2. Materials and Methods
The metal stacks of copper and zinc with the ratio of Cu/Zn of 1.6 were electrodeposited on a molybdenum layer by the constant current method using a two-electrode system at room temperature. A 4 cm
2 × 4 cm
2 piece of soda-lime glass on which a 1200 nm-thick double Mo layer was deposited by DC–magnetron sputtering was used as the cathode in the electrodeposition process. Copper was electrodeposited from a solution that was freshly prepared and contained 187.5 g/L of CuSO
4·5H
2O and 75 g/L of H
2SO
4. The solution of zinc was prepared in-house using 0.2 M zinc vitriol that was dissolved in 0.5 M methane sulfuric acid [
11]. The pH of the solution was adjusted to 2.2 by adding sodium hydroxide. The copper and zinc layers were electrodeposited using direct current with current densities of 50 mA/cm
2 and 20 mA/cm
2, respectively. The typical deposition time was about 15 seconds. Samples of the metallic precursors (4 cm
2 × 4 cm
2 in area) were reactively annealed in a laboratory-made furnace capable of working in vacuum (1 × 10
−4 Pa) or an inert gas (Ar) atmosphere [
13]. The sample temperature, Sn temperature and the Se temperature are controlled individually. The elements Sn and Se are placed in the selenization furnace, under heating. Se evaporates into the selenization furnace and reacts with Sn to form Sn–Se binary phases, which evaporate easily due to a high saturated vapor pressure. The Se and SnSe
x partial pressures are controlled by three parameters: the Sn and Se temperature, and the total pressure.
The electrodeposited metal stacks were annealed at 300 °C in inert gas at 1000 Pa for 30 min. Subsequently, the Se and Sn were heated to 270 °C and 300 °C respectively, then the tube was purged with inert gas to 1 × 10
−3 Pa and the valve was closed. These samples were followed by different selenization processes. Two sets of experiments are listed in
Table 1. The parameters of the first set of experiments are: total pressure of 15 Pa with Sn temperature of 550 °C, named as A1; total pressure of 15 Pa with Sn temperature of 520 °C, named A2; and total pressure of 25 Pa with Sn temperature of 520 °C, named A3. The substrate temperature was kept at 570 °C and the Se temperature was kept at 270 °C. The total pressure was adjusted by the valve of the vacuum pump manually with accuracy of ± 1 Pa. The parameters of the second set of experiments are: sample temperatures of 570 °C, 600 °C and 630 °C, named as a1, a2 and a3, respectively; tin source temperature of 550 °C in a sealed vacuum with maximum total pressure of about 40 Pa.
A standard procedure was applied to fabricate solar cells based on the CZTSe thin film without an etching process. First, a 50 nm thick CdS was deposited using chemical bath deposition. Next, 50 nm i-ZnO and 450 nm Al-ZnO were deposited by RF sputtering. Finally, a 50 nm Ni/2 μm Al metal grid was deposited on top of the device via electron-beam evaporation through a metal mask to create a metallic grid pattern. The active area of the CZTSe solar cell was 0.358 cm2.
The structures of the selenized samples were characterized using a Philips X-pert Pro diffractometer (PANalytical Ltd., EA Almelo, the Netherlands) with Cu radiation and a Renishaw Invia Raman spectroscope (Renishaw Ltd., Gloucestershire UK) The excitation wavelength of the Raman was 514 nm. The penetration depth of this laser is around 100 nm into the absorber. The composition of the CZTSe thin film was measured using a Magix PW2403 X-ray fluorescent spectrometer (XRF, (PW2403 (PANalytical Ltd., EA Almelo, the Netherlands)) with a Rh-anode, which was calibrated by inductively coupled plasma spectroscopy (ICP, PANalytical Ltd., EA Almelo, the Netherlands). Surface and cross-sectional images were taken using a scanning electron microscope (SEM) (JEOL JSM-6700, (JEOL Ltd., Akishima-shi, Japan)). The depth profiles of the elements were obtained by line scanning energy dispersive spectroscopy (EDS). Phase identity and determination were made by Raman spectroscopy (Renishaw + Nanonics). Current–voltage (J–V) measurements of CZTSe solar cells were made under illumination by a standard AM1.5 spectrum of 1000 W/m2 at room temperature with a constant light solar simulator, which was calibrated using a standard mono-crystalline Si solar cell.
3. Results
As shown in
Figure 1, depth distribution of elements in CuZn precursors was measured by line scanning energy dispersive spectroscopy (EDS) along the depth direction to estimate the effect of thermal treatment on the elemental distribution (
Figure 1a,b), and the XRD pattern was used to analyze the phase transition (
Figure 1c). Three peaks were observed in
Figure 1a, corresponding to the elements Mo, Cu and Zn from the bottom to the surface in the Mo/Cu/Zn stacked structure, fairly consistent with the electrodeposition sequence. The overlap of Cu and Zn signals in
Figure 1b, obviously in contrast to
Figure 1a, reveals a more uniform distribution of CuZn after annealing. The CuZn alloy was the main phase of the electrodeposited layer in
Figure 1c (black line), corresponding to the CuZn/Mo stacked structure. After thermal treatment, the Cu
0.64Zn
0.36 brass phase is the main phase along with some minor Cu and Zn phases. Two unknown peaks at 35.8° and 43.97° are labeled as “*”, which could be copper oxide phases. These oxides may come from the electrodeposition process. The copper has an obvious volume variation in the process of selenization, owing to the great disparities in the volume of Cu
2Se and CuSe
2 per copper atom [
20]. The copper distribution at the bottom will inevitably create pin holes at the back-contact interface during the reactive annealing, which is detrimental to the adhesion between the absorber layer and the molybdenum substrate. Furthermore, elemental distribution has great influence on the selenization reaction process as well as the quality of absorber layer, which lead to diverse device performance [
9]. We have done many experiments to selenize the Mo/Cu/Zn metal precursor directly (without annealing to form a metal alloy) in an Se + SnSe atmosphere for preparing CZTSe thin films. We found that the adhesion between the CZTSe thin film and the Mo layer was a big problem. Therefore, the formation of a CuZn alloy was essential for the follow-up selenization experiments.
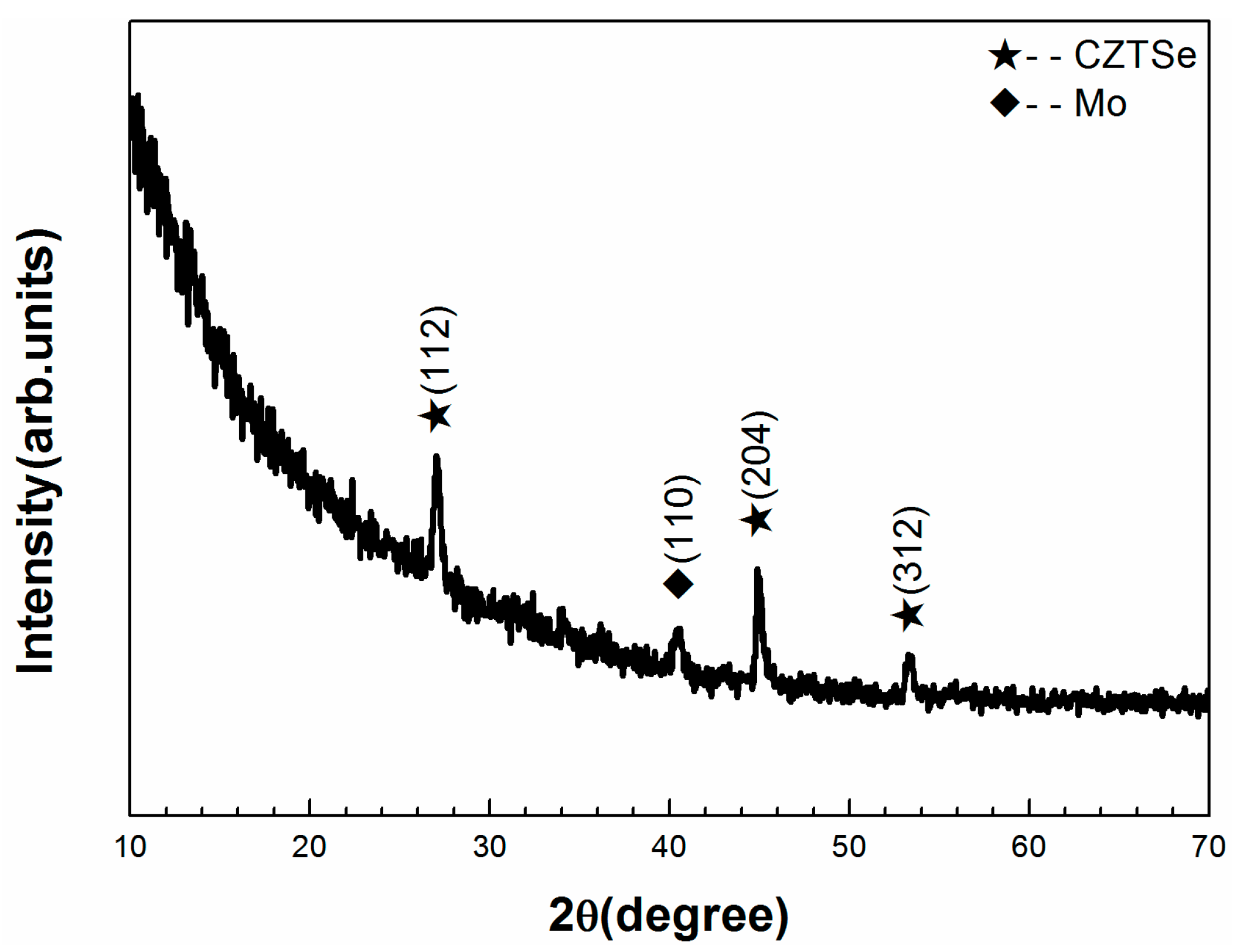
XRD patterns of the electrodeposited CZTSe absorber layers are shown in
Figure 2. The CZTSe phases are identified for all samples [
21]. All samples have peaks at 17.44°, 22.05°, 42.8°, 63.83°, and 68° and those labeled as “*”. The strongest CZTSe peak is (112), in agreement with other literature data [
21]. All samples except A3 have peaks at 24.5°, 29.2°, and 43.8°, which correspond to Cu
2Se
x (or Cu
2–x Se) phases according to (ICCD#00-053-0523, ICCD#00-026-0557 and ICCD#00-027-1131). This indicates that sample A3 was more consistent with pure CZTSe phases. Binary secondary phases of Cu
xSe and SnSe were not easily observable because of the similarity of their crystal structures. No MoSe
x related diffraction peaks are observed as expected because of the low Se partial pressure employed in this work.
To further confirm the minor MoSe
2 phase at the CZTSe and Mo interface, grazing incidence X-ray diffraction (GIXRD) was carried out on one of the samples with most of the CZTSe film mechanically stripped off from the sample, as shown in
Figure 3. Only CZTSe and Mo related diffraction peaks was present and no MoSe
2 related phase was observed (MoSe
2 with a main diffraction peak at 2θ = 31.7° and 55.8° according to ICCD#00-029-0914), which is consistent with the XRD measurement in
Figure 2.
Because of the similarity in diffraction patterns, XRD cannot unambiguously identify ZnSe, Cu
2SnSe
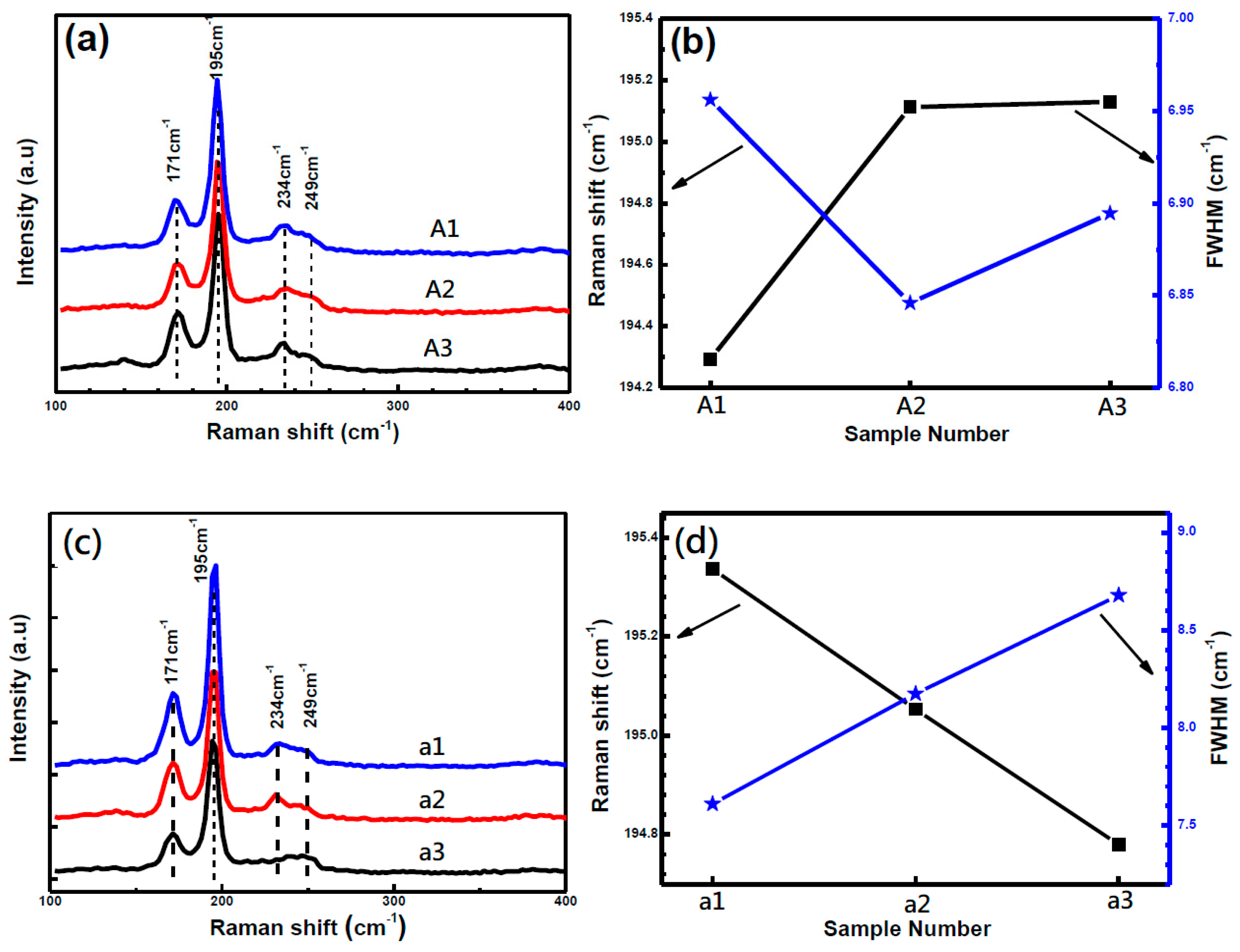
3 and CZTSe, and therefore it is necessary to use other characterization techniques to identify CZTSe such as Raman spectroscopy. As shown in
Figure 4, the Raman peak positions at 195 cm
−1,173 cm
−1 and 234 cm
−1 were identified as the main peaks of the CZTSe phase. The weak peak at 249 cm
−1 corresponds to ZnSe phases [
12], Cu
2SnSe
3 [
22] or disordered CZTSe phases [
23]. This is confirmed to be Cu
2SnSe
3 according to the Zn-poor surface confirmed by the follow-up line scanning EDS analysis of CZTSe thin films. For samples A1−A3, the intensity at 249 cm
−1 decreased with lower SnSe
x partial pressure, revealing that Cu
2SnSe
3 at the surface region is reduced. For samples a1-a3, sample a1 and a2 have an obvious peak intensity at 249 cm
−1, and a2 has a weak peak intensity here, revealing that annealing at 600 °C in a sealing atmosphere results in less Cu
2SnSe
3 phases. This will be discussed in the following sections.
The Raman spectrum with Lorenzian fitting was carried out and the peak position, peak intensity and full width half maximum (FWHM) data were analyzed. As listed in
Table 2, peak position and FWHM changed from 194.29 cm
−1 to 195.11 cm
−1 and from 6.96 cm
−1 to 6.85cm
−1, respectively, for samples A1 and A2 with lower SnSe
x partial pressure and higher Se partial pressure. However, it changed little with further adjustment to the Se and SnSe partial pressure for A2 and A3. This indicates that higher Se and lower SnSe partial pressure were preferred for the formation of pure CZTSe phases. However, excessive SnSe
x would cause secondary phases. Annealing temperature has an obvious influence on the Raman spectrum parameters. The peak position decreases and the FWHM increases with increasing annealing temperature from 570 °C to 630 °C. The broad CZTSe peaks are observed and Cu
2SnSe
3 was confirmed to be present at the surface layer. More information could be obtained from the Raman spectra, like the order–disorder character and the degree of crystallization. The broadening peak may arise from a high defect density and local compositional fluctuations. The local disorder in the cation sub-lattice could also be a reason for the peak shoulder [
23,
24]. A higher peak position accompanied with lower FWHM values corresponds to higher crystallization quality [
12].
To further analyze the effect of annealing condition on the morphology, secondary phases and elemental distribution, scanning electron microscopy (SEM) with line scanning EDS was applied. In order to facilitate the discussion, gas phase partial pressure analysis in the selenium atmosphere will be discussed first. It is well known that only SnSe
2 and SnSe phases were verified experimentally in the Sn–Se system, and no Sn
2Se
3 was found [
7]. The saturated vapor pressure (units in Pa) of Se, SnSe and SnSe
2 at the temperature adopted in this work are listed in
Table 3. The statured vapor pressure of SnSe
2 is two order of magnitudes higher than that of SnSe; therefore, SnSe
2 was considered as the main Sn-containing vapor gas in this work and the SnSe phase was neglected.
At the beginning of the selenization process, the annealing furnace was quickly injected with Se vapor and the Sn-containing vapor phase was negligible because the saturated vapor pressure of Sn was low [
25]. Subsequently, Sn reacts with Se to generate SnSe
2, and SnSe
2 evaporates into the annealing furnace and reacts with the precursor. The SnSe
2 partial pressure was affected by the reaction rate of Se with Sn, i.e., Sn temperature and the Se partial pressure. The analysis of partial pressure is listed in
Table 4 based on the data in table and the experimental conditions.
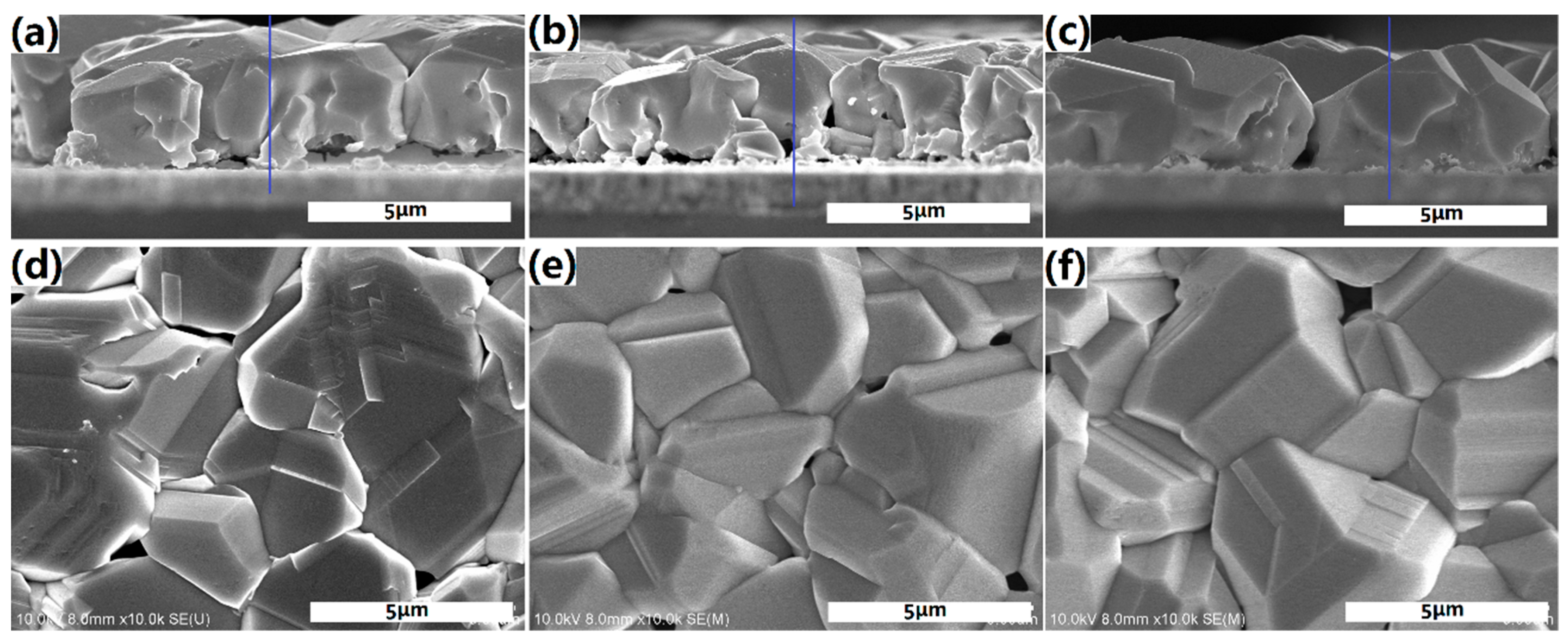
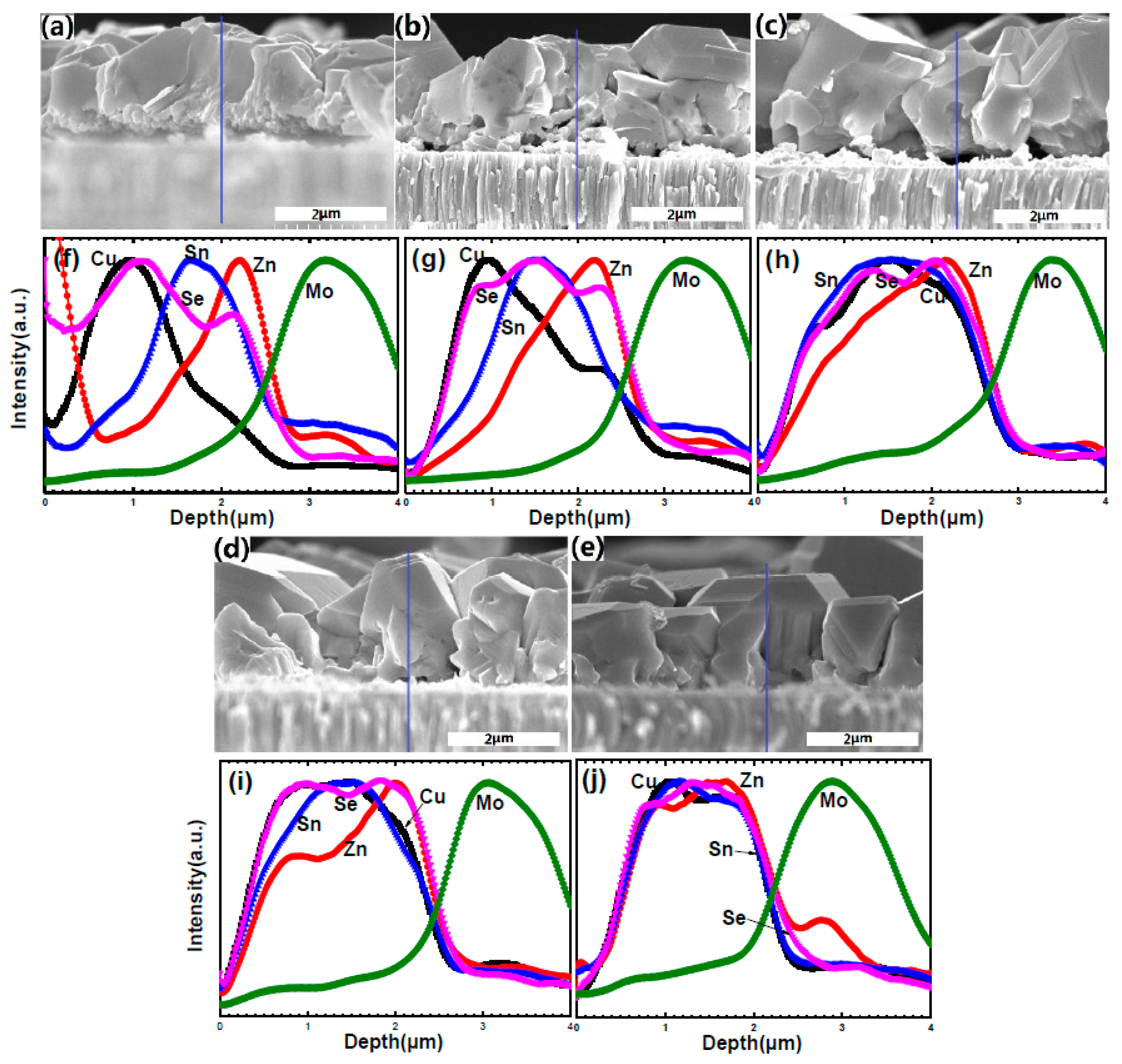
Figure 5 shows the cross-sectional and surface SEM micrographs of samples A1–A3. Large CZTSe grain with a smooth and compact surface was observed in
Figure 5a,d, accompanied by large holes and Zn-rich secondary phases at the CZTSe/Mo interface (which will be confirmed by EDS and shown herein later). The holes and Zn-rich secondary phases between CZTSe and Mo diminished with increasing Se partial pressure and decreasing SnSe
x partial pressure, as shown in
Figure 5b. The surface roughness increased, and grain size decreased in
Figure 5b,e. With further lower SnSe
x partial pressures and higher Se
x partial pressures, isolated hill-shaped grains were obtained and covered the whole film. Almost no holes and secondary phases existed at the interface in
Figure 5c,f. Unsurprisingly, the biggest surface roughness was observed in the trend of A1 and A2. It was obvious that with higher Se
x partial pressure and lower SnSe
x partial pressure, the grain tends grow longitudinally through the film. In contrast, with low Se partial pressure and higher SnSe partial pressure, the grain tends to growth laterally at the surface.
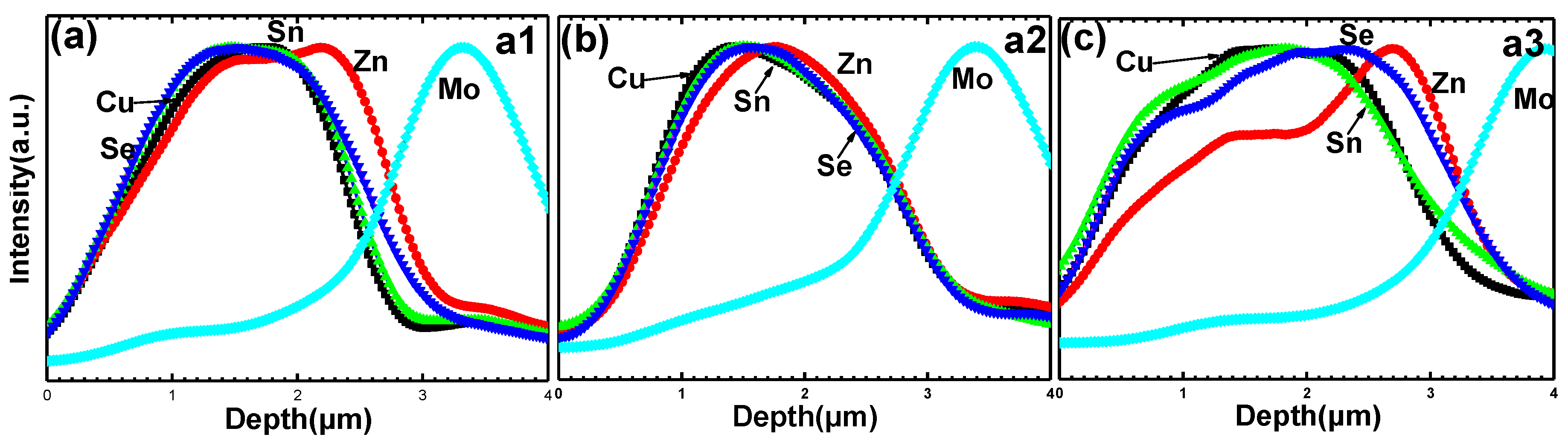
Figure 6 shows the line scanning EDS profiles of the samples A1–A3 (the scanning line (blue line) is indicated in
Figure 5a,b,c). The SnSe
x partial pressures from high to low for the preparation of sample A1, A2 and A3 were sample A1, A3 and A2, respectively. Sample A1 is Zn-poor at the surface region and Sn-poor at the bottom region. However, sample A2 is Sn-poor and Zn-rich at the surface region, indicating that lower SnSe
x partial pressures for sample A2 can result in better Sn in-diffusion than higher SnSe
x partial pressures for sample A1. Lower SnSe
x partial pressure is beneficial to the interdiffusion of Sn and Zn for a reactive annealing Cu/Zn metallic stack precursor. It is well known that a higher total pressure can prevent the Sn loss from the surface of CZTSe thin films owing to the high saturated vapor pressure of SnSe
x. Therefore, properly adjusting the annealing parameter by slightly increasing SnSe
x partial pressure and the total pressure of sample A3 leads to more uniform element distribution than those shown for samples A1 and A2, as shown in
Figure 6c.
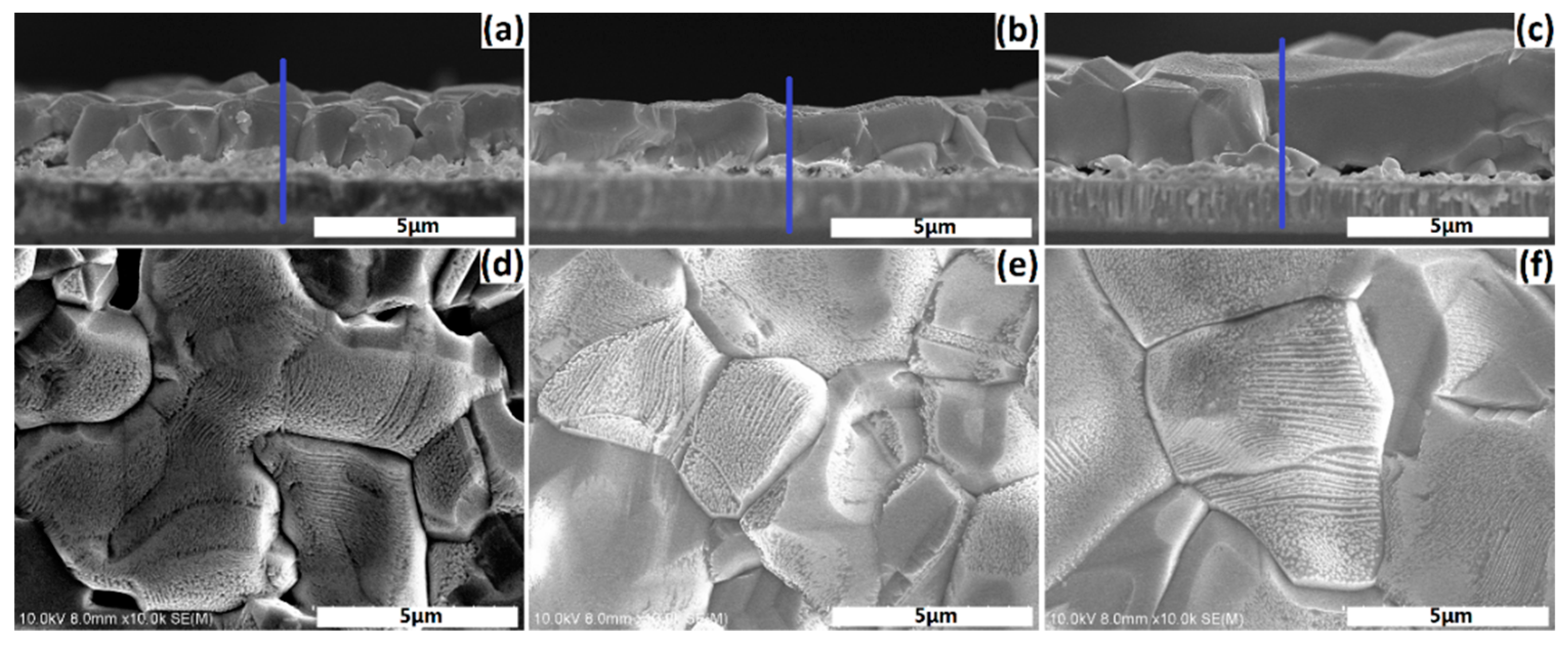
Figure 7 shows the cross-sectional and surface SEM micrographs for samples a1–a3. As shown in
Figure 7a, the 500 nm-thick secondary phase for sample a1 exists at the CZTSe/Mo interface, which is confirmed to be a Zn-rich phase by the line scanning EDS profiles of sample a1. It reveals an insufficient reaction of the phases at the interface region. No secondary phase, except a few pin holes at the CZTSe/Mo interface, is observed at an annealing temperature of 600 °C, as shown in
Figure 7b. It also reveals that a sufficient reaction of the phases occurred at the interface region. Secondary phases appeared with further increases of the annealing temperature to 630 °C, as shown in
Figure 7c, which will be discussed later. Coherent and compact grain of the upper layer with a flat surface was observed for all three samples. The grain boundary becomes more compact and the grain size increases with increasing temperature, as shown in
Figure 7d,e,f. The grain size exceeding 10 μm was obtained for sample a3.
Figure 8 shows the line scanning EDS profiles of samples a1–a3. The elemental signal intensity variation of the surface regions of samples a1–a3 was sharper than that of samples A2 and A3. They reveal a smooth surface in samples a1–a3. Sample a1 has a Sn-rich and Zn-poor composition at the surface region and a Zn-rich composition at the bottom region. This indicates that Sn and Zn diffusion is inadequate for samples annealed at 570 °C. However, for the sample annealed at 600 °C, the Zn-rich composition at the bottom region disappears which indicates that Zn diffusion is sufficient. A Sn-rich surface region still exists, which may originate from the sealed selenization conditions with relatively higher SnSe
x partial pressure compared to that of samples A1–A3. When further increasing the annealing temperature to 630 °C (sample a3), a thick Zn-poor surface region and interface region reveals that it is unfavorable to form CZTSe at 630 °C.
The composition of the samples is listed in
Table 5. The ratio of Cu/Zn was about 1.6 for all samples. For samples A1–A3, the Zn/Sn ratio is lower and the Sn content is higher at low SnSe
x partial pressure, in agreement with the observation of Sn diffusion in
Figure 6. For the samples a1–a3, the Zn/Sn ratio decreases and the Sn content increases with increasing annealing temperature. The Zn diffusion is more rapid for sample a2 in comparison with a1, and the higher Sn content is accessible. However, the Zn/Sn ratio for sample a3 with poor Zn diffusion is abnormal. Sample A1 and a3 have different compositions, although both of them have poor element diffusion. However, minor differences can be observed between
Figure 6a and
Figure 8c. Sample A1 has poor Sn and Zn diffusion, whereas sample a1 has good Sn diffusion and poor Zn diffusion.
As shown above, the formation process of CZTSe thin film from a CuZn precursor under Sn + Se atmosphere is affected by many factors, such as annealing temperature, Se partial pressure, SnSe
x partial pressure and total pressure. To verify the diffusion process, another set of experiments were carried out with annealing times of 1 min, 2 mins, 4 mins, 8 mins and 16 min, named as s1,s2, s4, s8 and s16, respectively. The cross-section SEM images and line scanning EDS profiles are shown in
Figure 9. Secondary phases at the CZTSe/Mo interface and the size of voids in the film both decrease and finally disappear with an increase in annealing time. The line scanning EDS profiles of samples s1 through s16 reveal the process of elemental diffusion. At the initial stage, as shown in
Figure 9f, Zn and Cu were the main elements and little Sn existed in the middle region. When increasing the annealing time, Sn diffuses to the bottom region and Zn diffuses to the surface, as shown in
Figure 9f,j. For all samples, a Cu-rich surface region is observed, owing to the surface tension of liquid phase Cu
2–x Se at above 570 °C.
It was reported that the formation of CZTSe thin films involves reaction (1) and (2) [
19,
26]. The sample temperature of the Cu
xZn
y precursor is heated to a higher temperature than 570 °C for 3 min. The main selenization reactions of the Cu
xZn
y precursor are [
19,
26]:
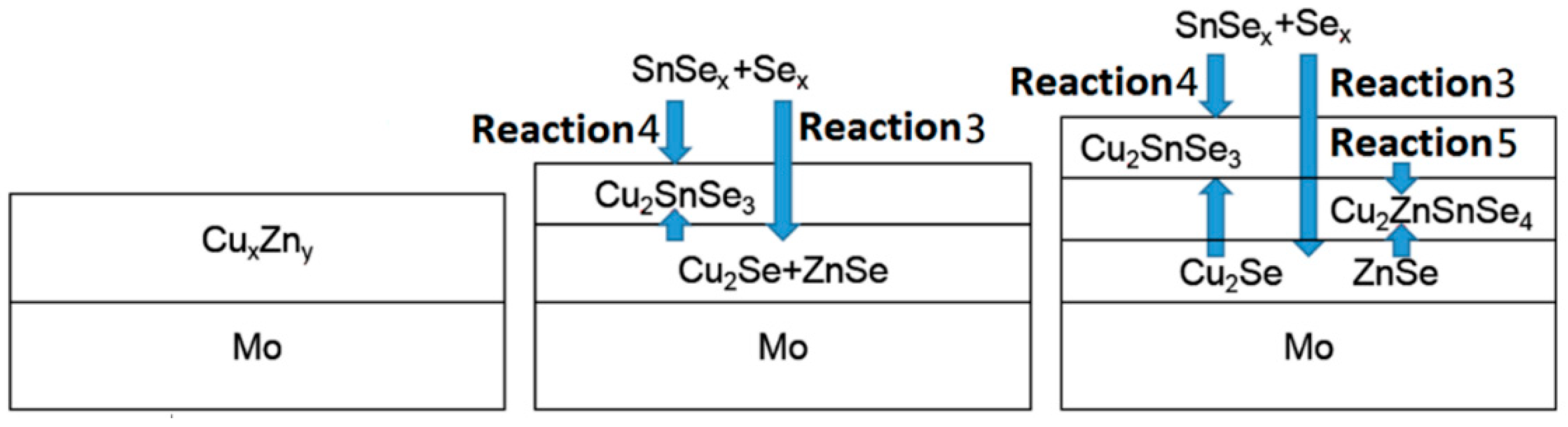
The selenization reaction process is proposed as shown in
Figure 10. The reaction of Cu
xZn
y with Se has been confirmed experimentally in the literature [
27] and adopted here as described in reaction (3). Sn reacts with Se to produce SnSe
x, and the evaporation of SnSe
x was feasible with temperatures higher than 400 °C [
12,
13]. Then, SnSe
x reacts with Cu
2Se to produce Cu
2SnSe
3 as described in reaction (4). At the same time, Cu
2SnSe
3 is consumed by the reaction with ZnSe and generates Cu
2ZnSnSe
4 as described in reaction (5). The rate of reaction (4) is affected by the partial pressure of SnSe
x, as well as the diffusion rate of copper. A higher Sn temperature leads to higher SnSe
x partial pressure, and a higher annealing temperature leads to a faster copper diffusion rate. In a similar way, a higher reaction rate (5) can be obtained at higher annealing temperatures.
The equilibrium between the production and the consumption of Cu2SnSe3 results in a significant effect on the morphology, crystallization and distribution of the elements. In sample A1 (high SnSex partial pressure), the production reaction of Cu2SnSe3 is in the ascendant, i.e. reaction (4) may be faster than reaction (5). Cu2SnSe3 grain grows fast and leads to a compact surface at the upper layer and Zn-rich layer at the bottom. However, in sample A3 (low SnSex partial pressure), the Cu2SnSe3 reaction is slower than that of sample 1. In addition, the overall pressure can also enhance these reactions. Reaction (4) may be slower than reaction (5), i.e., the formation of Cu2ZnSnSe4 thin films is faster than the formation of Cu2SnSe3 thin films. The large grain through the whole film is expected in this situation. With increasing annealing temperature from 570 °C to 600 °C, the consumption reaction Cu2SnSe3 is enhanced, the elemental distribution is uniform and secondary phases can be avoided. The production of Cu2SnSe3 is significantly affected by SnSex partial pressure when considering the liquid phase Cu2–xSe. However, the consumption rate is a solid-phase reaction greatly affected by temperature. Therefore, an increased annealing temperature can promote reaction (5) and avoid the secondary phases at the CZTSe/Mo interface, which is in agreement with experiment a2. With high annealing temperature, good crystallization at the upper layer is formed quickly with grain size exceeding 10 μm, which impedes the solid phase reaction (5), and therefore the Zn-rich region is left at the bottom.
The current–voltage (J–V) curves of samples A1–A3 and a1–a3 are shown in
Figure S1.
Table S1 lists the performance parameters of these solar cells. The highest efficiency open circuit voltage and short current density of these samples is reached with samples A2, A2, and a2, respectively. This means that the annealing parameters of the devices that perform the best may be in the range of sample temperatures from 570 °C to 600 °C, Sn temperatures from 520 °C to 550 °C and total pressures from 15 Pa to 40 Pa. The equilibrium between the production and the consumption can be obtained by carefully adjusting the experimental parameters, such as SnSe
x partial pressure and annealing temperature. Optimized annealing parameters were obtained based on the analysis above, and a CZTSe solar cell was fabricated at 580 °C with a Sn temperature of 550 °C and a total pressure of 30 Pa.
Figure 11a,b shows the cross-sectional and surface images of the CZTSe thin films. A compact surface and columnar-like grain are observed. The current–voltage (J–V) curve of this CZTSe solar cell is shown in
Figure 11c. It presents an efficiency of 7.26% with V
OC = 394 mV, J
SC = 32.27 mA/cm
2, FF = 57.1%.