Self-Assembly Motifs of Water in Crystals of Palladium β-Amino Acid Complexes Influenced by Methyl Substitution on the Amino Acid Backbone
Abstract
1. Introduction
2. Results and Discussion
2.1. Complex Syntheses
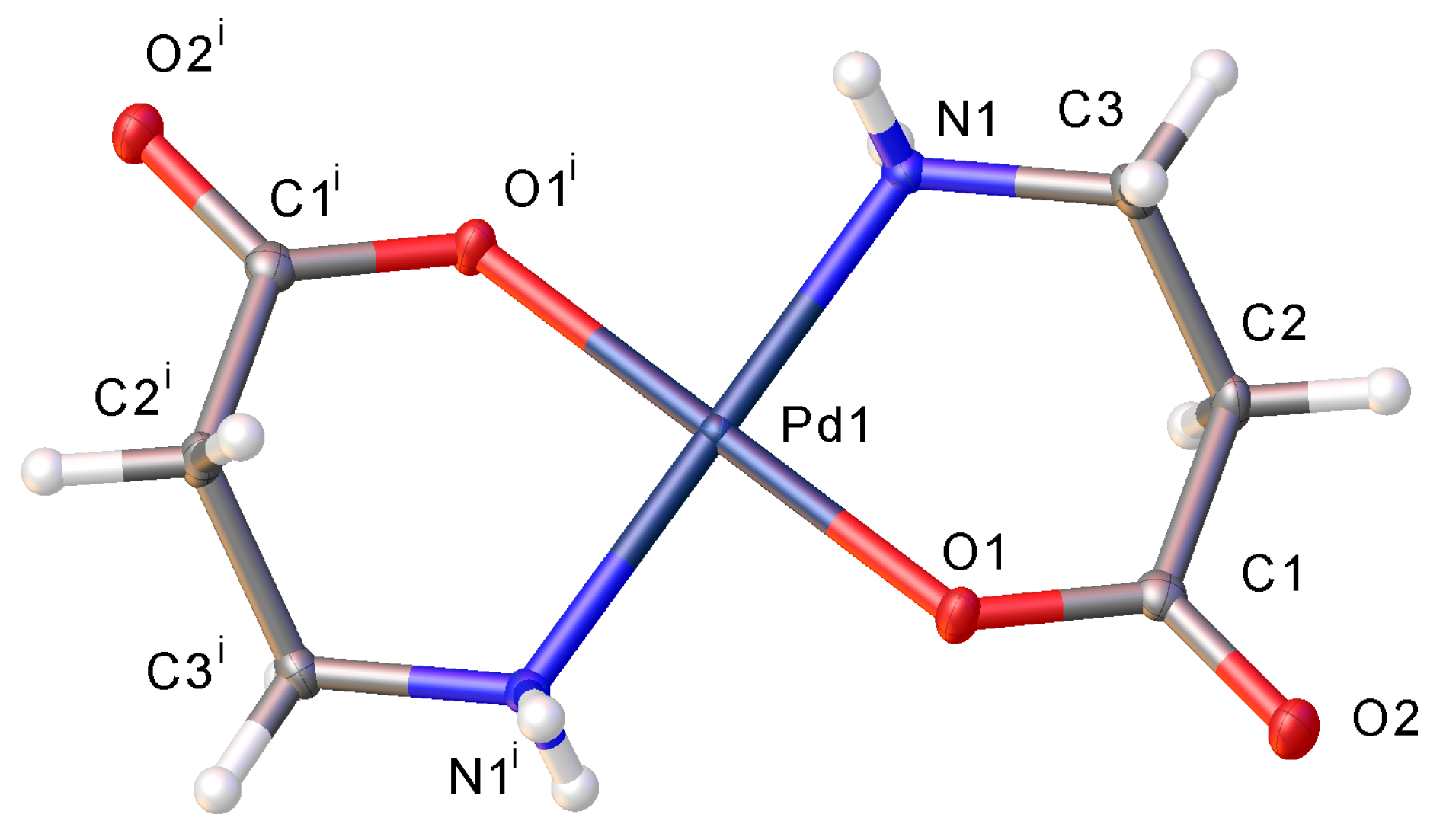
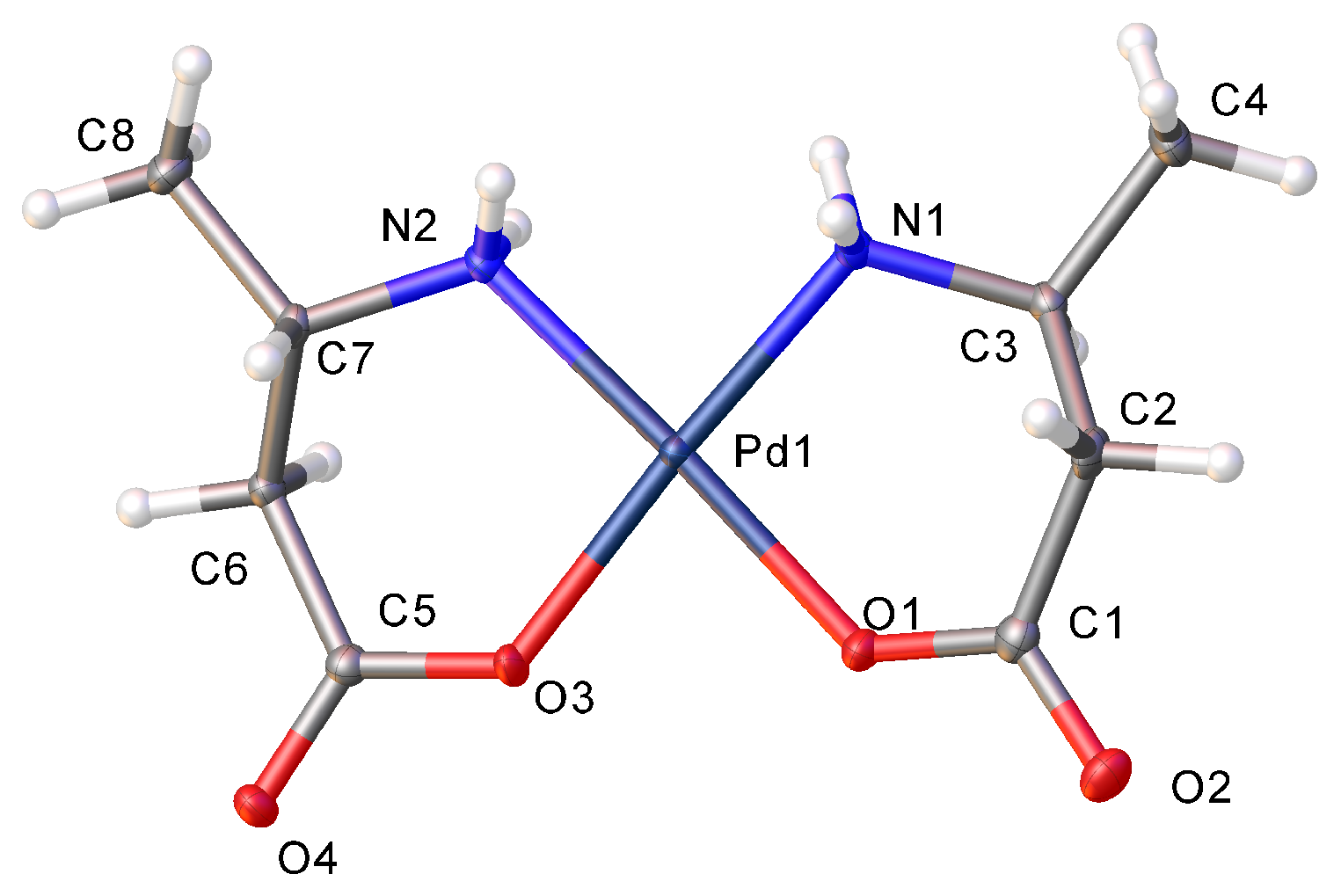
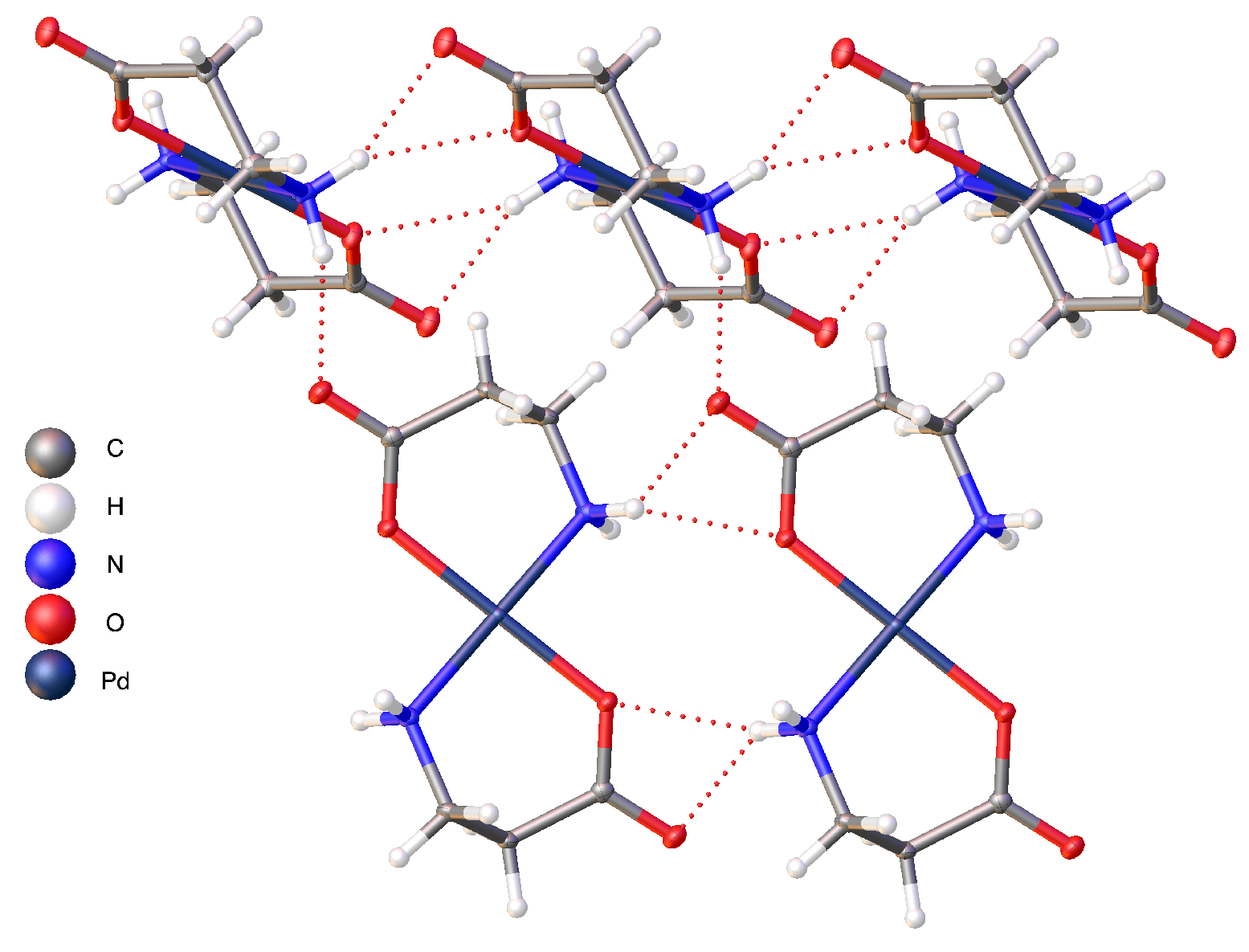
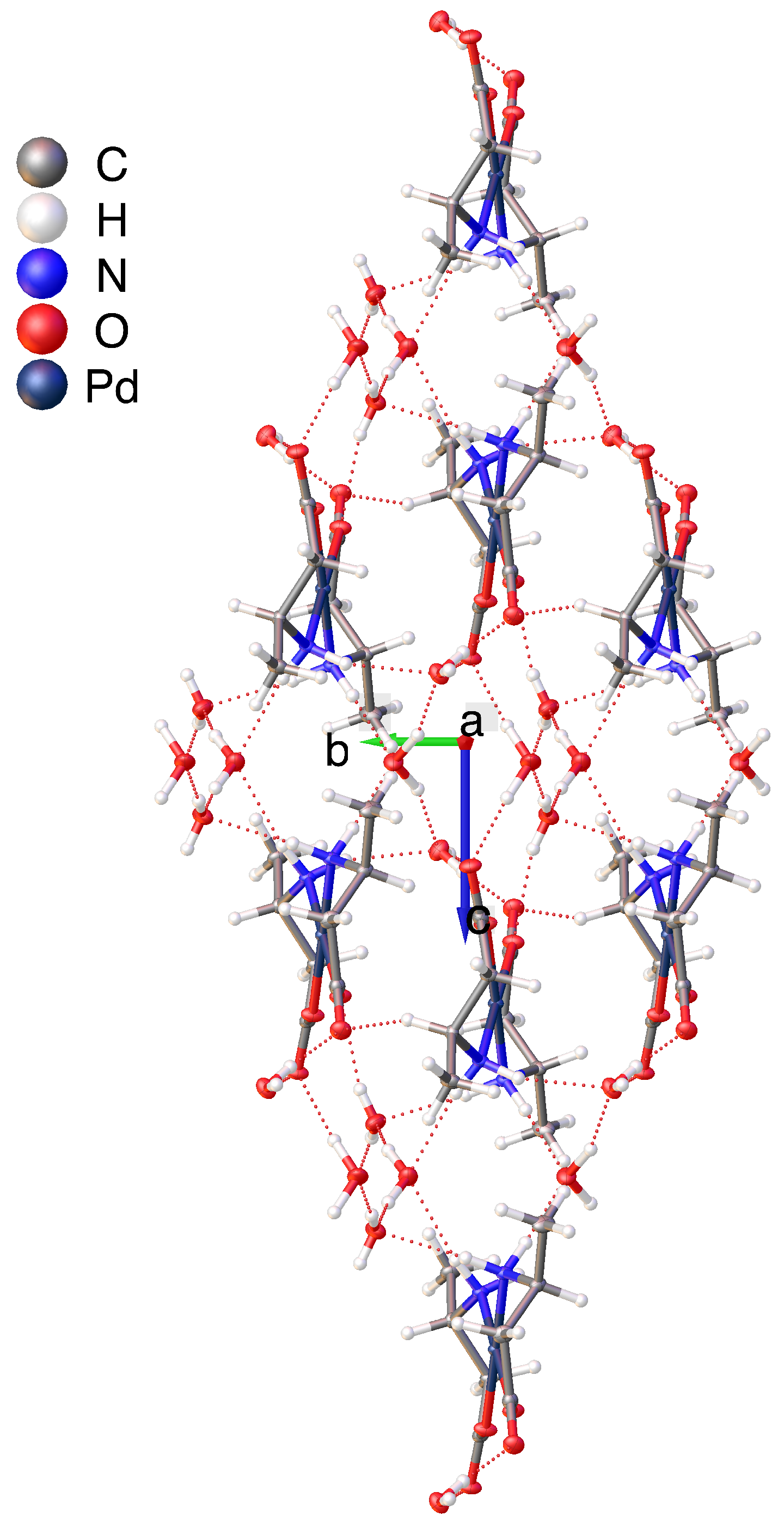
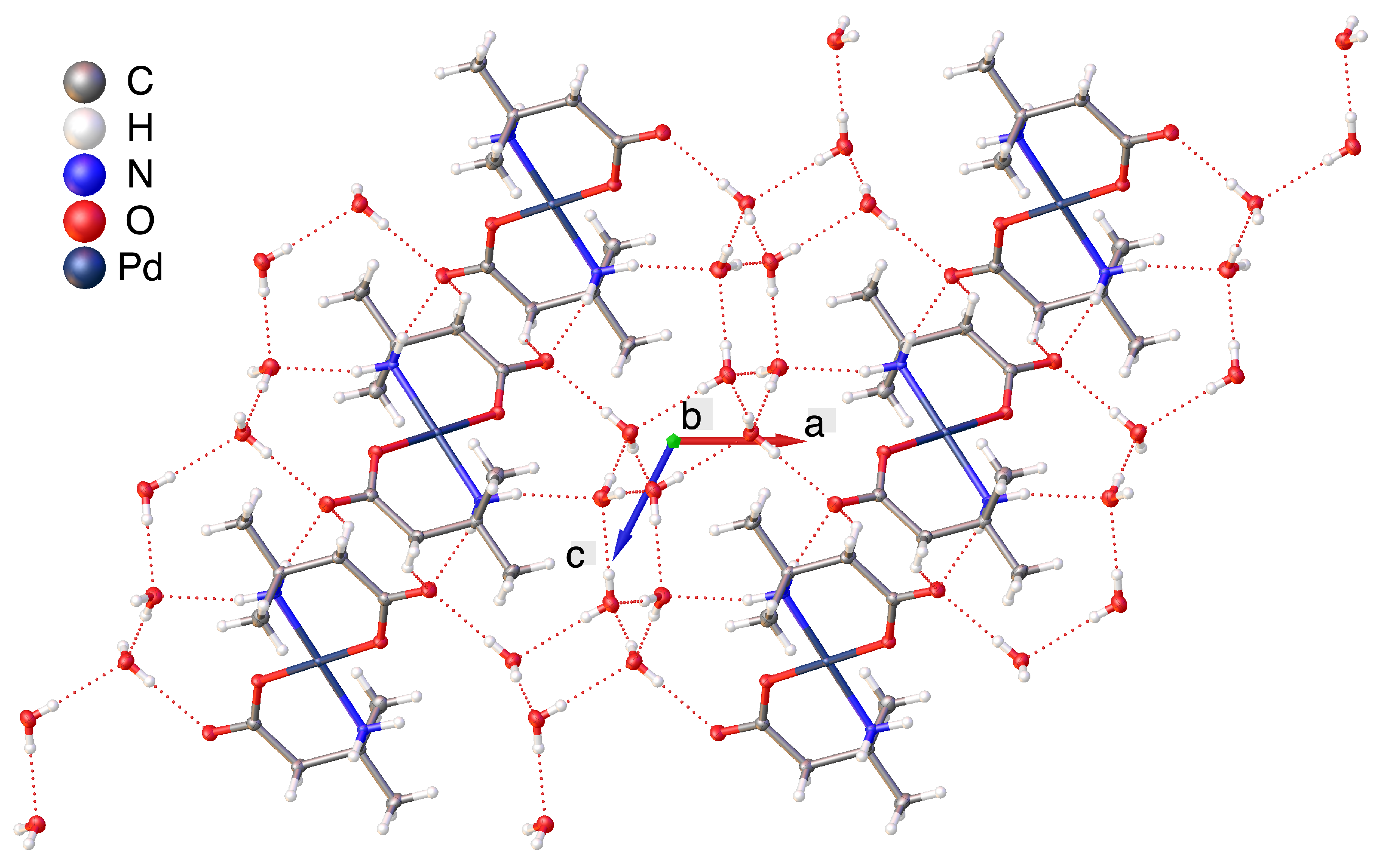
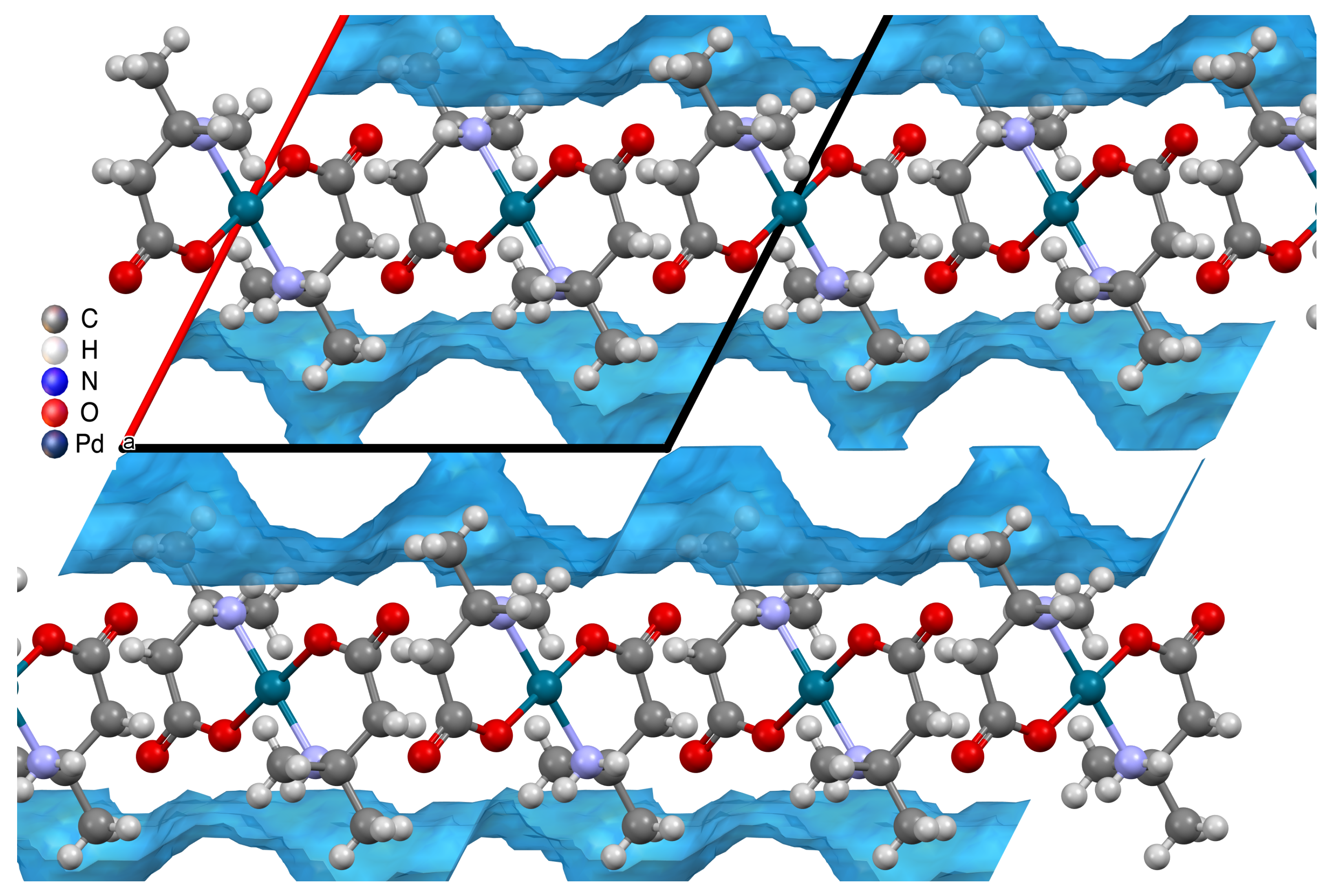
2.2. Hydrogen Bonding Motifs
3. Conclusions
4. Experimental
4.1. General Synthetic Techniques
4.2. Synthesis of Complexes
4.2.1. Synthesis of trans-bis-(3-aminopropionato)palladium(II)
4.2.2. Synthesis of cis-bis-((S)-3-aminobutanoato)palladium(II)
4.2.3. Synthesis of trans-bis-(3-amino-3-methylbutanoato)palladium(II)
4.3. X-ray Crystallography
5. Associated Content
Accession Codes
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Severin, K.; Bergs, R.; Beck, W. Bioorganometallic Chemistry ± Transition Metal Complexes with. Angew. Chem. Int. Ed. Engl. 1998, 37, 1634–1654. [Google Scholar] [CrossRef]
- Carmona, D.; Pilar Lamata, M.; Viguri, F.; San José, E.; Mendoza, A.; Lahoz, F.J.; García-Orduña, P.; Atencio, R.; Oro, L.A. Half-sandwich organometallic complexes with stereogenic metal centres: Synthesis and characterization of diastereomeric [(η-ring)M(Aa)X] (Aa = α-amino carboxylate) compounds. J. Organomet. Chem. 2012, 717, 152–163. [Google Scholar] [CrossRef]
- Biancalana, L.; Abdalghani, I.; Chiellini, F.; Zacchini, S.; Pampaloni, G.; Crucianelli, M.; Marchetti, F. Ruthenium Arene Complexes with α-Aminoacidato Ligands: New Insights into Transfer Hydrogenation Reactions and Cytotoxic Behaviour. Eur. J. Inorg. Chem. 2018, 2018, 3041–3057. [Google Scholar] [CrossRef]
- Roy, C.P.; Huff, L.A.; Barker, N.A.; Berg, M.A.G.; Merola, J.S. Iridium(III) hydrido amino acid compounds: Chiral complexes and a helical extended lattice. J. Organomet. Chem. 2006, 691, 2270–2276. [Google Scholar] [CrossRef]
- Merola, J.S.; Slebodnick, C.; Berg, M.; Ritchie, M.K. mer-Hydridotris(trimethylphosphane-κP)(d-valinato-κ2 N,O)iridium hexafluoridophosphate dichloromethane 0.675-solvate. Acta Crystallogr. Sect. E Struct. Rep. Online 2014, 70, m82. [Google Scholar] [CrossRef]
- Karpin, G.; Merola, J.; Falkinham, J., III. Transition metal-α-amino acid complexes with antibiotic activity against Mycobacterium spp. Antimicrob. Agents Chemother. 2013, 57. [Google Scholar] [CrossRef]
- Morris, D.M.; McGeagh, M.; De Peña, D.; Merola, J.S. Extending the range of pentasubstituted cyclopentadienyl compounds: The synthesis of a series of tetramethyl(alkyl or aryl)cyclopentadienes (Cp*R ), their iridium complexes and their catalytic activity for asymmetric transfer hydrogenation. Polyhedron 2014, 84, 120–135. [Google Scholar] [CrossRef]
- Wagner-Schuh, B.; Beck, W. Metal Complexes of Biologically Important Ligands, CLXXVII. Dichlorido Platinum(II) and Palladium(II) Complexes with Long Chain Amino Acids and Amino Acid Amides. Z. Anorg. Allg. Chem. 2017, 643, 632–635. [Google Scholar] [CrossRef]
- Cai, J.; Hu, X.; Feng, X.; Ji, L.; Bernal, I. Structures of three cis-β1 and three cis-β2 isomers of [Co(trien)(aminoacidato)]2+ complexes. Acta Crystallogr. Sect. B Struct. Sci. 2001, B57, 45–53. [Google Scholar] [CrossRef]
- Ng, C.H.; Ong, H.K.A.; Ngai, K.S.; Tan, W.T.; Lim, L.P.; Teoh, S.G.; Chong, T.S. mer-Tris(β-alaninato)cobalt(III): Crystal structure, solution properties and its DNA cleavage in the presence of ascorbic acid. Polyhedron 2005, 24, 1503–1509. [Google Scholar] [CrossRef]
- Garg, B.S.; Dwivedi, P. Solution studies on ternary complex formation of bivalent metal ions with cephalexine and amino acids, β-alanine and cysteine. J. Indian Chem. Soc. 2004, 81, 239–241. [Google Scholar]
- Gaizer, F.; Gondos, G.; Gera, L. Protonation and complex formation of the cis and trans isomers of alicyclic β-amino acids. Polyhedron 1986, 5, 1149–1156. [Google Scholar] [CrossRef]
- Juranic, N.; Prelesnik, B.; Manojlovic-Muir, L.; Andjelkovic, K.; Niketic, S.R.; Celap, M.B. Geometrical isomerism of mixed tris(amino carboxylato)cobalt(III) complexes containing glycinato and β-alaninato ligands. Crystal structure of trans(O5)-(β-alaninato)bis(glycinato)cobalt(III) trihydrate. Inorg. Chem. 2005, 29, 1491–1495. [Google Scholar] [CrossRef]
- Gridchin, S.N.; Shekhanov, R.F.; Bychkova, S.A. Stability constants of cobalt (II) complexes of taurine and β-alanine. Izv. Vyss. Uchebnykh Zaved. Khimiya I Khimicheskaya Tekhnol. 2016, 59, 95–96. [Google Scholar] [CrossRef]
- Sharma, V.S.; Mathur, H.B. Thermodynamic properties of coordination complexes of transition metal ions with amino acids. Indian J. Chem. 1965, 3, 475–479. [Google Scholar]
- Skoulika, S.; Michaelides, A.; Aubry, A. Habit modification of coordination compounds in the presence of additives; a study of the system Ni(β−ala)2·2H2O-glycine. J. Cryst. Growth 1991, 108, 285–289. [Google Scholar] [CrossRef]
- Böhm, A.; Seebach, D. Determination of Enantiomer Purity of β- and γ-Amino Acids by NMR Analysis of Diastereoisomeric Palladium Complexes. Helv. Chim. Acta 2000, 83, 3262–3278. [Google Scholar] [CrossRef]
- Cui, X.; Qin, T.; Wang, J.R.; Liu, L.; Guo, Q.X. Pd(N, N -Dimethyl β-alaninate) 2 as a High-Turnover-Number, Phosphine-Free Catalyst for the Suzuki Reaction. Synth. (Stuttg.) 2007, 2007, 393–399. [Google Scholar] [CrossRef]
- Krylova, L.F.; Kovtunova, L.M.; Romanenko, G.V. Pt(II) and Pd(II) Complexes with β-Alanine. Bioinorg. Chem. Appl. 2008, 2008, 1–10. [Google Scholar] [CrossRef]
- Ferraris, G.; Franchini-Angela, M. Survey of the geometry and environment of water molecules in crystalline hydrates studied by neutron diffraction. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1972, 28, 3572–3583. [Google Scholar] [CrossRef]
- Braga, D.; Grepioni, F.; Desiraju, G.R. Hydrogen bonding in organometallic crystals—A survey. J. Organomet. Chem. 1997, 548, 33–43. [Google Scholar] [CrossRef]
- Fucke, K.; Steed, J.W. X-ray and neutron diffraction in the study of organic crystalline hydrates. Water 2010, 2, 333–350. [Google Scholar] [CrossRef]
- Infantes, L.; Motherwell, S. Water clusters in organic molecular crystals. CrystEngComm 2002, 4, 454–461. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Braga, D.; Grepioni, F. Intermolecular interactions in nonorganic crystal engineering. Acc. Chem. Res. 2000, 33, 601–608. [Google Scholar] [CrossRef]
- Desiraju, G.R. Hydrogen Bridges in Crystal Engineering: Interactions without Borders. Acc. Chem. Res. 2002, 35, 565–573. [Google Scholar] [CrossRef]
- Brammer, L.; Mareque Rivas, J.C.; Reinaldo Atencio, J.; Fang, S.; Christopher Pigge, F. Combining hydrogen bonds with coordination chemistry or organometallic π-arene chemistry: Strategies for inorganic crystal engineering. J. Chem. Soc. Dalt. Trans. 2000, 3855–3867. [Google Scholar] [CrossRef]
- Pal, S.; Sankaran, N.B.; Samanta, A. Structure of a self-assembled chain of water molecules in a crystal host. Angew. Chem. Int. Ed. 2003, 42, 1741–1743. [Google Scholar] [CrossRef]
- Infantes, L.; Chisholma, J.; Motherwell, S. Extended motifs from water and chemical functional groups in organic molecular crystals. CrystEngComm 2003, 5, 480–486. [Google Scholar] [CrossRef]
- Mascal, M.; Infantes, L.; Chisholm, J. Water oligomers in crystal hydrates—What’s news and what isn’t? Angew. Chem. Int. Ed. 2005, 45, 32–36. [Google Scholar] [CrossRef]
- Galek, P.T.; Fábián, L.; Motherwell, W.D.; Allen, F.H.; Feeder, N. Knowledge-based model of hydrogen-bonding propensity in organic crystals. Acta Crystallogr. Sect. B Struct. Sci. 2007, 63, 768–782. [Google Scholar] [CrossRef] [PubMed]
- Galek, P.T.; Fábián, L.; Allen, F.H. Truly prospective prediction: Inter- and intramolecular hydrogen bonding. CrystEngComm 2010, 12, 2091–2099. [Google Scholar] [CrossRef]
- Cruz Cabeza, A.J.; Day, G.M.; Motherwell, W.D.; Jones, W. Importance of molecular shape for the overall stability of hydrogen bond motifs in the crystal structures of various carbamazepine-type drug molecules. Cryst. Growth Des. 2007, 7, 100–107. [Google Scholar] [CrossRef]
- Desiraju, G.R. Crystal engineering: From molecule to crystal. J. Am. Chem. Soc. 2013, 135, 9952–9967. [Google Scholar] [CrossRef]
- Hobart, D.B.; Berg, M.A.G.; Merola, J.S. Bis-glycinato complexes of palladium(II): Synthesis, structural determination, and hydrogen bonding interactions. Inorg. Chim. Acta 2014, 423, 21–30. [Google Scholar] [CrossRef]
- Hobart, D.B.; Merola, J.S.; Rogers, H.M.; Saghal, S.; Mitchell, J.; Florio, J.; Merola, J.W. Synthesis, Structure, and Catalytic Reactivity of Pd(II) Complexes of Proline and Proline Homologs. Catalysts 2019, 9, 515. [Google Scholar] [CrossRef]
- Dexter, C.S.; Jackson, R.F.W. Efficient synthesis of β- and γ-amino acid derivatives using new functionalized zinc reagents: Enhanced stability and reactivity of β-amido zinc reagents in dimethylformamide. Chem. Commun. 1998, 75–76. [Google Scholar] [CrossRef]
- Abdel-Magid, A.F.; Cohen, J.H.; Maryanoff, C.A. Chemical process synthesis of β-amino acids and esters. Curr. Med. Chem. 1999, 6, 955–970. [Google Scholar]
- Hoen, R.; Tiemersma-Wegman, T.; Procuranti, B.; Lefort, L.; de Vries, J.G.; Minnaard, A.J.; Feringa, B.L. Enantioselective synthesis of β2-amino acids using rhodium-catalyzed hydrogenation. Org. Biomol. Chem. 2007, 5, 267–275. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hobart, D.B.; Patel, V.G.; Pendergrass, H.; Florio, J.; Merola, J.S. Self-Assembly Motifs of Water in Crystals of Palladium β-Amino Acid Complexes Influenced by Methyl Substitution on the Amino Acid Backbone. Crystals 2019, 9, 590. https://doi.org/10.3390/cryst9110590
Hobart DB, Patel VG, Pendergrass H, Florio J, Merola JS. Self-Assembly Motifs of Water in Crystals of Palladium β-Amino Acid Complexes Influenced by Methyl Substitution on the Amino Acid Backbone. Crystals. 2019; 9(11):590. https://doi.org/10.3390/cryst9110590
Chicago/Turabian StyleHobart, David B., Vraj G. Patel, Heather Pendergrass, Jacqueline Florio, and Joseph S. Merola. 2019. "Self-Assembly Motifs of Water in Crystals of Palladium β-Amino Acid Complexes Influenced by Methyl Substitution on the Amino Acid Backbone" Crystals 9, no. 11: 590. https://doi.org/10.3390/cryst9110590
APA StyleHobart, D. B., Patel, V. G., Pendergrass, H., Florio, J., & Merola, J. S. (2019). Self-Assembly Motifs of Water in Crystals of Palladium β-Amino Acid Complexes Influenced by Methyl Substitution on the Amino Acid Backbone. Crystals, 9(11), 590. https://doi.org/10.3390/cryst9110590






