Tracing Phase Transformation and Lattice Evolution in a TRIP Sheet Steel under High-Temperature Annealing by Real-Time In Situ Neutron Diffraction
Abstract
:1. Introduction
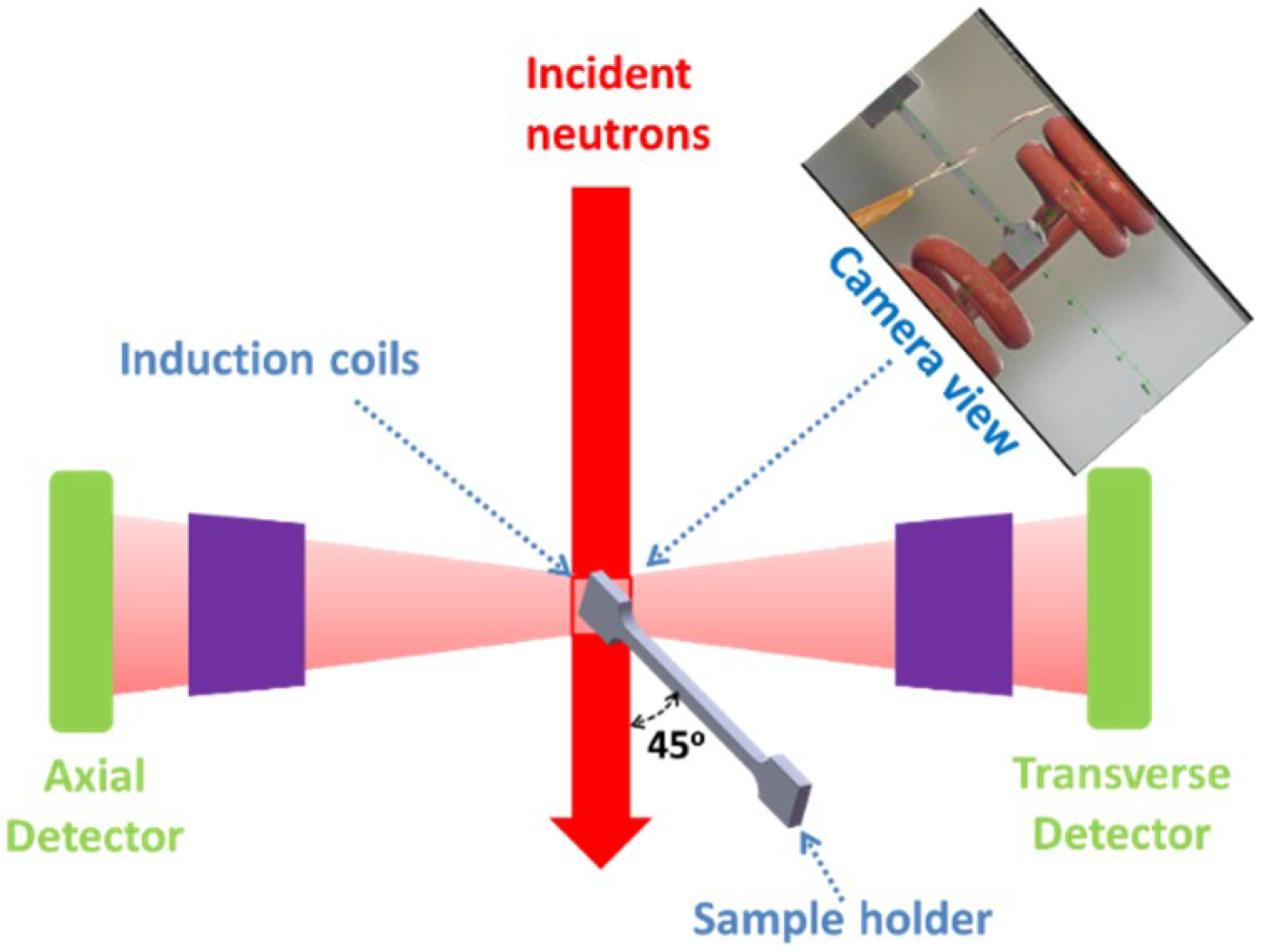
2. Material and Experiment
3. Results and Discussion
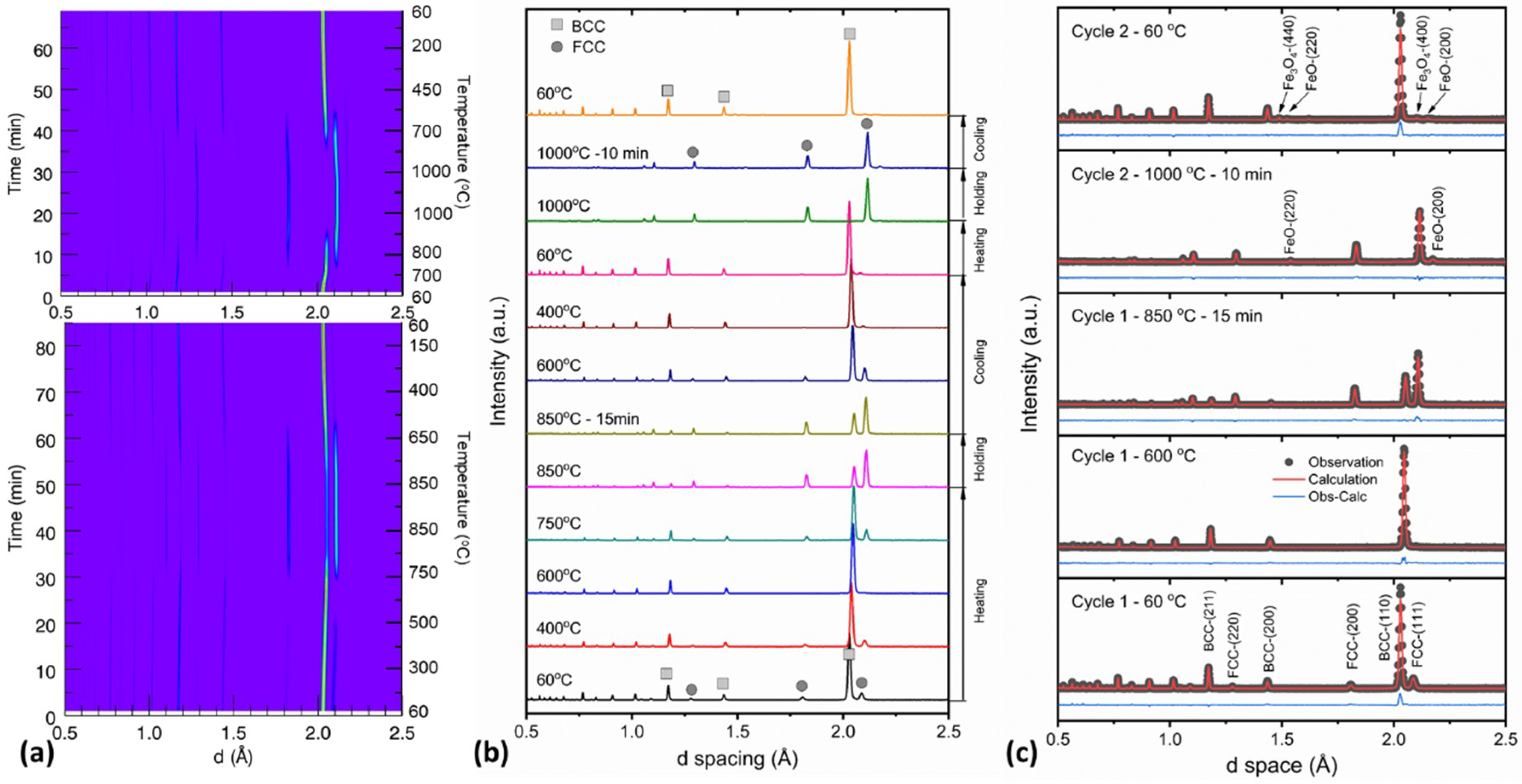
3.1. Phase Transformation
3.2. Lattice Parameter Evolution
4. Conclusions
- (1)
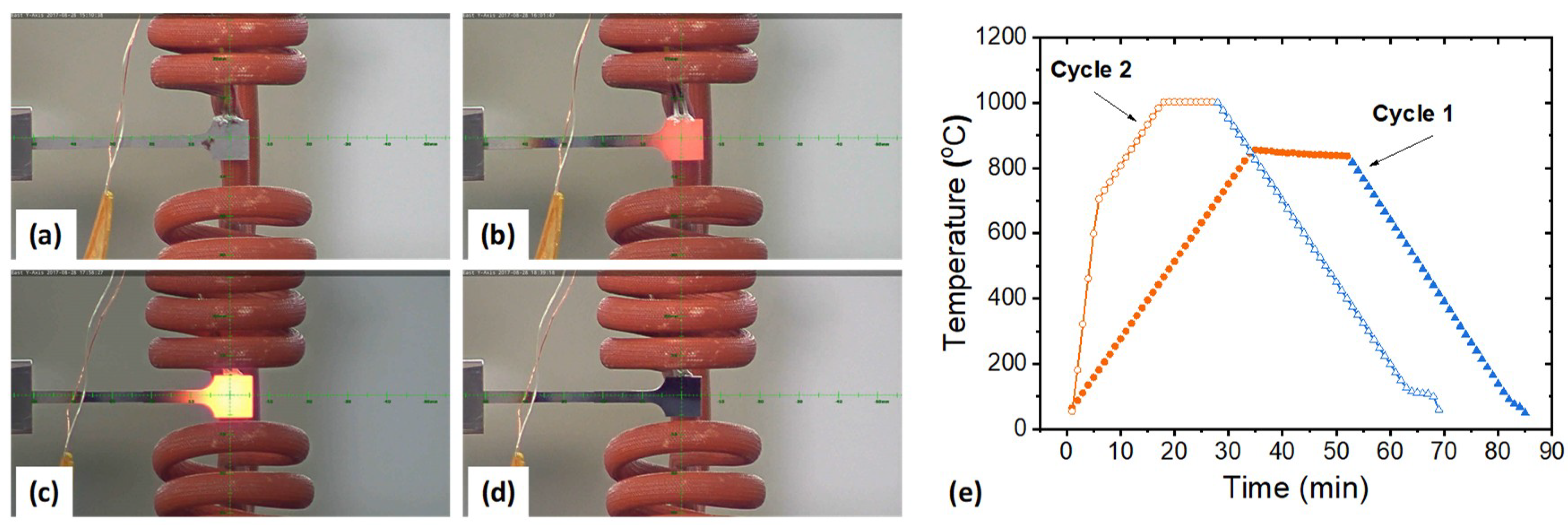
- Upon heating, the RA is stable in the temperature region below 300 °C and starts to transform to ferrite drastically in the temperature region between 500 and 600 °C. Carbon diffusion occurs in between 300 and 500 °C accompanied by a gradual phase transformation.
- (2)
- Upon further heating, the transformation of ferrite to austenite starts at ~730 °C accompanied by a lattice contraction of FCC due to carbon depletion in austenite. The finish temperature could be dependent on the heating history. In this study, the heating scheme is composed of one cycle of annealing up to 850 °C and then a rapid heating up to 700 °C before slow heating to the finish temperature of ~1000 °C.
- (3)
- Upon cooling from the finish temperature, the transformation of austenite to ferrite starts at ~850 °C and finishes at ~500 °C. The thermal contractions of FCC and BCC with decreasing temperature are retarded due to carbon-concentration induced expansion. Restrained phase transformation is also observed to some degree starting at ~700 °C.
- (4)
- The CTE of FCC in the temperature region below 550 °C is much lower than that above 900 °C, which is considered as the result of thermal constrain induced by the mismatch of CTE of BCC and FCC. The RA could be under a tensile hydrostatic stress state at room temperature.
- (5)
- The above results prove that the technique of real-time in situ neutron diffraction can be a powerful tool for heat treatment design of novel metallic materials.
Author Contributions
Acknowledgments
Conflicts of Interest
Disclaimer
Notice of Copyright
References
- De Cooman, B.C. Structure–properties relationship in TRIP steels containing carbide-free bainite. Curr. Opin. Solid State Mater. Sci. 2004, 8, 285–303. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, Z. Thermomechanical processing of advanced high strength steels. Prog. Mater. Sci. 2018, 94, 174–242. [Google Scholar] [CrossRef]
- Mahieu, J.; De Cooman, B.C.; Claessens, S. Galvanizability of high-strength steels for automotive applications. Metall. Mater. Trans. A 2001, 32, 2905–2908. [Google Scholar] [CrossRef]
- Mintz, B. Hot dip galvanising of transformation induced plasticity and other intercritically annealed steels. Int. Mater. Rev. 2001, 46, 169–197. [Google Scholar] [CrossRef]
- Choi, S.D.; Kim, H.S.; Je, J.H.; Park, S.H. Annealing behavior of retained austenite in low carbon steel: Real time synchrotron X-ray scattering study. J. Mater. Sci. Lett. 2002, 21, 353–355. [Google Scholar] [CrossRef]
- Babu, S.S.; Specht, E.D.; David, S.A.; Karapetrova, E.; Zschack, P.; Peet, M.; Bhadeshia, H.K.D.H. In-situ observations of lattice parameter fluctuations in austenite and transformation to bainite. Metall. Mater. Trans. A 2005, 36, 3281–3289. [Google Scholar] [CrossRef]
- Van Dijk, N.H.; Butt, A.M.; Zhao, L.; Sietsma, J.; Offerman, S.E.; Wright, J.P.; Van Der Zwaag, S. Thermal stability of retained austenite in TRIP steels studied by synchrotron X-ray diffraction during cooling. Acta Mater. 2005, 53, 5439–5447. [Google Scholar] [CrossRef]
- Xu, P.G.; Tomota, Y.; Lukáš, P.; Muránsky, O.; Adachi, Y. Austenite-to-ferrite transformation in low alloy steels during thermomechanically controlled process studied by in situ neutron diffraction. Mater. Sci. Eng. A 2006, 435–436, 46–53. [Google Scholar] [CrossRef]
- Jimenez-Melero, E.; Van Dijk, N.H.; Zhao, L.; Sietsma, J.; Offerman, S.E.; Wright, J.P.; Van Der Zwaag, S. Characterization of individual retained austenite grains and their stability in low-alloyed TRIP steels. Acta Mater. 2007, 55, 6713–6723. [Google Scholar] [CrossRef]
- Christien, F.; Telling, M.T.F.; Knight, K.S. A comparison of dilatometry and in-situ neutron diffraction in tracking bulk phase transformations in a martensitic stainless steel. Mater. Charact. 2013, 82, 50–57. [Google Scholar] [CrossRef]
- Allain, S.Y.P.; Geandier, G.; Hell, J.-C.; Soler, M.; Danoix, F.; Gouné, M. Effects of Q&P processing conditions on austenite carbon enrichment studied by in situ high-energy X-ray diffraction experiments. Metals 2017, 7, 232. [Google Scholar]
- Allain, S.Y.P.; Geandier, G.; Hell, J.C.; Soler, M.; Danoix, F.; Gouné, M. In-situ investigation of quenching and partitioning by high energy X-ray diffraction experiments. Scr. Mater. 2017, 131, 15–18. [Google Scholar] [CrossRef]
- Allain, S.Y.P.; Gaudez, S.; Geandier, G.; Hell, J.-C.; Gouné, M.; Danoix, F.; Soler, M.; Aoued, S.; Poulon-Quintin, A. Internal stresses and carbon enrichment in austenite of quenching and partitioning steels from high energy X-ray diffraction experiments. Mater. Sci. Eng. A 2018, 710, 245–250. [Google Scholar] [CrossRef]
- Liss, K.-D.; Yan, K. Thermo-mechanical processing in a synchrotron beam. Mater. Sci. Eng. A 2010, 528, 11–27. [Google Scholar] [CrossRef]
- Wu, W.; An, K.; Huang, L.; Lee, S.Y.; Liaw, P.K. Deformation dynamics study of a wrought magnesium alloy by real-time in situ neutron diffraction. Scr. Mater. 2013, 69, 358–361. [Google Scholar] [CrossRef]
- Yu, D.; Bei, H.; Chen, Y.; George, E.P.; An, K. Phase-specific deformation behavior of a relatively tough NiAl–Cr(Mo) lamellar composite. Scr. Mater. 2014, 84–85, 59–62. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, D.; An, K. Stress-induced charge-ordering process in LiMn2O4. Mater. Res. Lett. 2017, 5, 89–94. [Google Scholar] [CrossRef]
- Yang, H.; Yu, D.; Chen, Y.; Mu, J.; Wang, Y.D.; An, K. In-situ TOF neutron diffraction studies of cyclic softening in superelasticity of a NiFeGaCo shape memory alloy. Mater. Sci. Eng. A 2017, 680, 324–328. [Google Scholar] [CrossRef]
- Wu, W.; Liaw, P.K.; An, K. Unraveling cyclic deformation mechanisms of a rolled magnesium alloy using in situ neutron diffraction. Acta Mater. 2015, 85, 343–353. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; An, K. Understanding low-cycle fatigue life improvement mechanisms in a pre-twinned magnesium alloy. J. Alloy Compd. 2016, 656, 539–550. [Google Scholar] [CrossRef]
- Wu, W.; Qiao, H.; An, K.; Guo, X.; Wu, P.; Liaw, P.K. Investigation of deformation dynamics in a wrought magnesium alloy. Int. J. Plast. 2014, 62, 105–120. [Google Scholar] [CrossRef]
- Lee, S.Y.; Wang, H.; Gharghouri, M.A.; Nayyeri, G.; Woo, W.; Shin, E.; Wu, P.D.; Poole, W.J.; Wu, W.; An, K. Deformation behavior of solid-solution-strengthened Mg–9 wt.% Al alloy: In situ neutron diffraction and elastic–viscoplastic self-consistent modeling. Acta Mater. 2014, 73, 139–148. [Google Scholar] [CrossRef]
- Cakmak, E.; Kirka, M.M.; Watkins, T.R.; Cooper, R.C.; An, K.; Choo, H.; Wu, W.; Dehoff, R.R.; Babu, S.S. Microstructural and micromechanical characterization of IN718 theta shaped specimens built with electron beam melting. Acta Mater. 2016, 108, 161–175. [Google Scholar] [CrossRef] [Green Version]
- Huang, E.W.; Barabash, R.I.; Wang, Y.; Clausen, B.; Li, L.; Liaw, P.K.; Ice, G.E.; Ren, Y.; Choo, H.; Pike, L.M.; et al. Plastic behavior of a nickel-based alloy under monotonic-tension and low-cycle-fatigue loading. Int. J. Plast. 2008, 24, 1440–1456. [Google Scholar] [CrossRef]
- Chen, Y.; Rangasamy, E.; dela Cruz, C.R.; Liang, C.; An, K. A study of suppressed formation of low-conductivity phases in doped Li7La3Zr2O12 garnets by in situ neutron diffraction. J. Mater. Chem. A 2015, 3, 22868–22876. [Google Scholar] [CrossRef]
- Jin, K.; Mu, S.; An, K.; Porter, W.D.; Samolyuk, G.D.; Stocks, G.M.; Bei, H. Thermophysical properties of Ni-containing single-phase concentrated solid solution alloys. Mater. Des. 2017, 117, 185–192. [Google Scholar] [CrossRef]
- Wang, X.-L.; An, K.; Cai, L.; Feng, Z.; Nagler, S.E.; Daniel, C.; Rhodes, K.J.; Stoica, A.D.; Skorpenske, H.D.; Liang, C.; et al. Visualizing the chemistry and structure dynamics in lithium-ion batteries by in-situ neutron diffraction. Sci. Rep. 2012, 2, 747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Chen, Y.; Hy, S.; An, K.; Venkatachalam, S.; Qian, D.; Zhang, M.; Meng, Y.S. Operando lithium dynamics in the Li-Rich layered oxide cathode material via neutron diffraction. Adv. Energy Mater. 2016, 6, 1502143. [Google Scholar] [CrossRef]
- Liu, T.; Gao, Y.; Bei, H.; An, K. In situ neutron diffraction study on tensile deformation behavior of carbon-strengthened CoCrFeMnNi high-entropy alloys at room and elevated temperatures. J. Mater. Res. 2018, 1–12. [Google Scholar] [CrossRef]
- Yu, D.; An, K.; Chen, X.; Bei, H. Phase-specific deformation behavior of a NiAl–Cr(Mo) lamellar composite under thermal and mechanical loads. J. Alloy Compd. 2016, 65, 481–490. [Google Scholar] [CrossRef]
- Benafan, O.; Noebe, R.D.; Padula II, S.A.; Gaydosh, D.J.; Lerch, B.A.; Garg, A.; Bigelow, G.S.; An, K.; Vaidyanathan, R. Temperature-dependent behavior of a polycrystalline NiTi shape memory alloy around the transformation regime. Scr. Mater. 2013, 68, 571–574. [Google Scholar] [CrossRef]
- Huang, S.; Gao, Y.; An, K.; Zheng, L.; Wu, W.; Teng, Z.; Liaw, P.K. Deformation mechanisms in a precipitation-strengthened ferritic superalloy revealed by in situ neutron diffraction studies at elevated temperatures. Acta Mater. 2015, 83, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.M.; Chen, Y.; Mu, J.; Zhu, Z.W.; Zhang, H.F.; Wang, Y.D.; An, K. An in situ neutron diffraction study of plastic deformation in a Cu46.5Zr46.5Al7 bulk metallic glass composite. Scr. Mater. 2018, 153, 118–121. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, L.; Ren, F.; An, K. Visualizing the structural evolution of LSM/xYSZ composite cathodes for SOFC by in-situ neutron diffraction. Sci. Rep. 2014, 4, 5179. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ma, D.; Li, Q.K.; Stoica, A.D.; Song, W.L.; Wang, H.; Liu, X.J.; Stoica, G.M.; Wang, G.Y.; An, K.; et al. Transformation-induced plasticity in bulk metallic glass composites evidenced by in-situ neutron diffraction. Acta Mater. 2017, 124, 478–488. [Google Scholar] [CrossRef]
- Huang, H.; Wu, Y.; He, J.; Wang, H.; Liu, X.; An, K.; Wu, W.; Lu, Z. Phase-Transformation Ductilization of Brittle High-Entropy Alloys via Metastability Engineering. Adv. Mater. 2017, 29, 1701678. [Google Scholar] [CrossRef] [PubMed]
- An, K.; Yuan, L.; Dial, L.; Spinelli, I.; Stoica, A.D.; Gao, Y. Neutron residual stress measurement and numerical modeling in a curved thin-walled structure by laser powder bed fusion additive manufacturing. Mater. Des. 2017, 135, 122–132. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, Y.; Li, J.; Feygenson, M.; Heller, W.T.; Liang, C.; An, K. Lattice-Cell Orientation Disorder in Complex Spinel Oxides. Adv. Energy Mater. 2017, 7, 1601950. [Google Scholar] [CrossRef]
- Yu, D.; An, K.; Chen, Y.; Chen, X. Revealing the cyclic hardening mechanism of an austenitic stainless steel by real-time in situ neutron diffraction. Scr. Mater. 2014, 89, 45–48. [Google Scholar] [CrossRef]
- Stoica, G.M.; Stoica, A.D.; An, K.; Ma, D.; Vogel, S.; Carpenter, J.; Wang, X.-L. Extracting grain-orientation-dependent data from in situ time-of-flight neutron diffraction. I. Inverse pole figures. J. Appl. Crystallogr. 2014, 47, 2019–2029. [Google Scholar] [CrossRef]
- Chen, Y.; Rangasamy, E.; Liang, C.; An, K. Origin of high Li+ conduction in doped Li7La3Zr2O12 garnets. Chem. Mater. 2015, 27, 5491–5494. [Google Scholar] [CrossRef]
- Cai, L.; Liu, Z.; An, K.; Liang, C. Unraveling structural evolution of LiNi0.5Mn1.5O4 by in situ neutron diffraction. J. Mater. Chem. A 2013, 1, 6908–6914. [Google Scholar] [CrossRef]
- Xie, Q.; Chen, Y.; Yang, P.; Zhao, Z.; Wang, Y.D.; An, K. In-situ neutron diffraction investigation on twinning/detwinning activities during tension-compression load reversal in a twinning induced plasticity steel. Scr. Mater. 2018, 150, 168–172. [Google Scholar] [CrossRef]
- Wu, W.; Gao, Y.; Li, N.; Parish, C.M.; Liu, W.; Liaw, P.K.; An, K. Intragranular twinning, detwinning, and twinning-like lattice reorientation in magnesium alloys. Acta Mater. 2016, 121, 15–23. [Google Scholar] [CrossRef] [Green Version]
- United States Steel Corporation. Transformation Induced Plasticity 780 Grades. Available online: https://www.ussteel.com/products-solutions/products/transformation-induced-plasticity-780 (accessed on 6 September 2018).
- Yu, D.; Huang, L.; Chen, Y.; Komolwit, P.; An, K. Real-time in situ neutron diffraction investigation of phase-specific load sharing in a cold-rolled TRIP sheet steel. JOM 2018, 70, 1576–1586. [Google Scholar] [CrossRef]
- An, K.; Skorpenske, H.D.; Stoica, A.D.; Ma, D.; Wang, X.-L.; Cakmak, E. First in situ lattice strains measurements under load at VULCAN. Metall. Mater. Trans. A 2011, 42, 95–99. [Google Scholar] [CrossRef]
- An, K. VDRIVE-Data Reduction and Interactive Visualization Software for Event Mode Neutron Diffraction; ORNL Report No. ORNL-TM-2012-621; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2012. [Google Scholar]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- De Meyer, M.; Vanderschueren, D.; De Blauwe, K.; De Cooman, B.C. Presented at the Characterization of Retained Austenite in TRIP Steels by X-ray Diffraction, ISS, 41st Mechanical Working and Steel Processing Conference, Baltimore, MD, USA, 24–27 October 1999; pp. 483–491.
- Onink, M.; Brakman, C.M.; Tichelaar, F.D.; Mittemeijer, E.J.; Van Der Zwaag, S.; Root, J.H.; Konyer, N.B. The lattice parameters of austenite and ferrite in Fe-C alloys as functions of carbon concentration and temperature. Scr. Metall. Mater. 1993, 29, 1011–1016. [Google Scholar] [CrossRef]
- Huang, J.; Vogel, S.C.; Poole, W.J.; Militzer, M.; Jacques, P. The study of low-temperature austenite decomposition in a Fe–C–Mn–Si steel using the neutron Bragg edge transmission technique. Acta Mater. 2007, 55, 2683–2693. [Google Scholar] [CrossRef]
- Lee, S.-J.; Lusk, M.T.; Lee, Y.-K. Conversional model of transformation strain to phase fraction in low alloy steels. Acta Mater. 2007, 55, 875–882. [Google Scholar] [CrossRef]
- Lee, S.-J.; Lee, S.; De Cooman, B.C. Mn partitioning during the intercritical annealing of ultrafine-grained 6% Mn transformation-induced plasticity steel. Scr. Mater. 2011, 64, 649–652. [Google Scholar] [CrossRef]
- Li, C.-M.; Sommer, F.; Mittemeijer, E.J. Characteristics of the γ→α transformation in Fe–Mn alloys. Mater. Sci. Eng. A 2002, 325, 307–319. [Google Scholar] [CrossRef]
- Lu, X.-G.; Selleby, M.; Sundman, B. Assessments of molar volume and thermal expansion for selected bcc, fcc and hcp metallic elements. Calphad 2005, 29, 68–89. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, D.; Chen, Y.; Huang, L.; An, K. Tracing Phase Transformation and Lattice Evolution in a TRIP Sheet Steel under High-Temperature Annealing by Real-Time In Situ Neutron Diffraction. Crystals 2018, 8, 360. https://doi.org/10.3390/cryst8090360
Yu D, Chen Y, Huang L, An K. Tracing Phase Transformation and Lattice Evolution in a TRIP Sheet Steel under High-Temperature Annealing by Real-Time In Situ Neutron Diffraction. Crystals. 2018; 8(9):360. https://doi.org/10.3390/cryst8090360
Chicago/Turabian StyleYu, Dunji, Yan Chen, Lu Huang, and Ke An. 2018. "Tracing Phase Transformation and Lattice Evolution in a TRIP Sheet Steel under High-Temperature Annealing by Real-Time In Situ Neutron Diffraction" Crystals 8, no. 9: 360. https://doi.org/10.3390/cryst8090360
APA StyleYu, D., Chen, Y., Huang, L., & An, K. (2018). Tracing Phase Transformation and Lattice Evolution in a TRIP Sheet Steel under High-Temperature Annealing by Real-Time In Situ Neutron Diffraction. Crystals, 8(9), 360. https://doi.org/10.3390/cryst8090360





