Information Capacity of Positron Emission Tomography Scanners
Abstract
1. Introduction
2. Materials and Methods
2.1. Modeled PET System Geometry
2.2. Software Customization and Plane Source Test Object
2.3. Noise Equivalent Quanta
2.4. Information Capacity
3. Results
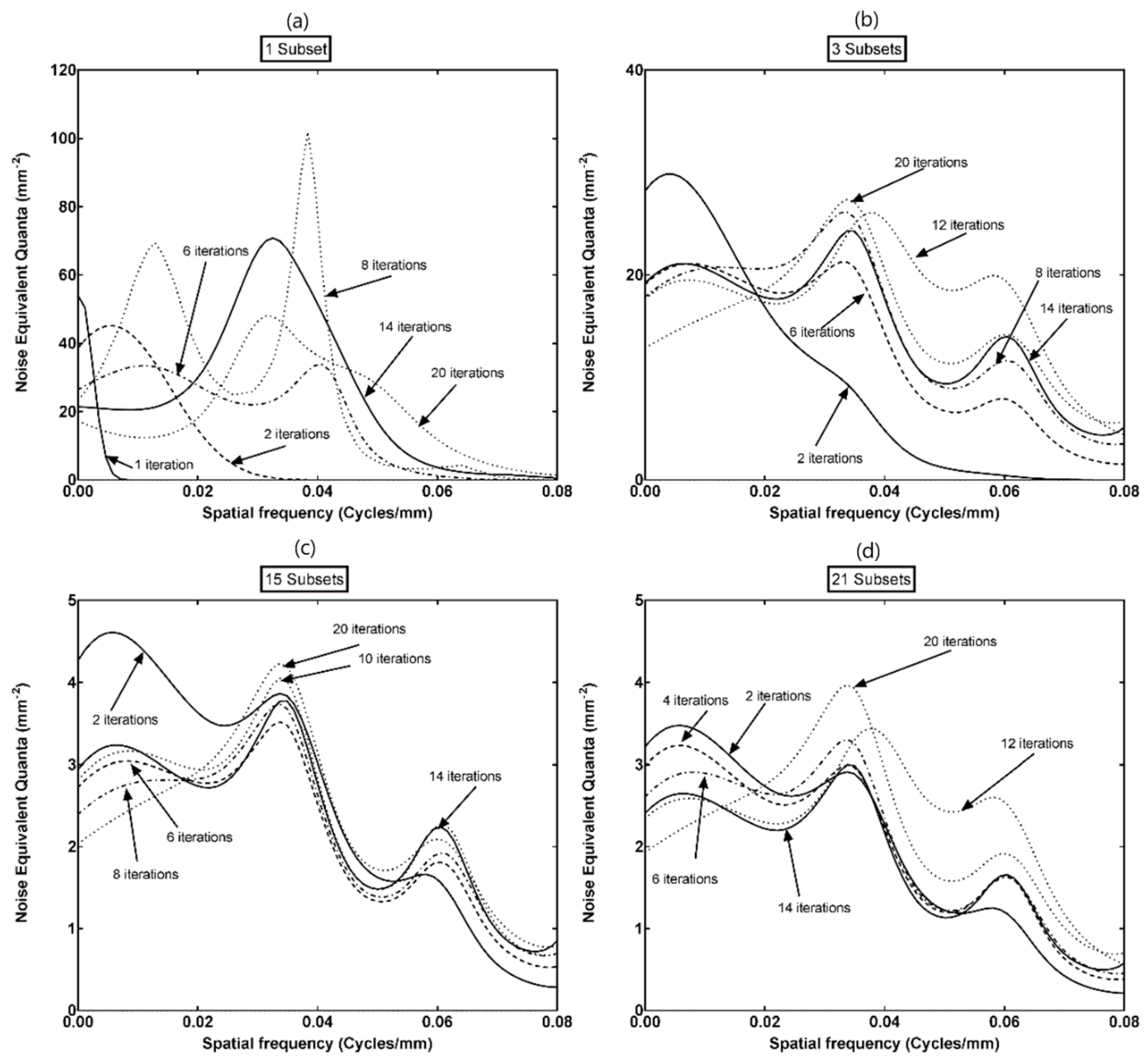
3.1. NEQ
3.2. Information Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dolazza, E.; Poulo, L. Optimal Quantization of Noisy Signals. Proc. SPIE Instrum. Med. XII 1984, 454, 403–417. [Google Scholar]
- Dobbins, J., III. Image Quality Metrics for Digital Systems. In Handbook of Medical Imaging Physics and Psychophysics; Beutel, J., Kundel, H., Van Metter, R., Eds.; SPIE: Bellingham, WA, USA, 2000; Volume 1, pp. 161–222. ISBN 9780819477729. [Google Scholar]
- Goodenough, D. Theoretical Limitations of Tumor Imaging. In Radiolabeled Monoclonal Antibodies for Imaging and Therapy NATO ASI Series (Series A: Life Sciences); Srivastava, S., Ed.; Springer: Boston, MA, USA, 1988; pp. 494–512. ISBN 978-1-4684-5538-0. [Google Scholar]
- González, A.; Majewski, S.; Sánchez, F.; Aussenhofer, S.; Aguilar, A.; Conde, P.; Hernández, L.; Vidal, L.; Pani, R.; Bettiol, M.; et al. The MINDView brain PET detector, feasibility study based on SiPM arrays. Nucl. Instrum. Meth. Phys. Res. A 2016, 818, 82–90. [Google Scholar] [CrossRef]
- Salomoni, M.; Pots, R.; Auffray, E.; Lecoq, P. Enhancing Light Extraction of Inorganic Scintillators Using Photonic Crystals. Crystals 2018, 8, 78. [Google Scholar] [CrossRef]
- Brookeman, V. Computers and quality control in nuclear medicine. Semin. Nucl. Med. 1978, 8, 113–124. [Google Scholar] [CrossRef]
- Brownell, G. Information capacity as a criterion for comparing imaging systems. In Fundamental Problems in Scanning; Gottschalk, A., Beck, R., Eds.; Thomas: Springfield, IL, USA, 1968; pp. 339–347. [Google Scholar]
- Schmidtlein, C.; Kirov, A.; Nehmeh, S.; Erdi, Y.; Humm, J.; Amols, H.; Bidaut, L.; Ganin, A.; Stearns, C.; McDaniel, D.; et al. Validation of GATE Monte Carlo simulations of the GE Advance/Discovery LS PET scanners. Med. Phys. 2006, 33, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Seco, J.; Clasie, B.; Partridge, M. Review on the characteristics of radiation detectors for dosimetry and imaging. Phys. Med. Biol. 2014, 59, R303–R347. [Google Scholar] [CrossRef] [PubMed]
- Grammaticos, P.; Fountos, G. The physician should benefit, not harm the patient. Hell. J. Nucl. Med. 2006, 9, 82–84. [Google Scholar]
- Aarsvold, J.; Barrett, H.; Chen, J.; Landesman, A.; Milster, T.; Patton, D.; TRoney, T.; Rowe, R.; Seacat, R.; Strimbu, L. Modular Scintillation Cameras: A Progress Report. Proc. SPIE Med. Imaging 1988, 0914, 319–325. [Google Scholar]
- Gregg, E. Modulation Transfer Function, Information Capacity and Performance Criteria of Scintiscans. J. Nucl. Med. 1967, 9, 116–127. [Google Scholar]
- Killat, U. Modern Optical Methods for the Storage of Radiographs. In Progress in Medical Radiation Physics; Orton, C., Ed.; Springer: Boston, MA, USA, 1982; Volume 1, pp. 323–376. ISBN 978-1-4615-7693-8. [Google Scholar]
- Sharp, P. Physical Limitations to the Quality of X- And Gamma Ray Images. In Technical Advances in Biomedical Physics, NATO Science Series E; Dendy, P., Ernst, D., Sengün, A., Eds.; Springer: Hague, The Netherlands, 1984; pp. 219–234. [Google Scholar]
- Shannon, C. A mathematical theory of communication. Bell. Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Shannon, C. Communication in the Presence of Noise. Proc. IEEE 1998, 86, 447–457. [Google Scholar] [CrossRef]
- Jones, R. Information capacity of radiation detectors I. J. Opt. Soc. Am. 1961, 50, 1166–1170. [Google Scholar] [CrossRef]
- Jones, R. Information capacity of radiation detectors II. J. Opt. Soc. Am. 1962, 52, 1193–1200. [Google Scholar] [CrossRef]
- Kanamori, H. Information capacity of radiographic films. Jpn. J. App. Phys. 1968, 7, 414–421. [Google Scholar] [CrossRef]
- Dainty, J.; Shaw, R. Image Science; Academic Press: London, UK, 1974; pp. 152–188. [Google Scholar]
- Maiorchuk, M.; Nikitin, V.; Samoilov, V. Possible recording of Fourier holograms with an information capacity of the order of 107 bit/mm2. Sov. J. Quantum Electron. 1974, 4, 68–171. [Google Scholar] [CrossRef]
- Wagner, R.; Brown, D.; Paster, M. Application of information theory to the assessment of computed tomography. Med. Phys. 1979, 6, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.; Anderson, M.; Wagner, R. Information capacity considerations in medical imaging. SPIE 1979, 206, 77–82. [Google Scholar]
- Shaw, R. The Physics of Medical Imaging: Recording System, Measurements and Techniques; American Association of Physicists in Medicine: New York, NY, USA, 1979; pp. 524–545. [Google Scholar]
- Evans, A. The Evaluation of Medical Images; Adam Hilger: Bristol, UK, 1981. [Google Scholar]
- Kanamori, H.; Matsuoto, M. The information spectrum as a measure of radiographic image quality and system performance. Phys. Med. Biol. 1984, 29, 303–313. [Google Scholar] [CrossRef]
- Kandarakis, I.; Cavouras, D.; Panayiotakis, G.; Nomicos, C. Experimental investigation of the optical signal, gain, signal to noise ratio and information content of X-ray phosphor screens. Appl. Phys. B 2001, 72, 877–883. [Google Scholar] [CrossRef]
- Michail, C.; Kalyvas, N.; Valais, I.; Fudos, I.; Fountos, G.; Dimitropoulos, N.; Koulouras, G.; Kandris, D.; Samarakou, M.; Kandarakis, I. Figure of Image Quality and Information Capacity in Digital Mammography. Biomed. Res. Int. 2014, 2014, 634856. [Google Scholar] [CrossRef]
- Seferis, I.; Michail, C.; Valais, I.; Fountos, G.; Kalyvas, N.; Stromatia, F.; Oikonomou, G.; Kandarakis, I.; Panayiotakis, G. On the response of a europium doped phosphor-coated CMOS digital imaging detector. Nucl. Instrum. Meth. Phys. Res. A 2013, 729, 307–315. [Google Scholar] [CrossRef]
- Gallager, R. Information Theory and Reliable Communication; John Wiley: New York, NY, USA, 1968. [Google Scholar]
- Gregg, E. Information capacity of scintiscans. J. Nucl. Med. 1967, 6, 441–458. [Google Scholar]
- Barrett, H.; Swindell, W. Radiological Imaging. The Theory of Image Formation, Detection and Processing; Academic Press: London, UK, 2012. [Google Scholar]
- Michail, C.; Spyropoulou, V.; Fountos, G.; Kalyvas, N.; Valais, I.; Kandarakis, I.; Panayiotakis, G. Experimental and theoretical evaluation of a high resolution CMOS based detector under X-ray imaging conditions. IEEE Trans. Nucl. Sci. 2011, 58, 314–322. [Google Scholar] [CrossRef]
- Starck, S.; Bath, M.; Carlsson, S. The use of detective quantum efficiency (DQE) in evaluating the performance of gamma camera systems. Phys. Med. Biol. 2005, 50, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Karpetas, G.; Michail, C.; Fountos, G.; Valsamaki, P.; Kandarakis, I.; Panayiotakis, G. Towards the optimization of nuclear medicine procedures for better spatial resolution, sensitivity, scan image quality and quantitation measurements by using a new Monte Carlo model featuring PET imaging. Hell. J. Nucl. Med. 2013, 16, 111–120. [Google Scholar] [PubMed]
- Michail, C.; Karpetas, G.; Fountos, G.; Kalyvas, N.; Valais, I.; Fountzoula, C.; Zanglis, A.; Kandarakis, I.; Panayiotakis, G. A novel method for the Optimization of Positron Emission Tomography Scanners Imaging Performance. Hell. J. Nucl. Med. 2016, 19, 231–240. [Google Scholar]
- Jan, S.; Santin, G.; Strul, D.; Staelens, S.; Assie, K.; Autret, D.; Avner, S.; Barbier, R.; Bardies, M.; Bloomfield, P.M.; et al. GATE: A simulation toolkit for PET and SPECT. Phys. Med. Biol. 2004, 49, 4543–4561. [Google Scholar] [CrossRef]
- Strulab, D.; Santin, G.; Lazaro, D.; Breton, V.; Morel, C. (Geant4 application for tomographic emission): A PET/SPECT general purpose simulation platform. Nucl. Phys. B 2003, 125, 75–79. [Google Scholar] [CrossRef]
- Agostinelli, S.; Allison, J.; Amako, K.A.; Apostolakis, J.; Araujo, H.; Arce, P.; Asai, M.; Axen, D.; Banerjee, S.; Barrand, G.; et al. Geant4-a simulation toolkit. Nucl. Instrum. Meth. Phys. Res. A 2003, 506, 250–303. [Google Scholar] [CrossRef]
- Karpetas, G.; Michail, C.; Fountos, G.; Kalyvas, N.; Valais, I.; Kandarakis, I.; Panayiotakis, G. Detective Quantum Efficiency (DQE) in PET Scanners: A Simulation Study. Appl. Radiat. Isot. 2017, 125, 154–162. [Google Scholar] [CrossRef]
- Thielemans, K.; Tsoumpas, C.; Mustafovic, S.; Beisel, T.; Aguiar, P.; Dikaios, N.; Jacobson, M.W. STIR: Software for tomographic image reconstruction release 2. Phys. Med. Biol. 2012, 57, 867–883. [Google Scholar] [CrossRef] [PubMed]
- Visvikis, D.; Bardies, M.; Chiavassa, S.; Danford, C.; Kirov, A.; Lamare, F.; Maigne, L.; Staelens, S.; Taschereau, R. Use of the GATE Monte Carlo package for dosimetry applications. Nucl. Instrum. Meth. A 2006, 569, 335–340. [Google Scholar] [CrossRef]
- Valais, I.; Michail, C.; David, S.; Nomicos, C.; Panayiotakis, G.; Kandarakis, I. A Comparative Study of the Luminescence Properties of LYSO:Ce, LSO:Ce, GSO:Ce and BGO Single Crystal Scintillators for Use in Medical X-Ray Imaging. Phys. Med. 2008, 24, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Van Eijk, C. Inorganic scintillators in medical imaging. Phys. Med. Biol. 2002, 47, R85–R106. [Google Scholar] [CrossRef] [PubMed]
- Michail, C.; Fountos, G.; Liaparinos, P.; Kalyvas, N.; Valais, I.; Kandarakis, I.; Panayiotakis, G. Light emission efficiency and imaging performance of Gd2O2S:Eu powder scintillator under X-ray Radiography conditions. Med. Phys. 2010, 37, 3694–3703. [Google Scholar] [CrossRef] [PubMed]
- Valais, I.; Michail, C.; David, S.; Liaparinos, P.; Fountos, G.; Paschalis, T.; Kandarakis, I.; Panayiotakis, G. Comparative Investigation of Ce3+ doped Scintillators in a wide Range of Photon Energies covering X-ray CT, Nuclear Medicine and Megavoltage Radiation Therapy Portal Imaging applications. IEEE Trans. Nucl. Sci. 2010, 57, 3–7. [Google Scholar] [CrossRef]
- Michail, C.; David, S.; Liaparinos, P.; Valais, I.; Nikolopoulos, D.; Kalivas, N.; Toutountzis, A.; Cavouras, D.; Kandarakis, I.; Panayiotakis, G. Evaluation of the imaging performance of LSO powder scintillator for use in x-ray mammography. Nucl. Instrum. Meth. Phys. Res. A 2007, 580, 558–561. [Google Scholar] [CrossRef]
- Michail, C.; Fountos, G.; David, S.; Valais, I.; Toutountzis, A.; Kalyvas, N.; Kandarakis, I.; Panayiotakis, G. A comparative investigation of Lu2SiO5:Ce and Gd2O2S:Eu powder scintillators for use in X-ray mammography detectors. Meas. Sci. Technol. 2009, 20, 104008. [Google Scholar] [CrossRef]
- Michail, C.; Toutountzis, A.; David, S.; Kalivas, N.; Valais, I.; Kandarakis, I.; Panayiotakis, G. Imaging performance and light emission efficiency of Lu2SiO5:Ce (LSO:Ce) powder scintillator under x-ray mammographic conditions. App. Phys. B 2009, 95, 131–139. [Google Scholar] [CrossRef]
- Kilian, A.; Bilski, P.; Gorbenko, V.; Zorenko, T.; Witkiewicz, S.; Paprocki, K.; Zorenko, Y. Thermoluminescent Properties of Cerium-Doped Lu2SO5 and Y2SiO5 Single Crystalline Films Scintillators Grown from PbO-B2O3 and Bi2O3 Fluxes. Crystals 2018, 8, 120. [Google Scholar] [CrossRef]
- Valais, I.; David, S.; Michail, C.; Konstantinidis, A.; Kandarakis, I.; Panayiotakis, G. Investigation of luminescence properties of the LSO:Ce, LYSO:Ce and GSO:Ce crystal scintillators under low-energy γ-ray excitation used in nuclear imaging. Nucl. Instrum. Meth. Phys. Res. A 2007, 581, 99–102. [Google Scholar] [CrossRef]
- GATE Simulations of Preclinical and Clinical Scans in Emission Tomography, Transmission Tomography and Radiation Therapy. Available online: http://www.opengatecollaboration.org/ (accessed on 2 November 2018).
- Boone, J. Determination of the presampled MTF in computed tomography. Med. Phys. 2001, 28, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Fountos, G.; Michail, C.; Zanglis, A.; Samartzis, A.; Martini, N.; Koukou, V.; Kalatzis, I.; Kandarakis, I. A novel easy-to-use phantom for the determination of MTF in SPECT scanners. Med. Phys. 2012, 39, 1561–1570. [Google Scholar] [CrossRef] [PubMed]
- Thielemans, K.; Tsoubas, C.; Sauge, D.; Sauge, D.; Labbe, C.; Morel, C.; Jacobson, M.; Zverovich, A.; Beisel, T.; Falcon, C. STIR Software for Tomographic Image Reconstruction User’s Guide Version 3.0. (2012) pp. 1–71. Available online: http://stir.sourceforge.net/documentation/STIR-UsersGuide.pdf (accessed on 2 November 2018).
- Kinahan, P.; Rogers, J. Analytic 3D image reconstruction using all detected events. IEEE Trans. Nucl. Sci. 1989, 36, 964–968. [Google Scholar] [CrossRef]
- Labbe, C.; Zaidi, H.; Morel, C. (Updated by Thielemans, K.; Imanet). STIR Description of the STIR implementation of FBP 3DRP Version 0.91. (2004) pp. 1–10. Available online: http://stir.sourceforge.net/documentation/STIR-FBP3DRP.pdf (accessed on 2 November 2018).
- Dobbins, J., III. Effects of undersampling on the proper interpretation of modulation transfer function, noise power spectra, and noise equivalent quanta of digital systems. Med. Phys. 1995, 22, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.; Brown, D. Unified SNR analysis of medical imaging systems. Phys. Med. Biol. 1985, 30, 489–518. [Google Scholar] [CrossRef] [PubMed]
- Hillen, W.; Schiebel, U.; Zaengel, T. Imaging performance of a digital storage phosphor system. Med. Phys. 1987, 14, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Suryanarayanan, S.; Karellas, A.; Vedantham, S.; Onishi, S. High-resolution imager for digital mammography: Physical characterization of a prototype sensor. Phys. Med. Biol. 2005, 50, 3957–3969. [Google Scholar] [CrossRef]
- Gagne, R.; Boswell, J.; Myers, K. Signal detectability in digital radiography: Spatial domain figures of merit. Med. Phys. 2003, 30, 2180–2193. [Google Scholar] [CrossRef]
- Bosmans, H.; Carton, A.; Rogge, F.; Zanca, F.; Jacobs, J.; Van Ongeval, C.; Nijs, K.; Van Steen, A.; Marchal, G. Image quality measurements and metrics in full field digital mammography: An overview. Radiat. Protect. Dosim. 2005, 117, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Russo, P. Handbook of X-ray Imaging: Physics and Technology; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Gureyev, T.; Hoog, F.; Nesterets, Y.; Paganin, D. On the noise-resolution duality, Heisenberg uncertainty and Shannon’s information. ANJIAM J. 2015, 56, C1–C15. [Google Scholar] [CrossRef]
- Gureyev, T.; Nesterets, Y.; De Hoog, F. Spatial resolution, signal-to-noise and information capacity of linear imaging systems. Opt. Express. 2016, 24, 17168–17182. [Google Scholar] [CrossRef] [PubMed]
- Rogachov, A.; Cheng, J.; Erpelding, N.; Hemington, K.; Crawley, A.; Davis, K. Regional brain signal variability: A novel indicator of pain sensitivity and coping. Pain 2016, 157, 2483–2492. [Google Scholar] [CrossRef]
- Norman, R.; Dunning-Davies, J.; Heredia-Rojas, J.; Foletti, A. Quantum Information Medicine: Bit as It-The Future Direction of Medical Science: Antimicrobial and Other Potential Nontoxic Treatments. World J. Neurosci. 2016, 6, 193–207. [Google Scholar] [CrossRef]
- Shew, W.; Yang, H.; Yu, S.; Roy, R.; Plenz, D. Information capacity and transmission are maximized in balanced cortical networks with neuronal avalanches. J. Neurosci. 2011, 31, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Fagerholm, E.; Scott, G.; Shew, W.; Song, C.; Leech, R.; Knopfel, T.; Sharp, D. Cortical Entropy, Mutual Information and Scale-Free Dynamics in Waking Mice. Cereb. Cortex. 2016, 26, 3945–3952. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lauer, K.; Ward, B.; Roberts, C.; Liu, S.; Gollapudy, S.; Rohloff, R.; Gross, W.; Xu, Z.; Chen, S.; et al. Regional entropy of functional imaging signals varies differently in sensory and cognitive systems during propofol-modulated loss and return of behavioral responsiveness. Brain Imaging Behav. 2018, 1–12. [Google Scholar] [CrossRef]
- Sitek, A.; Celler, A. Limitations of Poisson statistics in describing radioactive decay. Phys. Med. 2015, 31, 1105–1107. [Google Scholar] [CrossRef]
- Prokushkin, S.; Galil, E. Information theoretic methods for image processing algorithm optimization. Proc. SPIE 2015, 9396, 939604. [Google Scholar]
- Watson, C.; Casey, M.; Bendriem, B.; Carney, J.; Townsend, D.; Eberl, S.; Meikle, S.; DiFilippo, F. Optimizing injected dose in clinical PET by accurately modeling the counting-rate response functions specific to individual patient scans. J. Nucl. Med. 2005, 46, 1825–1834. [Google Scholar]
- Soret, M.; Bacharach, S.; Buvat, I. Partial-Volume Effect in PET Tumor Imaging. J. Nucl. Med. 2007, 48, 932–945. [Google Scholar] [CrossRef] [PubMed]
- Karpetas, G.; Michail, C.; Fountos, G.; Kandarakis, I.; Panayiotakis, G. A new PET resolution measurement method through Monte Carlo simulations. Nucl. Med. Commun. 2014, 35, 967–976. [Google Scholar] [CrossRef]
- Karakatsanis, N.; Fokou, E.; Tsoumpas, C. Dosage optimization in positron emission tomography: State-of-the-art methods and future prospects. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 527–547. [Google Scholar]
- Levin, C.; Hoffman, E. Calculation of positron range and its effect on the fundamental limit of positron emission tomography system spatial resolution. Phys. Med. Biol. 1999, 44, 781–799. [Google Scholar] [CrossRef] [PubMed]
- Rose, A. Vision: Human and Electronic; Plenum Press: New York, NY, USA, 1973. [Google Scholar]
- Motz, J.; Danos, M. Image information content and patient exposure. Med. Phys. 1978, 5, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Wickham, F.; McMeekin, H.; Burniston, M.; McCool, D.; Pencharz, D.; Skillen, A.; Wagner, T. Patient-specific optimisation of administered activity and acquisition times for 18F-FDG PET imaging. EJNMMI Res. 2017, 7, 3. [Google Scholar] [CrossRef]

| Iterations | Subsets | |||
|---|---|---|---|---|
| 1 | 3 | 15 | 21 | |
| Information Capacity (bits/mm2) | ||||
| 1 | 0.0217 | - | - | - |
| 2 | 0.5577 | 1.3774 | 1.6869 | 1.4100 |
| 4 | - | - | - | 1.6770 |
| 6 | 2.2899 | 3.8744 | 1.9202 | 1.7926 |
| 8 | 2.8716 | 4.5902 | 1.9510 | - |
| 12 | - | 4.7432 | 2.2109 | 1.7690 |
| 14 | 3.5305 | 4.7080 | 1.9586 | 1.6097 |
| 20 | 4.0222 | 5.1947 | 2.1936 | 2.0781 |
| PET Module/Scintillating Crystal Combination | Algorithm | ||
|---|---|---|---|
| FBP2D | FBP3DRP | OS-MAP-OSL | |
| Information Capacity (bits/mm2) | |||
| BGO | 0.7197 | 2.4829 | 1.6042 |
| Gd2SiO5:Ce | 0.2481 | 1.4735 | 0.8864 |
| Lu2SiO5:Ce | 0.3215 | 1.7611 | 1.1651 |
| LuAP:Ce | 0.8648 | 1.8757 | 0.5875 |
| LuYAP:Ce—70% | 0.1242 | 0.9860 | 0.7620 |
| LuYAP:Ce—80% | 0.2265 | 1.2798 | 1.4028 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michail, C.; Karpetas, G.; Kalyvas, N.; Valais, I.; Kandarakis, I.; Agavanakis, K.; Panayiotakis, G.; Fountos, G. Information Capacity of Positron Emission Tomography Scanners. Crystals 2018, 8, 459. https://doi.org/10.3390/cryst8120459
Michail C, Karpetas G, Kalyvas N, Valais I, Kandarakis I, Agavanakis K, Panayiotakis G, Fountos G. Information Capacity of Positron Emission Tomography Scanners. Crystals. 2018; 8(12):459. https://doi.org/10.3390/cryst8120459
Chicago/Turabian StyleMichail, Christos, George Karpetas, Nektarios Kalyvas, Ioannis Valais, Ioannis Kandarakis, Kyriakos Agavanakis, George Panayiotakis, and George Fountos. 2018. "Information Capacity of Positron Emission Tomography Scanners" Crystals 8, no. 12: 459. https://doi.org/10.3390/cryst8120459
APA StyleMichail, C., Karpetas, G., Kalyvas, N., Valais, I., Kandarakis, I., Agavanakis, K., Panayiotakis, G., & Fountos, G. (2018). Information Capacity of Positron Emission Tomography Scanners. Crystals, 8(12), 459. https://doi.org/10.3390/cryst8120459







