A Crystallographic Study of a Novel Tetrazolyl-Substituted Nitronyl Nitroxide Radical
Abstract
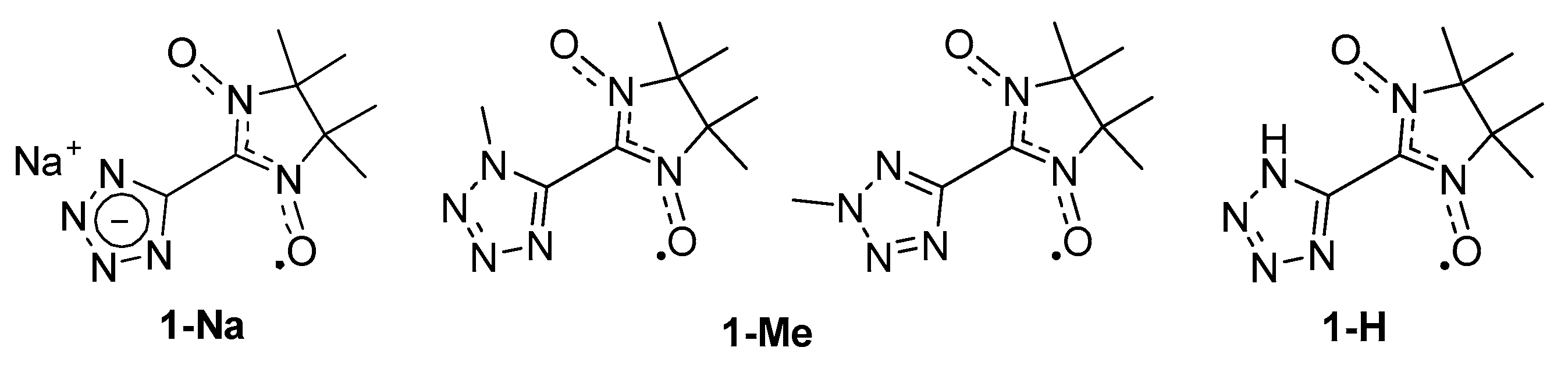
:1. Introduction
2. Materials and Methods
2.1. General Notes
2.2. 2-(1H-Tetrazol-5-Yl)-4,4,5,5-Tetramethyl-4,5-Dihydro-1H-Imidazol-3-Oxide-1-Oxyl (1-H)
2.3. Data Collection and Refinement
2.4. Computational Methods
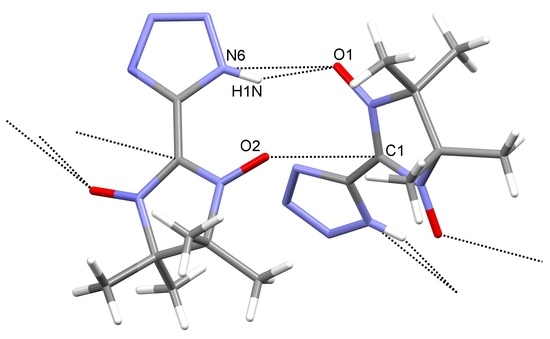
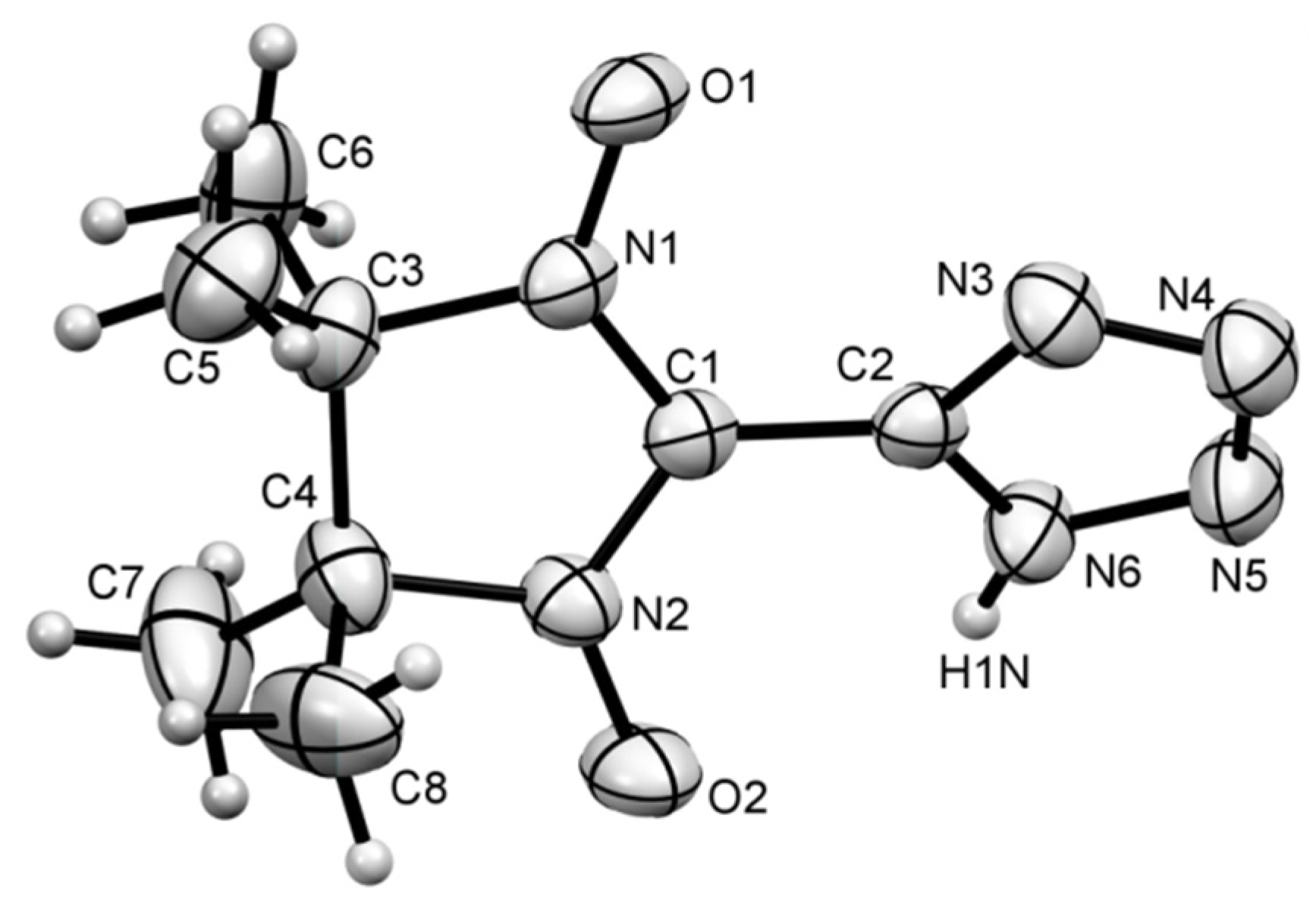
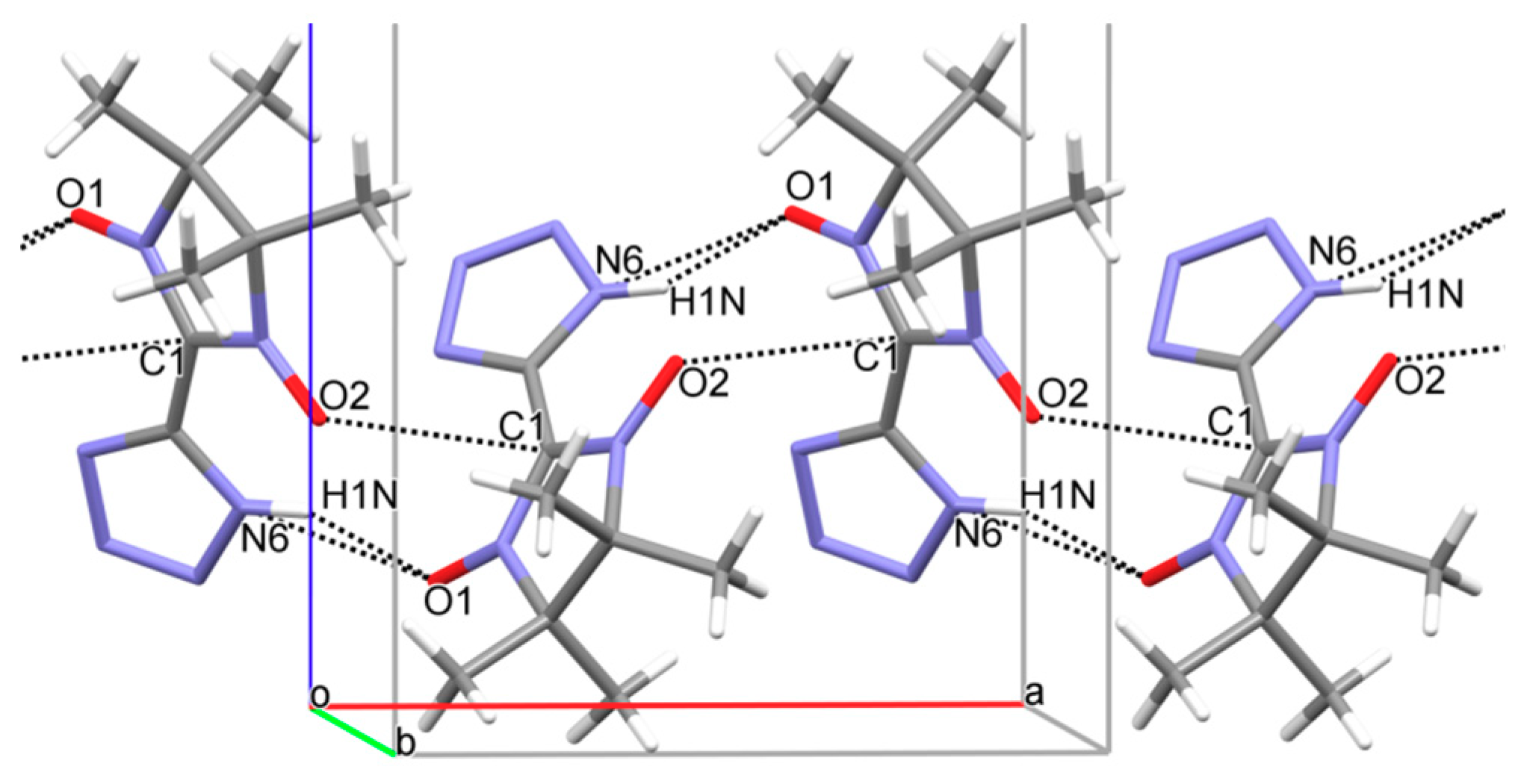
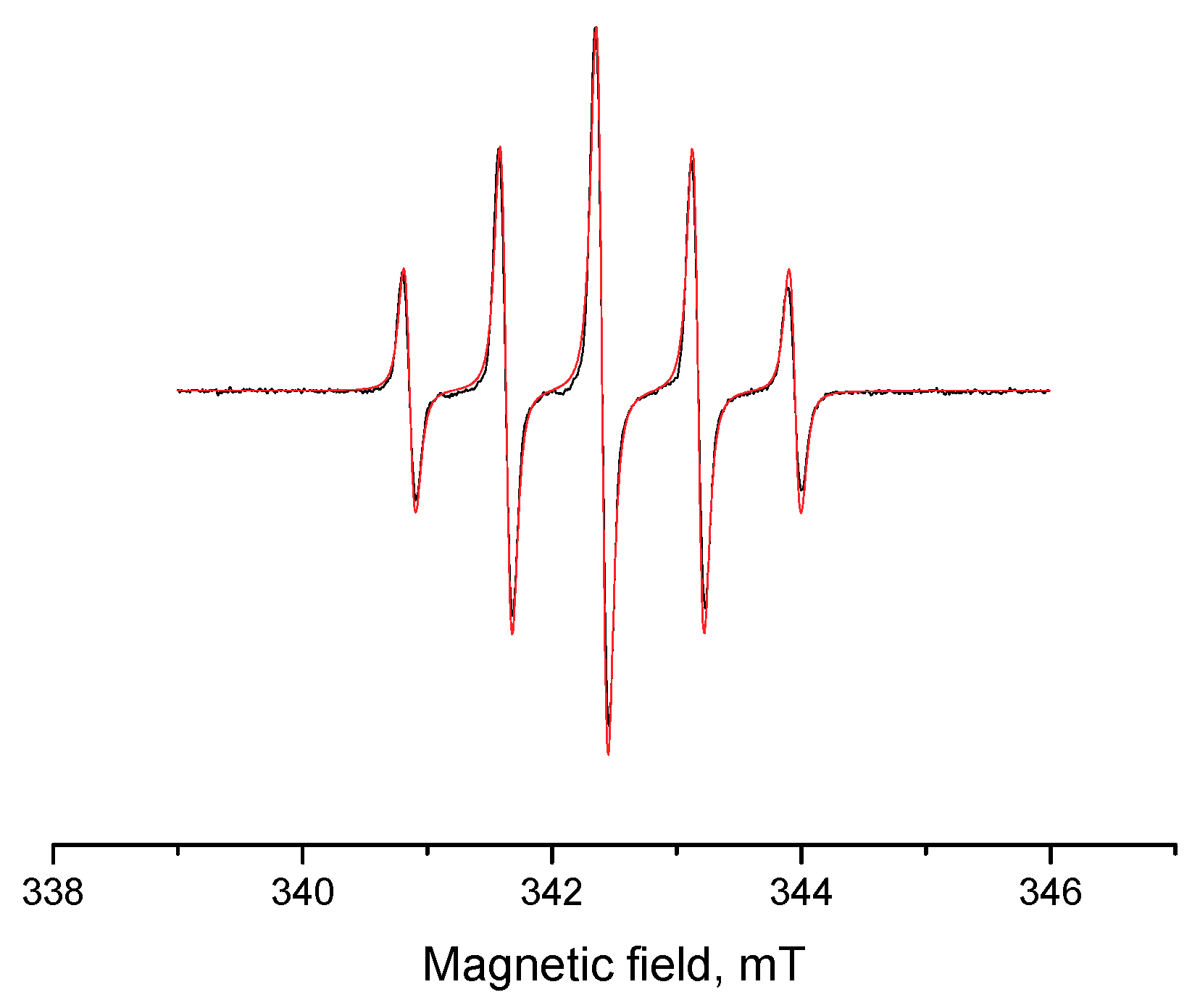
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Awaga, K.; Maruyama, Y. Ferromagnetic intermolecular interaction of the organic radical, 2-(4-nitrophenyl)-4,4,5,5-tetramethyl-4,5-dihydro-1H-imidazolyl-1-oxy 3-oxide. Chem. Phys. Lett. 1989, 158, 556–558. [Google Scholar] [CrossRef]
- Awaga, K.; Maruyama, Y. Ferromagnetic and antiferromagnetic intermolecular interactions of organic radicals, u-nitronyl nitroxides. J. Chem. Phys. 1989, 91, 2743–2747. [Google Scholar] [CrossRef]
- Awaga, K.; Inabe, T.; Nagashima, U.; Maruyama, Y. Two-dimensional network of the ferromagnetic organic radical, 2-(4-nitrophenyl)-4,4,5,5-tetramethyl-4,5-dihydro-1H-imidazol-1-oxyl 3-N-oxide. J. Chem. Soc. Chem. Commun. 1989, 1617–1618. [Google Scholar] [CrossRef]
- Sugimoto, T.; Tsujii, M.; Suga, T.; Hosoito, N.; Ishikawa, M.; Takeda, N.; Shiro, M. New Charge-Transfer Complex-Based Organic Ferromagnets: Pyridinium-Substitued Imidazolin-1-Oxyl/Tetrafluorotetracyanoquinodimethanide or Hexacyanobutadienide Salts. Mol. Cryst. Liq. Cryst. 1995, 272, 183–194. [Google Scholar] [CrossRef]
- Caneschi, A.; Gatteschi, D.; Rey, P.; Sessoli, R. Structure and magnetic properties of ferrimagnetic chains formed by manganese (II) and nitronyl nitroxides. Inorg. Chem. 1988, 27, 1756–1761. [Google Scholar] [CrossRef]
- Caneschi, A.; Gatteschi, D.; Renard, J.P.; Rey, P.; Sessoli, R. Magnetic phase transition and low-temperature EPR spectra of a one-dimensional ferrimagnet formed by manganese (II) and a nitronyl nitroxide. Inorg. Chem. 1989, 28, 1976–1980. [Google Scholar] [CrossRef]
- Caneschi, A.; Gatteschi, D.; Renard, J.P.; Rey, P.; Sessoli, R. Ferromagnetic phase transitions of two one-dimensional ferrimagnets formed by manganese (II) and nitronyl nitroxides cis octahedrally coordinated. Inorg. Chem. 1989, 28, 3314–3319. [Google Scholar] [CrossRef]
- Caneschi, A.; Gatteschi, D.; Sessoli, R.; Rey, P. Toward molecular magnets: the metal-radical approach. Acc. Chem. Res. 1989, 22, 392–398. [Google Scholar] [CrossRef]
- Lanfranc de Panthou, F.; Belorizky, E.; Calemczuk, R.; Luneau, D.; Marcenat, C.; Ressouche, E.; Turek, P.; Rey, P. A New Type of Thermally Induced Spin Transition Associated with an Equatorial .dblarw. Axial Conversion in a Copper(II)-Nitroxide Cluster. J. Am. Chem. Soc. 1995, 117, 11247–11253. [Google Scholar] [CrossRef]
- Likhtenshtein, G.I.; Yamauchi, J.; Nakatsuji, S.; Smirnov, A.I.; Tamura, R. Nitroxides: Applications in Chemistry, Biomedicine, and Materials Science, 1st ed.; Wiley-VCH: Weinheim, Germany, 2008; ISBN 978-3527318896. [Google Scholar]
- Rowen, S. Concepts and Applied Principles of Nitroxides; Research Press: New York, NY, USA, 2015. [Google Scholar]
- Romanov, V.; Vorob’ev, A.; Bagryanskaya, I.; Parkhomenko, D.; Tretyakov, E. 1,3-Dipolar Cycloaddition of a Nitronyl Nitroxide-Substituted Alkyne to Heteroaromatic N-Imines. Aust. J. Chem. 2017, 70, 1317–1320. [Google Scholar] [CrossRef]
- Tretyakov, E.V.; Fedyushin, P.A.; Panteleeva, E.V.; Stass, D.V.; Bagryanskaya, I.Yu.; Beregovaya, I.V.; Bogomyakov, A.S. Substitution of a Fluorine Atom in Perfluorobenzonitrile by a Lithiated Nitronyl Nitroxide. J. Org. Chem. 2017, 82, 4179–4185. [Google Scholar] [CrossRef] [PubMed]
- Tretyakov, E.V.; Peshkov, R.Yu.; Panteleeva, E.V.; Scrypnik, A.S.; Stass, D.V.; Romanenko, G.V.; Ovcharenko, V.I. Addition of Cyanomethyl Anion to the Cyano Group of 2-Cyano-4,4,5,5-tetramethyl-4,5-dihydro-1H-imidazol-3-oxide-1-oxyl, a Nitronyl Nitroxide. Tetrahedron Lett. 2016, 57, 2327–2330. [Google Scholar] [CrossRef]
- Depperman, E.C.; Bodnar, S.H.; Vostrikova, K.E.; Shultz, D.A.; Kirk, M.L. Spin Robustness of a New Hybrid Inorganic−Organic High-Spin Molecule. J. Am. Chem. Soc. 2001, 123, 3133–3134. [Google Scholar] [CrossRef] [PubMed]
- Gaponik, P.N.; Voitekhovich, S.V.; Ivashkevich, О.А. Metal Derivatives of Tetrazoles. Russ. Chem. Rev. 2006, 75, 507–539. [Google Scholar] [CrossRef]
- Aromi, G.; Barrios, L.A.; Roubeau, O.; Gamez, P. Triazoles and Tetrazoles: Prime Ligands to Generate Remarkable Coordination Materials. Coord. Chem. Rev. 2011, 255, 485–546. [Google Scholar] [CrossRef]
- Massi, M.; Stagni, S.; Ogden, M.I. Lanthanoid Tetrazole Coordination Complexes. Coord. Chem. Rev. 2017, in press. [Google Scholar] [CrossRef]
- Tretyakov, E.V.; Fokin, S.V.; Romanenko, G.V.; Ovcharenko, V.I. Nitronyl Nitroxides Containing Tetrazole Substituents and Metal Complexes with Spin-Labeled Tetrazole. Polyhedron 2003, 22, 1965–1972. [Google Scholar] [CrossRef]
- Tretyakov, E.V.; Fokin, S.V.; Romanenko, G.V.; Ikorskii, V.N.; Podoplelov, A.V.; Ovcharenko, V.I. Copper(II) bis(hexafluoroacetylacetonate) complexes with 2-(2-methyltetrazolyl)-4,4,5,5-tetramethyl-4,5-dihydro-1H-imidazole 1-oxides. Russ. Chem. Bull. 2006, 55, 66–73. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS. Version 2.01; Bruker AXS Inc.: Madison, WI, USA, 2004. [Google Scholar]
- Sheldrick, G.M. SHELXTL. Version 6.10; Bruker AXS Inc.: Madison, WI, USA, 2004. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Clarke, C.S.; Jornet-Somoza, J.; Mota, F.; Novoa, J.J.; Deumal, M. Origin of the Magnetic Bistability in Molecule-Based Magnets: A First-Principles Bottom-Up Study of the TTTA Crystal. J. Am. Chem. Soc. 2010, 132, 17817–17830. [Google Scholar] [CrossRef] [PubMed]
- Tolstikov, S.E.; Tretyakov, E.V.; Gorbunov, D.E.; Zhurko, I.F.; Fedin, M.V.; Romanenko, G.V.; Bogomyakov, A.S.; Gritsan, N.P.; Mazhukin, D.G. Reaction of Paramagnetic Synthon, Lithiated 4,4,5,5-Tetramethyl-4,5-dihydro-1H-imidazol-1-oxyl 3-oxide, with Cyclic Aldonitrones of the Imidazole Series. Chem. Eur. J. 2016, 22, 14598–14604. [Google Scholar] [CrossRef] [PubMed]
- Gritsan, N.P.; Zibarev, A.V. Chalcogen-Nitrogen Π-Heterocyclic Radical Anion Salts: The Synthesis and Properties. Russ. Chem. Bull. 2011, 60, 2131–2140. [Google Scholar] [CrossRef]
- Soda, T.; Kitagawa, Y.; Onishi, T.; Takano, Y.; Shigeta, Y.; Nagao, H.; Yoshioka, Y.; Yamaguchi, K. Ab Initio Computations of Effective Exchange Integrals for H–H, H–He–H And Mn2o2 Complex: Comparison of Broken-Symmetry Approaches. Chem. Phys. Lett. 2000, 319, 223–230. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. ORCA–An Ab Initio, Density Functional and Semiempirical Program Package. Version 2008, 2, 35. [Google Scholar]
- Allen, F.H.; Kenard, O.; Watson, D.G.; Bramer, L.; Orpen, A.G.; Taylor, R. Tables of Bond Lengths Determined By X-ray and Neutron Diffraction. Part 1. Bond Lengths in Organic Compounds. J. Chem. Soc. Perkin Trans. II 1987, 0, S1–S19. [Google Scholar] [CrossRef]
- McConnell, H.M. Ferromagnetism in Solid Free Radicals. J. Chem. Phys. 1963, 39, 1910. [Google Scholar] [CrossRef]
- Naoki, Y.; Munetoshi, I.; Yuichiro, M.; Takanari, K.; Hidenari, I.; Shigeru, O. Unusually Large Magnetic Interactions Observed in Hydrogen-Bonded Nitronyl Nitroxides. Chem. Lett. 1997, 26, 251–252. [Google Scholar]
- Inoue, K.; Iwamura, H. Magnetic Properties of the Crystals of p-(1-oxyl-3-oxido-4,4,5,5-tetramethyl-2-imidazolin-2-yl)benzoic acid and Its Alkali Metal Salts. Chem. Phys. Lett. 1993, 207, 551–554. [Google Scholar] [CrossRef]
- Zheludev, A.; Barone, V.; Bonnet, M.; Delley, B.; Grand, A.; Ressouche, E.; Rey, P.; Subra, R.; Schweizer, J. Spin Density in a Nitronyl Nitroxide Free Radical. Polarized Neutron Diffraction Investigation and Ab Initio Calculations. J. Am. Chem. Soc. 1994, 116, 2019–2027. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanov, V.E.; Bagryanskaya, I.Y.; Gorbunov, D.E.; Gritsan, N.P.; Zaytseva, E.V.; Luneau, D.; Tretyakov, E.V. A Crystallographic Study of a Novel Tetrazolyl-Substituted Nitronyl Nitroxide Radical. Crystals 2018, 8, 334. https://doi.org/10.3390/cryst8090334
Romanov VE, Bagryanskaya IY, Gorbunov DE, Gritsan NP, Zaytseva EV, Luneau D, Tretyakov EV. A Crystallographic Study of a Novel Tetrazolyl-Substituted Nitronyl Nitroxide Radical. Crystals. 2018; 8(9):334. https://doi.org/10.3390/cryst8090334
Chicago/Turabian StyleRomanov, Vasily E., Irina Yu. Bagryanskaya, Dmitry E. Gorbunov, Nina P. Gritsan, Elena V. Zaytseva, Dominique Luneau, and Evgeny V. Tretyakov. 2018. "A Crystallographic Study of a Novel Tetrazolyl-Substituted Nitronyl Nitroxide Radical" Crystals 8, no. 9: 334. https://doi.org/10.3390/cryst8090334
APA StyleRomanov, V. E., Bagryanskaya, I. Y., Gorbunov, D. E., Gritsan, N. P., Zaytseva, E. V., Luneau, D., & Tretyakov, E. V. (2018). A Crystallographic Study of a Novel Tetrazolyl-Substituted Nitronyl Nitroxide Radical. Crystals, 8(9), 334. https://doi.org/10.3390/cryst8090334