Two Interpenetrated Zn(II) Coordination Polymers: Synthesis, Topological Structures, and Property
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Techniques
2.2. Synthesis of {[Zn1(L)(NO2pbda)]n[Zn2(L)(NO2pbda)]n} (1)
2.3. Synthesis of [Zn2(L)(Brpbda)2]n (2)
2.4. Crystallographic Data Collection and Refinements
3. Results
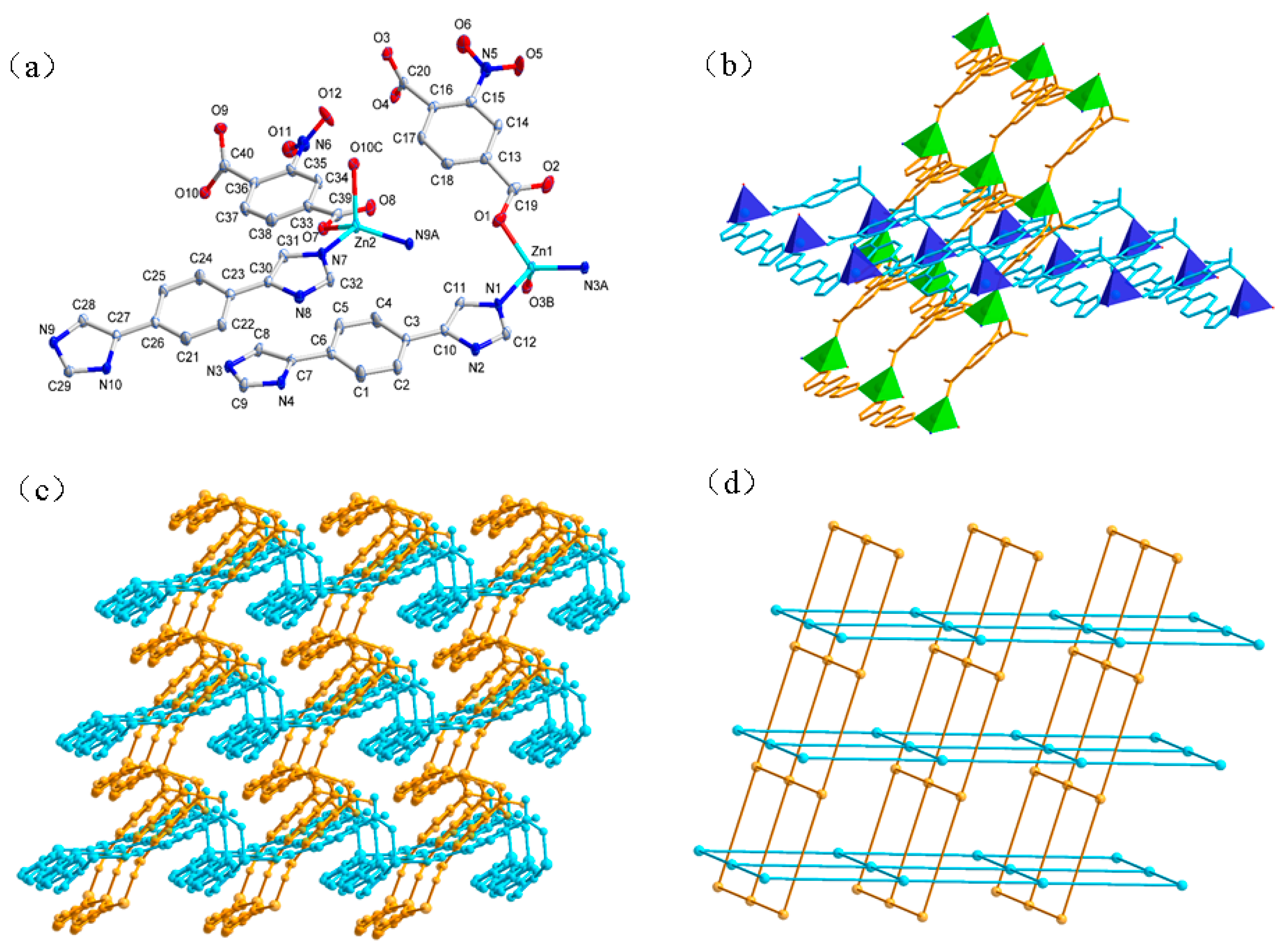
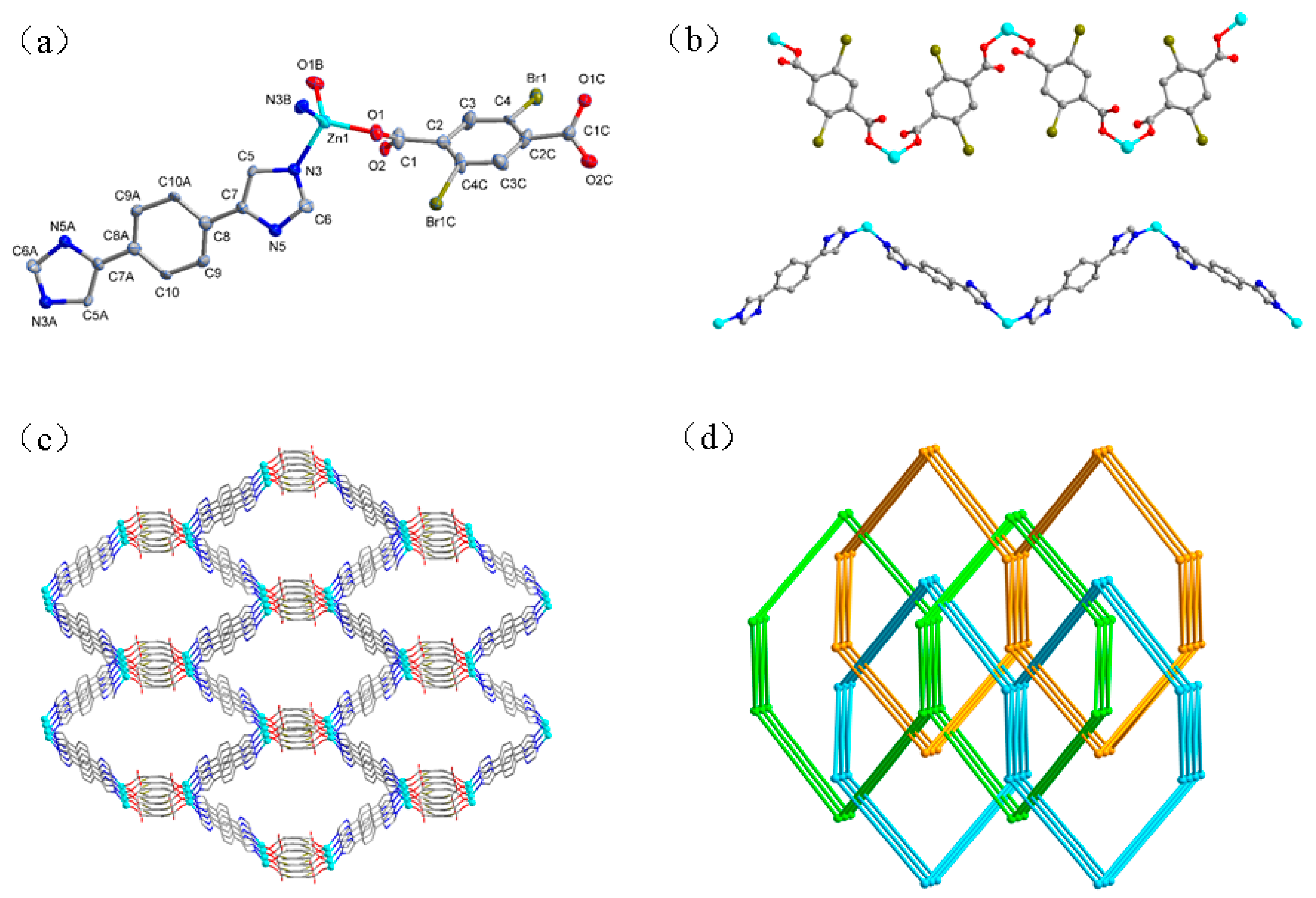
3.1. Structural Descriptions
3.1.1. Structure of {[Zn1(L)(NO2pbda)]n[Zn2(L)(NO2pbda)]n} (1)
3.1.2. Structure of [Zn(L)(Brpbda)]n (2)
3.2. Thermal Analyses and X-Ray Power Diffraction Analyses
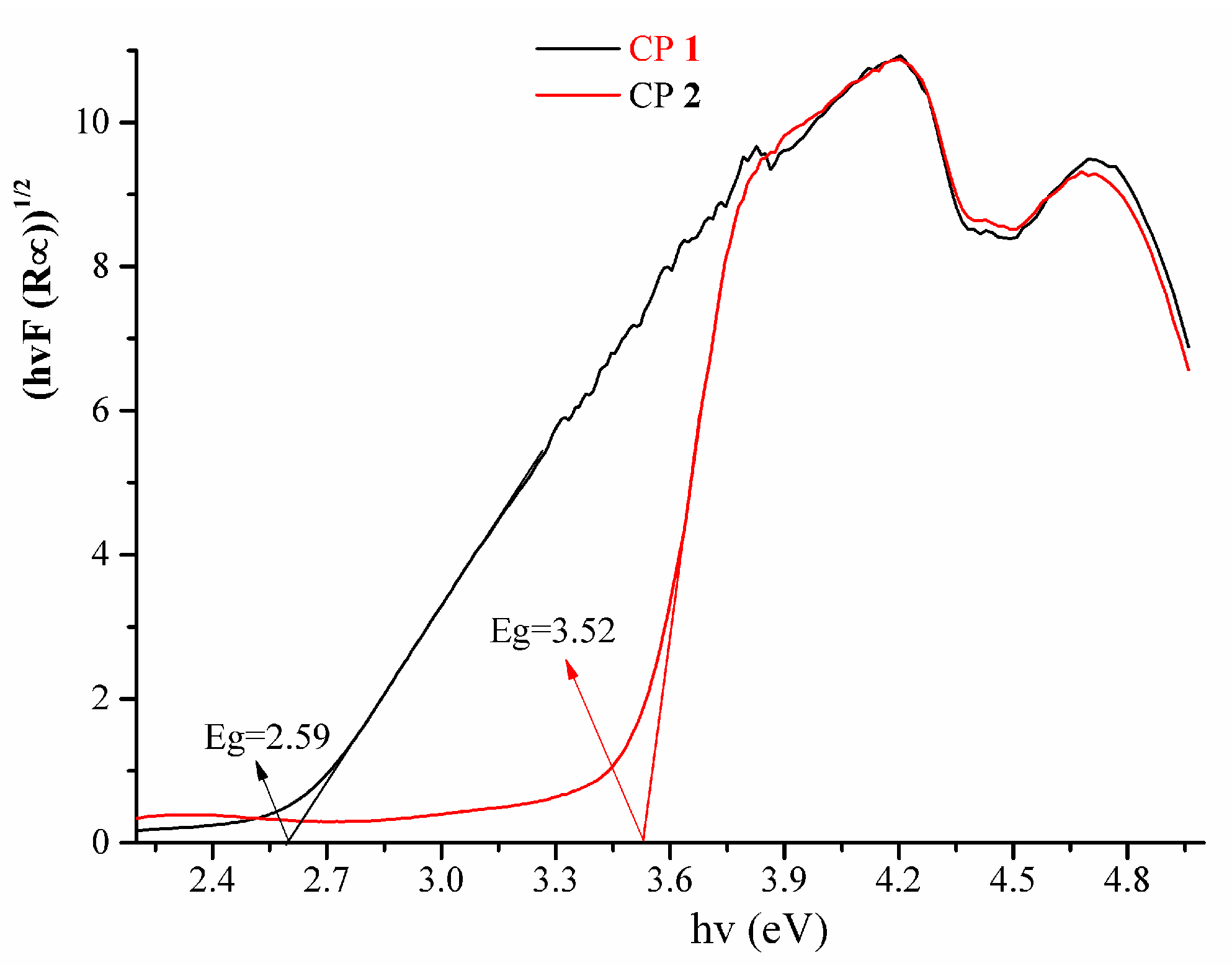
3.3. IR and Diffuse Reflectance Spectra
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kang, Y.S.; Lu, Y.; Chen, K.; Zhao, Y.; Wang, P.; Sun, W.Y. Metal-organic frameworks with catalytic centers: From synthesis to catalytic application. Coord. Chem. Rev. 2019, 378, 262–280. [Google Scholar] [CrossRef]
- Fu, H.R.; Wang, N.; Qin, J.H.; Han, M.L.; Ma, L.F.; Wang, F. Spatial confinement of a cationic MOF: A SC-SC approach for high capacity Cr(VI)-oxyanion capture in aqueous solution. Chem. Commun. 2018, 54, 11645–11648. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, L.; Fan, N.N.; Han, M.L.; Yang, G.P.; Ma, L.F. Porous Zn(II)-based metal–organic frameworks decorated with carboxylate groups exhibiting high gas adsorption and separation of organic dyes. Cryst. Growth Des. 2018, 18, 7114–7121. [Google Scholar] [CrossRef]
- Yang, X.G.; Ma, L.F.; Yan, D.P. Facile synthesis of 1D organic–inorganic perovskite micro-belts with high water stability for sensing and photonic applications. Chem. Sci. 2019, 10, 4567–4572. [Google Scholar] [CrossRef]
- Seidi, F.; Jenjob, R.; Crespy, D. Designing smart polymer conjugates for controlled release of payloads. Chem. Rev. 2018, 118, 3965–4036. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.G.; Lu, X.M.; Yang, C.D.; Fan, N.N.; Yang, Z.T.; Wang, L.Y.; Ma, L.F. {Zn6} cluster based metal–organic framework with enhanced room-temperature phosphorescence and optoelectronic performances. Inorg. Chem. 2019, 58, 6215–6221. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Wang, R.; Wang, S.; Xi, X.J.; Ma, L.F.; Zang, S.Q. Encapsulating [Mo3S13]2− clusters in cationic covalent organic frameworks: Enhancing stability and recyclability by converting a homogeneous photocatalyst to a heterogeneous photocatalyst. Chem. Commun. 2018, 54, 13563–13566. [Google Scholar] [CrossRef]
- Wang, C.X.; Xia, Y.P.; Yao, Z.Q.; Xu, J.; Chang, Z.; Bu, X.H. Two luminescent coordination polymers as highly selective and sensitive chemosensors for CrVI-anions in aqueous medium. Dalton Trans. 2019, 48, 387–394. [Google Scholar] [CrossRef]
- Han, M.L.; Chang, X.H.; Feng, X.; Ma, L.F.; Wang, L.Y. Temperature and pH driven self-assembly of Zn(II) coordination polymers: Crystal structures, supramolecular isomerism, and photoluminescence. CrystEngComm 2014, 16, 1687–1695. [Google Scholar] [CrossRef]
- Li, X.Q.; Zhou, Z.; Zhang, C.C.; Zheng, Y.H.; Gao, J.W.; Wang, Q.M. Modulation of assembly and disassembly of a new tetraphenylethene based nanosensor for highly selective detection of hyaluronidase. Sens. Actuators B Chem. 2018, 276, 95–100. [Google Scholar] [CrossRef]
- Zhou, Z.; Han, M.L.; Fu, H.R.; Ma, L.F.; Luo, F.; Li, D.S. Engineering design toward exploring the functional group substitution in 1D channels of Zn-organic frameworks upon nitro explosives and antibiotics detection. Dalton Trans. 2018, 47, 5359–5365. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.Y.; Si, C.D.; Fan, Y.; Hu, D.C.; Yao, X.Q.; Yang, Y.X.; Liu, J.C. Effect of N-donor ligands and metal ions on the coordination polymers based on a semirigid carboxylic acid ligand: Structures analysis, magnetic properties, and photoluminescence. Cryst. Growth Des. 2016, 16, 2062–2073. [Google Scholar] [CrossRef]
- Yang, X.G.; Lu, X.M.; Zhai, Z.M.; Zhao, Y.; Liu, X.-Y.; Ma, L.-F.; Zang, S.-Q. Facile synthesis of micro-scale MOF host-guest with long-last phosphorescence and enhanced optoelectronic performance. Chem. Commun. 2019, 55, 11099–11102. [Google Scholar] [CrossRef] [PubMed]
- Roztocki, K.; Jedrzejowski, D.; Hodorowicz, M.; Senkovska, I.; Kaskel, S.; Matoga, D. Effect of linker substituent on layers arrangement, stability, and sorption of Zn-isophthalate/acylhydrazone frameworks. Cryst. Growth Des. 2018, 18, 488–497. [Google Scholar] [CrossRef]
- Singh, R.; Bharadwaj, P.K. Coordination polymers built with a linear bis-imidazole and different dicarboxylates: Unusual entanglement and emission properties. Cryst. Growth Des. 2013, 13, 3722–3733. [Google Scholar] [CrossRef]
- Guo, X.Z.; Li, J.L.; Shi, S.S.; Zhou, H.; Han, S.S.; Chen, S.S. Synthesis, structure and luminescent property of a Zn(II) complex with mixed multi-N donor and 2,5-dihydroxy-terephthalic acid ligands. Chin. J. Struct. Chem. 2018, 37, 1117–1124. [Google Scholar]
- Cui, Y.; Li, B.; He, H.; Zhou, W.; Chen, B.; Qian, G. Metal-organic frameworks as platforms for functional materials. Acc. Chem. Res. 2016, 49, 483–493. [Google Scholar] [CrossRef]
- Wang, S.L.; Hu, F.L.; Zhou, J.Y.; Zhou, Y.; Huang, Q.; Lang, J.P. Rigidity versus flexibility of ligands in the assembly of entangled coordination polymers based on Bi-and tetra carboxylates and N-Donor ligands. Cryst. Growth Des. 2015, 15, 4087–4097. [Google Scholar] [CrossRef]
- Wang, S.; Yun, R.; Peng, Y.; Zhang, Q.; Lu, J.; Dou, J.; Bai, J.; Li, D.; Wang, D. A series of four-connected entangled metal–organic frameworks assembled from pamoic acid and pyridine-containing ligands: Interpenetrating, self-penetrating, and supramolecular isomerism. Cryst. Growth Des. 2012, 12, 79–92. [Google Scholar] [CrossRef]
- Chang, X.H.; Qin, J.H.; Ma, L.F.; Wang, J.G.; Wang, L.Y. Two- and three-dimensional divalent metal coordination polymers constructed from a new tricarboxylate linker and dipyridyl ligands. Cryst. Growth Des. 2012, 12, 4649–4657. [Google Scholar] [CrossRef]
- Hernández-Maldonado, A.J.; Arrieta-Pérez, R.R.; Primera-Pedrozo, J.N.; Exley, J. Structure of a porous Cu2(pzdc)2(bpp) (pzdc: Pyrazine-2,3-dicarboxylate, bpp: 1,3-Bis(4-pyridyl)propane) coordination polymer and flexibility upon concomitant hysteretic CO2 adsorption. Cryst. Growth Des. 2015, 15, 4123–4131. [Google Scholar] [CrossRef]
- Chen, S.S.; Chen, M.; Takamizawa, S.; Chen, M.S.; Su, Z.; Sun, W.Y. Temperature dependent selective gas sorption of the microporous metal-imidazolate framework [Cu(L)] [H2L = 1,4-di(1H-imidazol-4-yl)benzene]. Chem. Commun. 2011, 47, 752–754. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.S.; Chen, M.; Takamizawa, S.; Wang, P.; Lv, G.C.; Sun, W.Y. Porous cobalt(II)-imidazolate supramolecular isomeric frameworks with selective gas sorption property. Chem. Commun. 2011, 47, 4902–4904. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.S.; Wang, P.; Takamizawa, S.; Okamura, T.A.; Chen, M.; Sun, W.Y. Zinc(II) and cadmium(II) metal–organic frameworks with 4-imidazole containing tripodal ligand: Sorption and anion exchange properties. Dalton Trans. 2014, 43, 6012–6020. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.X.; Sun, W.Y. Zinc(II)– and cadmium(II)–organic frameworks with 1-imidazole-containing and 1-imidazole-carboxylate ligands. CrystEngComm 2015, 17, 4045–4063. [Google Scholar] [CrossRef]
- Chen, S.S.; Sheng, L.Q.; Zhao, Y.; Liu, Z.D.; Qiao, R.; Yang, S. Syntheses, structures, and properties of a series of polyazaheteroaromatic core-based Zn(II) coordination polymers together with carboxylate auxiliary ligands. Cryst. Growth Des. 2016, 16, 229–241. [Google Scholar] [CrossRef]
- Have, R.T.; Huisman, M.; Meetsma, A.; van Leusen, A.M. Novel synthesis of 4(5)-monosubstituted imidazoles via cycloaddition of tosylmethyl isocyanide to aldimines. Tetrahedron 1997, 53, 11355–11368. [Google Scholar] [CrossRef]
- SAINT; Version 6.2; Bruker AXS, Inc.: Madison, WI, USA, 2001.
- Sheldrick, G.M. SADABS; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXTL; Version 6.10; Bruker Analytical Xray Systems: Madison, WI, USA, 2001. [Google Scholar]
- Wang, C.Y.; Wilseck, Z.M.; LaDuca, R.L. 1D + 1D → 1D polyrotaxane, 2D + 2D → 3D interpenetrated, and 3D self-penetrated divalent metal terephthalate bis(pyridylformyl)piperazine coordination polymers. Inorg. Chem. 2011, 50, 8997–9003. [Google Scholar] [CrossRef]
- Chen, S.S.; Li, J.L.; Li, W.D.; Guo, X.Z.; Zhao, Y. Four new transition metal coordination polymers based on mixed 4-imidazole and carboxylate–sulfonate ligands: Syntheses, structures, and properties. J. Solid State Chem. 2019, 277, 510–518. [Google Scholar] [CrossRef]
- Xue, L.P.; Li, Z.H.; Ma, L.F.; Wang, L.Y. Crystal engineering of cadmium coordination polymers decorated with nitro-functionalized thiophene-2,5-dicarboxylate and structurally related bis(imidazole) ligands with varying flexibility. CrystEngComm 2015, 17, 6441–6449. [Google Scholar] [CrossRef]
- Liu, X.M.; Xie, L.H.; Lin, J.B.; Lin, R.B.; Zhang, J.P.; Chen, X.M. Flexible porous coordination polymers constructed from 1,2-bis(4-pyridyl)hydrazine via solvothermal in situ reduction of 4,4′-azopyridine. Dalton Trans. 2011, 40, 8549–8554. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.A.; Guo, X.Z.; Xiao, L.; Chen, S.S. A new Cd(II) coordination compound based on 4-(1,2,4-Triazol-4-yl)phenylacetic acid: Synthesis, structure and photoluminescence property. Chin. J. Struct. Chem. 2018, 37, 437–444. [Google Scholar]
- Shi, Z.; Pan, Z.; Qin, L.; Zhou, J.; Zheng, H. Five new transition metal coordination polymers based on V-shaped bis-triazole ligand with aromatic dicarboxylates: Syntheses, structures, and properties. Cryst. Growth Des. 2017, 17, 2757–2766. [Google Scholar] [CrossRef]
- Liu, L.; Huang, C.; Xue, X.; Li, M.; Hou, H.; Fan, Y. Ni(II) coordination polymers constructed from the flexible tetracarboxylic acid and different N-donor ligands: Structural diversity and catalytic activity. Cryst. Growth Des. 2015, 15, 4507–4517. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Zhao, M.; Su, J.; Xu, S.; Hu, L.; Liu, H.; Zhang, Q.; Zhang, J.; Wu, J.; Tian, Y. Three coordination polymers based on a star-like geometry 4,4′,4″-nitrilotribenzoic acid ligand and their framework dependent luminescent properties. J. Solid State Chem. 2018, 258, 328–334. [Google Scholar] [CrossRef]

| 1 | 2 | |
|---|---|---|
| Empirical formula | C20H13N5O6Zn | C20H10Br2N4O4Zn |
| Formula weight | 484.72 | 595.51 |
| Temperature/K | 296(2) | 296(2) |
| Crystal system | Triclinic | Orthorhombic |
| Space group | P 1 | P nna |
| a/Å | 9.037(2) | 11.0101(10) |
| b/Å | 9.141(2) | 14.6802(14) |
| c/Å | 12.948(3 | 14.8721(13) |
| α/° | 107.832(3) | 90 |
| β/° | 95.123(3) | 90 |
| γ/° | 108.233(3) | 90 |
| V (Å3) | 946.6(4) | 2403.8(4) |
| Z, Dcalc/(Mg/m3) | 2, 1.701 | 4, 1.646 |
| F(000) | 492 | 1160 |
| θ range/° | 1.69–25.99 | 3.59–25.01 |
| Reflections collected | 7131 | 26144 |
| Independent reflections | 5942 | 2088 |
| Goodness-of-fit on F2 | 0.997 | 1.094 |
| R1 [I > 2σ (I)] a | 0.0398 | 0.0989 |
| wR2 [I > 2σ (I)] b | 0.0819 | 0.2401 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.-W.; Liu, C.-J.; Li, W.-D.; Han, S.-S.; Chen, S.-S. Two Interpenetrated Zn(II) Coordination Polymers: Synthesis, Topological Structures, and Property. Crystals 2019, 9, 601. https://doi.org/10.3390/cryst9110601
He Z-W, Liu C-J, Li W-D, Han S-S, Chen S-S. Two Interpenetrated Zn(II) Coordination Polymers: Synthesis, Topological Structures, and Property. Crystals. 2019; 9(11):601. https://doi.org/10.3390/cryst9110601
Chicago/Turabian StyleHe, Zi-Wei, Chang-Jie Liu, Wei-Dong Li, Shuai-Shuai Han, and Shui-Sheng Chen. 2019. "Two Interpenetrated Zn(II) Coordination Polymers: Synthesis, Topological Structures, and Property" Crystals 9, no. 11: 601. https://doi.org/10.3390/cryst9110601
APA StyleHe, Z.-W., Liu, C.-J., Li, W.-D., Han, S.-S., & Chen, S.-S. (2019). Two Interpenetrated Zn(II) Coordination Polymers: Synthesis, Topological Structures, and Property. Crystals, 9(11), 601. https://doi.org/10.3390/cryst9110601





