Google Scholar]
- Wang, J.; Yang, J.; Hu, T.; Chen, X.; Lang, J.; Wu, X.; Zhang, J.; Zhao, H.; Yang, J.; Cui, Q. Structural Phase Transition and Compressibility of CaF2 Nanocrystals under High Pressure. Crystals 2018, 8, 199. [Google Scholar] [CrossRef]
- Daví, F. Decay Time Estimates by a Continuum Model for Inorganic Scintillators. Crystals 2019, 9, 41. [Google Scholar] [CrossRef]
- Hu, T.; Cui, X.; Wang, J.; Zhong, X.; Chen, Y.; Zhang, J.; Li, X.; Yang, J.; Gao, C. The Electrical Properties of Tb-Doped CaF2 Nanoparticles under High Pressure. Crystals 2018, 8, 98. [Google Scholar] [CrossRef]
- Di Tommaso, D.; Prakash, M.; Lemaire, T.; Lewerenz, M.; De Leeuw, N.; Naili, S. Molecular Dynamics Simulations of Hydroxyapatite Nanopores in Contact with Electrolyte Solutions: The Effect of Nanoconfinement and Solvated Ions on the Surface Reactivity and the Structural, Dynamical, and Vibrational Properties of Water. Crystals 2017, 7, 57. [Google Scholar] [CrossRef]
- Advatech UK. CaF2(Eu)—Calcium Fluoride (Eu). Available online: https://www.advatech-uk.co.uk/caf2_eu.html (accessed on 25 January 2019).
- Michail, C.; Valais, I.; Fountos, G.; Bakas, A.; Fountzoula, C.; Kalyvas, N.; Karabotsos, A.; Sianoudis, I.; Kandarakis, I. Luminescence Efficiency of Calcium Tungstate (CaWO4) under X-ray radiation: Comparison with Gd2O2S:Tb. Measurement 2018, 120, 213–220. [Google Scholar] [CrossRef]
- Michail, C.; Valais, I.; Seferis, I.; Kalyvas, N.; David, S.; Fountos, G.; Kandarakis, I. Measurement of the Luminescence properties of Gd2O2S:Pr,Ce,F Powder Scintillators under X-ray radiation. Radiat. Meas. 2014, 70, 59–64. [Google Scholar] [CrossRef]
- Hubbell, J.; Seltzer, S. Tables of X-ray Mass Attenuation Coefficients and Mass Energy Absorption Coefficients 1 to 20 MeV for Elements Z = 1 to 92 and 48 Additional Substances of Dosimetric Interest; NISTIR 5632; US Department of Commerce: Washington, DC, USA, 1995.
- Hamamatsu Photonics, MPPC (Multi-Pixel Photon Counters). Available online: http://www.hamamatsu.com/us/en/product/category/3100/4004/4113/index.html# (accessed on 3 December 2018).
- SensL. Silicon Photomultipliers. Available online: http://sensl.com/products/silicon-photomultipliers/ (accessed on 3 December 2018).
- Rowlands, J.A.; Yorkston, J. Flat Panel Detectors for Digital Radiography. In Handbook of Medical Imaging Physics and Psychophysics; Beutel, J., Kundel, H., Van Metter, R., Eds.; SPIE: Bellingham, WA, USA, 2000; Volume 1, pp. 223–328. ISBN 9780819477729. [Google Scholar]
- Magnan, P. Detection of visible photons in CCD and CMOS: A comparative view. Nucl. Instrum. Meth. Phys. Res. A 2003, 504, 199–212. [Google Scholar] [CrossRef]
- Michail, C.; Kalyvas, N.; Valais, I.; David, S.; Seferis, I.; Toutountzis, A.; Karabotsos, A.; Liaparinos, P.; Fountos, G.; Kandarakis, I. On the response of GdAlO3:Ce powder scintillators. J. Lumin. 2013, 144, 45–52. [Google Scholar] [CrossRef]
- Evans, R.D. The Atomic Nucleus; McGraw-Hill: New York, NY, USA, 1955. [Google Scholar]
- Seibert, J.; Boone, J. X-ray imaging physics for nuclear medicine technologists. Part 2: X-ray interactions and image formation. J. Nucl. Med. Technol. 2005, 33, 3–18. [Google Scholar] [PubMed]
- Michail, C.; David, S.; Bakas, A.; Kalyvas, N.; Fountos, G.; Kandarakis, I.; Valais, I. Luminescence Efficiency of (Lu,Gd)2SiO5:Ce (LGSO:Ce) crystals under X-ray radiation. Radiat. Meas. 2015, 80, 1–9. [Google Scholar] [CrossRef]
- Storm, E.; Israel, H. Report LA-3753, Los Alamos Scientific Laboratory; University of California: Oakland, CA, USA, 1967. [Google Scholar]
- Yu, H.; Zhang, B.; Chen, X.; Qian, X.; Jiang, D.; Wu, Q.; Wang, J.; Xu, J.; Su, L. Color-tunable visible photoluminescence of Eu:CaF2 single crystals: Variations of valence state and local lattice environment of Eu ions. Opt. Express 2019, 27, 523–532. [Google Scholar] [CrossRef]
- Vartika, S.; Singh, V.; Joshi, C.; Moharil, S.; Muthal, P.; Dhopte, S. Modification of luminescence spectra of CaF2:Eu2+. Luminescence 2015, 30, 1101–1105. [Google Scholar]
- Aiga, F.; Hiramatsu, R.; Ishida, K. Ab initio theoretical study of 4f→5d transitions in Eu2+-doped CaF2: (2) Augmented-basis-set-study. J. Lumin. 2016, 169, 601–605. [Google Scholar] [CrossRef]
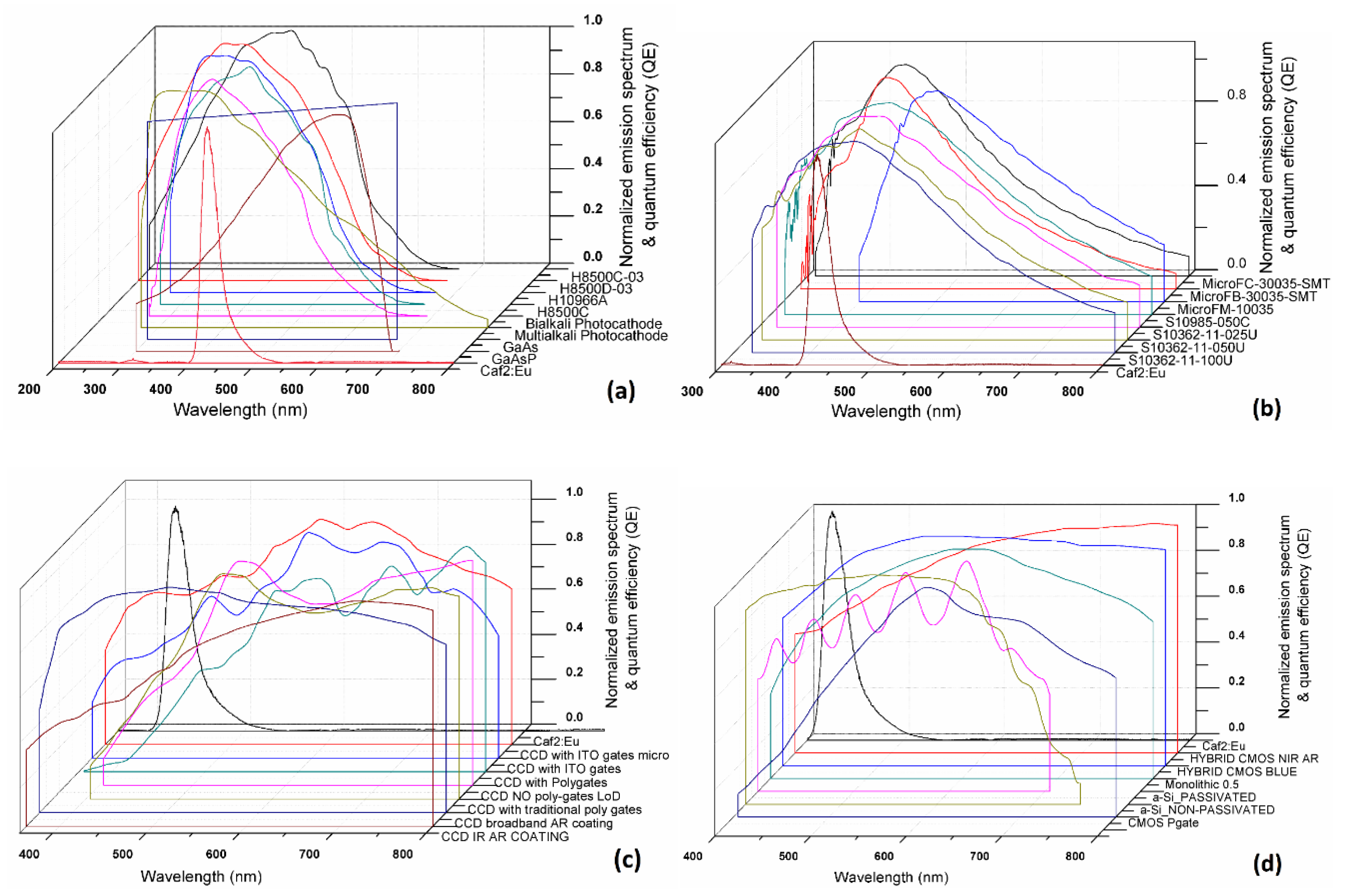

| Light Sensors | CaF2:Eu | Light Sensors | CaF2:Eu |
|---|---|---|---|
| CCD broadband AR coating | 0.94 | GaAsP phosphor photocathode | 0.52 |
| CCD infrared (IR) anti-reflection (AR) coating | 0.54 | Extended photocathode (E-S20) | 0.94 |
| CMOS hybrid with blue anti-reflection (AR) coating | 0.60 | Si PM MicroFC-30035-SMT | 0.94 |
| Hybrid CMOS blue | 0.79 | Si PM MicroFB-30035-SMT | 0.92 |
| CMOS (monolithic 0.25 μm) | 0.64 | Si PM MicroFM-10035 | 0.61 |
| a-Si:H passivated | 0.63 | Si PM S10985-050C | 0.95 |
| a-Si:H_non-passivated | 0.92 | Si PM S10362-11-025U | 0.96 |
| CCD with indium tin oxide (ITO) gates with microlenses | 0.68 | Si PM S10362-11-050U | 0.95 |
| CCD with indium tin oxide (ITO) gates | 0.51 | Si PM S10362-11-100U | 0.96 |
| CCD with poly gates | 0.18 | Flat panel PS-PMT H8500C-03 | 0.91 |
| CCD no poly gates LoD | 0.34 | Flat panel PS-PMT H8500D-03 | 0.78 |
| CCD with traditional poly gates | 0.34 | Flat panel PS-PMT H10966A | 0.79 |
| CMOS (photogate array 0.5 μm) | 0.26 | Flat panel PS-PMT H8500C | 0.86 |
| CMOS RadEye HR | 0.68 | Bialkali Photocathode | 0.78 |
| GaAs Photocathode | 0.95 | Multialkali Photocathode | 0.81 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michail, C.; Kalyvas, N.; Bakas, A.; Ninos, K.; Sianoudis, I.; Fountos, G.; Kandarakis, I.; Panayiotakis, G.; Valais, I. Absolute Luminescence Efficiency of Europium-Doped Calcium Fluoride (CaF2:Eu) Single Crystals under X-ray Excitation. Crystals 2019, 9, 234. https://doi.org/10.3390/cryst9050234
Michail C, Kalyvas N, Bakas A, Ninos K, Sianoudis I, Fountos G, Kandarakis I, Panayiotakis G, Valais I. Absolute Luminescence Efficiency of Europium-Doped Calcium Fluoride (CaF2:Eu) Single Crystals under X-ray Excitation. Crystals. 2019; 9(5):234. https://doi.org/10.3390/cryst9050234
Chicago/Turabian StyleMichail, Christos, Nektarios Kalyvas, Athanasios Bakas, Konstantinos Ninos, Ioannis Sianoudis, George Fountos, Ioannis Kandarakis, George Panayiotakis, and Ioannis Valais. 2019. "Absolute Luminescence Efficiency of Europium-Doped Calcium Fluoride (CaF2:Eu) Single Crystals under X-ray Excitation" Crystals 9, no. 5: 234. https://doi.org/10.3390/cryst9050234
APA StyleMichail, C., Kalyvas, N., Bakas, A., Ninos, K., Sianoudis, I., Fountos, G., Kandarakis, I., Panayiotakis, G., & Valais, I. (2019). Absolute Luminescence Efficiency of Europium-Doped Calcium Fluoride (CaF2:Eu) Single Crystals under X-ray Excitation. Crystals, 9(5), 234. https://doi.org/10.3390/cryst9050234









