PbI2 Single Crystal Growth and Its Optical Property Study
Abstract
1. Introduction
2. Materials and Methods
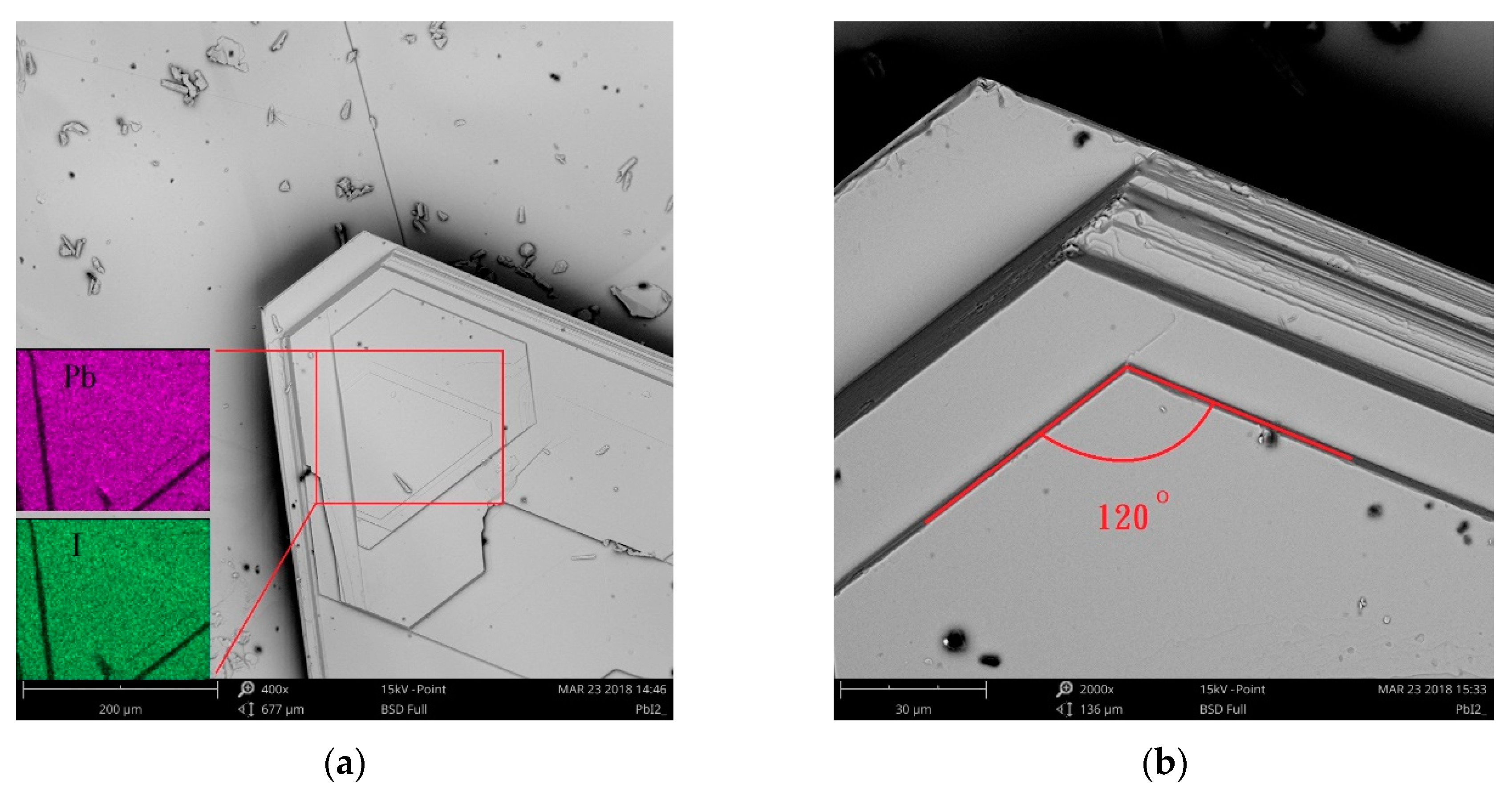
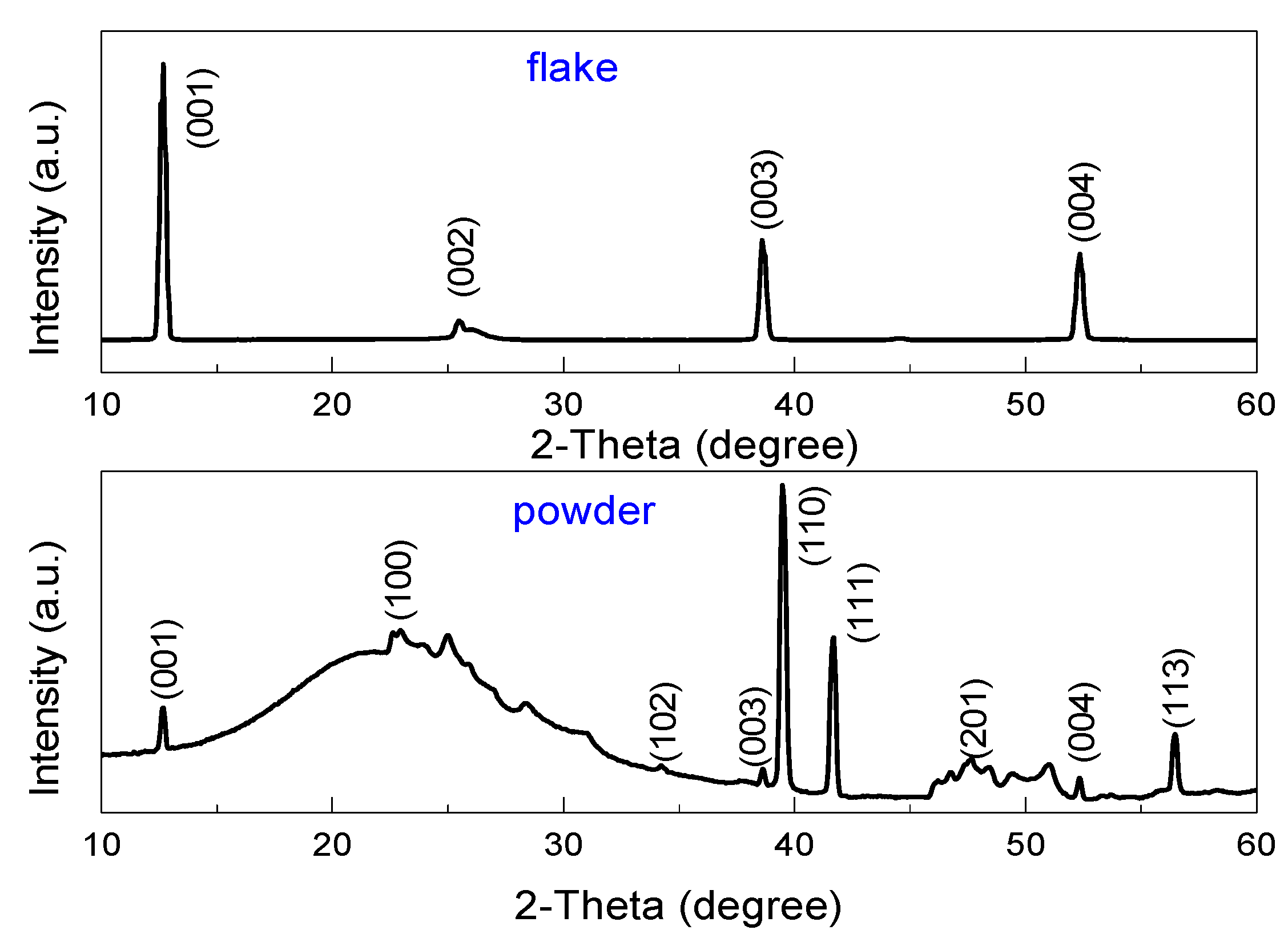
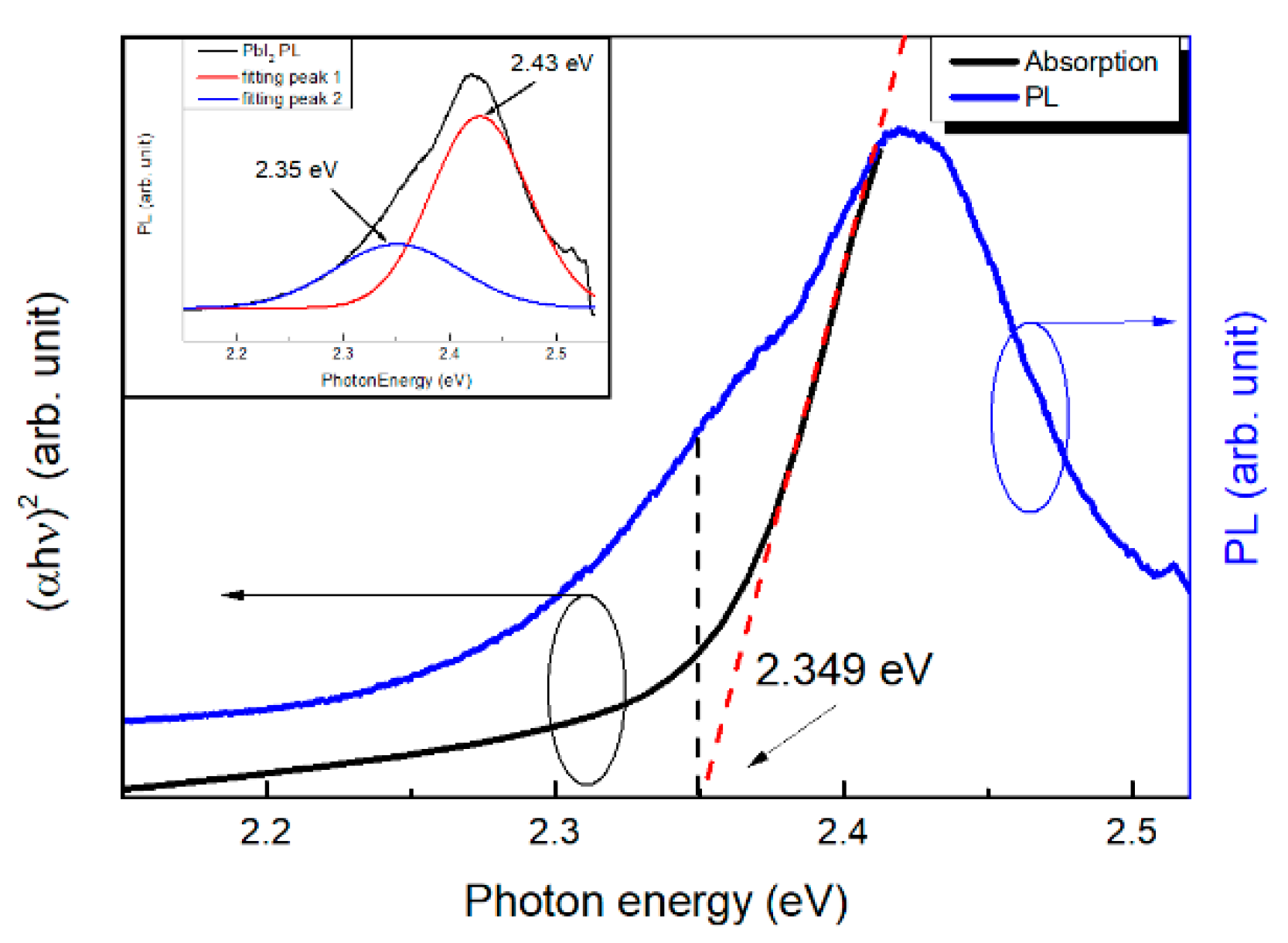
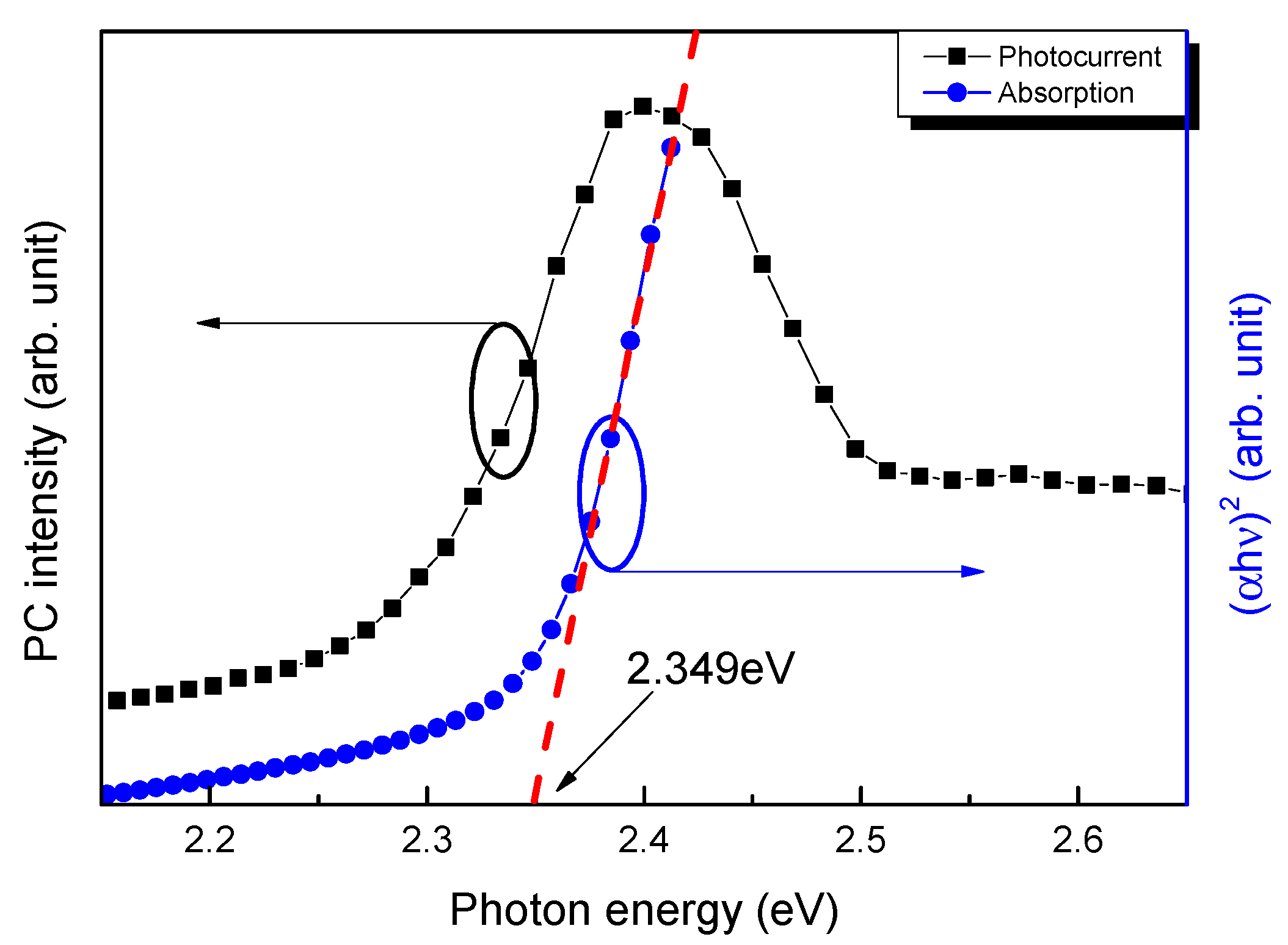
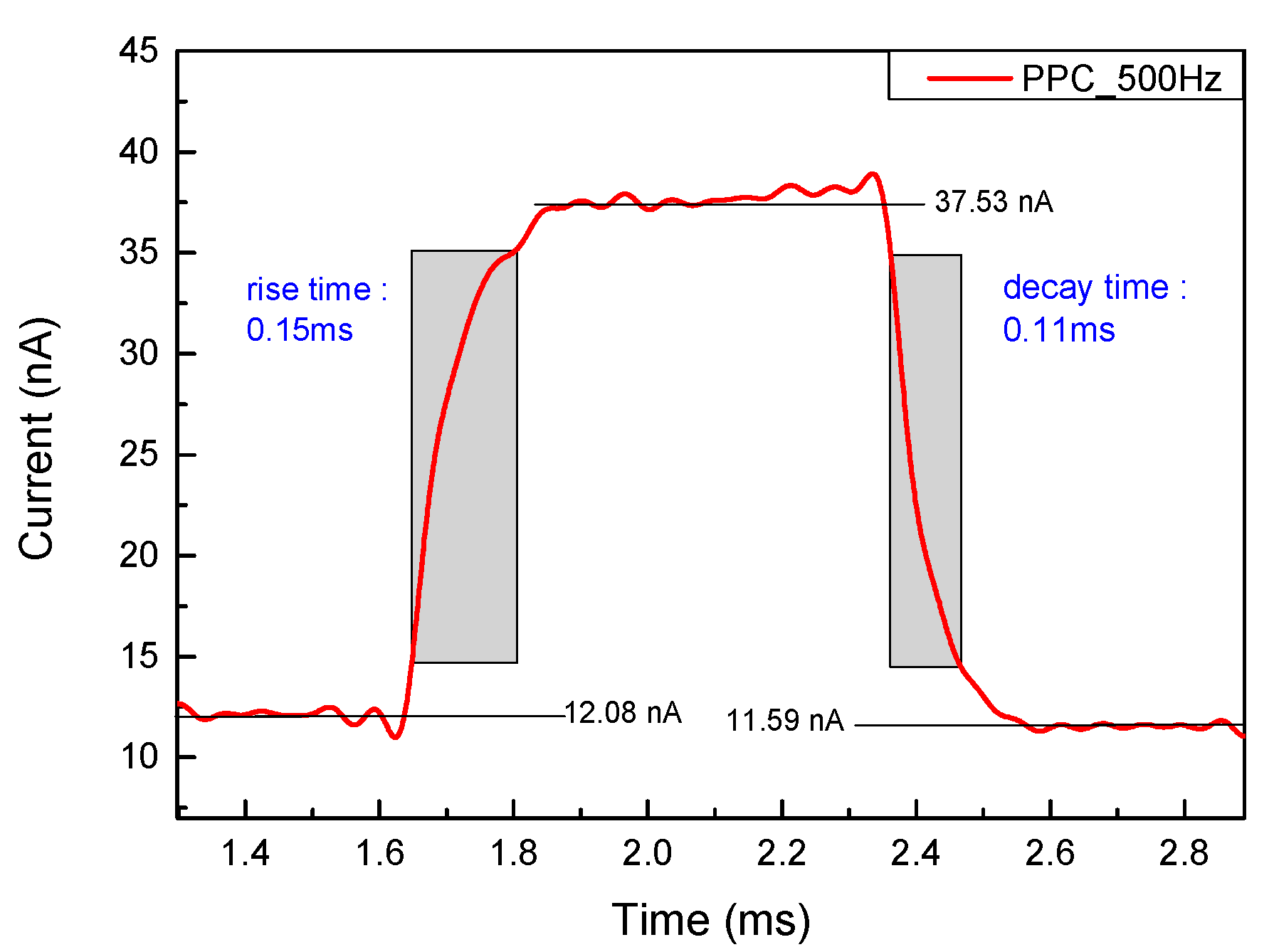
3. Results
Subsection
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147. [Google Scholar] [CrossRef] [PubMed]
- Velusamy, D.B.; Kim, R.H.; Cha, S.; Huh, J.; Khazaeinezhad, R.; Kassani, S.H.; Song, G.; Cho, S.M.; Cho, S.H.; Hwang, I. Flexible transition metal dichalcogenide nanosheets for band-selective photodetection. Nat. Commun. 2015, 6, 8063. [Google Scholar] [CrossRef] [PubMed]
- Huo, N.; Yang, S.; Wei, Z.; Li, S.-S.; Xia, J.-B.; Li, J. Photoresponsive and gas sensing field-effect transistors based on multilayer WS2 nanoflakes. Sci. Rep. 2014, 4, 5209. [Google Scholar] [CrossRef] [PubMed]
- Svatek, S.A.; Antolin, E.; Lin, D.-Y.; Frisenda, R.; Reuter, C.; Molina-Mendoza, A.J.; Muñoz, M.; Agraït, N.; Ko, T.-S.; De Lara, D.P. Gate tunable photovoltaic effect in MoS2 vertical p–n homostructures. J. Mater. Chem. C 2017, 5, 854–861. [Google Scholar] [CrossRef]
- Donarelli, M.; Ottaviano, L. 2D materials for gas sensing applications: A review on graphene oxide, MoS2, WS2 and phosphorene. Sensors 2018, 18, 3638. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-T.; Liang, Y.-C.; Lin, H.-H.; Li, C.-H.; Lu, S.-Y. Exfoliated SnS2 nanoplates for enhancing direct electrochemical glucose sensing. Electrochim. Acta 2016, 219, 241–250. [Google Scholar] [CrossRef]
- Birowosuto, M.D.; Cortecchia, D.; Drozdowski, W.; Brylew, K.; Lachmanski, W.; Bruno, A.; Soci, C. X-ray scintillation in lead halide perovskite crystals. Sci. Rep. 2016, 6, 37254. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Huang, L.; Deng, H.-X.; Wang, X.; Li, B.; Wei, Z.; Li, J. Flexible photodetectors based on phase dependent PbI2 single crystals. J. Mater. Chem. C 2016, 4, 6492–6499. [Google Scholar] [CrossRef]
- Hayashi, T.; Kinpara, M.; Wang, J.; Mimura, K.; Isshiki, M. Growth of PbI2 single crystals from stoichiometric and Pb excess melts. J. Cryst. Growth 2008, 310, 47–50. [Google Scholar] [CrossRef]
- Tonn, J.; Danilewsky, A.; Cröll, A.; Matuchova, M.; Maixner, J. Czochralski growth of lead iodide single crystals: Investigations and comparison with the Bridgman method. J. Cryst. Growth 2011, 318, 558–562. [Google Scholar] [CrossRef]
- Han, M.; Sun, J.; Bian, L.; Wang, Z.; Zhang, L.; Yin, Y.; Gao, Z.; Li, F.; Xin, Q.; He, L. Two-step vapor deposition of self-catalyzed large-size PbI2 nanobelts for high-performance photodetectors. J. Mater. Chem. C 2018, 6, 5746–5753. [Google Scholar] [CrossRef]
- Wang, R.; Li, S.; Wang, P.; Xiu, J.; Wei, G.; Sun, M.; Li, Z.; Liu, Y.; Zhong, M. PbI2 Nanosheets for Photodetectors via the Facile Cooling Thermal Supersaturation Solution Method. J. Phys. Chem. C 2019, 123, 9609–9616. [Google Scholar] [CrossRef]
- Meyers, J.K.; Kim, S.; Hill, D.J.; Cating, E.E.; Williams, L.J.; Kumbhar, A.S.; McBride, J.R.; Papanikolas, J.M.; Cahoon, J.F. Self-catalyzed vapor–liquid–solid growth of lead halide nanowires and conversion to hybrid perovskites. Nano Lett. 2017, 17, 7561–7568. [Google Scholar] [CrossRef] [PubMed]
- Muralikrishna, S.; Manjunath, K.; Samrat, D.; Reddy, V.; Ramakrishnappa, T.; Nagaraju, D.H. Hydrothermal synthesis of 2D MoS 2 nanosheets for electrocatalytic hydrogen evolution reaction. RSC Adv. 2015, 5, 89389–89396. [Google Scholar] [CrossRef]
- Xue, X.; Zhou, Z.; Peng, B.; Zhu, M.; Zhang, Y.; Ren, W.; Ye, Z.; Chen, X.; Liu, M. Review on nanomaterials synthesized by vapor transport method: Growth and their related applications. RSC Adv. 2015, 5, 79249–79263. [Google Scholar] [CrossRef]
- Ponpon, J.; Amann, M. Preliminary characterization of PbI2 polycrystalline layers deposited from solution for nuclear detector applications. Thin Solid Film. 2001, 394, 276–282. [Google Scholar] [CrossRef]
- Mousa, A.M.; Al-rubaie, N.J. The influence of deposition conditions on structural properties of PbI2. Texture Stress Microstruct. 2009, 2009, 494537. [Google Scholar] [CrossRef]
- He, Y.; Zhu, S.; Zhao, B.; Jin, Y.; He, Z.; Chen, B. Improved growth of PbI2 single crystals. J. Cryst. Growth 2007, 300, 448–451. [Google Scholar] [CrossRef]
- Pałosz, B.; Salje, E. Lattice parameters and spontaneous strain in AX2 polytypes: CdI2, PbI2 SnS2 and SnSe2. J. Appl. Crystallogr. 1989, 22, 622–623. [Google Scholar] [CrossRef]
- Flahaut, E.; Sloan, J.; Friedrichs, S.; Kirkland, A.I.; Coleman, K.; Williams, V.; Hanson, N.; Hutchison, J.; Green, M.L. Crystallization of 2H and 4H PbI2 in carbon nanotubes of varying diameters and morphologies. Chem. Mater. 2006, 18, 2059–2069. [Google Scholar] [CrossRef]
- Du, L.; Wang, C.; Xiong, W.; Zhang, S.; Xia, C.; Wei, Z.; Li, J.; Tongay, S.; Yang, F.; Zhang, X. Perseverance of direct bandgap in multilayer 2D PbI2 under an experimental strain up to 7.69%. 2D Mater. 2019, 6, 025014. [Google Scholar] [CrossRef]
- Ahuja, R.; Arwin, H.; Ferreira da Silva, A.; Persson, C.; Osorio-Guillén, J.M.; Souza de Almeida, J.; Moyses Araujo, C.; Veje, E.; Veissid, N.; An, C. Electronic and optical properties of lead iodide. J. Appl. Phys. 2002, 92, 7219–7224. [Google Scholar] [CrossRef]
- Panish, M.; Casey, H., Jr. Temperature dependence of the energy gap in GaAs and GaP. J. Appl. Phys. 1969, 40, 163–167. [Google Scholar] [CrossRef]
- Burton, L.A.; Whittles, T.J.; Hesp, D.; Linhart, W.M.; Skelton, J.M.; Hou, B.; Webster, R.F.; O’Dowd, G.; Reece, C.; Cherns, D. Electronic and optical properties of single crystal SnS2: An earth-abundant disulfide photocatalyst. J. Mater. Chem. A 2016, 4, 1312–1318. [Google Scholar] [CrossRef]
- Sears, W.; Morrison, J. Low temperature thermal properties of PbI2. J. Phys. Chem. Solids 1979, 40, 503–508. [Google Scholar] [CrossRef]
- Daoud, S. Linear correlation between Debye temperature and lattice thermal conductivity in II-VI and III-V semiconductors. Int. J. Sci. World 2015, 3, 216–220. [Google Scholar] [CrossRef]
- Peng, B.; Mei, H.; Zhang, H.; Shao, H.; Xu, K.; Ni, G.; Jin, Q.; Soukoulis, C.M.; Zhu, H. High thermoelectric efficiency in monolayer PbI2 from 300 K to 900 K. Inorg. Chem. Front. 2019, 6, 920–928. [Google Scholar] [CrossRef]
- Cröll, A.; Tonn, J.; Post, E.; Böttner, H.; Danilewsky, A. Anisotropic and temperature-dependent thermal conductivity of PbI2. J. Cryst. Growth 2017, 466, 16–21. [Google Scholar] [CrossRef]
- O’Donnell, K.; Chen, X. Temperature dependence of semiconductor band gaps. Appl. Phys. Lett. 1991, 58, 2924–2926. [Google Scholar] [CrossRef]
- Varshni, Y.P. Temperature dependence of the energy gap in semiconductors. Physica 1967, 34, 149–154. [Google Scholar] [CrossRef]
- Manoogian, A.; Leclerc, A. Determination of the dilation and vibrational contributions to the energy band gaps in germanium and silicon. Phys. Status Solidi 1979, 92, 23–27. [Google Scholar] [CrossRef]
- Skolnick, M.; Bimberg, D. Angular-dependent magnetoluminescence study of the layer compound 2H-Pb I2. Phys. Rev. B 1978, 18, 7080. [Google Scholar] [CrossRef]
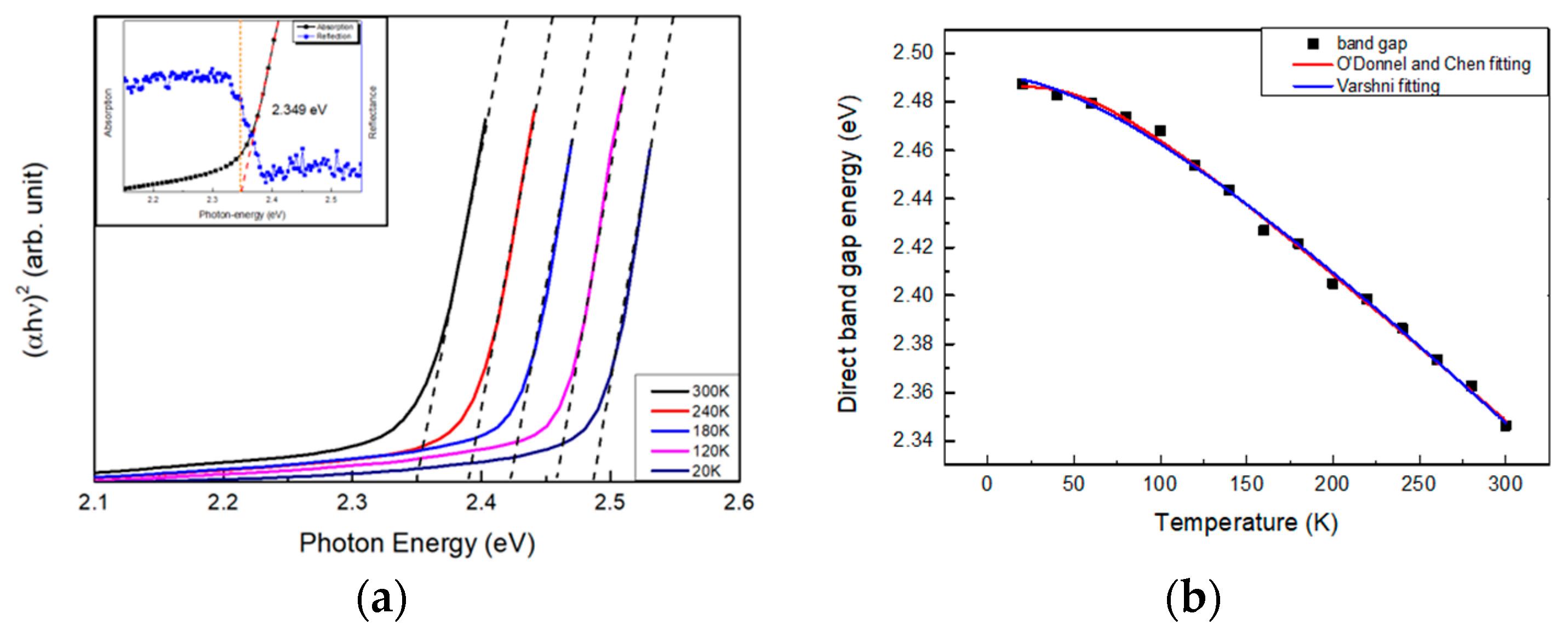
| Eg(0) (eV) | α (meV/K) | Β (K) | Ref. | |
|---|---|---|---|---|
| PbI2 | 2.491 | 0.7349 | 161.4 | This work |
| PbI2 | 2.49 | 0.705 | 130 | [22] |
| GaP | 2.338 | 0.62 | 460 | [23] |
| SnS2 | 2.56 | 2.12 | 797 | [24] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, D.-Y.; Guo, B.-C.; Dai, Z.-Y.; Lin, C.-F.; Hsu, H.-P. PbI2 Single Crystal Growth and Its Optical Property Study. Crystals 2019, 9, 589. https://doi.org/10.3390/cryst9110589
Lin D-Y, Guo B-C, Dai Z-Y, Lin C-F, Hsu H-P. PbI2 Single Crystal Growth and Its Optical Property Study. Crystals. 2019; 9(11):589. https://doi.org/10.3390/cryst9110589
Chicago/Turabian StyleLin, Der-Yuh, Bo-Cheng Guo, Zih-You Dai, Chia-Feng Lin, and Hung-Pin Hsu. 2019. "PbI2 Single Crystal Growth and Its Optical Property Study" Crystals 9, no. 11: 589. https://doi.org/10.3390/cryst9110589
APA StyleLin, D.-Y., Guo, B.-C., Dai, Z.-Y., Lin, C.-F., & Hsu, H.-P. (2019). PbI2 Single Crystal Growth and Its Optical Property Study. Crystals, 9(11), 589. https://doi.org/10.3390/cryst9110589





