Determination of Crystal Growth Geometry Factors and Nucleation Site Densities of Electrodeposited Ferromagnetic Cobalt Nanowire Arrays
Abstract
1. Introduction
2. Materials and Methods
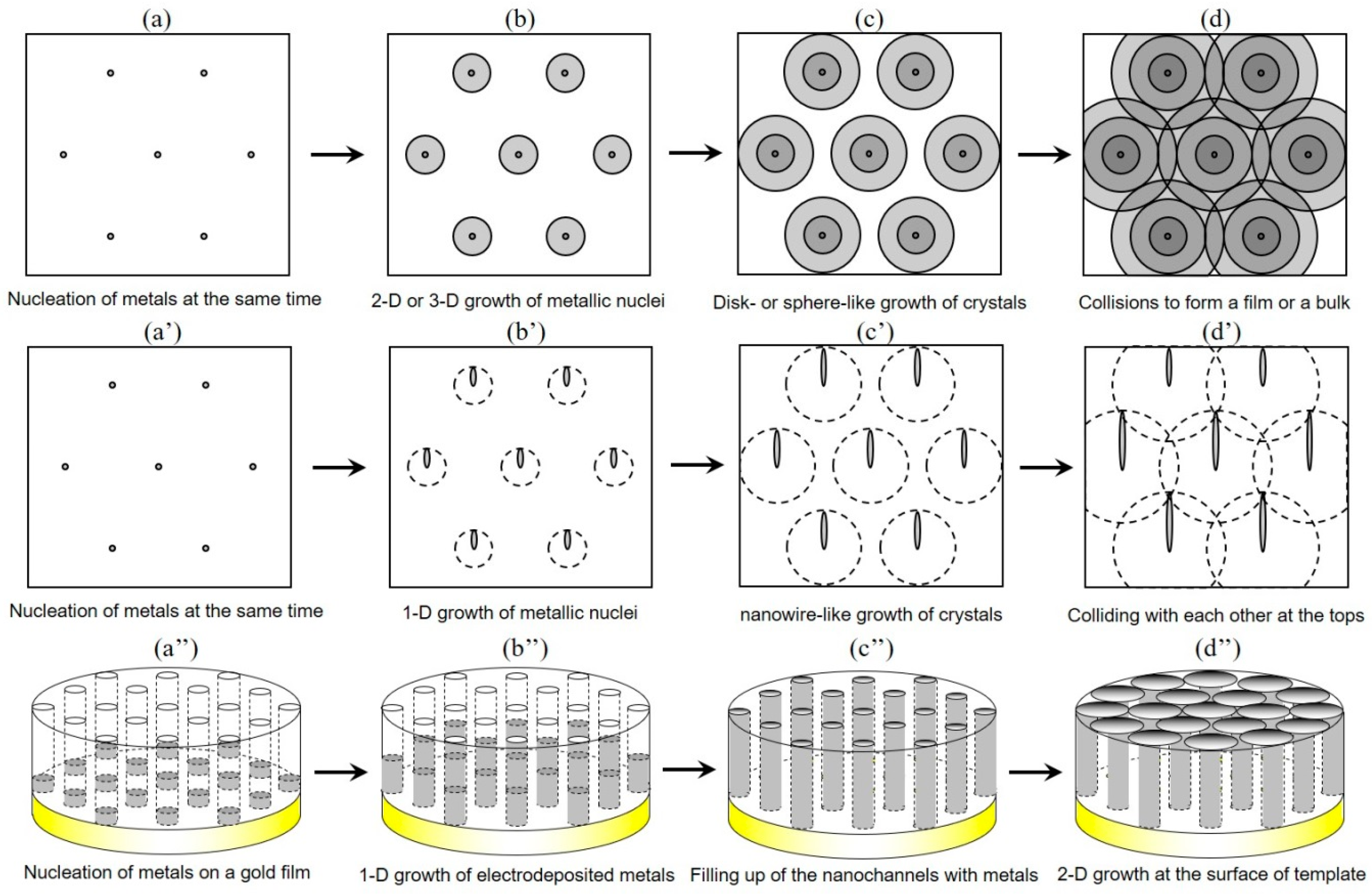
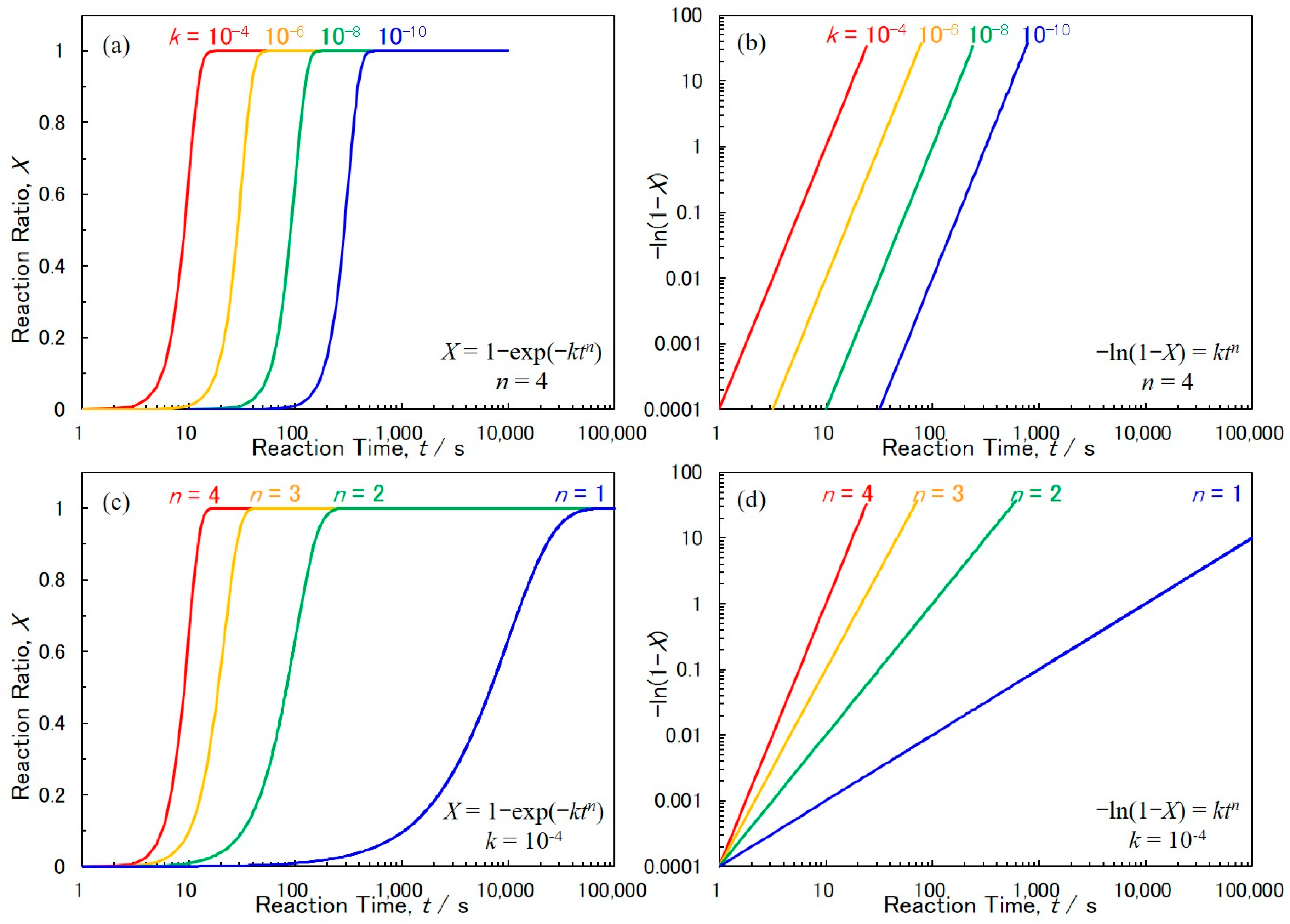
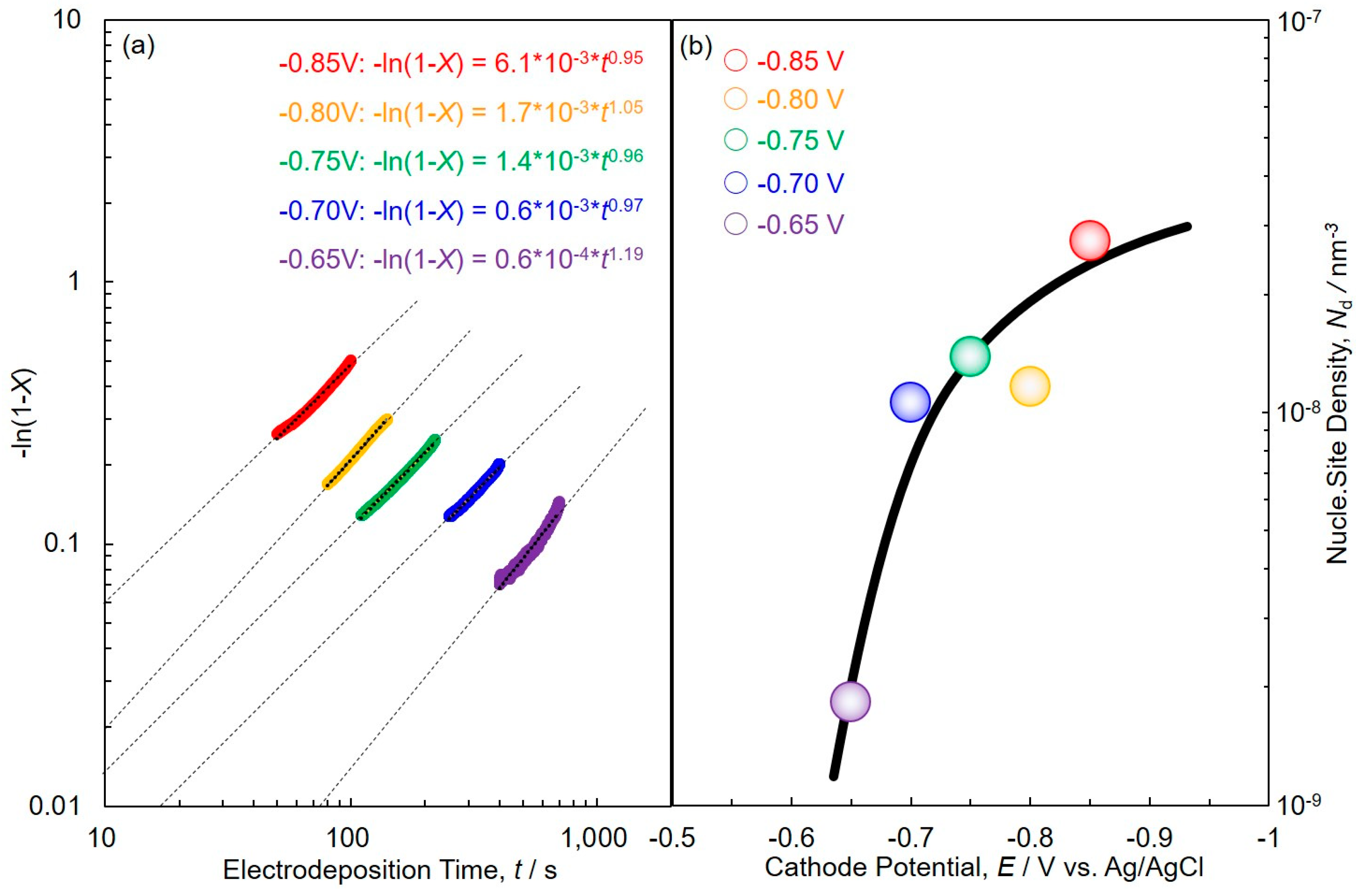
3. Theoretical Simulation of Crystal Growth Process Based on Johnson–Mehl–Avrami–Kolmogorov (JMAK) Theory
4. Results and Discussion
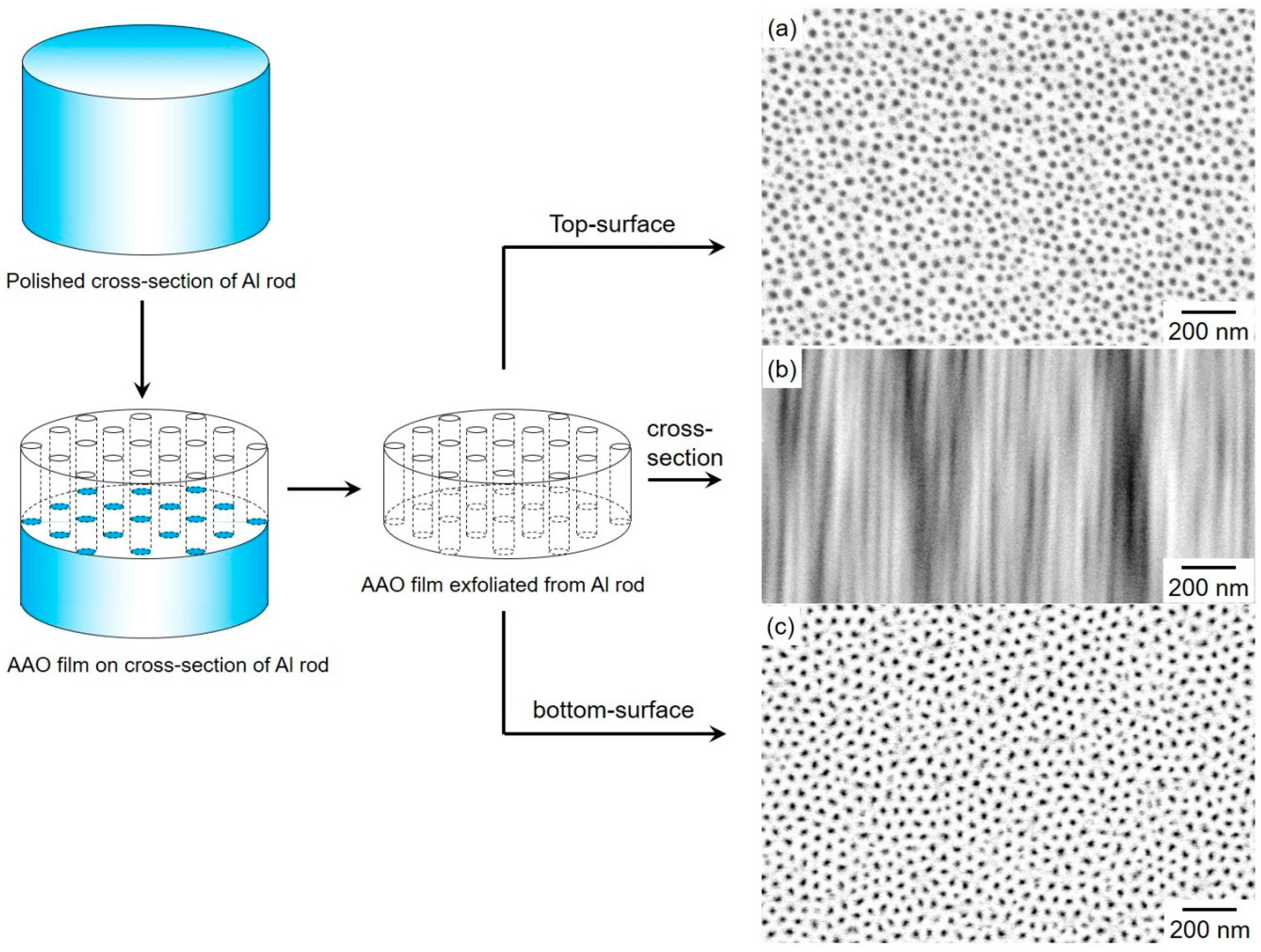
4.1. Structure of Anodized Aluminum Oxide (AAO) Nanochannel Films
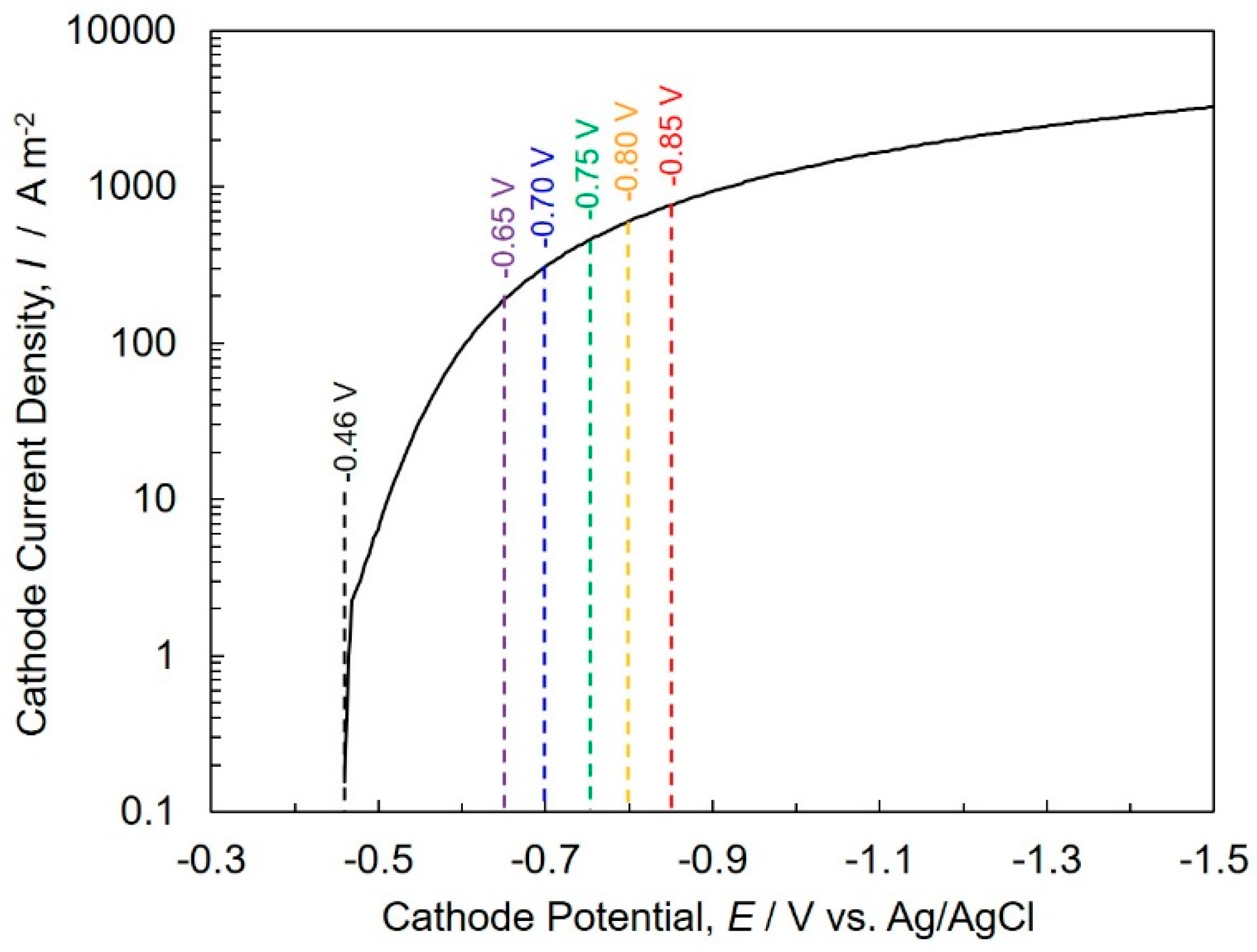
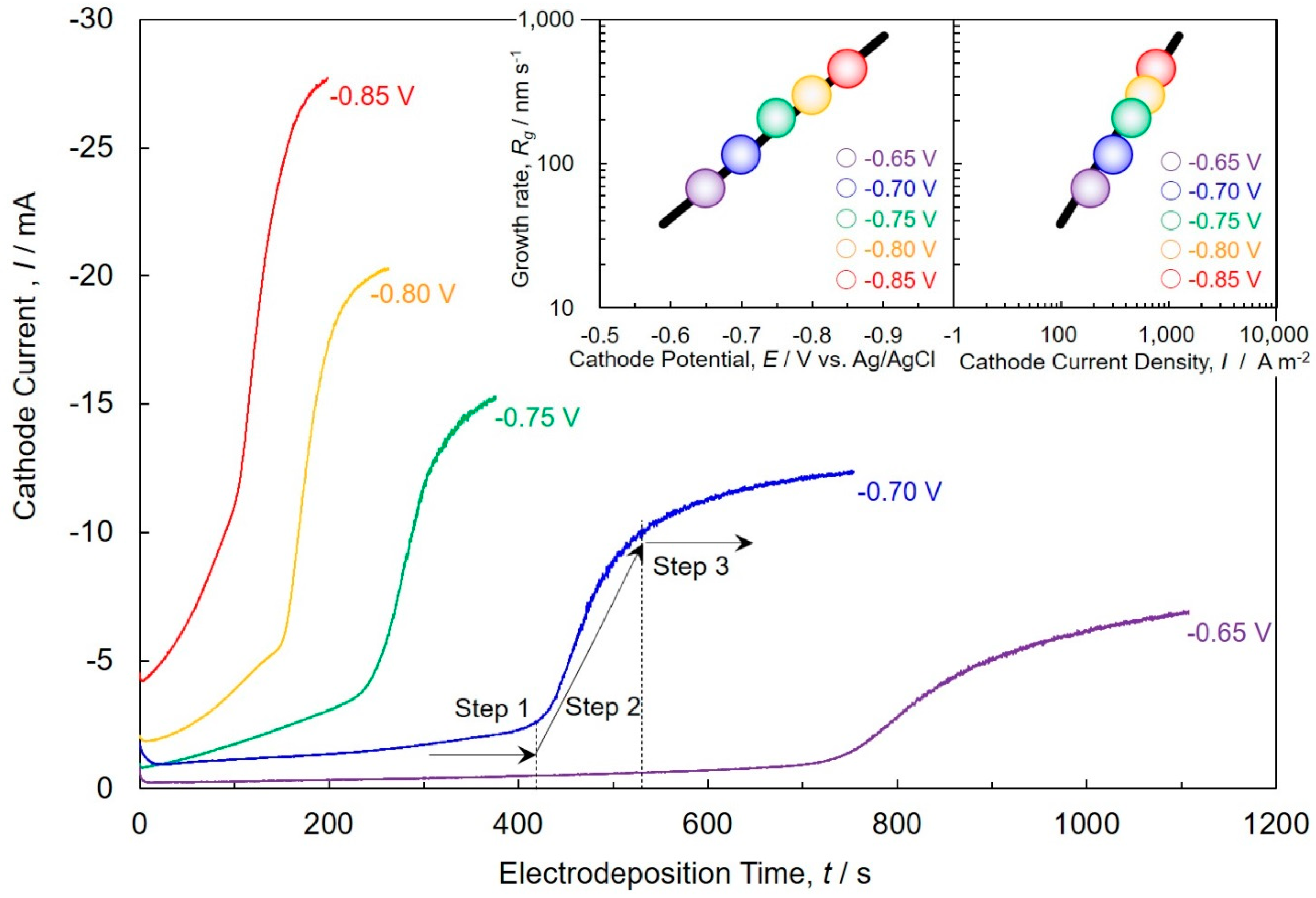
4.2. Cobalt Electrodeposition into AAO Nanochannel Films
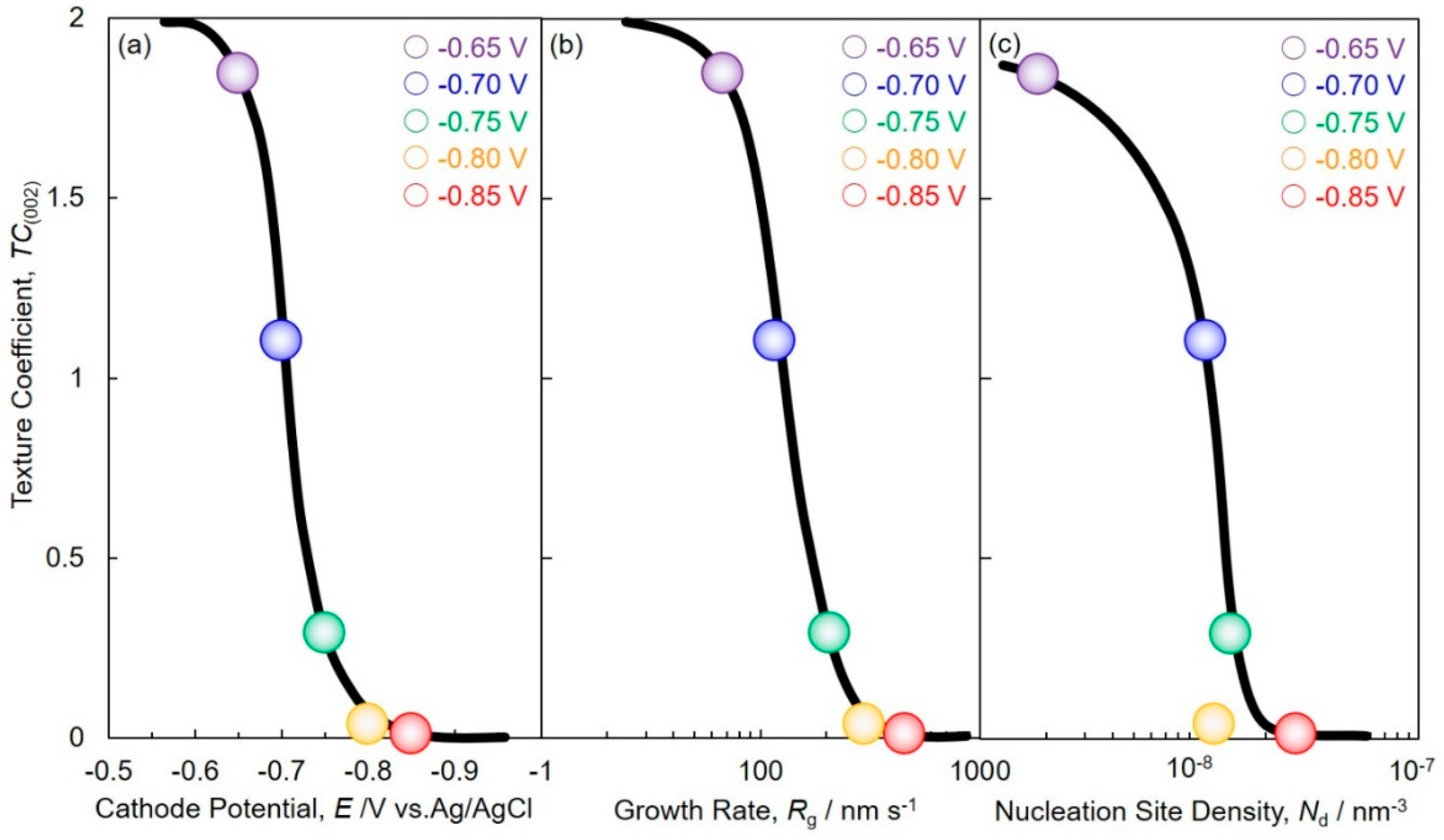
4.3. Electrochemical Crystal Growth Process of Cobalt Nanowire Arrays Analyzed by JMAK Theory
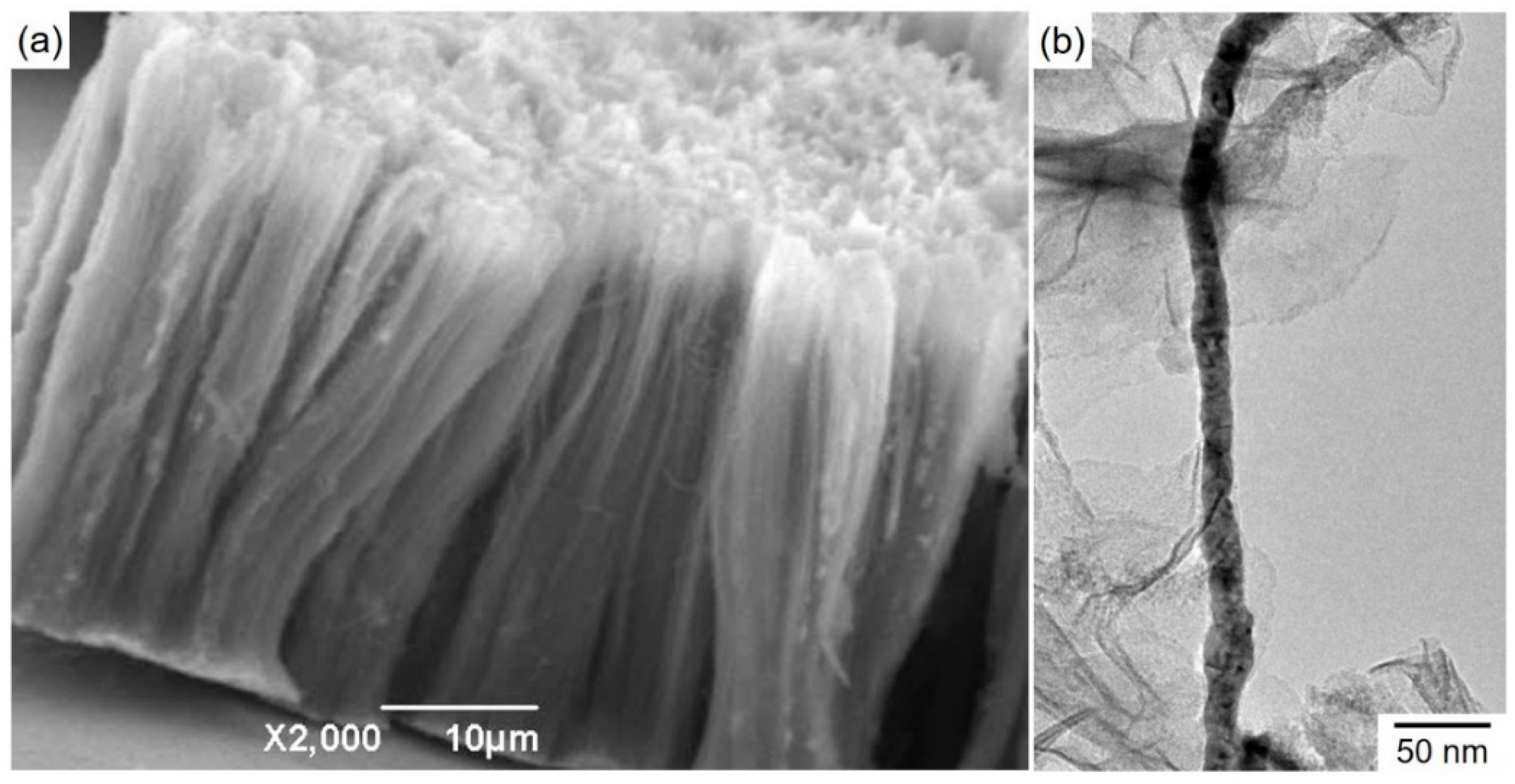
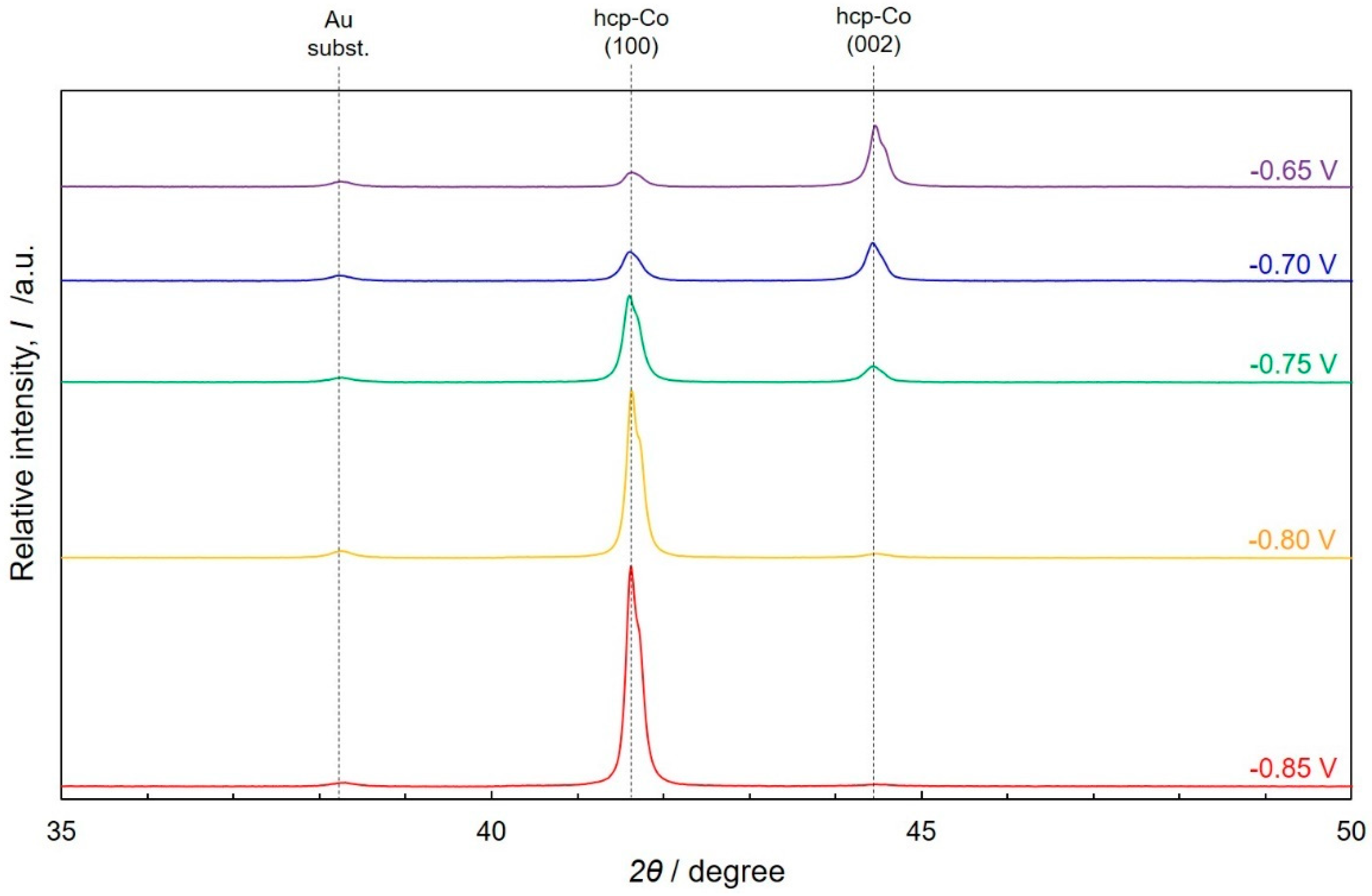
4.4. Morphological and Structural Studies of Cobalt Nanowire Arrays
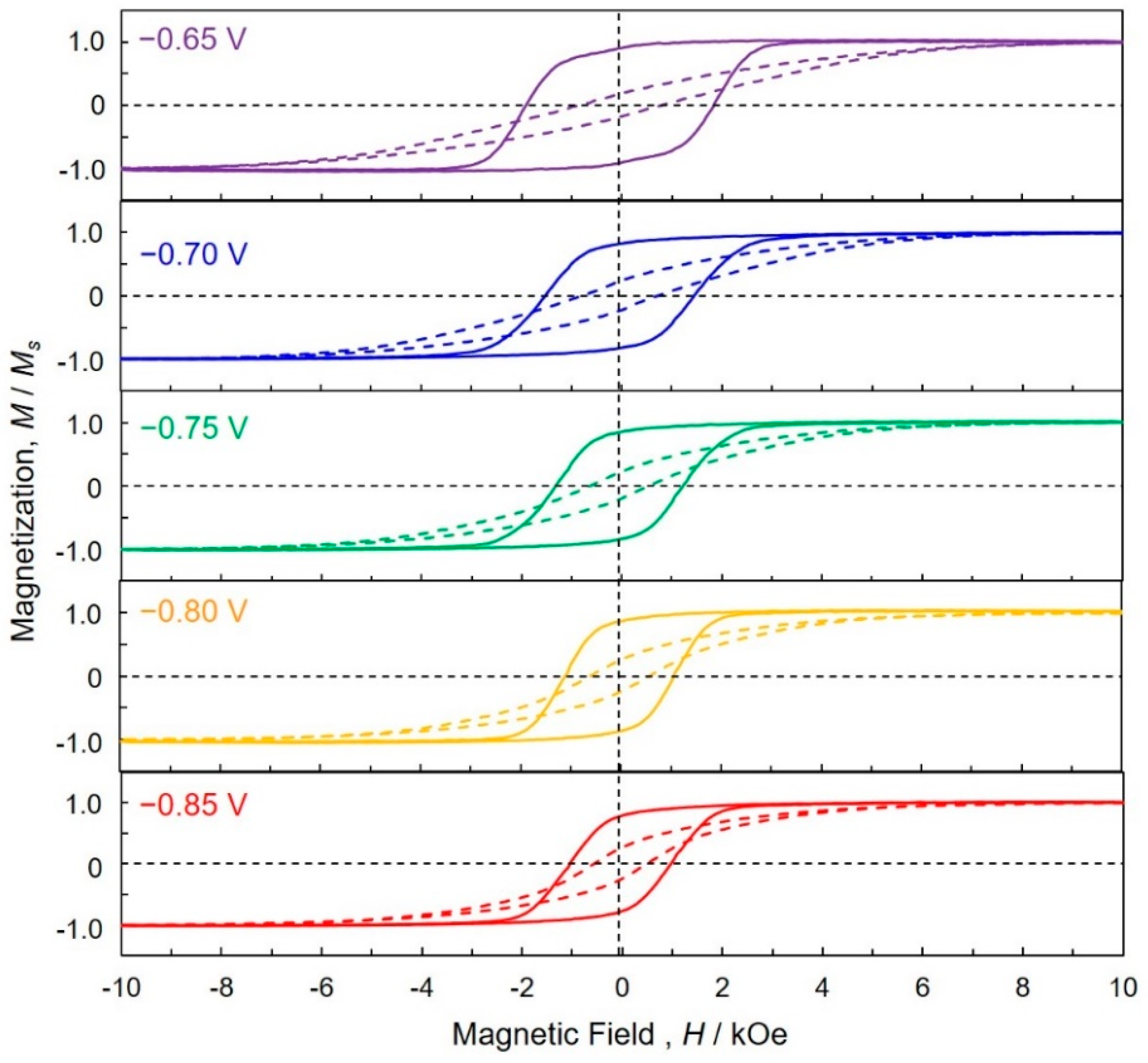
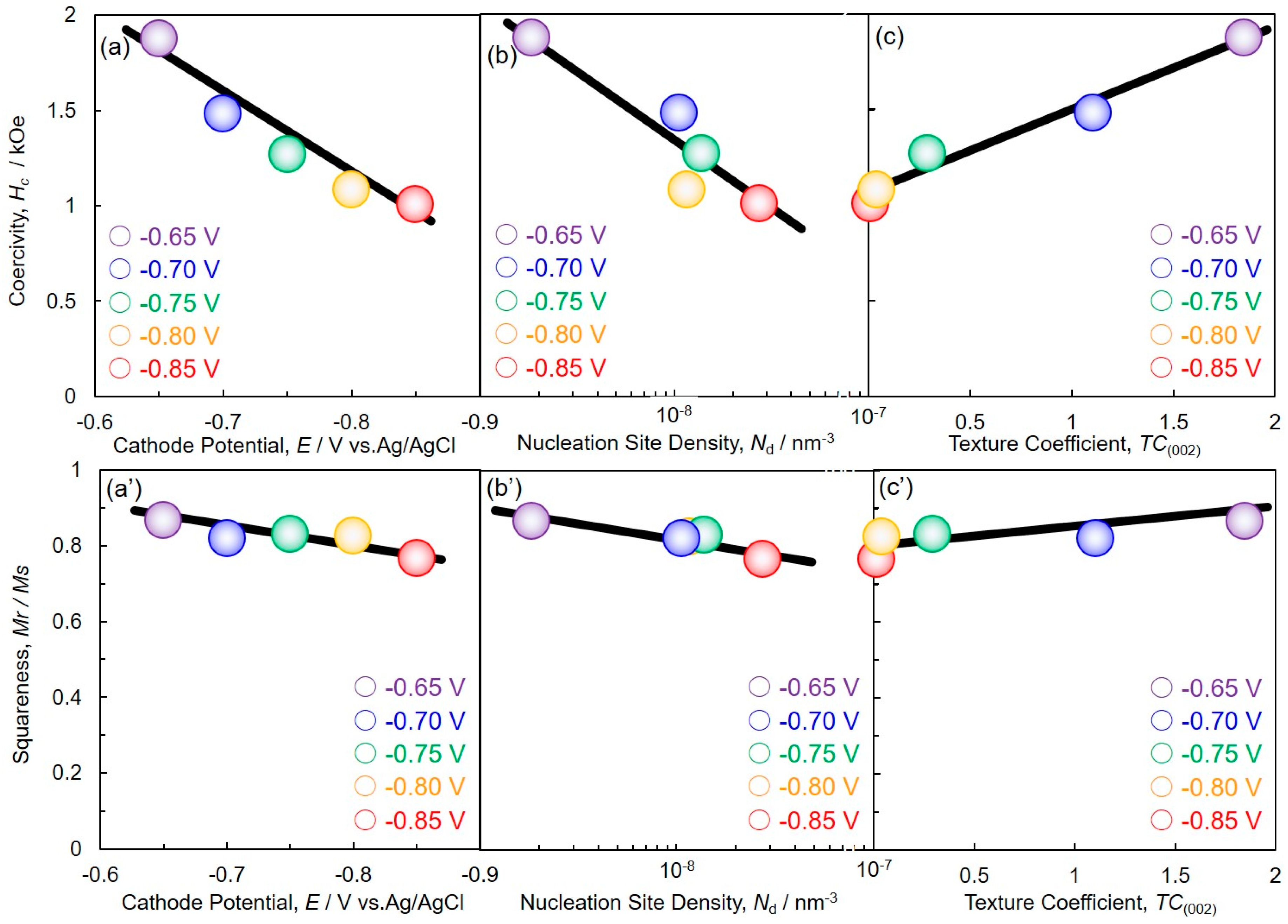
4.5. Magnetic Properties of Cobalt Nanowire Arrays
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| JMAK | Johnson–Mehl–Avrami–Kolmogorov |
| AAO | anodized aluminum oxide |
| SEM | scanning electron microscopy |
| hcp | hexagonal close packed structure |
| VSM | vibrating sample magnetometer |
References
- Widmer, J.D.; Martin, R.; Kimiabeigi, M. Electric vehicle traction motors without rare earth magnets. Sustain. Mater. Technol. 2015, 3, 7–13. [Google Scholar] [CrossRef]
- Srivastav, A.K.; Shekhar, R. Crystal anisotropy induced temperature dependent magnetization in cobalt nanowires electrodeposited within alumina template. J. Magn. Magn. Mater. 2014, 349, 21–26. [Google Scholar] [CrossRef]
- Bazzi, K.; Meka, V.M.; Rathi, A.; Jayaraman, T.V. Influence of temperature on the magnetic properties of nanostructured Fe-49 wt.% Co-2 wt.% V alloy powder synthesized by mechanically milling prealloyed gas-atomized powder. Mater. Chem. Phys. 2019, 227, 36–45. [Google Scholar] [CrossRef]
- Chen, W.; Han, M.; Deng, L. High frequency microwave absorbing properties of cobalt nanowires with transverse magnetocrystalline anisotropy. Phys. B 2010, 405, 1484–1488. [Google Scholar] [CrossRef]
- Gandha, K.; Elkins, K.; Poudyal, N.; Liu, X.; Liu, J.P. High energy product developed from cobalt nanowires. Sci. Rep. 2014, 4, 5345. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, Y.; Wu, Y.; Chen, X.; Kong, M. Diameter-Controllable Magnetic Properties of Co Nanowire Arrays by Pulsed Electrodeposition. J. Nanomater. 2010, 2010, 793854. [Google Scholar] [CrossRef]
- Sharma, S.; Barman, A.; Sharma, M.; Shelford, L.R.; Kruglyak, V.V.; Hicken, R.J. Structural and magnetic properties of electrodeposited Cobalt nanowire arrays. Solid State Commun. 2009, 149, 1650–1653. [Google Scholar] [CrossRef]
- Rani, V.S.; Anandakumar, S.; Lee, H.; Bang, W.; Hong, K.; Yoon, S.S.; Jeong, J.R.; Kim, C. Structural and magnetic properties of electrodeposited cobalt nanowires in polycarbonate membrane. Phys. Status Solidi A 2009, 206, 667–670. [Google Scholar] [CrossRef]
- Ohgai, T.; Fujimaru, T.; Tanaka, Y. Isotropic magnetization response of electrodeposited nanocrystalline Ni–W alloy nanowire arrays. J. Appl. Electrochem. 2014, 44, 301–307. [Google Scholar] [CrossRef]
- Ohgai, T.; Washio, R.; Tanaka, Y. Anisotropic magnetization behavior of electrodeposited nanocrystalline Ni-Mo alloy thin films and nanowires array. J. Electrochem. Soc. 2012, 159, H800–H804. [Google Scholar] [CrossRef]
- Masuda, H.; Fukuda, K. Ordered metal nanohole arrays made by a two-step replication of honeycomb structures of anodic alumina. Science 1995, 268, 1466–1468. [Google Scholar] [CrossRef] [PubMed]
- Shaterabadi, Z.; Soltanian, S.; Koohbor, M.; Salimi, A.; Servati, P. Modification of microstructure and magnetic properties of electrodeposited Co nanowire arrays: A study of the effect of external magnetic field, electrolyte acidity and annealing process. Mater. Chem. Phys. 2015, 160, 389–397. [Google Scholar] [CrossRef]
- Zhang, H.; Jia, W.; Sun, H.; Guo, L.; Sun, J. Growth mechanism and magnetic properties of Co nanowire arrays by AC electrodeposition. J. Magn. Magn. Mater. 2018, 468, 188–192. [Google Scholar] [CrossRef]
- Garcia, C.; Rosa, W.O.; Garcia, J.; Prida, V.M.; Hernando, B.; López, J.A.; Vargas, P.; Ross, C.A. Magnetization Reversal in Radially Distributed Nanowire Arrays. J. Phys. Chem. C 2018, 122, 5124−5130. [Google Scholar] [CrossRef]
- Saeki, R.; Ohgai, T. Effect of growth rate on the crystal orientation and magnetization performance of cobalt nanocrystal arrays electrodeposited from aqueous solution. Nanomaterials 2018, 8, 566. [Google Scholar] [CrossRef] [PubMed]
- Saeki, R.; Ohgai, T. Determination of activation overpotential during the nucleation of hcp-cobalt nanowires synthesized by potentio-static electrochemical reduction. Materials 2018, 11, 2355. [Google Scholar] [CrossRef] [PubMed]
- Kaur, D.; Chaudhary, S.; Pandya, D.K.; Gupta, R.; Kotnala, R.K. Magnetization reversal studies in structurally tailored cobalt nanowires. J. Magn. Magn. Mater. 2013, 344, 72–78. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, Q.F.; Li, S.L.; Wang, J.B.; Han, X.H. The effect of structure on magnetic properties of Co nanowire arrays. J. Magn. Magn. Mater. 2009, 321, 226–230. [Google Scholar] [CrossRef]
- Kolomogrov, A. A statistical theory for the recrystallisation of metals. Izv. Akad. Nauk SSSR. Ser. Matem. 1937, 1, 355. [Google Scholar]
- Avrami, M. Kinetics of Phase Change. I General Theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. II Transformation-Time Relations for Random Distribution of Nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Avrami, M. Granulation, Phase Change, and Microstructure Kinetics of Phase Change. III. J. Chem. Phys. 1941, 9, 177–184. [Google Scholar] [CrossRef]
- Johnson, W.A.; Mehl, R.F. Reaction Kinetics in Processes of Nucleation and Growth. Trans. AIME 1939, 135, 416–441. [Google Scholar]
- Zhou, Y.; Lin, W.; Yang, F.; Fang, W.; Huang, J.; Li, Q. Insights into formation kinetics of gold nanoparticles using the classical JMAK model. Chem. Phys. 2014, 441, 23–29. [Google Scholar] [CrossRef]
- Rios, P.R.; Oliveira, V.T.; Pereira, L.O.; Pereira, M.R.; Castro, J.A. Cellular Automata Simulation of Site-saturated and Constant Nucleation Rate Transformations in Three Dimensions. Mater. Res. 2006, 9, 223–230. [Google Scholar] [CrossRef]
- Luo, H.; Sietsma, J.; van der Zwaag, S. Effect of Inhomogeneous Deformation on the Recrystallization Kinetics of Deformed Metals. ISIJ Int. 2004, 44, 1931–1936. [Google Scholar] [CrossRef]
- Moghadam, M.M.; Voorhees, P.W. Thin film phase transformation kinetics: From theory to experiment. Scr. Mater. 2016, 124, 164–168. [Google Scholar] [CrossRef]
- Schlörb, H.; Haehnel, V.; Khatri, M.S.; Srivastav, A.; Kumar, A.; Schultz, L.; Fähler, S. Magnetic nanowires by electrodeposition within templates. Phys. Status Solidi B 2010, 247, 2364–2379. [Google Scholar]
- Kaur, D.; Pandya, D.K.; Chaudhary, S. Texture changes in electrodeposited cobalt nanowires with bath temperature. J. Electrochem. Soc. 2012, 159, D713–D716. [Google Scholar] [CrossRef]
- Duan, J.; Liu, J.; Cornelius, T.W.; Yao, H.; Mo, D.; Chen, Y.; Zhang, L.; Sun, Y.; Hou, M.; Trautmann, C.; et al. Magnetic and optical properties of cobalt nanowires fabricated in polycarbonate ion-track templates. Nucl. Instr. Meth. Phys. Res. B 2009, 267, 2567–2570. [Google Scholar] [CrossRef]
- Pangarov, N.A.; Vitkova, S.D. Preferred orientation of electrodeposited cobalt crystallites. Electrochim. Acta 1966, 11, 1733–1745. [Google Scholar] [CrossRef]
- Pangarov, N.A. The crystal orientation of electrodeposited metals. Electrochim. Acta 1962, 7, 139–146. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeki, R.; Ohgai, T. Determination of Crystal Growth Geometry Factors and Nucleation Site Densities of Electrodeposited Ferromagnetic Cobalt Nanowire Arrays. Crystals 2019, 9, 142. https://doi.org/10.3390/cryst9030142
Saeki R, Ohgai T. Determination of Crystal Growth Geometry Factors and Nucleation Site Densities of Electrodeposited Ferromagnetic Cobalt Nanowire Arrays. Crystals. 2019; 9(3):142. https://doi.org/10.3390/cryst9030142
Chicago/Turabian StyleSaeki, Ryusei, and Takeshi Ohgai. 2019. "Determination of Crystal Growth Geometry Factors and Nucleation Site Densities of Electrodeposited Ferromagnetic Cobalt Nanowire Arrays" Crystals 9, no. 3: 142. https://doi.org/10.3390/cryst9030142
APA StyleSaeki, R., & Ohgai, T. (2019). Determination of Crystal Growth Geometry Factors and Nucleation Site Densities of Electrodeposited Ferromagnetic Cobalt Nanowire Arrays. Crystals, 9(3), 142. https://doi.org/10.3390/cryst9030142




