Size Matters: New Zintl Phase Hydrides of REGa (RE = Y, La, Tm) and RESi (RE = Y, Er, Tm) with Large and Small Cations
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Thermal Analysis
2.3. X-ray Powder Diffraction (XRPD)
2.4. Neutron Powder Diffraction (NPD)
2.5. In Situ Neutron Powder Diffraction
2.6. Rietveld Refinement
2.7. Density Functional Theory (DFT) Calculations
3. Results
3.1. Hydrogenation Reactions
3.2. Crystal Structures of the Hydrides
3.3. Density Functional Theory (DFT) Calculations
4. Discussion
5. Summary
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kohlmann, H. Encyclopedia of Physical Science and Technology, 3rd ed.; Meyers, R.A., Ed.; Academic Press: Cambridge, MA, USA, 2002; Volume 9, pp. 441–458. [Google Scholar]
- Zintl, E.; Kaiser, H. Über die Fähigkeit der Elemente zur Bildung negativer Ionen. Zeitschrift Anorganische Allgemeine Chemie 1933, 211, 113–131. [Google Scholar] [CrossRef]
- Schäfer, H.; Eisenmann, B.; Müller, W. Zintl-Phasen: Übergangsformen zwischen Metall- und Ionenbindung. Angew. Chem. 1973, 85, 742–760. [Google Scholar] [CrossRef]
- Nesper, R. Chemische Bindungen—Intermetallische Verbindungen. Angew. Chem. 1991, 103, 805–834. [Google Scholar] [CrossRef]
- Kurylyshyn, I.M.; Fässler, T.F.; Fischer, A.; Hauf, C.; Eickerling, G.; Presnitz, M.; Scherer, W. Probing the Zintl-Klemm Concept: A Combined Experimental and Theoretical Charge Density Study of the Zintl Phase CaSi. Angew. Chem. Int. Ed. 2014, 53, 3029–3032. [Google Scholar] [CrossRef] [PubMed]
- Häussermann, U.; Kranak, V.F.; Puhakainen, K. Hydrogenous Zintl Phases: Interstitial Versus Polyanionic Hydrides. Struct. Bond. 2011, 139, 143–162. [Google Scholar] [CrossRef]
- Chotard, J.-N.; Tang, W.S.; Raybaud, P.; Janot, R. Potassium Silanide (KSiH3): A Reversible Hydrogen Storage Material. Chem. Eur. J. 2011, 44, 12302–12309. [Google Scholar] [CrossRef]
- Werwein, A.; Auer, H.; Kuske, L.; Kohlmann, H. From Metallic LnTt (Ln = La, Nd; Tt = Si, Ge, Sn) to Electron-precise Zintl Phase Hydrides LnTtH. Zeitschrift Anorganische Allgemeine Chemie 2018, 644, 1532–1539. [Google Scholar] [CrossRef]
- Huang, B.; Corbett, J.D. Intermetallic Hydrides as Zintl Phases: A3TtH2 Compounds (A = Ca, Yb; Tt = Sn, Pb) and Their Structural Relationship to the Corresponding Oxides. Inorg. Chem. 1997, 36, 3730–3734. [Google Scholar] [CrossRef]
- Auer, H.; Guehne, R.; Bertmer, M.; Weber, S.; Wenderoth, P.; Hansen, T.C.; Haase, J.; Kohlmann, H. Hydrides of Alkaline Earth−Tetrel (AeTt) Zintl Phases: Covalent Tt−H Bonds from Silicon to Tin. Inorg. Chem. 2017, 56, 1061–1071. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, W.; Udovic, T.J.; Rush, J.J.; Yildirim, T. Structure and hydrogen bonding in CaSiD1+x: Issues about covalent bonding. Phys. Rev. B 2006, 74, 224101. [Google Scholar] [CrossRef]
- Björling, T.; Noréus, D.; Häussermannm, U. Polyanionic Hydrides from Polar Intermetallics AeE2 (Ae = Ca, Sr, Ba; E = Al, Ga, In). J. Am. Chem. Soc. 2006, 128, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Björling, T.; Noréus, D.; Jansson, K.; Andersson, M.; Leonova, E.; Edén, M.; Hålenius, U.; Häussermann, U. SrAlSiH: A Polyanionic Semiconductor Hydride. Angew. Chem. Int. Ed. 2005, 44, 7269–7273. [Google Scholar] [CrossRef] [PubMed]
- Dwight, A.E.; Downey, J.W.; Conner, R.A. Equiatomic compounds of Y and the lanthanide elements with Ga. Acta Cryst. 1967, 23, 860–862. [Google Scholar] [CrossRef]
- Dürr, I.; Bauer, B.; Röhr, C. Lanthan-Triel/Tetrelide La(Al,Ga)x(Si,Ge)1−x. Experimentelle und theoretische Studien zur Stabilität intermetallischer 1:1-Phasen. Zeitschrift Naturforschung B 2011, 66, 1107–1115. [Google Scholar] [CrossRef]
- Yaropolov, Y.L.; Andreenko, A.S.; Nikitin, S.A.; Agafonov, S.S.; Glazkov, V.P.; Verbetsky, V.N. Structure and magnetic properties of RNi (R=Gd, Tb, Dy, Sm) and R6M1.67Si3 (R=Ce, Gd, Tb; M=Ni, Co) hydrides. J. Alloys Compd. 2011, 509, S830–S834. [Google Scholar] [CrossRef]
- Auer, H.; Schlegel, R.; Oeckler, O.; Kohlmann, H. Structural and Electronic Flexibility in Hydrides of Zintl Phases with Tetrel-Hydrogen and Tetrel-Tetrel Bonds. Angew. Chem. Int. Ed. 2017, 56, 12344–12347. [Google Scholar] [CrossRef] [PubMed]
- Westlake, D.G.; Shaked, H.; Mason, P.R.; Matsumoto, T.; Amano, M. Interstitial site occupation in ZrNiH. J. Less-Common Met. 1982, 88, 17–23. [Google Scholar] [CrossRef][Green Version]
- Korst, W.L. The crystal structure of NiZrH3. J. Phys. Chem. 1962, 66, 370–372. [Google Scholar] [CrossRef]
- Ångstrom, J.; Johansson, R.; Sarkar, T.; Sørby, M.H.; Zlotea, C.; Andersson, M.S.; Nordblad, P.; Scheicher, R.H.; Häussermann, U.; Sahlberg, M. Hydrogenation-Induced Structure and Property Changes in the Rare-Earth Metal Gallide NdGa: Evolution of a [GaH]2− Polyanion Containing Peierls-like Ga−H Chains. Inorg. Chem. 2016, 55, 345–352. [Google Scholar] [CrossRef]
- Nedumkandathil, R.; Kranak, V.F.; Johansson, R.; Ångström, J.; Balmes, O.; Andersson, M.S.; Nordblad, P.; Scheicher, R.H.; Sahlberg, M.; Häussermann, U. Hydrogenation induced structure and property changes in GdGa. J. Solid State Chem. 2016, 239, 184–191. [Google Scholar] [CrossRef]
- Fahlquist, H.; Noréus, D.; Sørby, M.H. Varying the Alkali-Metal Radii in (KxRb1−x)n[GaH2]n (0 ≤ x ≤ 1) Reorients a Stable Polyethylene-Structured [GaH2]nn− Anionic Chain. Inorg. Chem. 2013, 52, 4771–4773. [Google Scholar] [CrossRef] [PubMed]
- Werwein, A.; Benndorf, C.; Bertmer, M.; Franz, A.; Oeckler, O.; Kohlmann, H. Hydrogenation Properties of LnAl2 (Ln = La, Eu, Yb), LaGa2, LaSi2 and the Crystal Structure of LaGa2H0.71(2). Crystals 2019, 9, 193. [Google Scholar] [CrossRef]
- Hansen, T.C.; Henry, P.F.; Fischer, H.E.; Torregrossa, J.; Convert, P. The D20 instrument at the ILL: A versatile high-intensity two-axis neutron diffractometer. Meas. Sci. Technol. 2008, 19, 34001. [Google Scholar] [CrossRef]
- Götze, A.; Auer, H.; Finger, R.; Hansen, T.C.; Kohlmann, H. A sapphire single-crystal cell for in situ neutron powder diffraction of solid-gas reactions. Physica B (Amst. Neth.) 2018, 551, 395–400. [Google Scholar] [CrossRef]
- Kohlmann, H.; Finger, R.; Goetze, A.; Hansen, T.; Pflug, C.; Werwein, A. Hydrides of the Zintl Phase TmGa; Institut Laue-Langevin (ILL): Grenoble, France, 2018. [Google Scholar] [CrossRef]
- Rietveld, H.M. Line profiles of neutron powder-diffraction peaks for structure refinement. Acta Cryst. 1967, 22, 151–152. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Bruker AXS, TOPAS© Version 5. Available online: https://www.bruker-axs.com (accessed on 30 October 2018).
- Rodrıguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Physica B (Amst. Neth.) 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Rodrıguez-Carvajal, J. FullProf.2k, Version 5.30—Mar2012-ILL JRC; Institut Laue-Langevin: Grenoble, France, 2018. [Google Scholar]
- VESTA—Visualisation for Electronic and Structural Analysis, Version 3.3.1; Koichi Momma and Fujio Izumi: Ibaraki, Japan, 2018.
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Abinit v. 8.8.2, GNU General Public License. Available online: http://www.abinit.org (accessed on 14 June 2018).
- Gonze, X.; Beuken, J.-M.; Caracas, R.; Detraux, F.; Fuchs, M.; Rignanese, G.-M.; Sindic, L.; Verstraete, M.; Zerah, G.; Jollet, F.; et al. First-principles computation of material properties: The ABINIT software project. Comput. Mater. Sci. 2002, 25, 478–492. [Google Scholar] [CrossRef]
- Gonze, X. A brief introduction to the ABINIT software package. Zeitschrift Kristallographie Cryst. Mater. 2005, 220, 558–562. [Google Scholar] [CrossRef]
- Gonze, X.; Amadon, B.; Anglade, P.-M.; Beuken, J.-M.; Bottin, F.; Boulanger, P.; Bruneval, F.; Caliste, D.; Caracas, R.; Côté, M.; et al. ABINIT: First-principles approach to material and nanosystem properties. Comput. Phys. Commun. 2009, 180, 2582–2615. [Google Scholar] [CrossRef]
- Gonze, X.; Jollet, F.; Araujo, F.A.; Adams, D.; Amadon, B.; Applencourt, T.; Audouze, C.; Beuken, J.-M.; Bieder, J.; Bokhanchuk, A.; et al. Recent developments in the ABINIT software package. Comput. Phys. Commun. 2016, 205, 106–131. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- JTH PAW Atomic Datasets, Version 1.0. Available online: https://www.abinit.org/downloads/PAW2 (accessed on 28 March 2019).
- Jollet, F.; Torrent, M.; Holzwarth, N. Generation of Projector Augmented-Wave atomic data: A 71 element validated table in the XML format. Comput. Phys. Commun. 2014, 185, 1246–1254. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Fischer, P.; Haelg, W.; Schlapbach, L.; Yvon, K. Neutron and X-ray diffraction investigation of deuterium storage in La7Ni3. J. Less-Common Met. 1978, 60, 1–9. [Google Scholar] [CrossRef]
- Udovic, T.J.; Huang, Q.; Santoro, A.; Rush, J.J. The nature of deuterium arrangements in YD3 and other rare-earth trideuterides. Zeitschrift Kristallographie Cryst. Mater. 2008, 223, 687–704. [Google Scholar] [CrossRef]
- Gschneidner, K.A., Jr.; Eyring, L. (Eds.) Handbook on the Physics and Chemistry of Rare Earths; North-Holland Publishing: Amsterdam, The Netherlands, 1978. [Google Scholar]
- Gingl, F.; Yvon, K.; Vogt, T. Synthesis and crystal structure of BaMgH4: A centrosymmetric variant of SrMgH4. J. Alloys Compd. 1997, 256, 155–158. [Google Scholar] [CrossRef]
- Shashikalaa, K.; Sathyamoorthy, A.; Raj, P.; Dhar, S.K.; Malik, S.K. Structure and magnetic properties of CeGa2D0.6 system. J. Alloys Compd. 2007, 436, 19–22. [Google Scholar] [CrossRef]
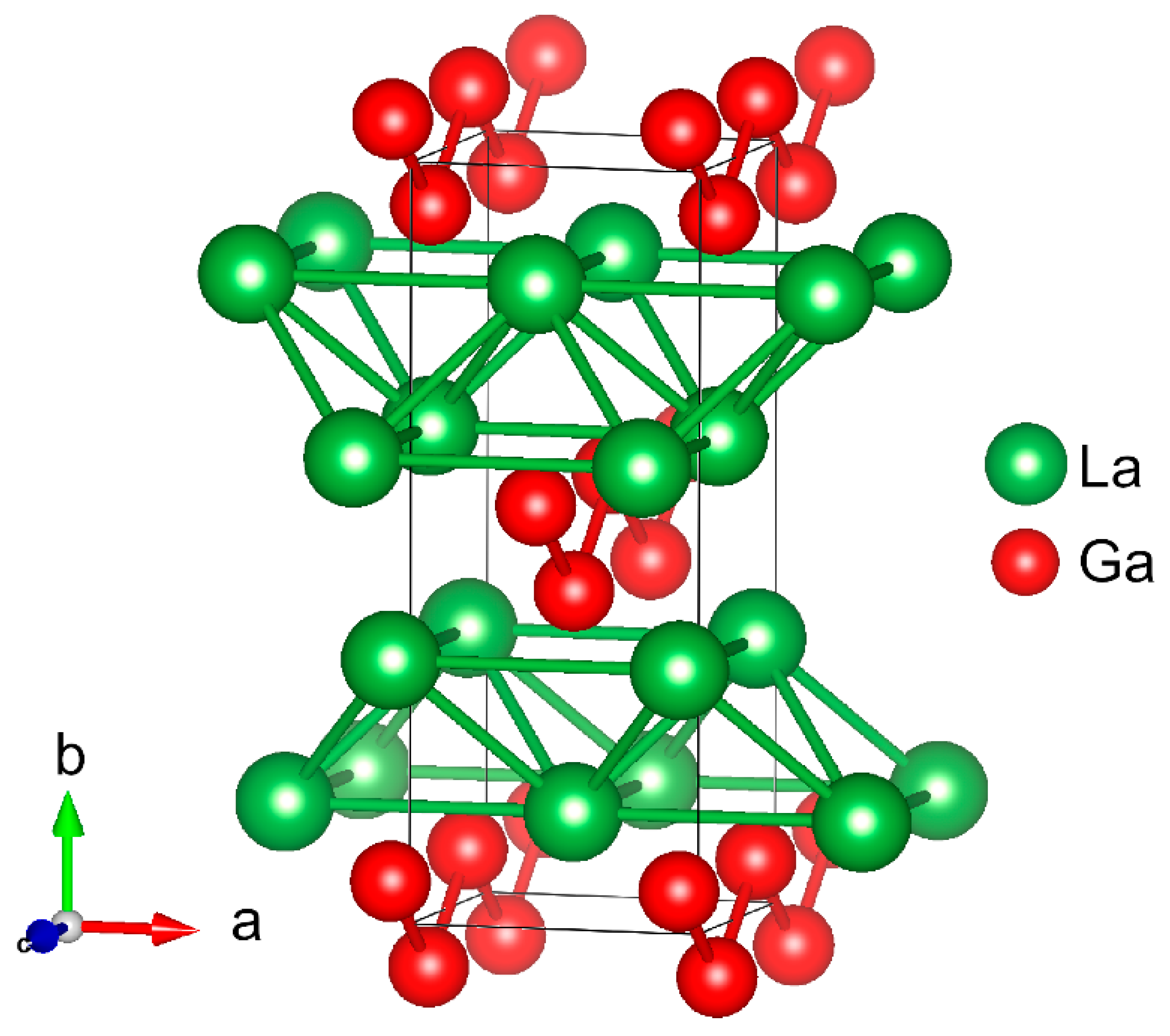







| Atom | Site | x | y | z | Biso/Å2 | s.o.f |
|---|---|---|---|---|---|---|
| La1 | 4c | 0 | 0.3366(7) | 1/4 | 1.07(8) | 1 |
| La2 | 8g | 0.3333(7) | 0.3651(4) | 1/4 | Biso(La1) | 1 |
| Ga1 | 4c | 0 | 0.0484(8) | 1/4 | 1.83(8) | 1 |
| Ga2 | 8g | 0.3456(7) | 0.0487(7) | 1/4 | Biso(Ga1) | 1 |
| D1 | 4c | 0 | 0.7627(9) | 1/4 | 2.32(9) | 1 |
| D2 | 8g | 0.1689(9) | 0.2549(7) | 1/4 | Biso(D1) | 1 |
| D3 | 8g | 0.1877(7) | 0.0596(7) | 1/4 | 3.1(2) | 0.946(14) |
| Atom | Site | x | y | z | Biso/Å2 | s.o.f |
|---|---|---|---|---|---|---|
| Tm1 | 4c | 0 | 0.3115(5) | 1/4 | 0.91(6) | 1 |
| Tm2 | 8g | 0.3190(4) | 0.3585(4) | 1/4 | Biso(Tm1) | 1 |
| Ga1 | 4c | 0 | 0.0592(5) | 1/4 | 1.32(7) | 1 |
| Ga2 | 8g | 0.3658(4) | 0.0575(4) | 1/4 | Biso(Ga1) | 1 |
| D1 | 4c | 0 | 0.7705(5) | 1/4 | 1.25(9) | 0.93(1) |
| D2 | 8g | 0.1751(5) | 0.2367(4) | 1/4 | Biso(D1) | 0.93(1) |
| Compound | a/Å | b/Å | c/Å | dGa-Ga/Å | V/Å3 |
|---|---|---|---|---|---|
| LaGa | 4.566 | 11.606 | 4.235 | 2.634 | 224.42 |
| LaGaH | 4.349 | 12.461 | 4.270 | 2.536 | 231.43 |
| LaGaH1.66 | 12.819/4.27 | 12.603 | 4.276 | 2.591 | 690.88/230.12 |
| YGa | 4.339 | 10.971 | 4.064 | 2.640 | 193.44 |
| YGaH | 4.064 | 11.514 | 4.076 | 2.471 | 190.75 |
| (C-LaGeH type) | |||||
| YGaH | 11.146/3.71 | 12.707 | 4.093 | 2.493 | 579.76/193.25 |
| (α-TmGaH type) | 2.516 |
| Compound | Structure Type | b/a | b/c | c/a |
|---|---|---|---|---|
| ZrNiH [18] | ZrNiH | 3.06 | 2.54 | 1.20 |
| ZrNiH3 [19] | ZrNiH3 | 2.96 | 2.44 | 1.22 |
| TbNiD3.3 [16] | TbNiD3.3 | 3.05 | 2.44 | 1.24 |
| BaMgD4 [47] | ReBiO4 | 3.27 | 2.41 | 1.35 |
| K0.5Rb0.5GaD2 [22] | K0.5Rb0.5GaD2 | 2.16 | 2.6 | 0.82 |
| NdGeD [8] | C-LaGeD | 2.81 | 2.93 | 0.95 |
| YSiHx | C-LaGeD | 2.90 | 3.02 | 0.96 |
| ErSiHx | C-LaGeD | 2.86 | 2.97 | 0.96 |
| TmSiHx | C-LaGeD | 2.85 | 2.96 | 0.96 |
| LaGaD1.66 | NdGaD1.66 | 2.97 | 2.96 | 0.99 |
| NdGaD1.66 [20] | NdGaD1.66 | 2.98 | 2.94 | 1.01 |
| α-YGaH | α-TmGaD | 3.36 | 3.09 | 1.08 |
| α-TmGaD | α-TmGaD | 3.38 | 3.06 | 1.10 |
| BaSiH2−x [10] | BaSiH2−x | 3.49 | 3.79 | 0.91 |
| SrSiH5/3−x [17] | SrSiH5/3−x | 3.66 | 3.80 | 0.96 |
| BaGeH5/3−x [17] | SrSiH5/3−x | 3.66 | 3.74 | 0.98 |
| BaSnH4/3−x [10] | CaSiH4/3−x | 3.78 | 3.58 | 1.05 |
| SrGeH4/3−x [10] | CaSiH4/3−x | 3.84 | 3.75 | 1.02 |
| CaSiH4/3−x [11] | CaSiH4/3−x | 3.89 | 3.81 | 1.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Werwein, A.; Hansen, T.C.; Kohlmann, H. Size Matters: New Zintl Phase Hydrides of REGa (RE = Y, La, Tm) and RESi (RE = Y, Er, Tm) with Large and Small Cations. Crystals 2019, 9, 600. https://doi.org/10.3390/cryst9110600
Werwein A, Hansen TC, Kohlmann H. Size Matters: New Zintl Phase Hydrides of REGa (RE = Y, La, Tm) and RESi (RE = Y, Er, Tm) with Large and Small Cations. Crystals. 2019; 9(11):600. https://doi.org/10.3390/cryst9110600
Chicago/Turabian StyleWerwein, Anton, Thomas C. Hansen, and Holger Kohlmann. 2019. "Size Matters: New Zintl Phase Hydrides of REGa (RE = Y, La, Tm) and RESi (RE = Y, Er, Tm) with Large and Small Cations" Crystals 9, no. 11: 600. https://doi.org/10.3390/cryst9110600
APA StyleWerwein, A., Hansen, T. C., & Kohlmann, H. (2019). Size Matters: New Zintl Phase Hydrides of REGa (RE = Y, La, Tm) and RESi (RE = Y, Er, Tm) with Large and Small Cations. Crystals, 9(11), 600. https://doi.org/10.3390/cryst9110600






