Structure, Optical, and Thermal Properties of 9, 10-Diphenylanthracene Crystals
Abstract
:1. Introduction
2. Experimental Methods
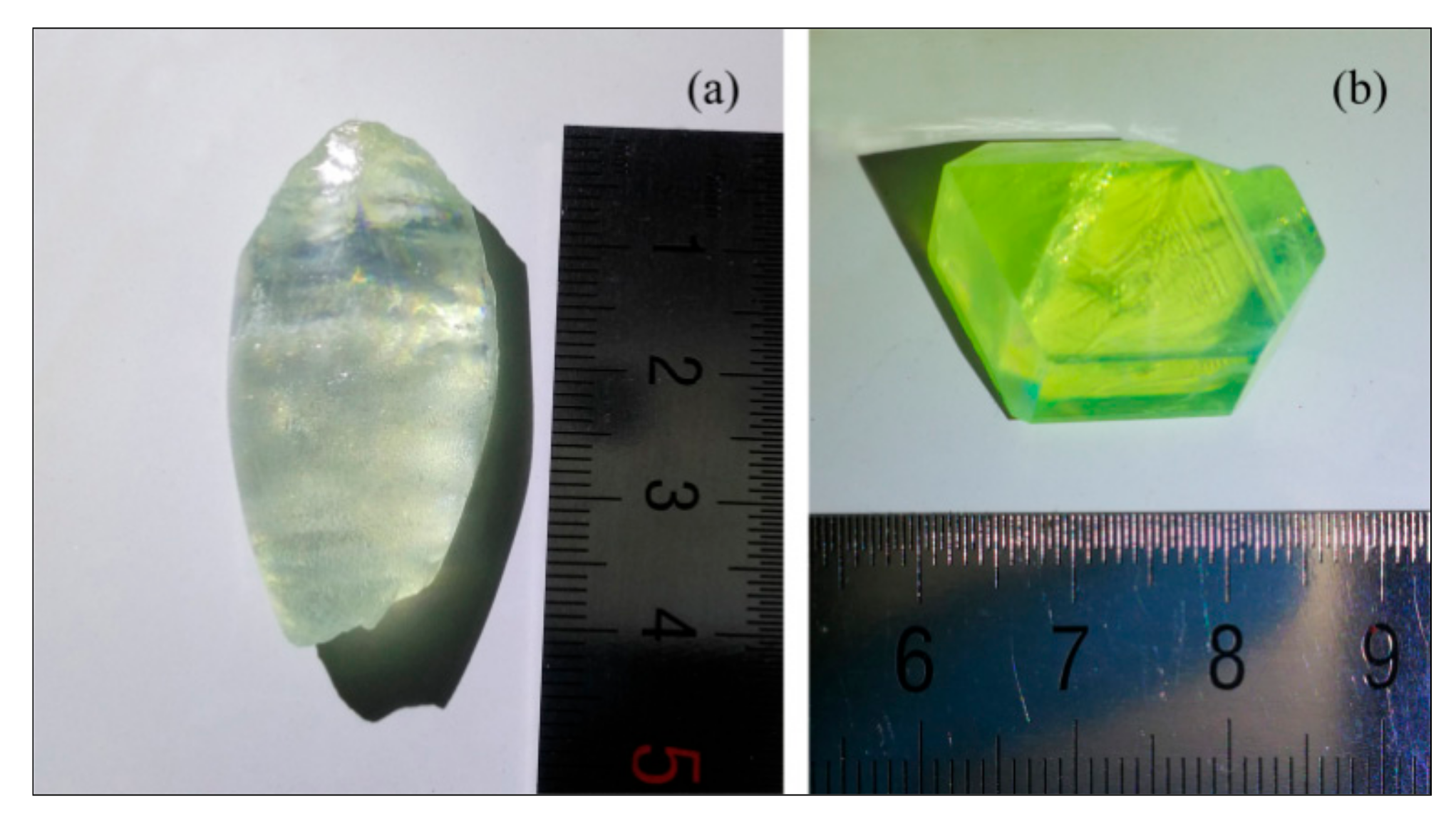
2.1. Growth of DPA Crystals
2.2. Characterisation of DPA Crystals
3. Results and Discussion
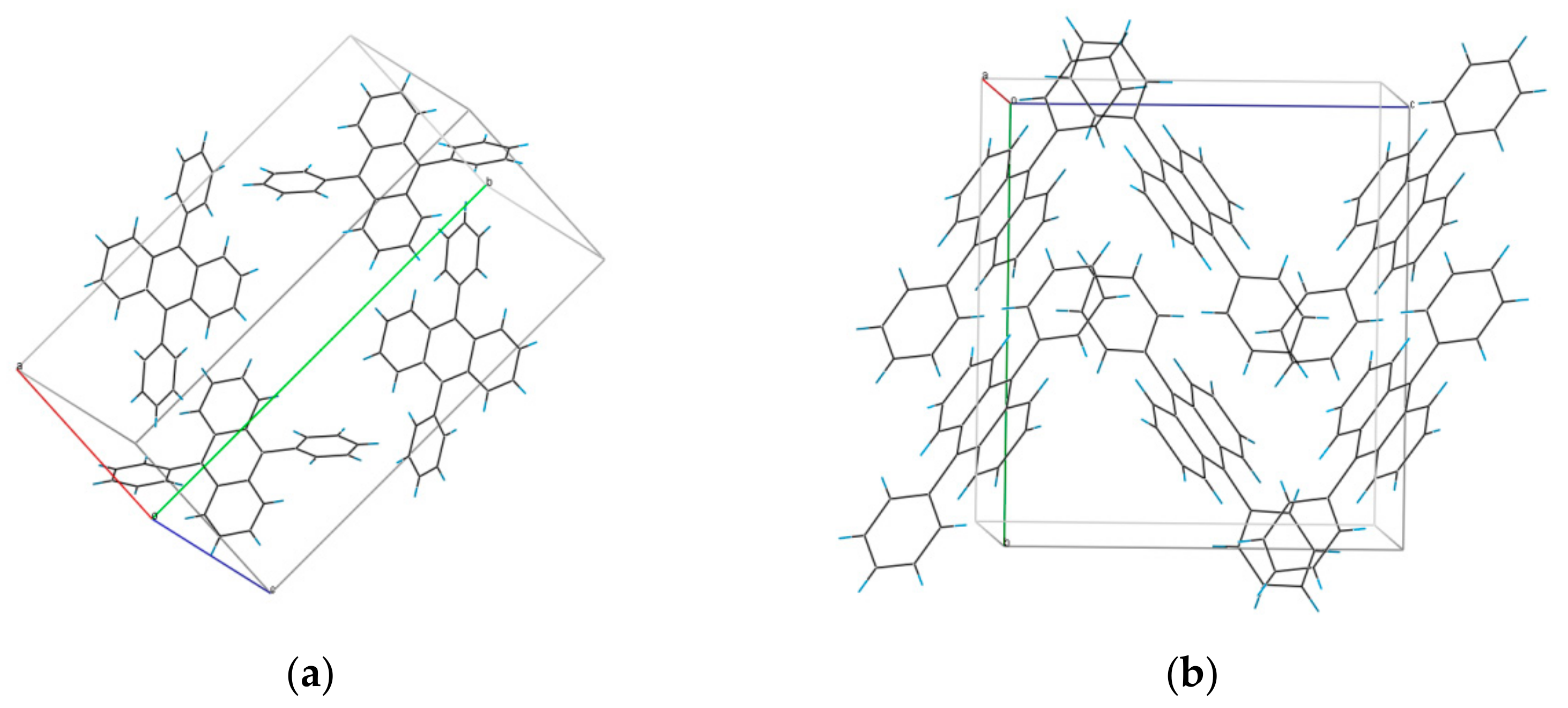
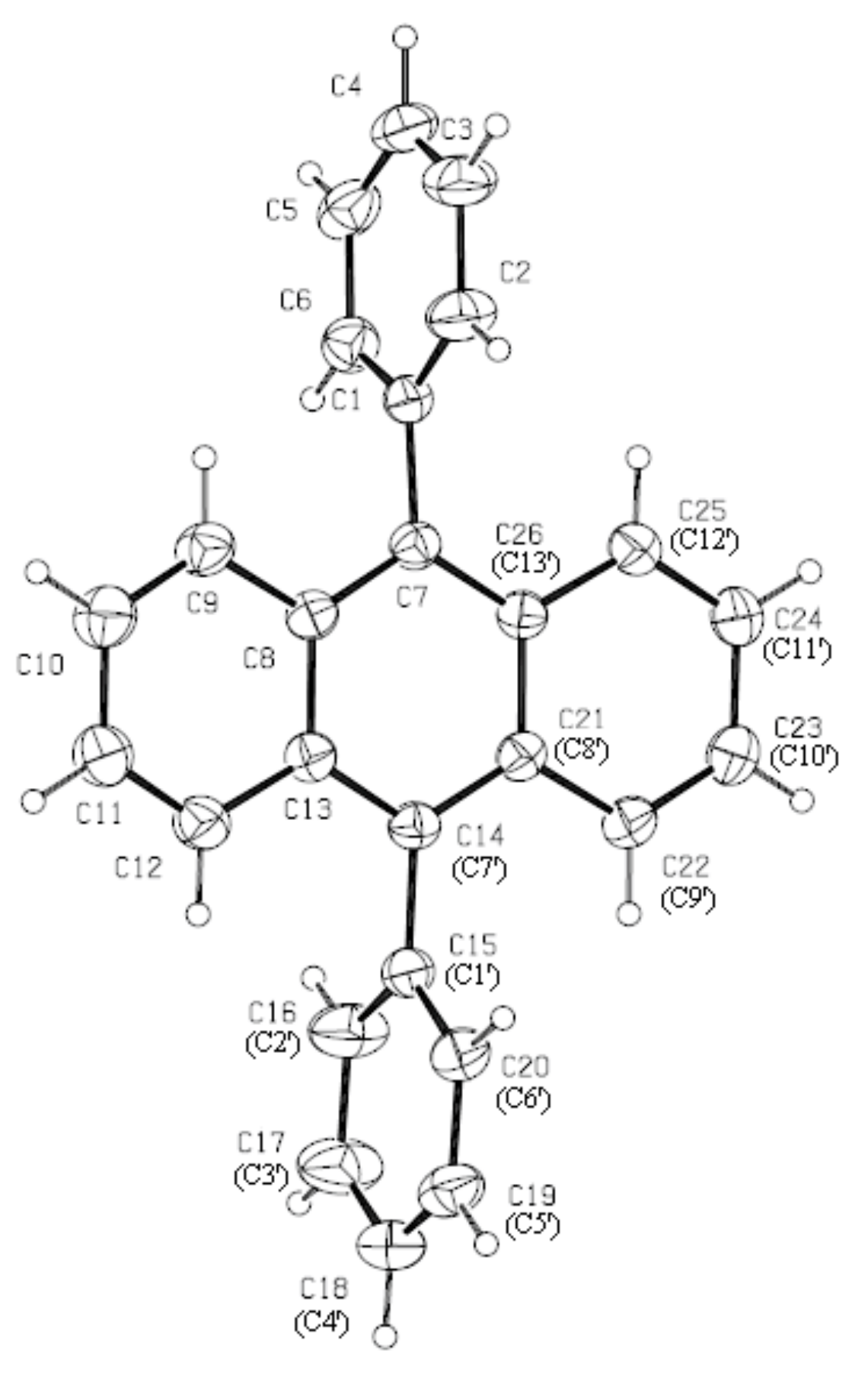
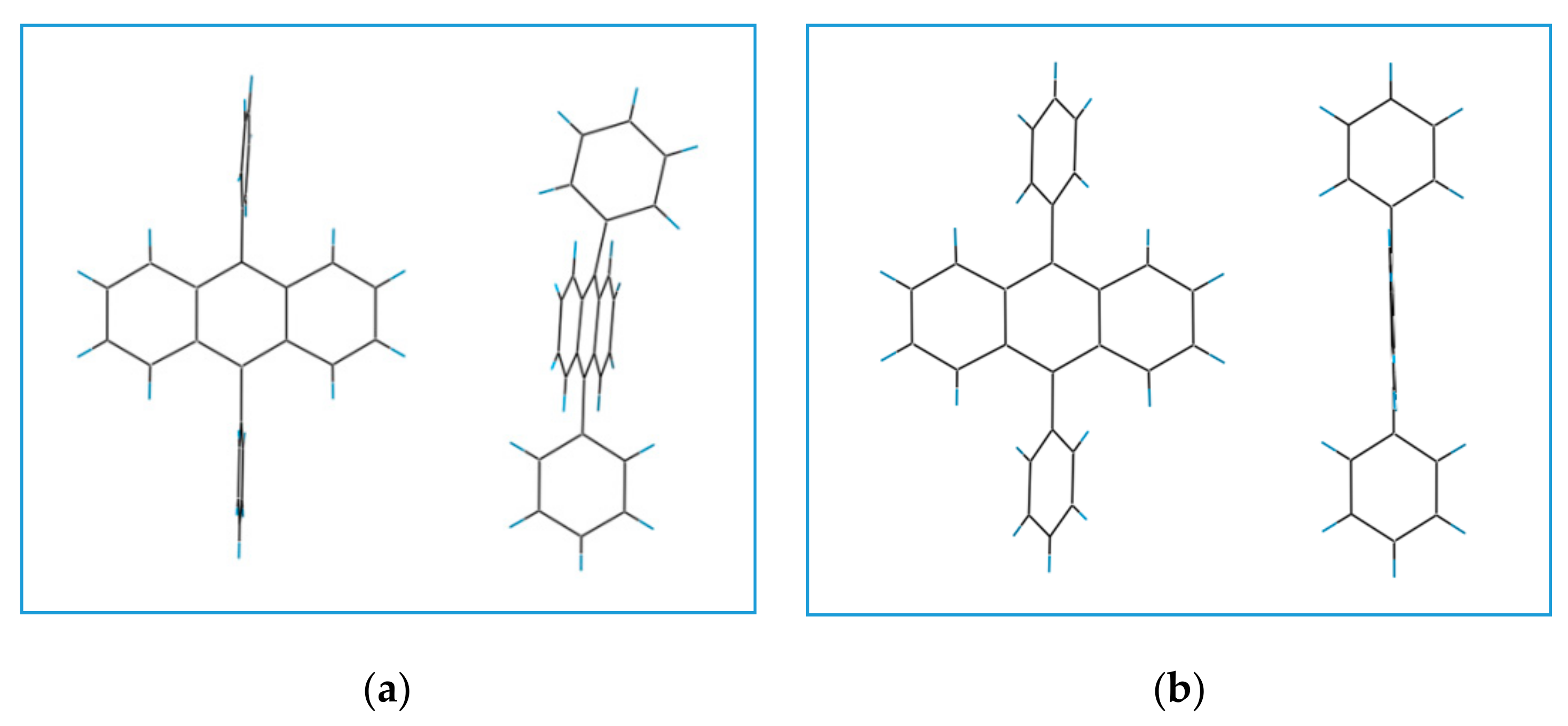
3.1. Structure of DPA Crystals
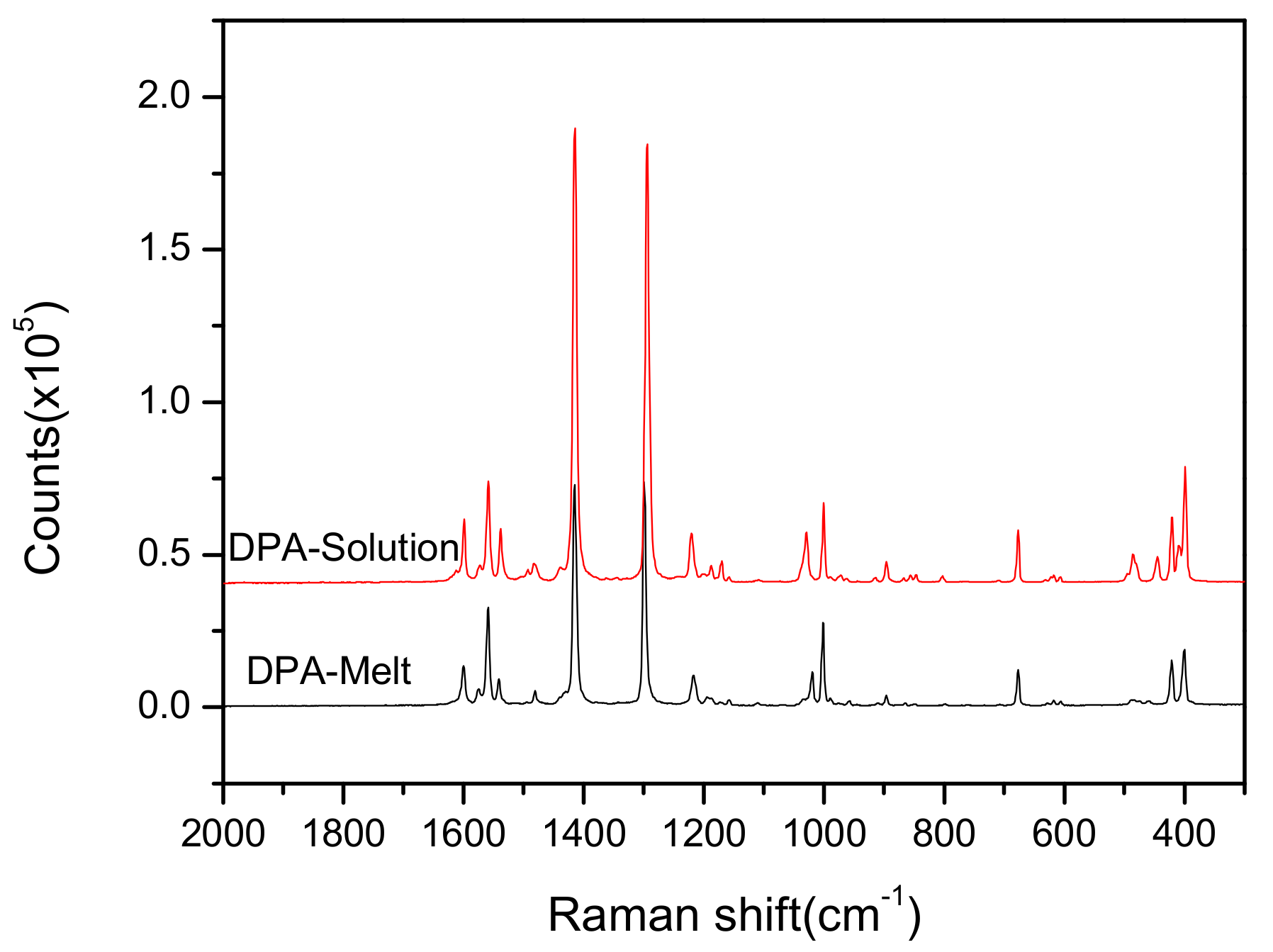
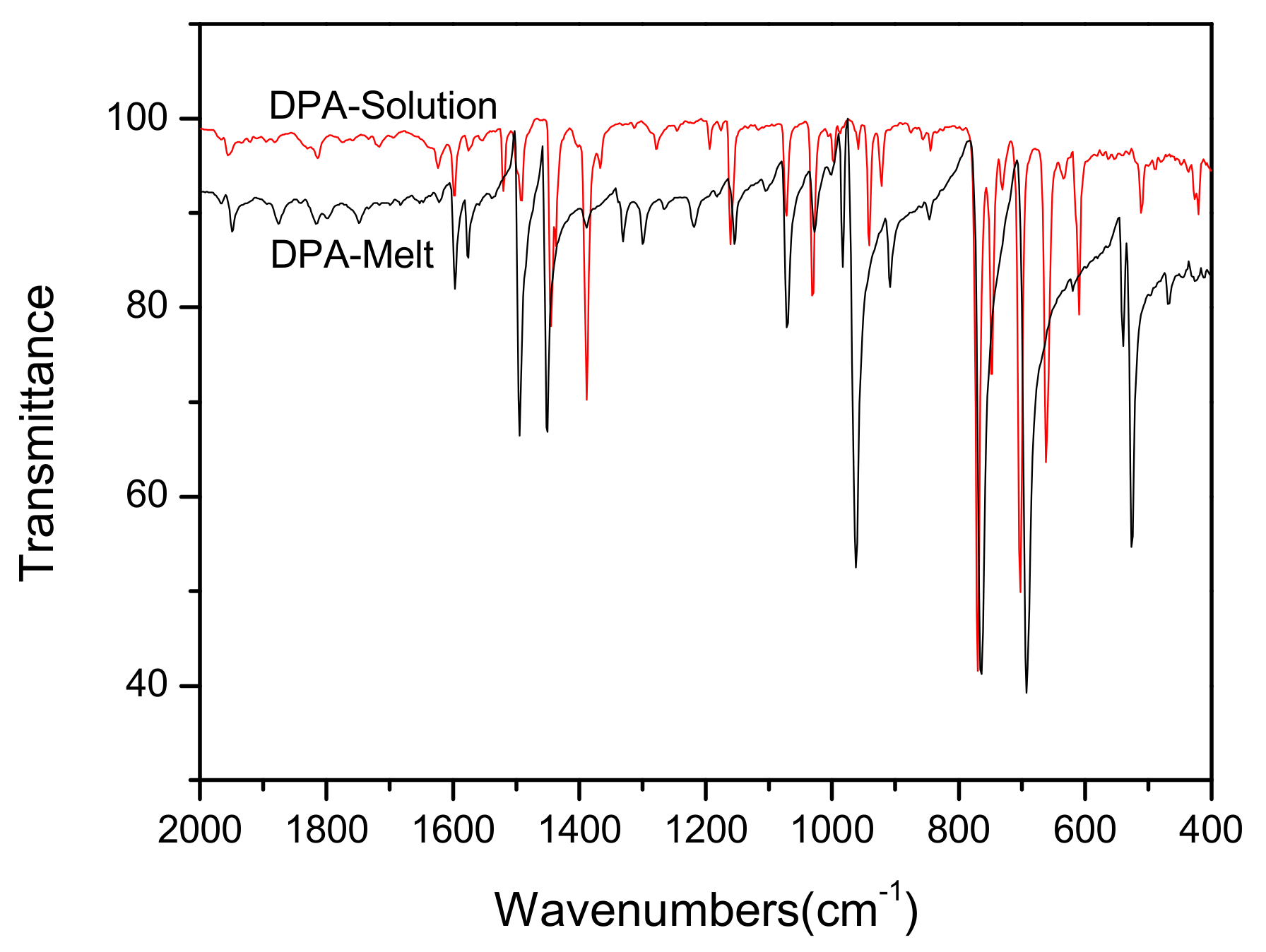
3.2. Analysis of Raman and IR Spectra
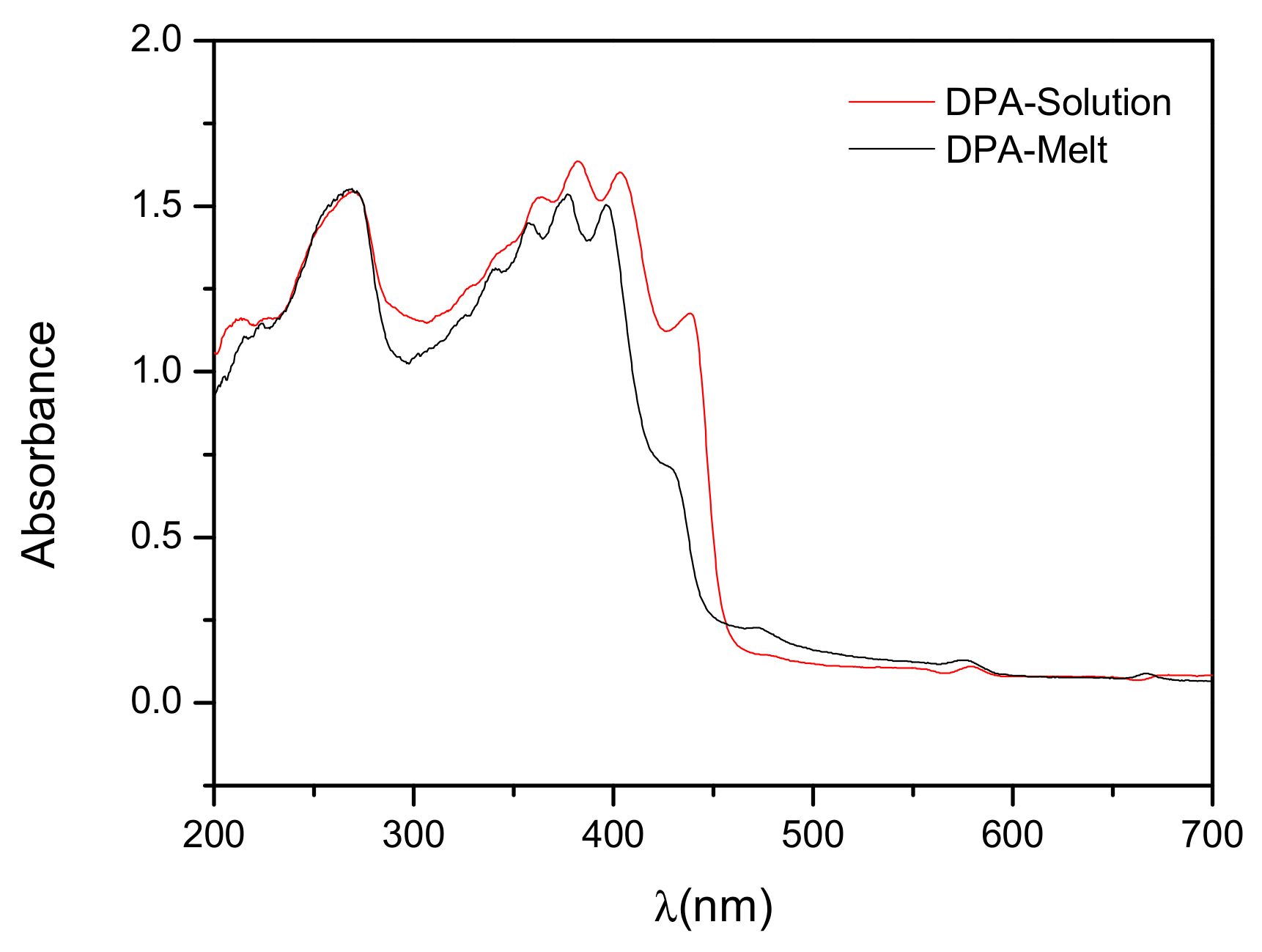
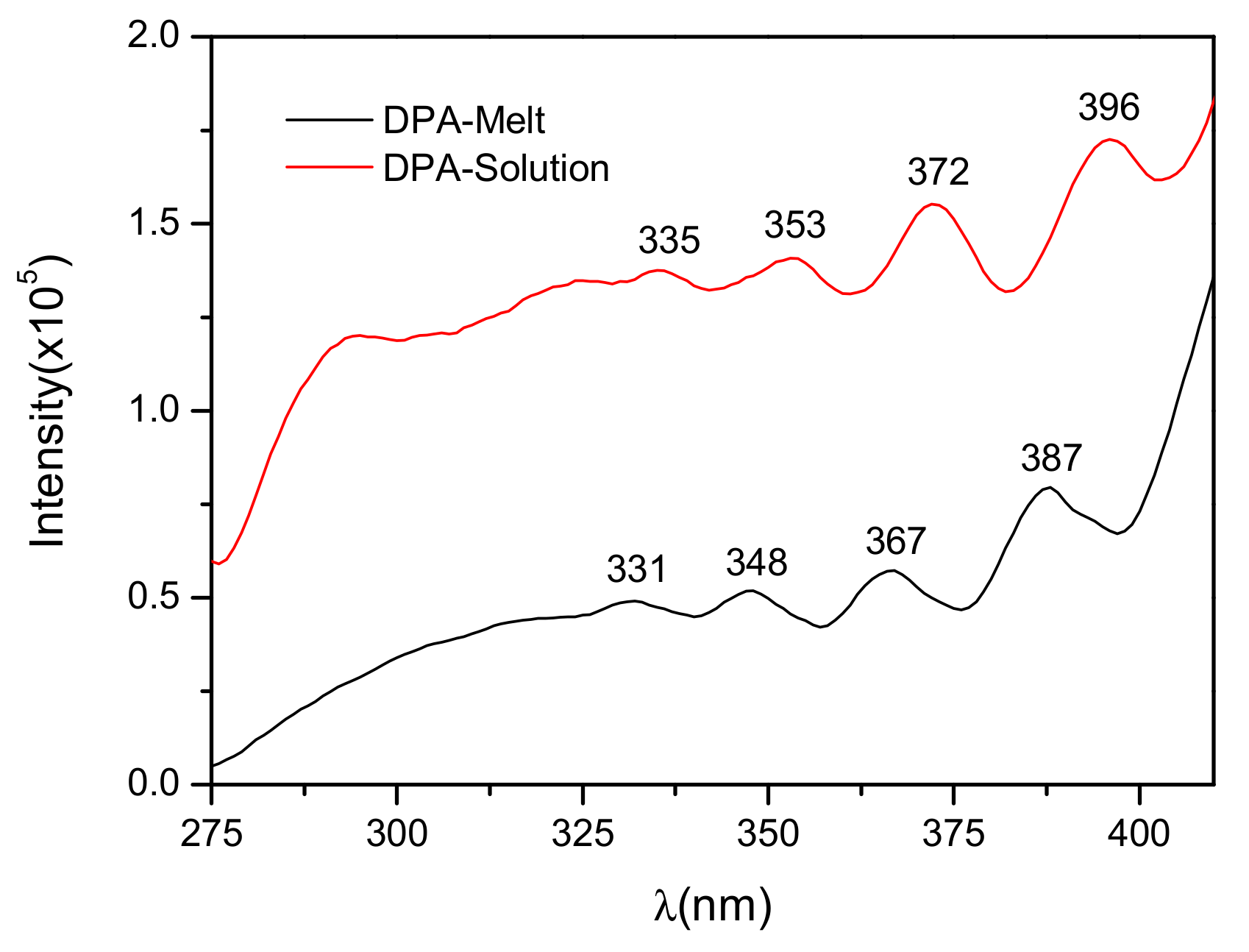
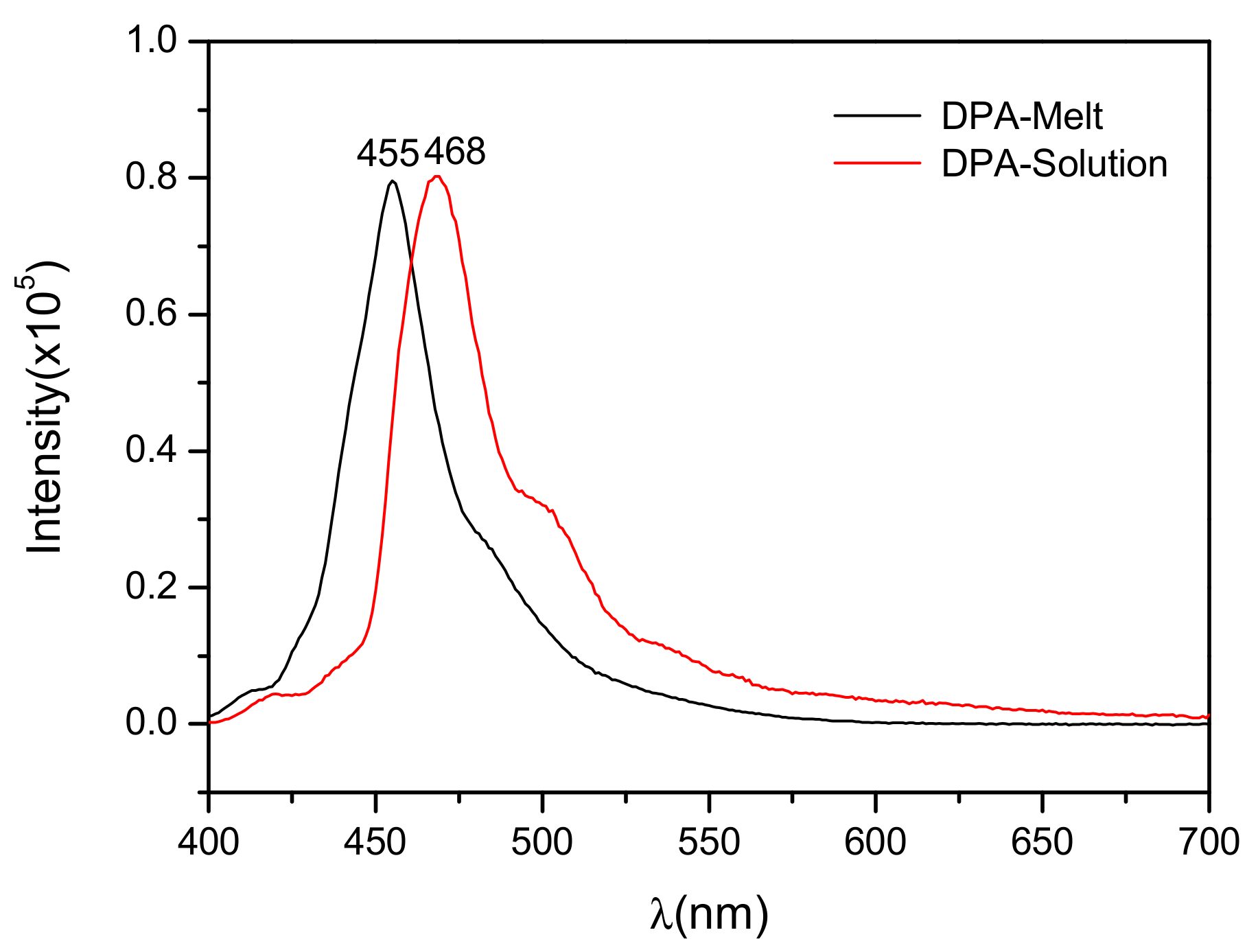
3.3. Optical Properties of DPA Crystals
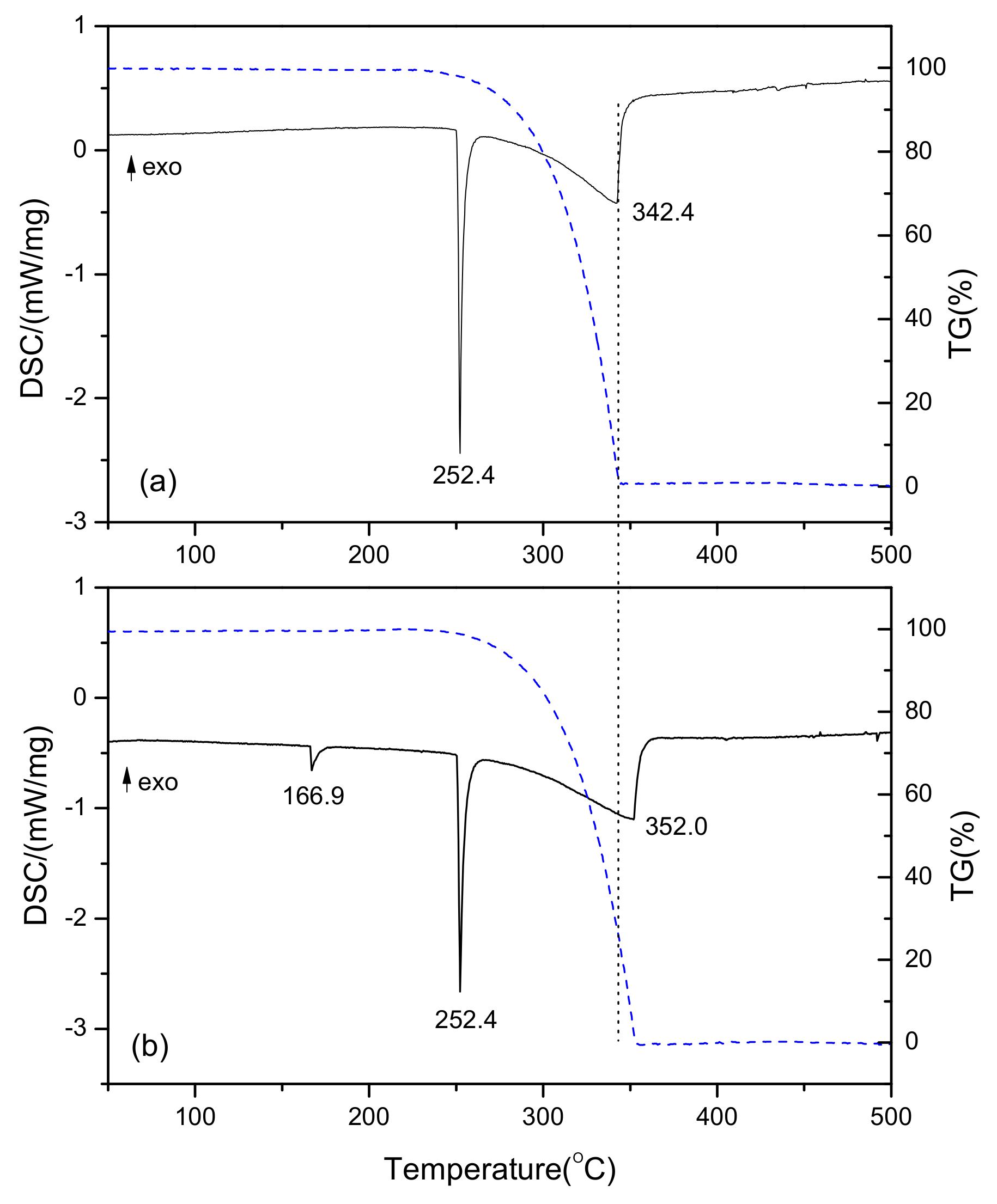
3.4. Analysis of Thermal Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cieślak, M.J.; Gamage, K.A.A.; Glover, R. Critical review of scintillating crystals for neutron detection. Crystals 2019, 9, 480–497. [Google Scholar]
- Vijayana, N.; Bhagavannarayana, G.; Maurya, K.K.; Haranath, D.; Rathi, B.; Balamurugan, N.; Sharma, Y.K.; Ramasamy, P. Effect of annealing on Bridgman grown organic scintillation material of trans-stilbene. Mater. Chem. Phys. 2012, 132, 453–457. [Google Scholar] [CrossRef]
- Carman, L.; Zaitseva, N.; Martinez, H.P.; Rupert, B.; Pawelczak, I.; Glenn, A.; Mulcahy, H.; Leif, R.; Lewis, K.; Payne, S. The effect of material purity on the optical and scintillation properties of solution-grown trans-stilbene crystals. J. Cryst. Growth. 2013, 368, 56–61. [Google Scholar] [CrossRef]
- Arulchakkaravarthi, A.; Santhanaraghavan, P.; Ramasamy, P. Crystal growth of Trans-stilbene by vertical Bridgman technique with modified growth vessels and its characterisation. J. Cryst. Growth. 2001, 224, 89–94. [Google Scholar] [CrossRef]
- Yamato, S.; Yamaji, A.; Kurosawa, S.; Yoshino, M.; Ohashi, Y.; Kamada, K.; Yokota, Y.; Yoshikawa, A. Crystal growth and luminescence properties of organic crystal scintillators for α-rays detection. Opt. Mater. 2019, 94, 58–63. [Google Scholar] [CrossRef]
- Glebov, V.Y.; Forrest, C.; Knauer, J.P.; Pruyne, A.; Romanofsky, M.; Sangster, T.C.; Shoup, M.J., 3rd; Stoeckl, C.; Caggiano, J.A.; Carman, M.L.; et al. Testing a new NIF neutron time-of-flight detector with a bibenzyl scintillatoron OMEGA. Rev. Sci. Instrum. 2012, 83, 10D309. [Google Scholar] [CrossRef] [PubMed]
- Zaitseva, N.; Glenn, A.; Carman, L.; Hatarik, R.; Hamel, S.; Faust, M. Brandon Schabes, Nerine Cherepy, and Stephen Payne, Pulse shape discrimination in impure and mixed single-crystal organic scintillators. Ieee T. Nucl. Sci. 2011, 58, 3411–3420. [Google Scholar] [CrossRef]
- Loef, E.V.V.; Glodo, J.; Shirwadkar, U.; Zaitseva, N.; Shah, K.S. Solution growth and scintillation properties of novel organic neutron detectors. Nucl. Instrum. Methods Phys. Res. Sect. A 2001, 652, 424–426. [Google Scholar]
- Hull, G.; Zaitseva, N.P.; Cherepy, N.J.; Newby, J.R.; Stoeffl, W.; Payne, A.S. New organic crystals for pulse shape discrimination. Ieee T. Nucl. Sci. 2009, 56, 899–903. [Google Scholar] [CrossRef]
- Loef, E.V.V.; Mukhopadhyay, S.; Zaitseva, N.; Payne, S.; Shah, K.S. Crystal growth and characterization of 9,10-diphenylanthracene. J. Cryst. Growth. 2012, 352, 103–105. [Google Scholar] [CrossRef]
- Ren, J.; Huang, B.N.; Chen, L.; Liu, Y.J.; Ye, T.; Liu, W.; Jin, X.Y.; Xu, Z.K.; Chen, H.Z.; Li, H.Y. Constructing bulk-contact inside single crystals of organic semiconductors through gel incorporation. CrystEngComm 2016, 18, 800–806. [Google Scholar] [CrossRef]
- Salzillo, T.; Valle, R.G.D.; Venuti, E.; Brillante, A.; Siegrist, T.; Masino, M.; Mezzadri, F.; Girlando, A. Two New Polymorphs of the Organic Semiconductor 9,10-Diphenylanthracene Raman and X-ray Analysis. J. Phys. Chem. C 2016, 120, 1831–1840. [Google Scholar] [CrossRef]
- Dolomanov; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.M.; Ramdas, S. The Crystal Structure of Solution-Grown 9,10-Diphenylanthracene. A Combined Computational and X-ray Study. Acta Cryst. B 1979, 35, 679–683. [Google Scholar] [CrossRef]
- Valovicova, V.; Plevová, E.; Vaculiková, L.; Ritz, M.; Vallova, S. Thermal polycondensation of anthracene for carbon precursors. J. Therm. Anal. Calorim. 2016, 124, 261–267. [Google Scholar] [CrossRef]
- Yükselici, M.H.; Aşıkoğlu, A. A vibrational study of liquid benzene through dispersive Raman spectroscopy using advanced optical elements. Opt. Laser Eng. 2009, 47, 842–846. [Google Scholar] [CrossRef]
- Bratschkov, C. FTIR spectroscopy study of the UV irradiation induced changes in an anthracene containing copolymer. Eur. Polym. J. 2001, 37, 1145–1149. [Google Scholar]
- Shinohara, H.; Yamakita, Y.; Ohno, K. Raman spectra of polycyclic aromatic hydrocarbons. Comparison of calculated Raman intensity distributions with observed spectra for naphthalene, anthracene, pyrene, and perylene. J. Mol. Struct. 1998, 442, 221–234. [Google Scholar] [CrossRef]
- Adichtchev, S.V.; Surovtsev, N.V. Raman line shape analysis as a mean characterizing molecular glass-forming liquids. J. Non-Cryst. Solids 2011, 357, 3058–3063. [Google Scholar] [CrossRef]
- Abasbegović, N.; Vukotić, N.; Colombo, L. Raman Spectrum of Anthracene. J. Chem. Phys. 1964, 41, 2575–2577. [Google Scholar]
- Chung, S.J.; Kim, K.K.; Jin, J.I. Fluorescing wholly aromatic polyesters containing diphenylanthracene fluorophores. Polymer 1999, 40, 1943–1953. [Google Scholar] [CrossRef]
- Dong, Y.K.; Kim, Y.S.; Song, E.L.; Kim, Y.K.; Yoon, S.S. 9,10-Diphenylanthracene derivative substituted with indole moiety for blue organic light-emitting diodes. Mol. Cryst. Liq. Cryst. 2017, 644, 197–204. [Google Scholar]
- Zhuang, S.; Shangguan, R.; Huang, H.; Tu, G.; Wang, L.; Zhu, X. Synthesis, characterization, physical properties, and blue electroluminescent device applications of phenanthroimidazole derivatives containing anthracene or pyrene moiety. Dye. Pigment. 2014, 101, 93–102. [Google Scholar] [CrossRef]
- Karasawa, S.; Hagihara, R.; Abe, Y.; Harada, N.; Todo, J.; Koga, N. Thermal properties, and emission behaviors of N,N-R-Phenyl-7-amino-2,4-trifluoromethylquinoline derivatives: Supercooled liquid-to-crystal transformation induced by mechanical stimuli. Cryst. Growth Design. 2014, 14, 2468–2478. [Google Scholar] [CrossRef]
| Crystal | DPA-Melt(γ-DPA) | DPA-Solution(α-DPA) |
|---|---|---|
| Empirical formula | C26H18 | C26H18 |
| Temperature/K | 293(2) | 293.15 |
| Formula weight | 330.40 | 330.40 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/n | C2/c |
| a/Å | 9.2004(4) | 10.6842(7) |
| b/Å | 21.0836(8) | 13.5461(8) |
| c/Å | 10.0367(4) | 12.2430(8) |
| α/° | 90 | 90 |
| β/° | 111.446(5) | 90.615(6) |
| γ/° | 90 | 90 |
| Volume/Å3 | 1812.10(14) | 1771.82(19) |
| Z | 4 | 4 |
| ρcalcg/cm3 | 1.211 | 1.239 |
| μ/mm−1 | 0.068 | 0.070 |
| F(000) | 696.0 | 696.0 |
| Crystal size/mm3 | 0.2 × 0.08 × 0.05 | 0.4 × 0.35 × 0.25 |
| Radiation | MoKα (λ = 0.71073) | MoKα (λ = 0.71073) |
| 2Θ range for data collection/° | 7.256 to 52.736 | 5.864 to 52.734 |
| Index ranges | −11 ≤ h ≤ 8, −26 ≤ k ≤ 20, −12 ≤ l ≤ 12 | −13 ≤ h ≤ 11, −16 ≤ k ≤ 16, −8 ≤ l ≤ 15 |
| Reflections collected | 11425 | 3856 |
| Independent reflections | 3699 [Rint = 0.0278, Rsigma = 0.0332] | 1823 [Rint = 0.0222, Rsigma = 0.0408] |
| Data/restraints/parameters | 3699/0/236 | 1823/0/118 |
| Goodness-of-fit on F2 | 1.068 | 1.073 |
| Final R indexes [I >= 2σ (I)] | R1 = 0.0523, wR2 = 0.1286 | R1 = 0.0533, wR2 = 0.1141 |
| Final R indexes [all data] | R1 = 0.0802, wR2 = 0.1470 | R1 = 0.0772, wR2 = 0.1320 |
| The Number of the Atom | Angle/° | ||||
|---|---|---|---|---|---|
| A | B | C | D | DPA-Melt | DPA-Solution |
| C8 | C7 | C1 | C6 | −89.0(2) | 113.15(16) |
| C8 | C7 | C1 | C2 | 93.1(2) | −67.5(2) |
| C26(C131) | C7 | C1 | C2 | −90.4(2) | −66.18(19) |
| C26(C131) | C7 | C1 | C6 | 87.5(2) | 113.22(17) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhu, T.; Hu, Q.; Dong, L.; Tan, Z. Structure, Optical, and Thermal Properties of 9, 10-Diphenylanthracene Crystals. Crystals 2019, 9, 512. https://doi.org/10.3390/cryst9100512
Liu X, Zhu T, Hu Q, Dong L, Tan Z. Structure, Optical, and Thermal Properties of 9, 10-Diphenylanthracene Crystals. Crystals. 2019; 9(10):512. https://doi.org/10.3390/cryst9100512
Chicago/Turabian StyleLiu, Xiuhua, Tonghua Zhu, Qingyuan Hu, Lan Dong, and Zhaoyi Tan. 2019. "Structure, Optical, and Thermal Properties of 9, 10-Diphenylanthracene Crystals" Crystals 9, no. 10: 512. https://doi.org/10.3390/cryst9100512
APA StyleLiu, X., Zhu, T., Hu, Q., Dong, L., & Tan, Z. (2019). Structure, Optical, and Thermal Properties of 9, 10-Diphenylanthracene Crystals. Crystals, 9(10), 512. https://doi.org/10.3390/cryst9100512




