Synthesis of Manganese Mononitride with Tetragonal Structure under Pressure
Abstract
:1. Introduction
2. Materials and Methods
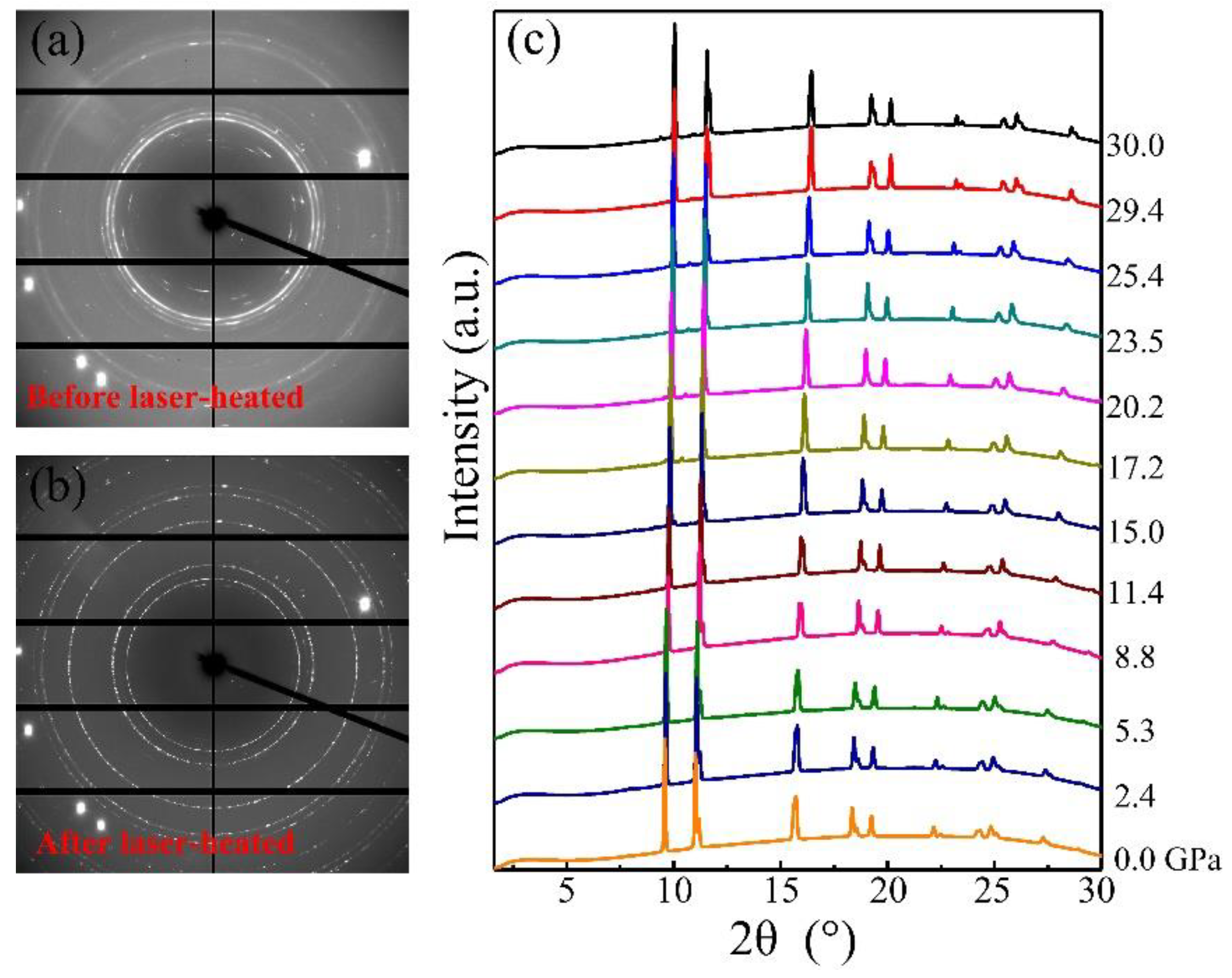
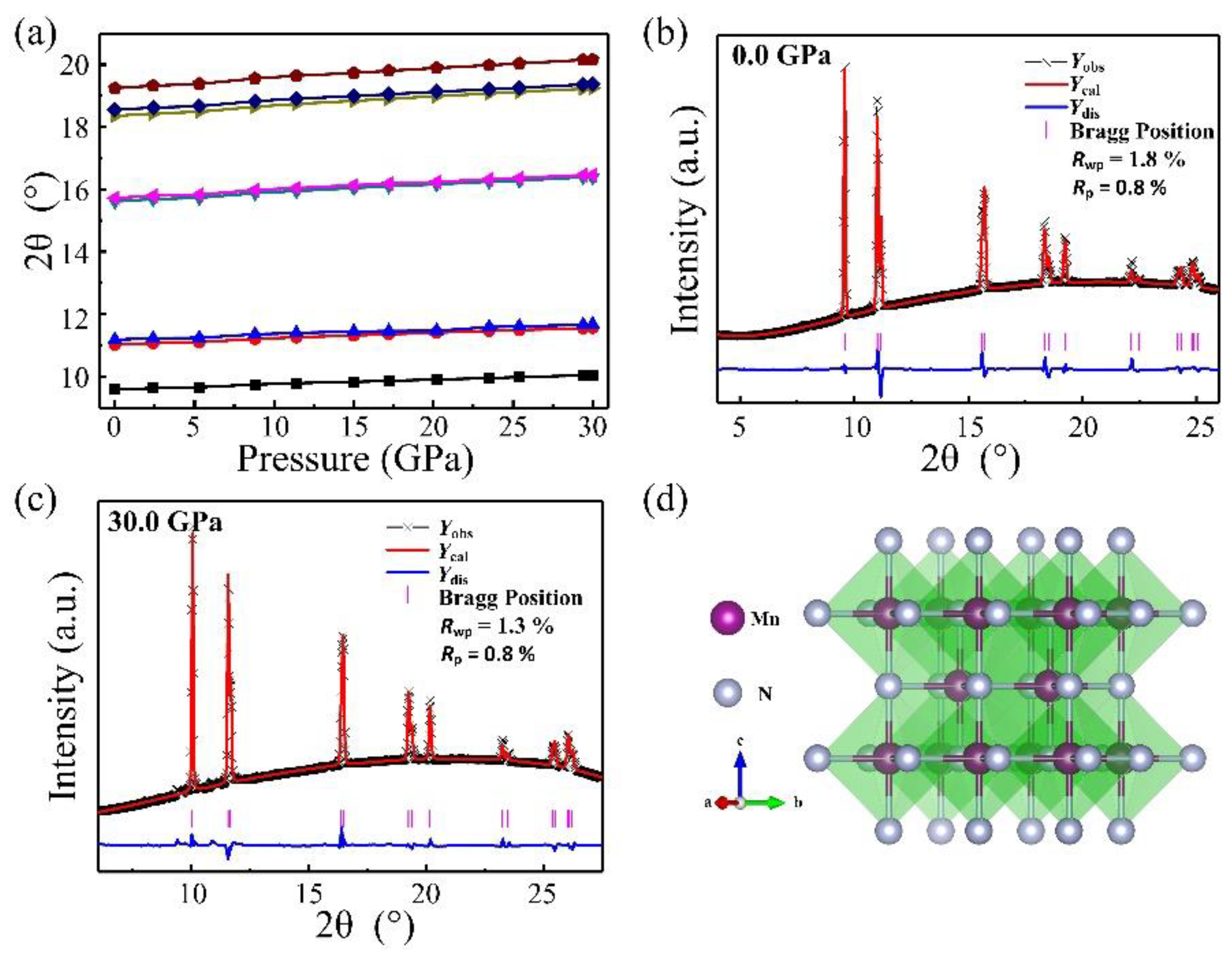
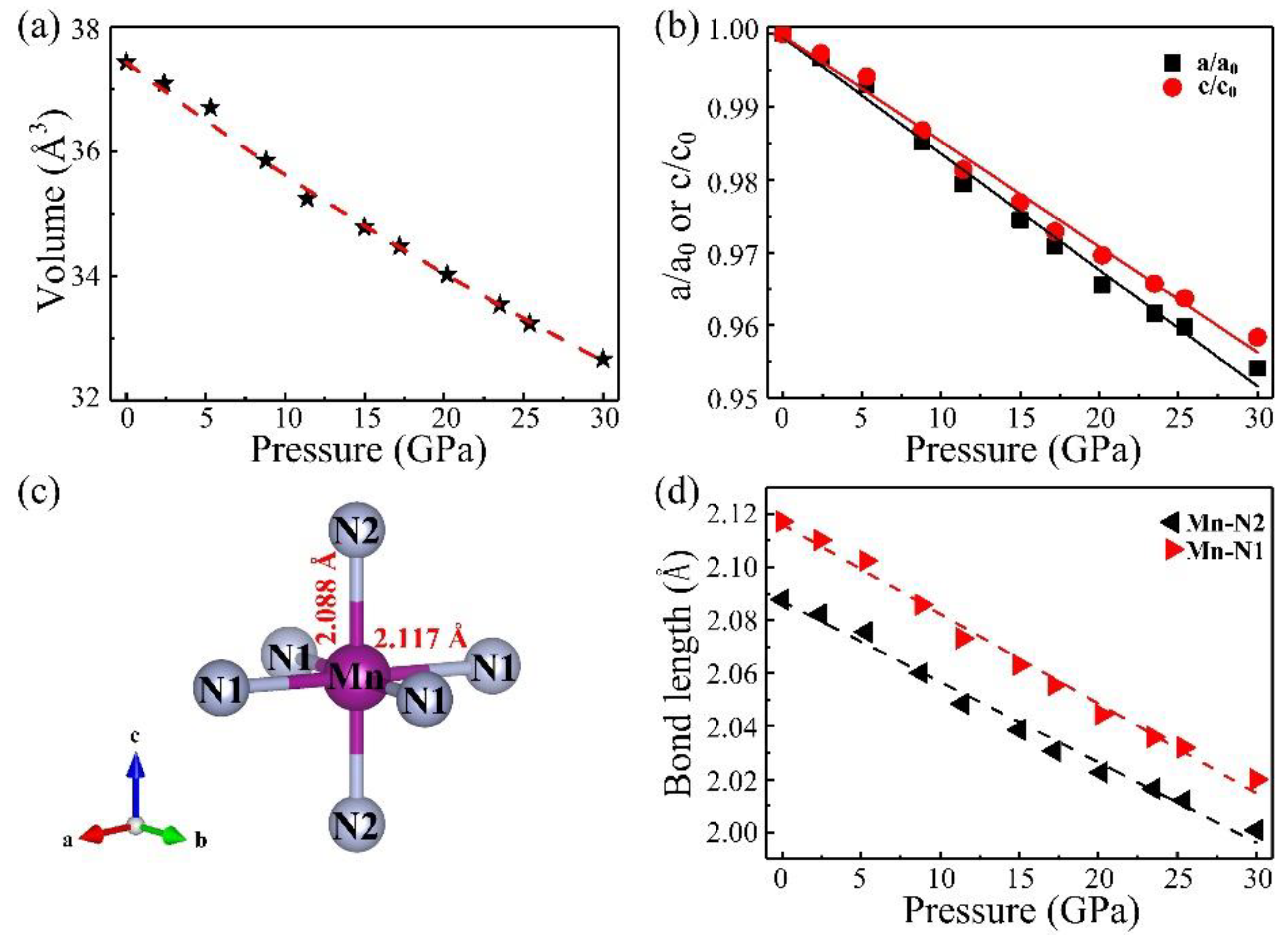
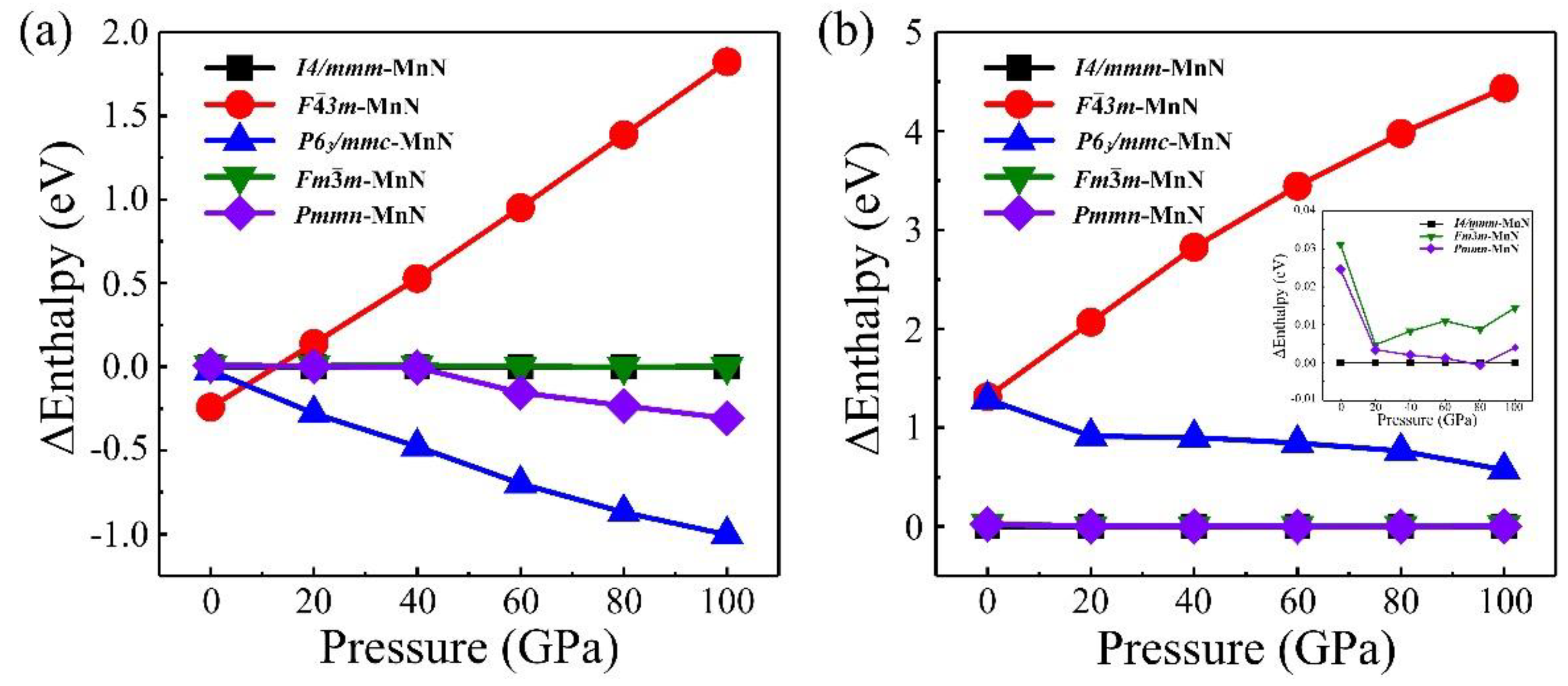
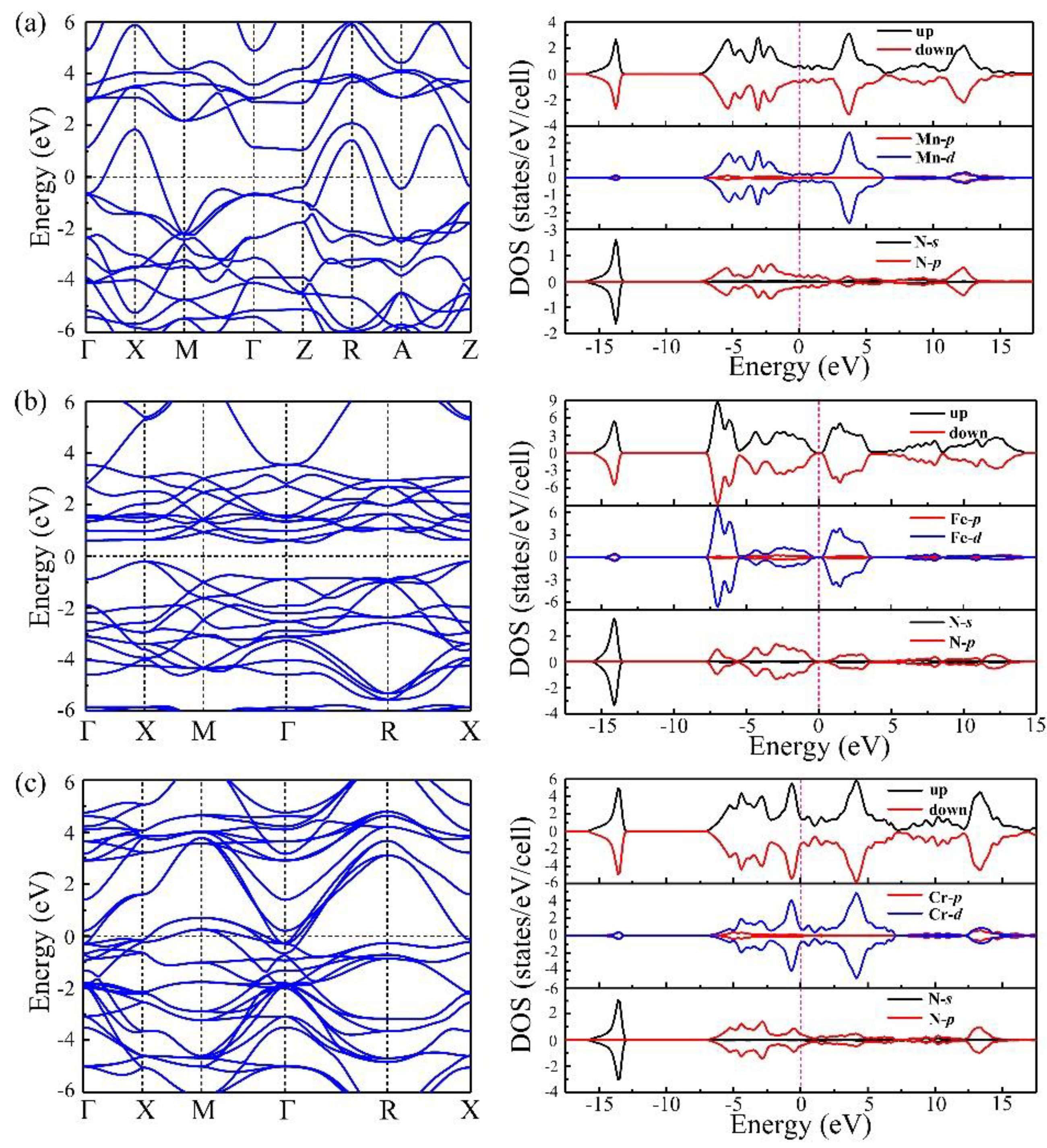
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaner, R.B.; Gilman, J.J.; Tolbert, S.H. Designing Superhard Materials. Science 2005, 308, 1268–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coey, J.M.D.; Smith, P.A.I. Magnetic Nitrides. J. Magn. Magn. Mater. 1999, 200, 405–424. [Google Scholar] [CrossRef]
- Oyama, S.T. Chemistry of Transition Metal Carbides and Nitrides; Springer: Berlin, Germany, 1996. [Google Scholar]
- Herle, P.S.; Hegde, M.S.; Vasathacharya, N.Y.; Philip, S.; Rao, M.V.R.; Sripathi, T. Synthesis of TiN, VN, and CrN from Ammonolysis of TiS2, VS2, and Cr2S3. J. Solid State Chem. 1997, 134, 120–127. [Google Scholar] [CrossRef]
- Kurtz, S.R.; Gordon, R.G. Chemical Vapor Deposition of Titanium Nitride at Low Temperatures. Thin Solid Films 1986, 140, 277–290. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Chawla, J.S.; Deng, R.P.; Gall, D. Epitaxial Suppression of the Metal-insulator Transition in CrN. Phys. Rev. B 2011, 84, 073101. [Google Scholar] [CrossRef]
- Bykov, M.; Bykova, E.; Aprilis, G.; Glazyrin, K.; Koemets, E.; Chuvashova, I.; Kupenko, I.; McCammon, C.; Mezouar, M.; Prakapenka, V.; et al. Fe-N System at High Pressure Reveals a Compound Featuring Polymeric Nitrogen Chains. Nat. Commun. 2018, 9, 2756. [Google Scholar] [CrossRef]
- Binns, J.; Donnelly, M.E.; Peña-Alvarez, M.; Wang, M.; Gregoryanz, E.; Hermann, A.; Dalladay-Simpson, P.; Howie, R.T. Direct Reaction between Copper and Nitrogen at High Pressures and Temperatures. J. Phys. Chem. Lett. 2019, 10, 1109–1114. [Google Scholar] [CrossRef] [Green Version]
- Salamat, A.; Hector, A.L.; Gray, B.M.; Kimber, S.A.J.; Bouvier, P.; McMillan, P.F. Synthesis of Tetragonal and Orthorhombic Polymorphs of Hf3N4 by High-Pressure Annealing of a Prestructured Nanocrystalline Precursor. J. Am. Chem. Soc. 2013, 135, 9503–9511. [Google Scholar] [CrossRef]
- Salamat, A.; Woodhead, K.; Shah, S.I.U.; Hector, A.L.; McMillan, P.F. Synthesis of U3Se5 and U3Te5 Type Polymorphs of Ta3N5 by Combining High Pressure–Temperature Pathways with a Chemical Precursor Approach. Chem. Commun. 2014, 50, 10041–10044. [Google Scholar] [CrossRef]
- Utsumi, W.; Saitoh, H.; Kaneko, H.; Watanuki, T.; Aoki, K.; Shimomura, O. Congruent Melting of Gallium Nitride at 6 GPa and its Application to Single-Crystal Growth. Nat. Mater. 2003, 2, 735–738. [Google Scholar] [CrossRef]
- Morio, K.; Koizumi, Y. Electronic Density of State of Mn-N Thin Films Measured by XPS. J. Surf. Sci. Soc. Jpn. 2003, 24, 480–484. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Al-Brithen, H.; Trifan, E.; Ingram, D.C.; Smith, A.R. Crystalline Phase and Orientation Control of Manganese Nitride Grown on MgO (001) by Molecular Beam Epitaxy. J. Appl. Phys. 2002, 91, 1053–1059. [Google Scholar] [CrossRef]
- Suzuki, K.; Kaneko, T.; Yoshida, H.; Obi, Y.; Fujimori, H.; Morita, H. Crystal Structure and Magnetic Properties of the Compound MnN. J. Alloys Compd. 2000, 306, 66–71. [Google Scholar] [CrossRef]
- Suzuki, K.; Suzuki, T.; Fujinaga, Y.; Kaneko, T.; Yoshida, H.; Obi, Y.; Tomiyoshi, S. A Nomalous Thermal Expansion of MnN. J. Alloys Compd. 2003, 360, 34–40. [Google Scholar] [CrossRef]
- Suzuki, K.; Yamaguchi, Y.; Kaneko, T.; Yoshida, H.; Obi, Y.; Fujimori, H.; Morita, H. Neutron Diffraction Studies of the Compounds MnN and FeN. J. Phys. Soc. Jpn. 2001, 70, 1084–1089. [Google Scholar] [CrossRef]
- Meng, Y.; Hrubiak, R.; Rod, E.; Boehler, R.; Shen, G.Y. New Developments in Laser-Heated Diamond Anvil Cell with In-Situ Synchrotron X-ray Diffraction at High Pressure Collaborative Access Team. Rev. Sci. Instrum. 2015, 86, 072201. [Google Scholar] [CrossRef] [PubMed]
- Prescher, C.; Prakapenka, V.B. DIOPTAS: A Program for Reduction of Two-Dimensional X-ray Diffraction Data and Data Exploration. High Pressure Res. 2015, 35, 223–230. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System (GSAS); 2004. Available online: https://11bm.xray.aps.anl.gov/documents/GSASManual.pdf (accessed on 30 September 2019).
- Toby, B.H. EXPGUI, a Graphical User Interface for GSAS. J. Appl. Cryst. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab Initio Molecular Dynamics for Open-Shell Transition Metals. Phys. Rev. B 1993, 48, 13115–13118. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blöchl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Baroni, S.; de Gironcoli, S.; Corso, A.D.; Giannozzi, P. Phonons and Related Crystal Properties from Density-functional Perturbation Theory. Rev. Mod. Phys. 2001, 73, 515–562. [Google Scholar] [CrossRef]
- Rivadulla, F.; Bañobre-López, M.; Quintela, C.X.; Piñeiro, A.; Pardo, V.; Baldomir, D.; López-Quintela, M.A.; Rivas, J.; Ramos, C.A.; Salva, H.; et al. Reduction of the Bulk Modulus at High Pressure in CrN. Nat. Mater. 2009, 8, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Soni, H.R.; Mankad, V.; Gupta, S.K.; Jha, P.K. A First Principles Calculations of Structural, Electronic, Magnetic and Dynamical Properties of Mononitrides FeN and CoN. J. Alloys Compd. 2012, 522, 106–113. [Google Scholar] [CrossRef]
| Compound | Space Group | Structure | Lattice Parameters (Å) | B0 (GPa) | Ref. | |
|---|---|---|---|---|---|---|
| CrN | Fm3m | RS | DFT: 340-430 | [27] | ||
| CrN | Pnma | Orthorhombic | 243(10) | [27] | ||
| MnN | I4/mmm | RS | a = 2.994(1) c = 4.175(1) | 173 DFT: 187 | 3.06 | Present |
| FeN | Fmm | RS | a = 4.1041 | 186 | 4.77 | [28] |
| FeN | F3m | ZB | a = 4.2250 | 266 | 6.65 | [28] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, D.; Niu, C.; Yan, B.; Gao, B.; Wu, L.; Zhang, D.; Wang, X.; Gou, H. Synthesis of Manganese Mononitride with Tetragonal Structure under Pressure. Crystals 2019, 9, 511. https://doi.org/10.3390/cryst9100511
Huang D, Niu C, Yan B, Gao B, Wu L, Zhang D, Wang X, Gou H. Synthesis of Manganese Mononitride with Tetragonal Structure under Pressure. Crystals. 2019; 9(10):511. https://doi.org/10.3390/cryst9100511
Chicago/Turabian StyleHuang, Dajian, Caoping Niu, Bingmin Yan, Bo Gao, Lailei Wu, Dongzhou Zhang, Xianlong Wang, and Huiyang Gou. 2019. "Synthesis of Manganese Mononitride with Tetragonal Structure under Pressure" Crystals 9, no. 10: 511. https://doi.org/10.3390/cryst9100511
APA StyleHuang, D., Niu, C., Yan, B., Gao, B., Wu, L., Zhang, D., Wang, X., & Gou, H. (2019). Synthesis of Manganese Mononitride with Tetragonal Structure under Pressure. Crystals, 9(10), 511. https://doi.org/10.3390/cryst9100511





