Radio-, Thermo- and Photoluminescence Properties of Lu2O3:Eu and Lu2O3:Tb Nanopowder and Film Scintillators
Abstract
:1. Introduction
2. Sample Preparation and Experimental Techniques
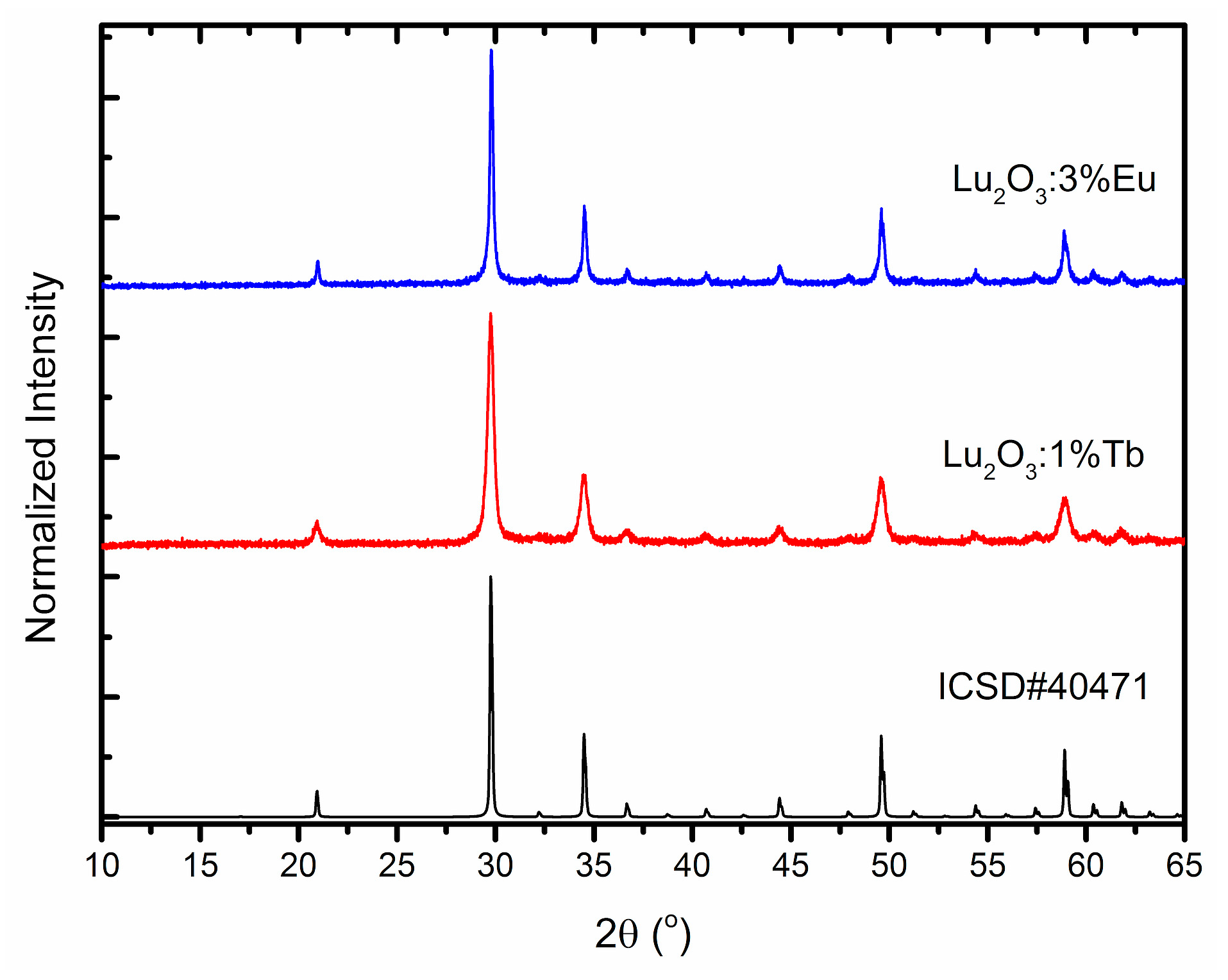
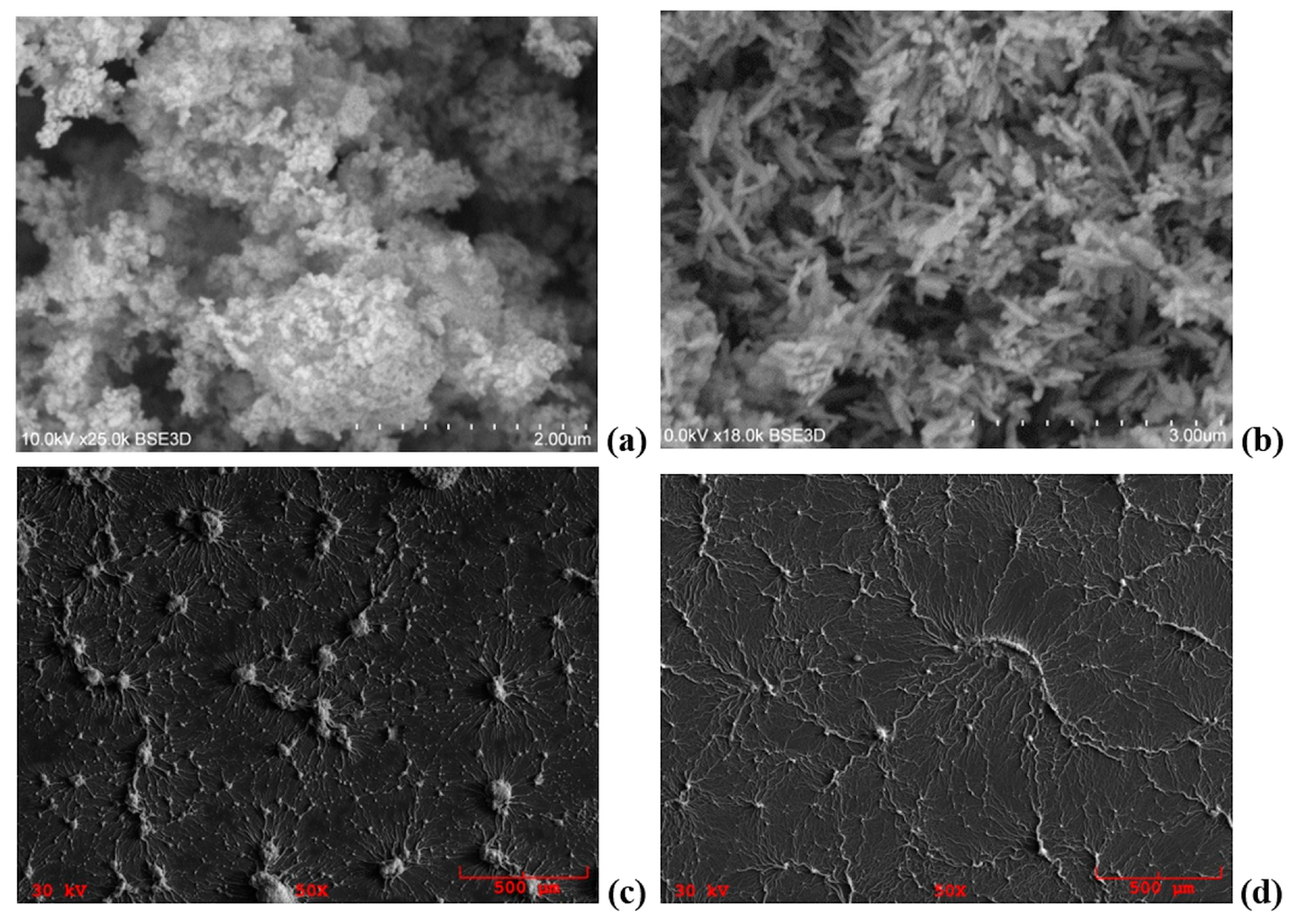
3. Structural and Morphological Properties of Lu2O3:Eu and Lu2O3:Tb NPs and Films
4. Luminescent Properties of Lu2O3:Eu and Lu2O3:Tb NPs and Films
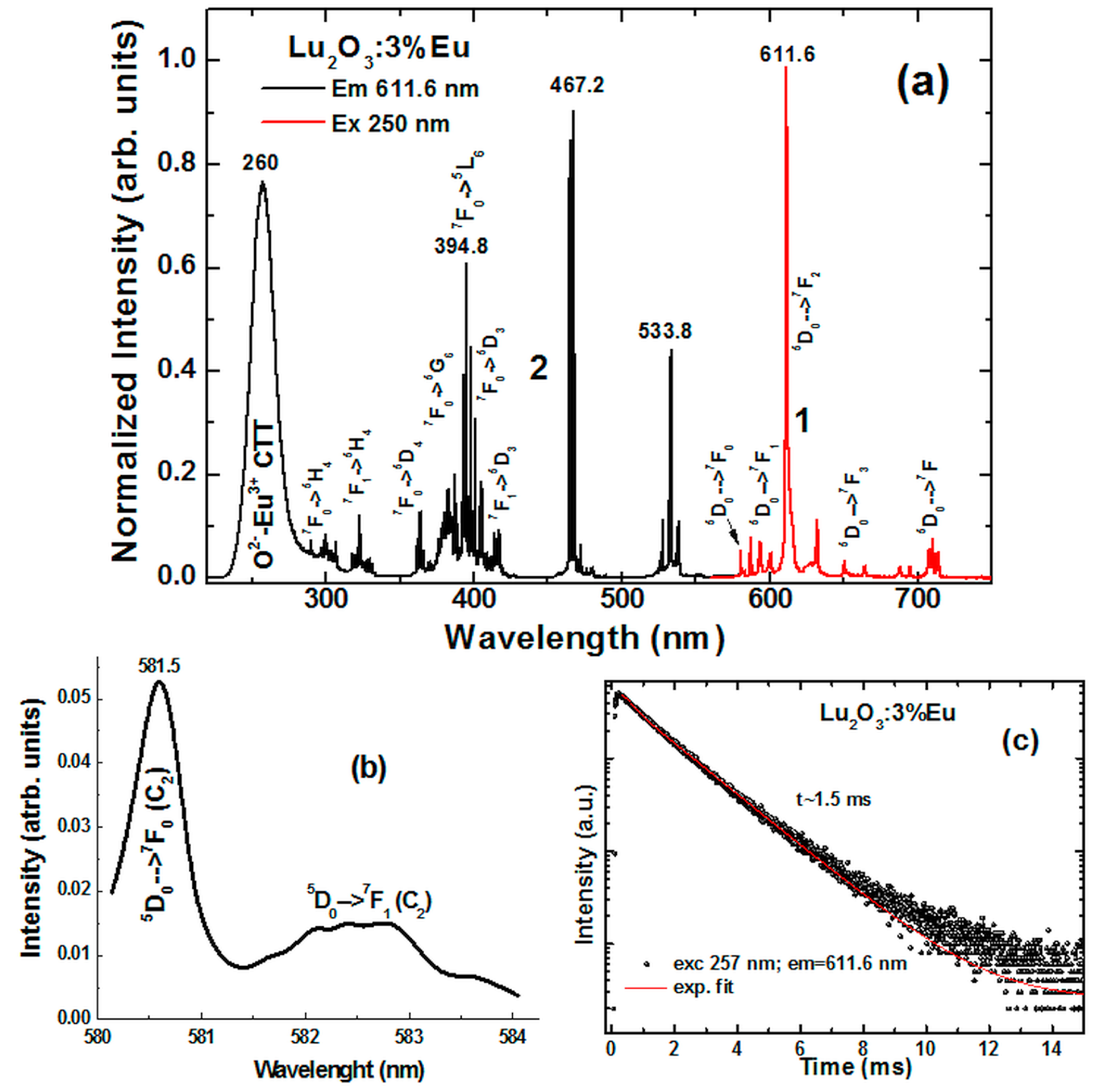
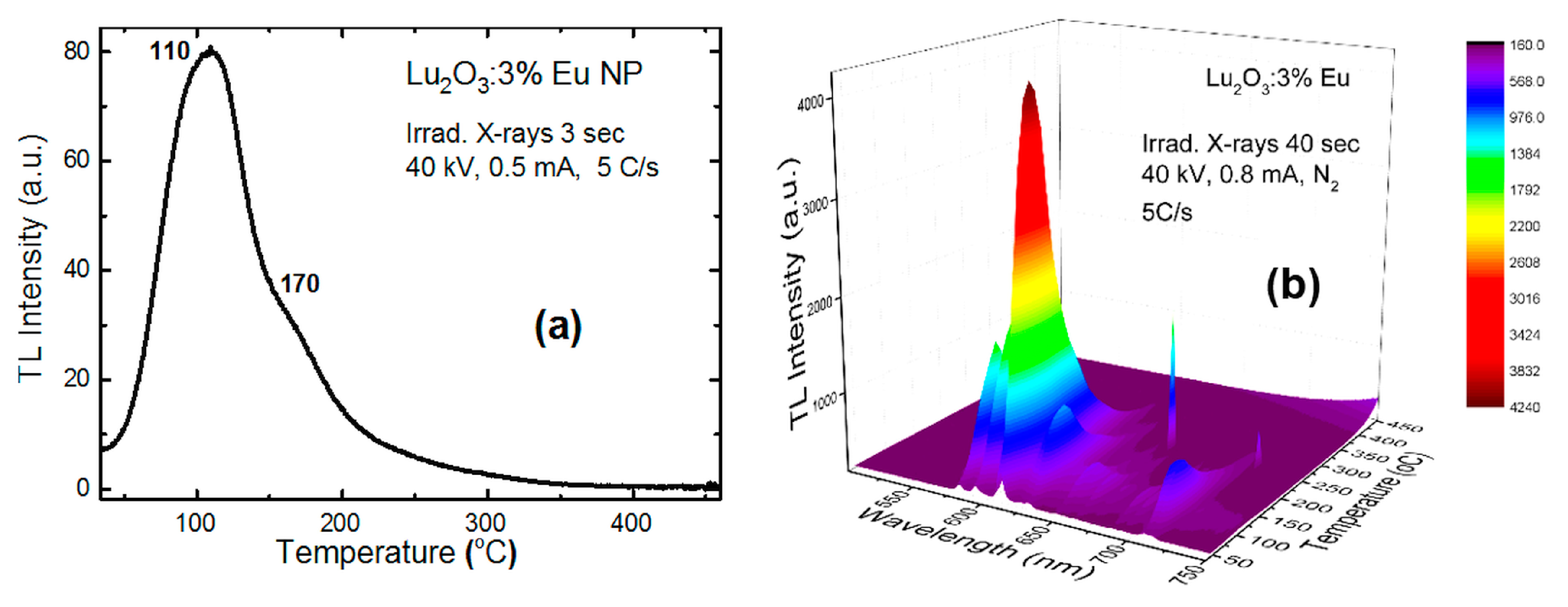
4.1. Lu2O3:Eu NPs and Films
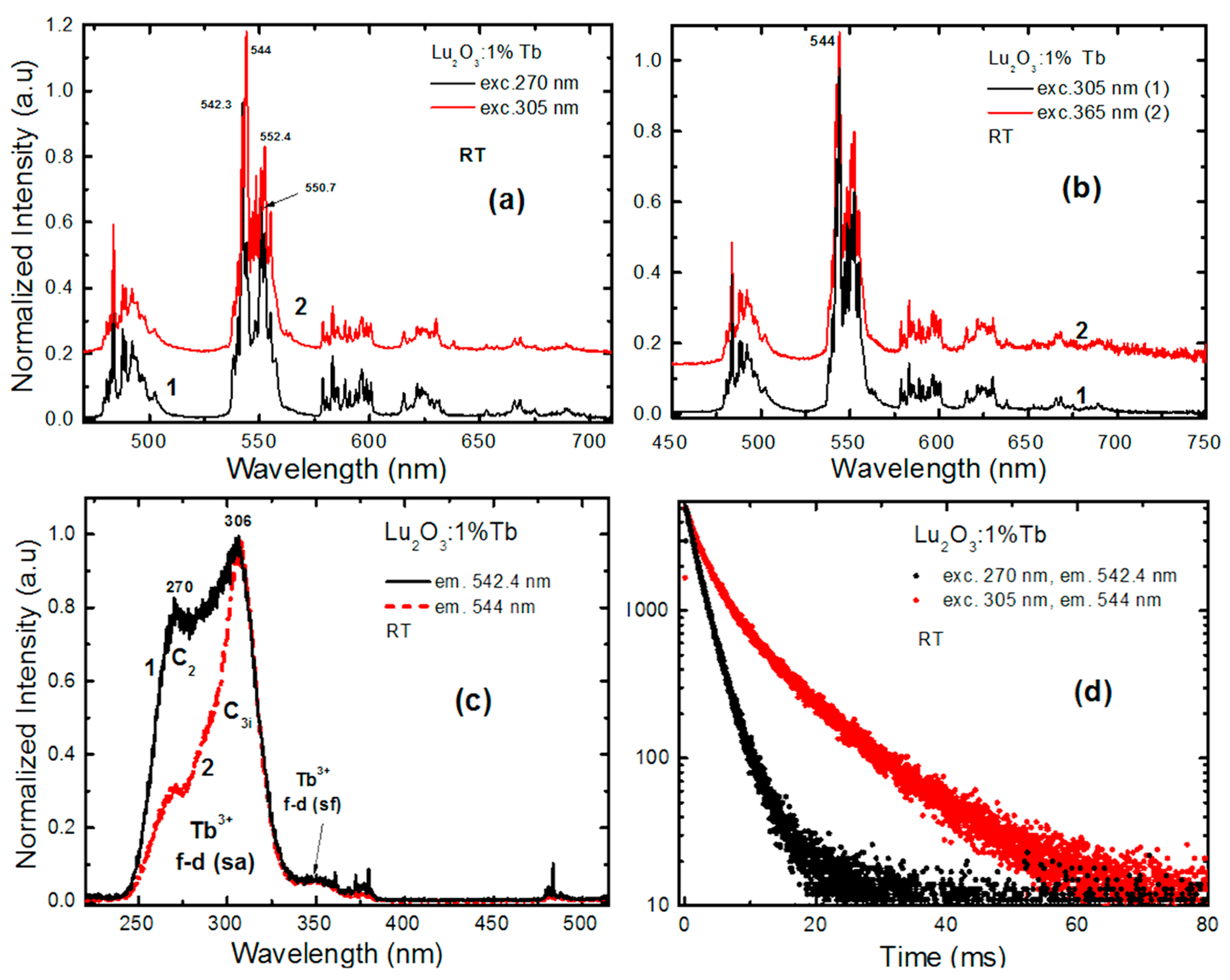
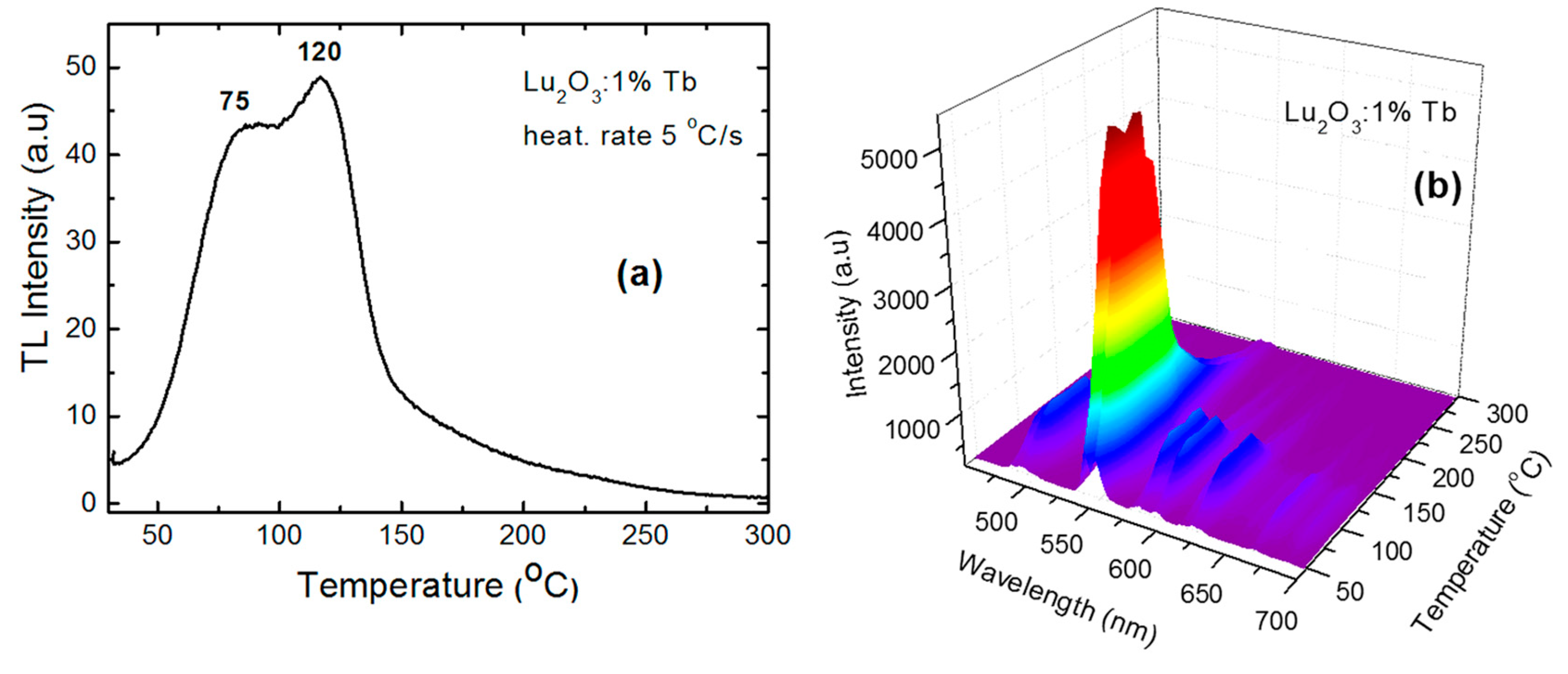
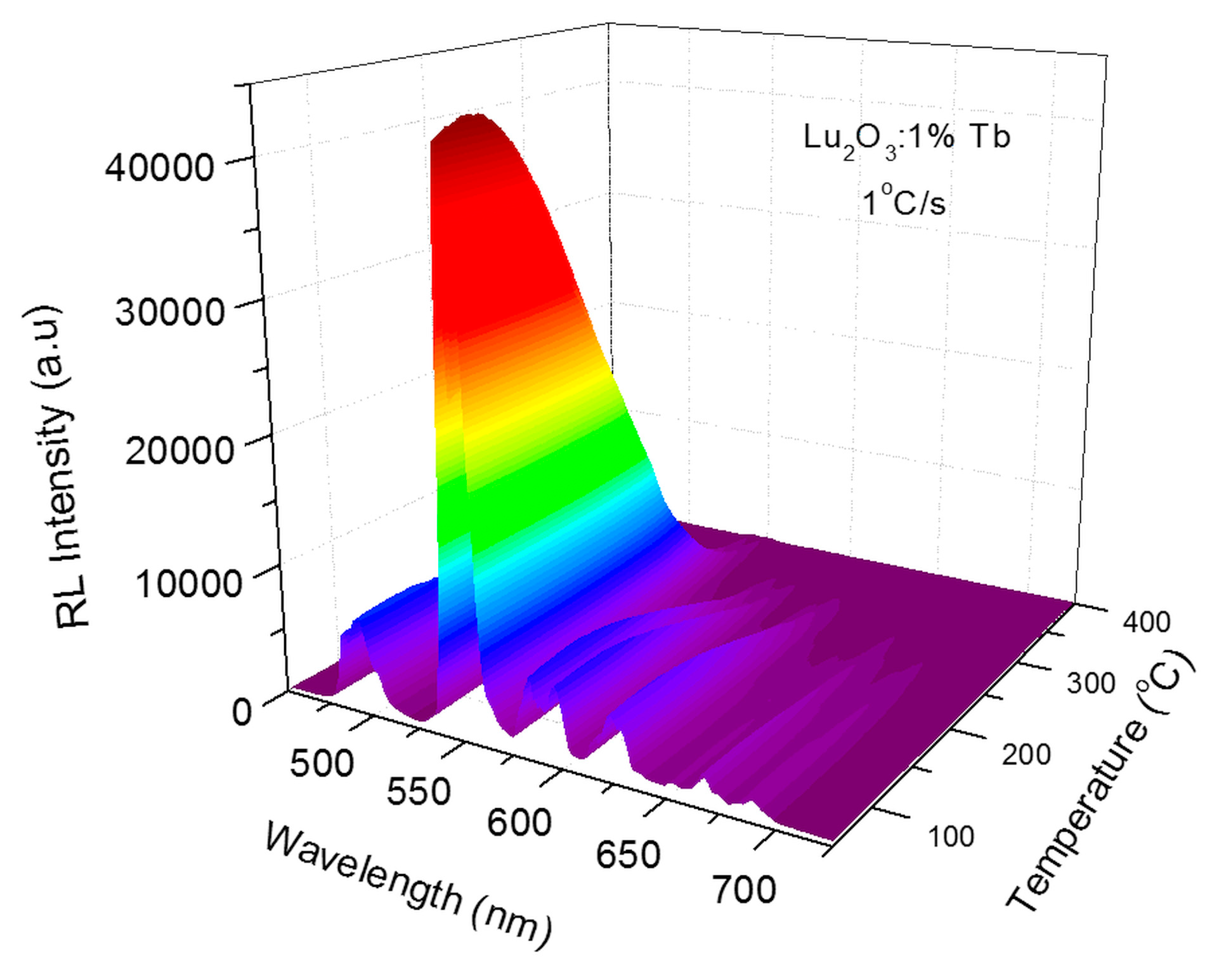
4.2. Lu2O3:Tb NPs and films
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lempicki, A.; Brecher, C.; Szupryczynski, P.; Lingertat, H.; Nagarkar, V.; Tipnis, S.; Miller, S. A new lutetia-based ceramic scintillator for X-ray imaging. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometersdetectors Assoc. Equip. 2002, 488, 579–590. [Google Scholar] [CrossRef]
- Zych, E. Concentration dependence of energy transfer between Eu3+ ions occupying two symmetry sites in Lu2O3. J. Phys. Condens. Matter 2002, 14, 5637. [Google Scholar] [CrossRef]
- Zeler, J.; Jerzykiewicz, L.B.; Zych, E. Flux-aided synthesis of Lu2O3 and Lu2O3: Eu—single crystal structure, morphology control and radioluminescence efficiency. Materials 2014, 7, 7059–7072. [Google Scholar] [CrossRef]
- Sengupta, D.; Miller, S.; Marton, Z.; Chin, F.; Nagarkar, V.; Pratx, G. Bright Lu2O3: Eu thin-film scintillators for high-resolution radioluminescence microscopy. Adv. Healthc. Mater. 2015, 4, 2064–2070. [Google Scholar] [CrossRef] [PubMed]
- Riva, F.; Martin, T.; Douissard, P.; Dujardin, C. Single crystal lutetium oxide thin film scintillators for X-ray imaging. J. Instrum. 2016, 11, C10010. [Google Scholar] [CrossRef]
- Zorenko, T.; Gorbenko, V.; Safronova, N.; Matveevskaya, N.; Yavetskiy, R.; Babayevska, N.; Zorenko, Y. Comparative study of the luminescent properties of oxide compounds under synchrotron radiation excitation: Lu2O3: Eu nanopowders, ceramics and films. J. Lumin. 2018, 199, 461–464. [Google Scholar] [CrossRef]
- Bellocchi, G.; Franzò, G.; Iacona, F.; Boninelli, S.; Miritello, M.; Cesca, T.; Priolo, F. Eu3+ reduction and efficient light emission in Eu2O3 films deposited on Si substrates. Opt. Express 2012, 20, 5501–5507. [Google Scholar] [CrossRef] [PubMed]
- Burmester, P.; Ishii, T.; Huber, G.; Kurfiss, M.; Schilling, M. Characterization of crystalline europium doped α-Y2O3 PLD-films grown on α-Al2O3. Mater. Sci. Eng. B 2003, 105, 25–29. [Google Scholar] [CrossRef]
- Gün, T.; Kahn, A.; İleri, B.; Petermann, K.; Huber, G. Two-dimensional growth of lattice matched Nd-doped (Gd, Lu)2O3 films on Y2O3 by pulsed laser deposition. Appl. Phys. Lett. 2008, 93, 053108. [Google Scholar] [CrossRef]
- Babaevskaya, N.; Kryzhanovskaya, A.; Matveevskaya, N.; Mateichenko, P.; Yavetskii, R.; Tolmachev, A.; Tret’yak, S. Composite phosphor films based on spherical Lu2O3: Eu3+ nanoparticles. Tech. Phys. Lett. 2011, 37, 174–177. [Google Scholar] [CrossRef]
- Babayevskaya, N.; Bezkrovnyi, A.; Mateychenko, P.; Vovk, O.; Yavetskiy, R. Sol-gel processing of transparent Lu2O3: Eu3+ phosphor films. Funct. Mater. 2010, 17, 537–542. [Google Scholar]
- Popielarski, P.; Paprocki, K.; Bala, W.; Banaszak-Piechowska, A.; Walczyk, K.; Fabisiak, K.; Szybowicz, M. Raman and impedance spectroscopy of blend polycarbonate and zinc oxide layers grown by sol-gel method. In Proceedings of the Solid State Phenomena, Lviv, Ukraine, 3–7 September 2012; pp. 22–26. [Google Scholar]
- Bala, W.; Zorenko, Y.; Savchyn, V.; Voznyak, T.; Paprocki, K.; Popielarski, P.; Szybowicz, M. Optical and electrical properties of ZnO thin films grown by sol-gel method. In Proceedings of the Solid State Phenomena, Lviv, Ukraine, 3–7 September 2012; pp. 14–21. [Google Scholar]
- Frackowiak, S.; Zych, E.; Kozlowski, M.; Kepinski, L. Modifying the luminescence characteristics of Lu2O3: Eu large nanocrystals with polycarbonate host. Polym. Compos. 2016, 37, 1330–1334. [Google Scholar] [CrossRef]
- Meltzer, R.; Feofilov, S.; Tissue, B.; Yuan, H. Dependence of fluorescence lifetimes of Y2O3: Eu3+ nanoparticles on the surrounding medium. Phys. Rev. B 1999, 60, R14012. [Google Scholar] [CrossRef]
- Meltzer, R.; Yen, W.; Zheng, H.; Feofilov, S.; Dejneka, M.; Tissue, B.; Yuan, H. Interaction of rare earth ions doped in nanocrystals embedded in amorphous matrices with two-level systems of the matrix. J. Lumin. 2001, 94, 221–224. [Google Scholar] [CrossRef]
- Vetrone, F.; Boyer, J.-C.; Capobianco, J.A.; Speghini, A.; Bettinelli, M. A spectroscopic investigation of trivalent lanthanide doped Y2O3 nanocrystals. Nanotechnology 2003, 15, 75. [Google Scholar] [CrossRef]
- Hreniak, D.; Gluchowski, P.; Strek, W.; Bettinelli, M.; Kozlowska, A.; Kozlowski, M. Preparation and upconversion properties of Er3+, Yb3+: Y2Si2O7 nanocrystallites embedded in PVA polymer nanocomposites. Mater. Sci. Wroc. 2006, 24, 405. [Google Scholar]
- Trojan-Piegza, J.; Zych, E.; Hreniak, D.; Stręk, W.; Kępiński, L. Structural and spectroscopic characterization of Lu2O3: Eu nanocrystalline spherical particles. J. Phys. Condens. Matter 2004, 16, 6983. [Google Scholar] [CrossRef]
- de Mello Donegá, C.; Meijerink, A.; Blasse, G. Saturation effects in the excitation spectra of rare-earth ions. J. Lumin. 1994, 62, 189–201. [Google Scholar] [CrossRef]
- Dorenbos, P. Relation between Eu2+ and Ce3+ f↔ d-transition energies in inorganic compounds. J. Phys. Condens. Matter 2003, 15, 4797. [Google Scholar] [CrossRef]
- Dorenbos, P. A review on how lanthanide impurity levels change with chemistry and structure of inorganic compounds. ECS J. Solid State Sci. Technol. 2013, 2, R3001–R3011. [Google Scholar] [CrossRef]
- Dorenbos, P. Charge transfer bands in optical materials and related defect level location. Opt. Mater. 2017, 69, 8–22. [Google Scholar] [CrossRef]
- Trojan-Piegza, J.; Zych, E. Afterglow luminescence of Lu2O3: Eu ceramics synthesized at different atmospheres. J. Phys. Chem. C 2010, 114, 4215–4220. [Google Scholar] [CrossRef]
- Trojan-Piegza, J.; Niittykoski, J.; Hölsä, J.; Zych, E. Thermoluminescence and kinetics of persistent luminescence of vacuum-sintered Tb3+-doped and Tb3+, Ca2+-codoped Lu2O3 materials. Chem. Mater. 2008, 20, 2252–2261. [Google Scholar] [CrossRef]
- Trojan-Piegza, J.; Zych, E.; Hölsä, J.; Niittykoski, J. Spectroscopic properties of persistent luminescence phosphors: Lu2O3: Tb3+, M2+ (M = Ca, Sr, Ba). J. Phys. Chem. C 2009, 113, 20493–20498. [Google Scholar] [CrossRef]
- Kulesza, D.; Bolek, P.; Bos, A.J.; Zych, E. Lu2O3-based storage phosphors. An (in) harmonious family. Coord. Chem. Rev. 2016, 325, 29–40. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popielarski, P.; Zeler, J.; Bolek, P.; Zorenko, T.; Paprocki, K.; Zych, E.; Zorenko, Y. Radio-, Thermo- and Photoluminescence Properties of Lu2O3:Eu and Lu2O3:Tb Nanopowder and Film Scintillators. Crystals 2019, 9, 148. https://doi.org/10.3390/cryst9030148
Popielarski P, Zeler J, Bolek P, Zorenko T, Paprocki K, Zych E, Zorenko Y. Radio-, Thermo- and Photoluminescence Properties of Lu2O3:Eu and Lu2O3:Tb Nanopowder and Film Scintillators. Crystals. 2019; 9(3):148. https://doi.org/10.3390/cryst9030148
Chicago/Turabian StylePopielarski, P., J. Zeler, P. Bolek, T. Zorenko, K. Paprocki, E. Zych, and Yu. Zorenko. 2019. "Radio-, Thermo- and Photoluminescence Properties of Lu2O3:Eu and Lu2O3:Tb Nanopowder and Film Scintillators" Crystals 9, no. 3: 148. https://doi.org/10.3390/cryst9030148
APA StylePopielarski, P., Zeler, J., Bolek, P., Zorenko, T., Paprocki, K., Zych, E., & Zorenko, Y. (2019). Radio-, Thermo- and Photoluminescence Properties of Lu2O3:Eu and Lu2O3:Tb Nanopowder and Film Scintillators. Crystals, 9(3), 148. https://doi.org/10.3390/cryst9030148






