Copper Delafossites under High Pressure—A Brief Review of XRD and Raman Spectroscopic Studies
Abstract
1. Introduction
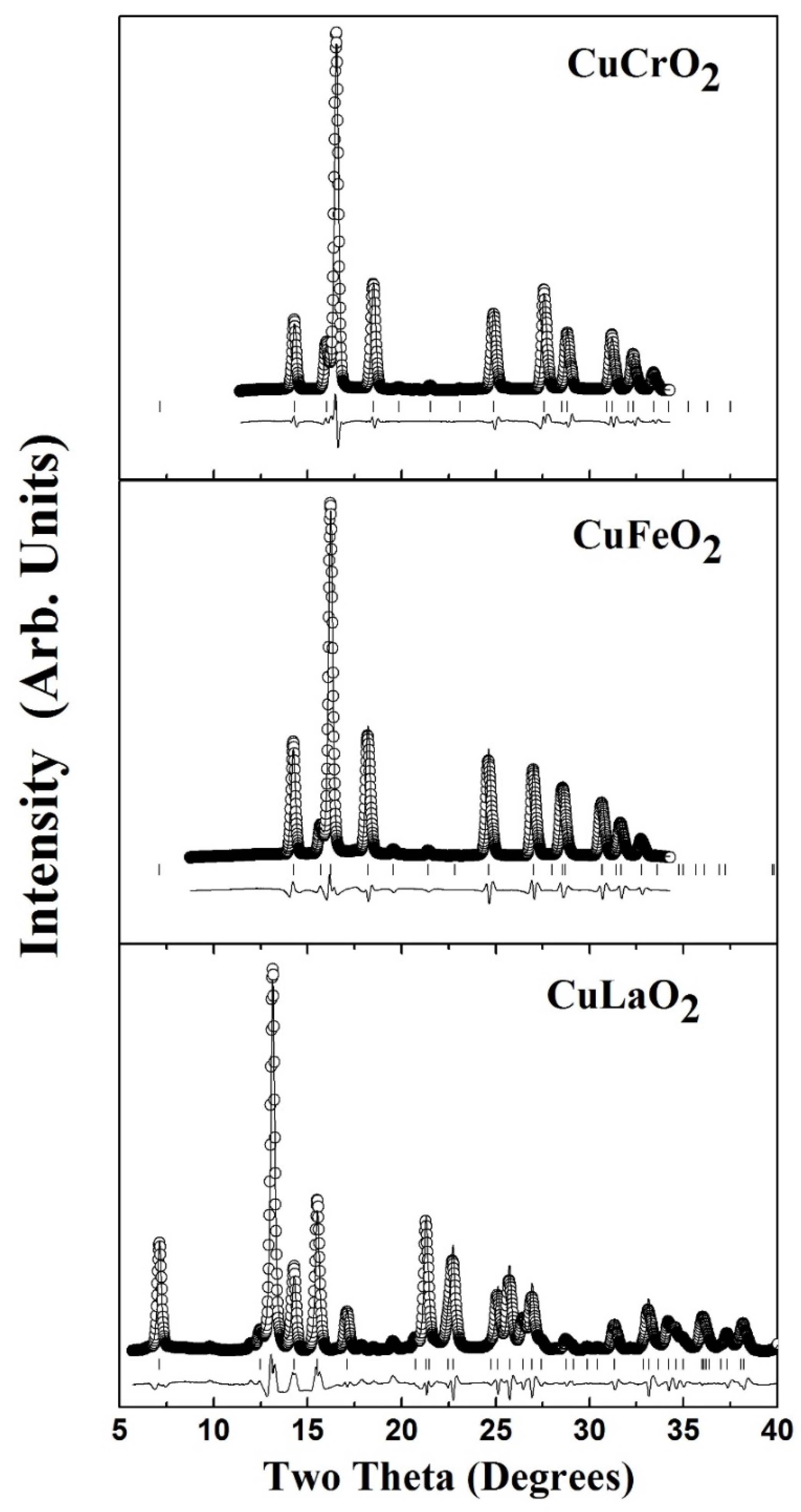
2. Ambient Crystal Structure and Vibrational Properties of ABO2
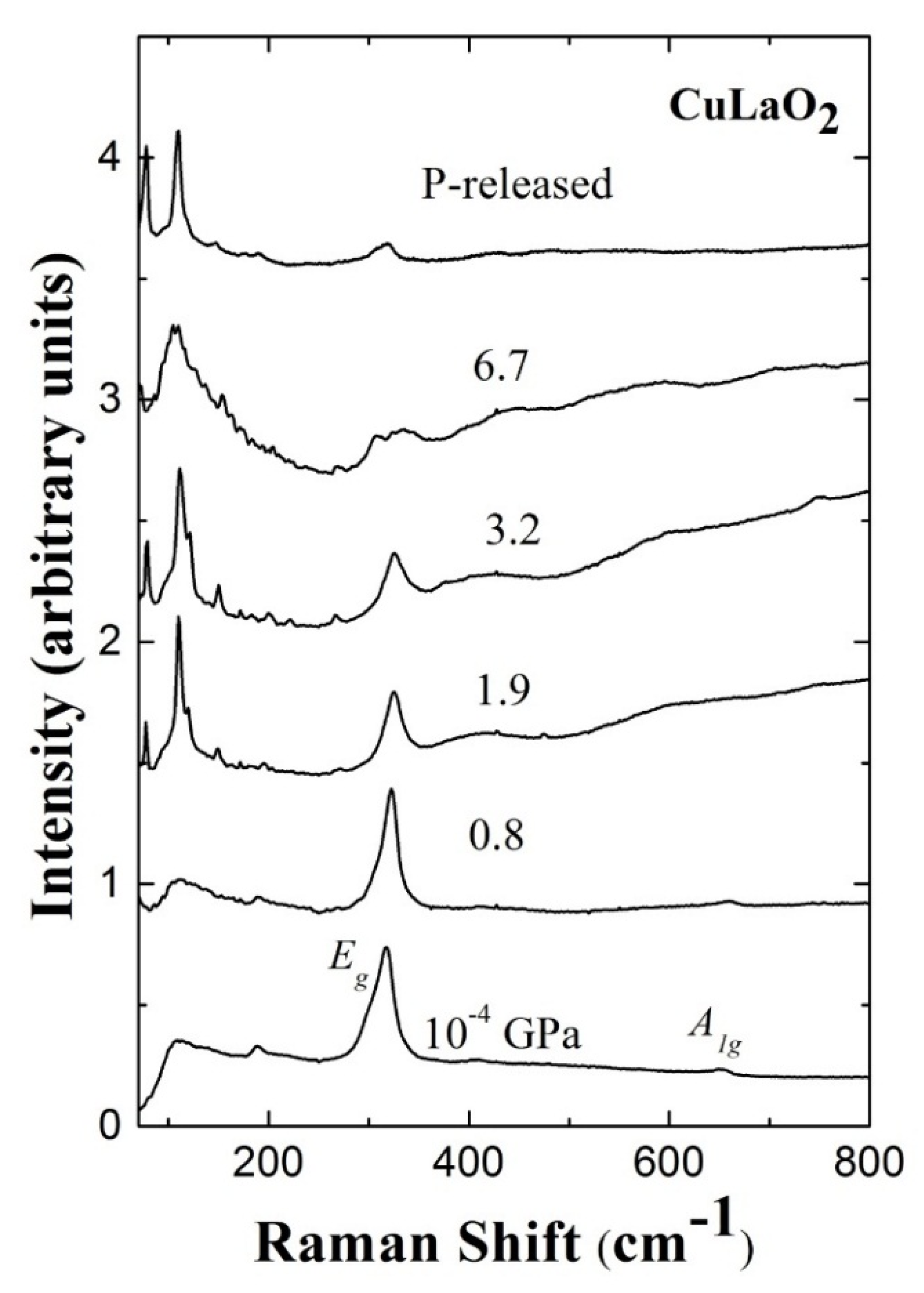
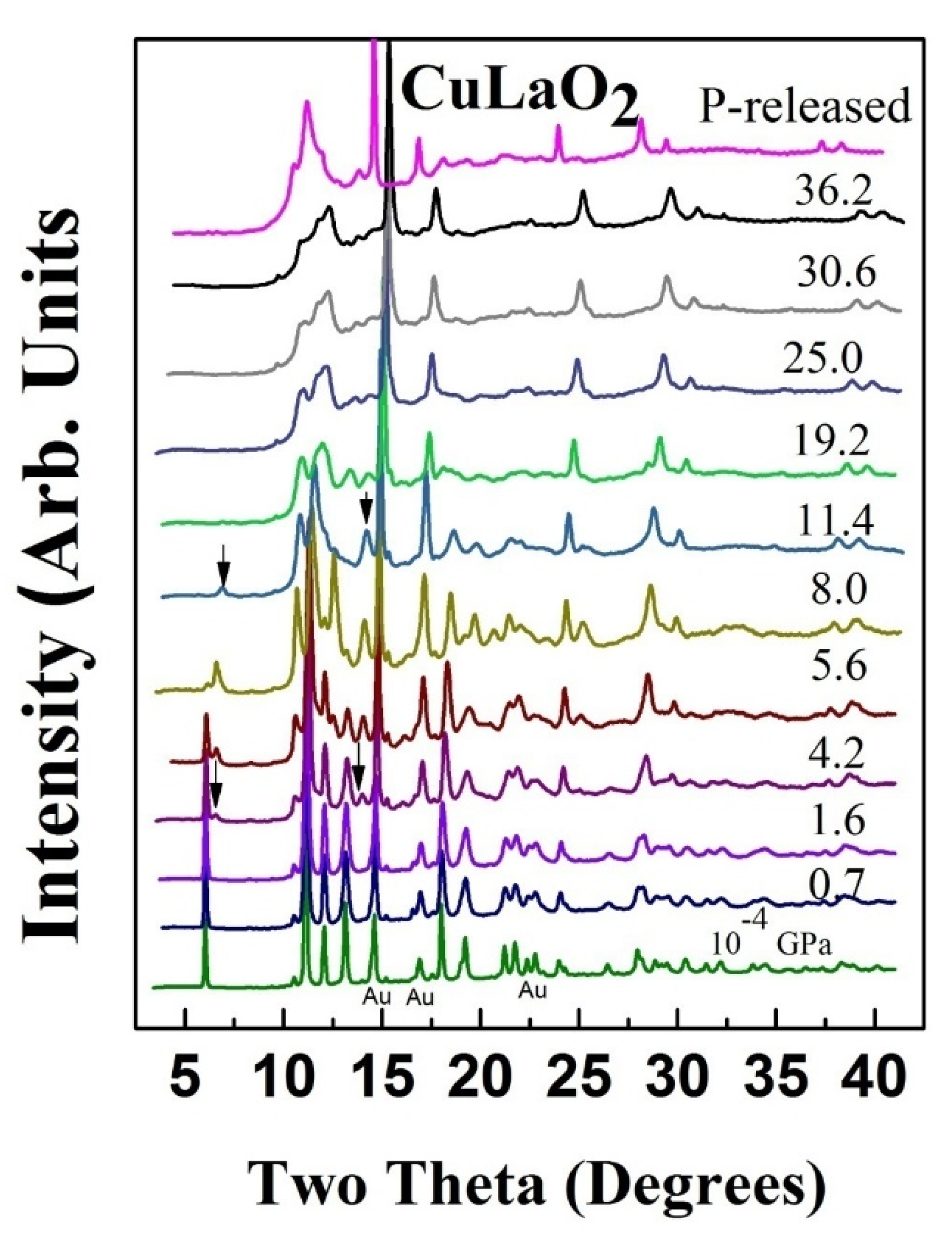
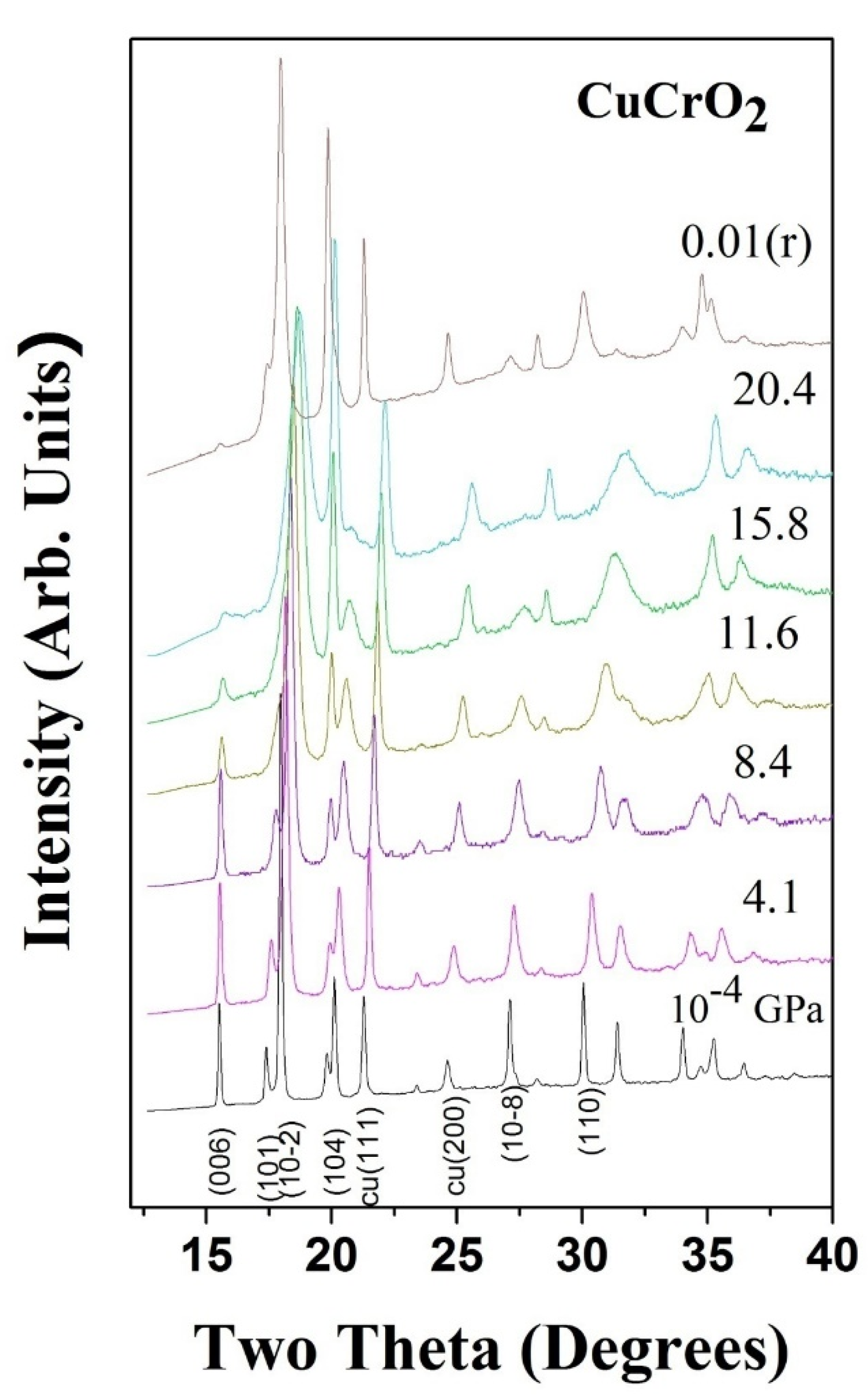
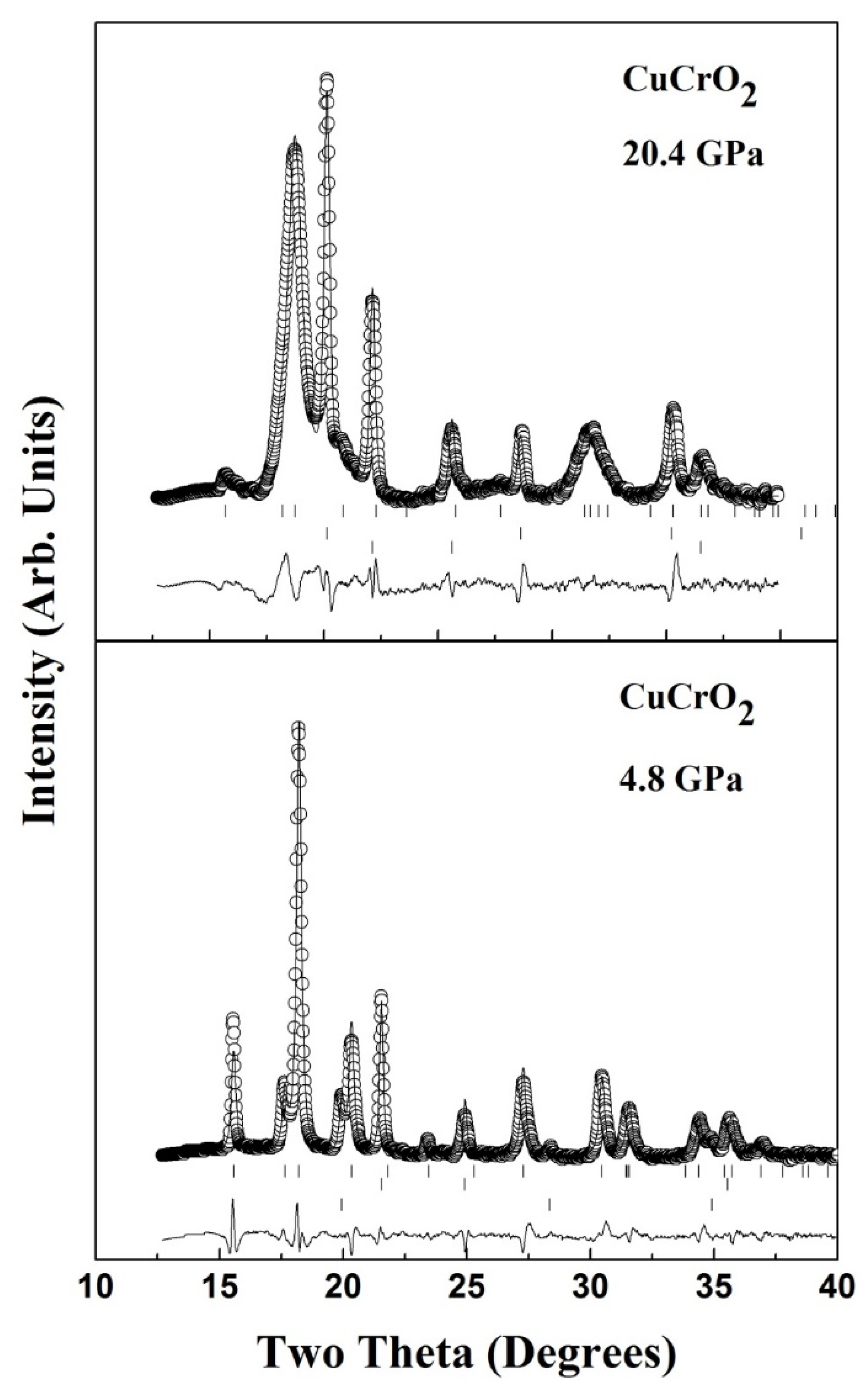
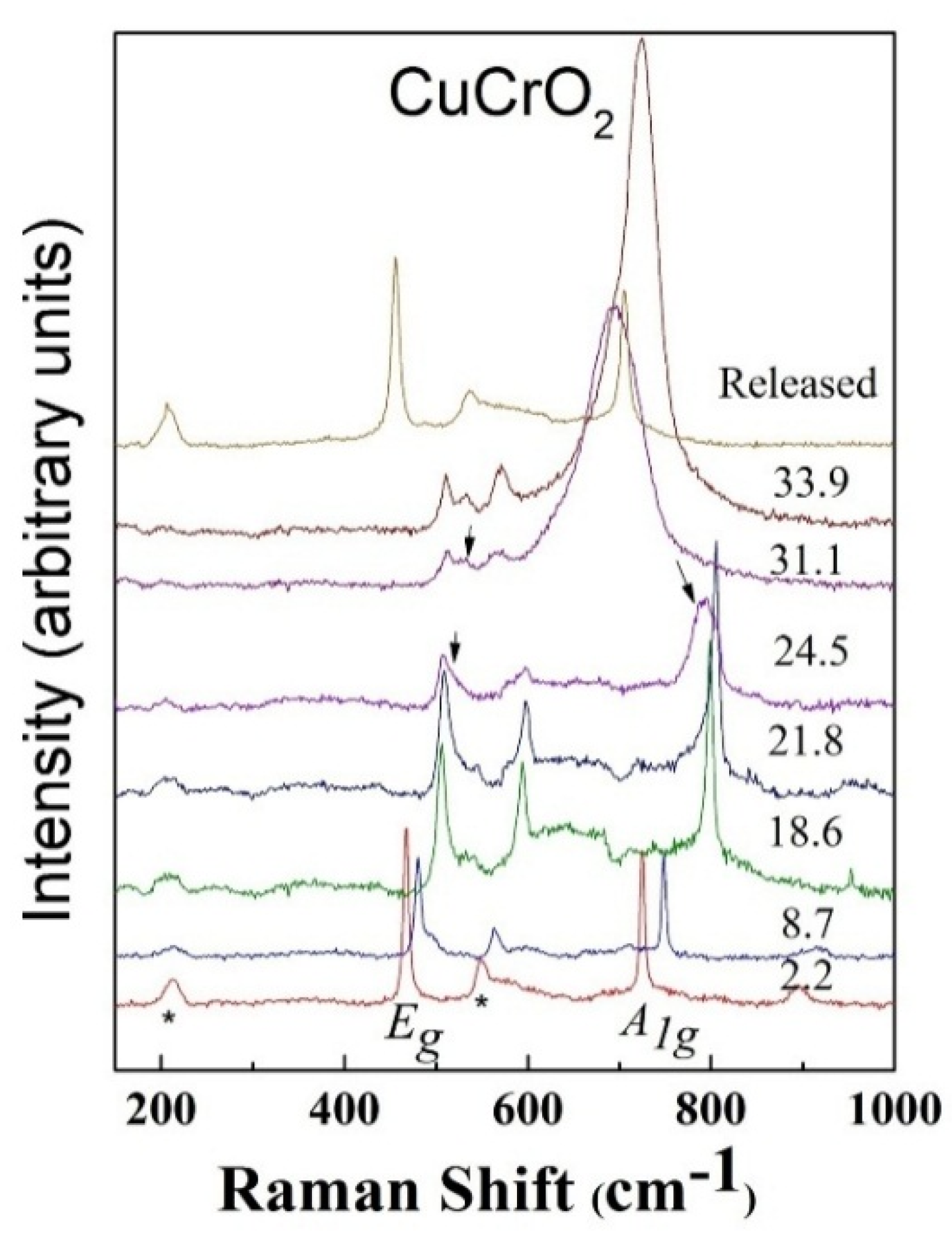
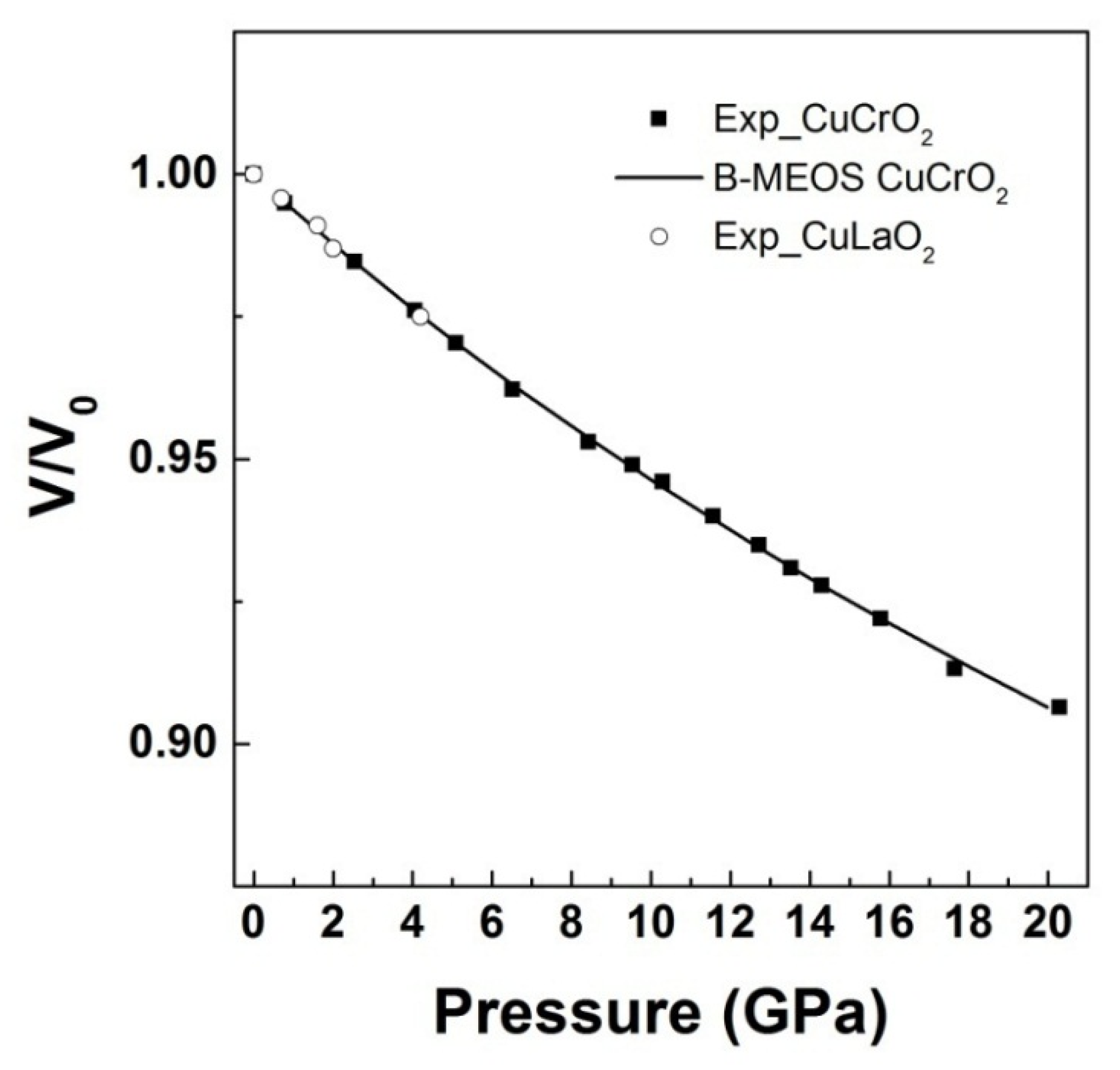
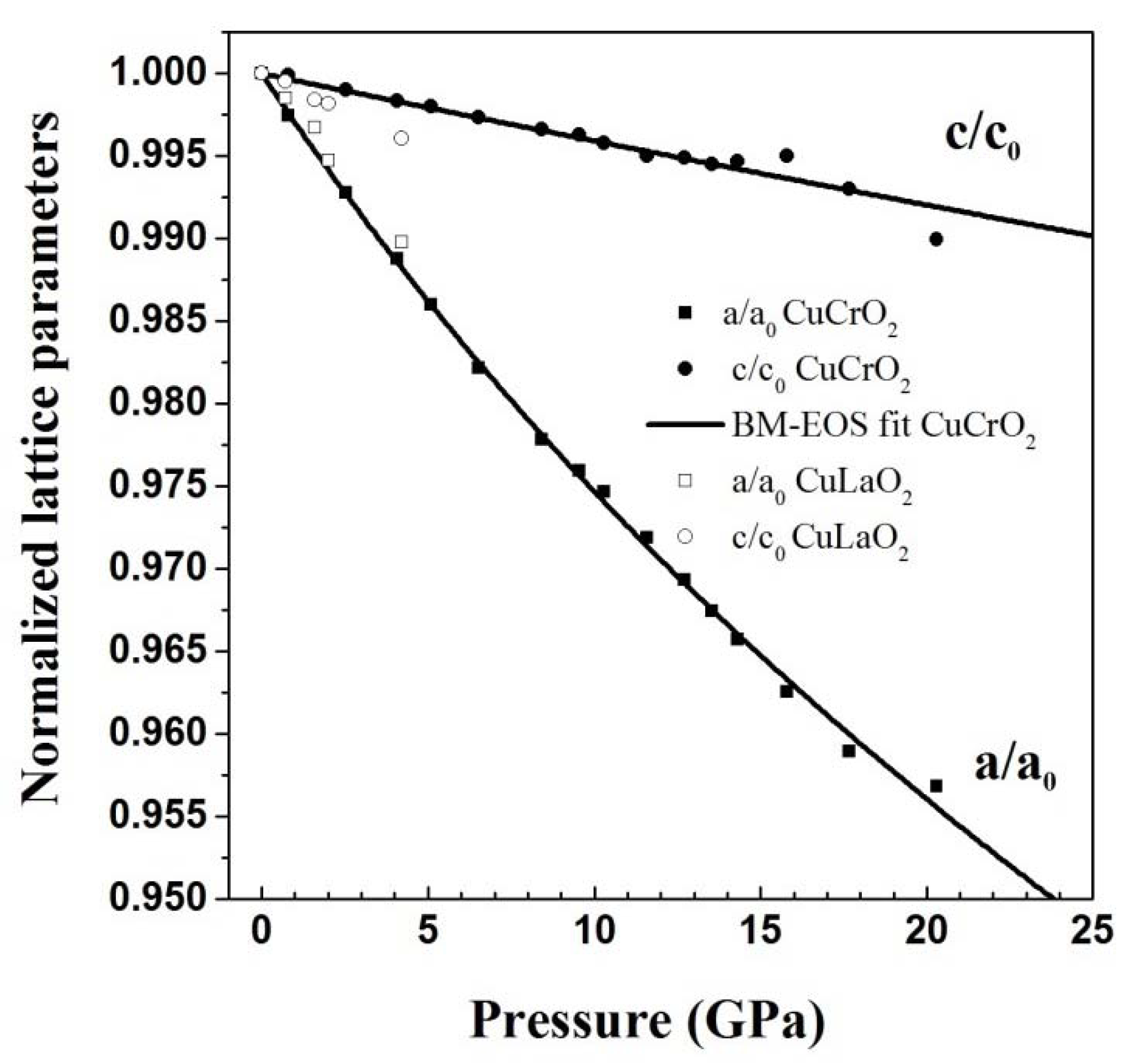
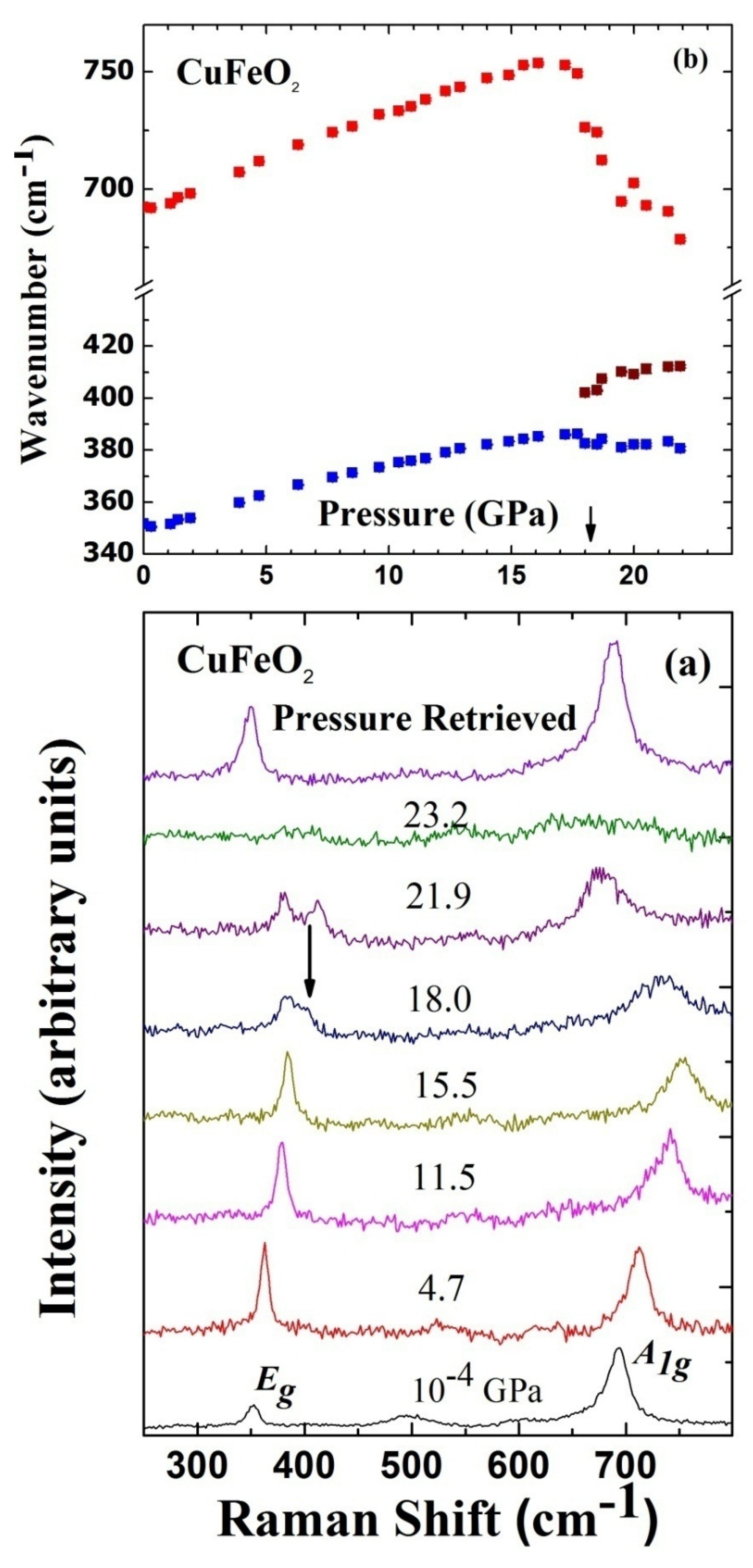
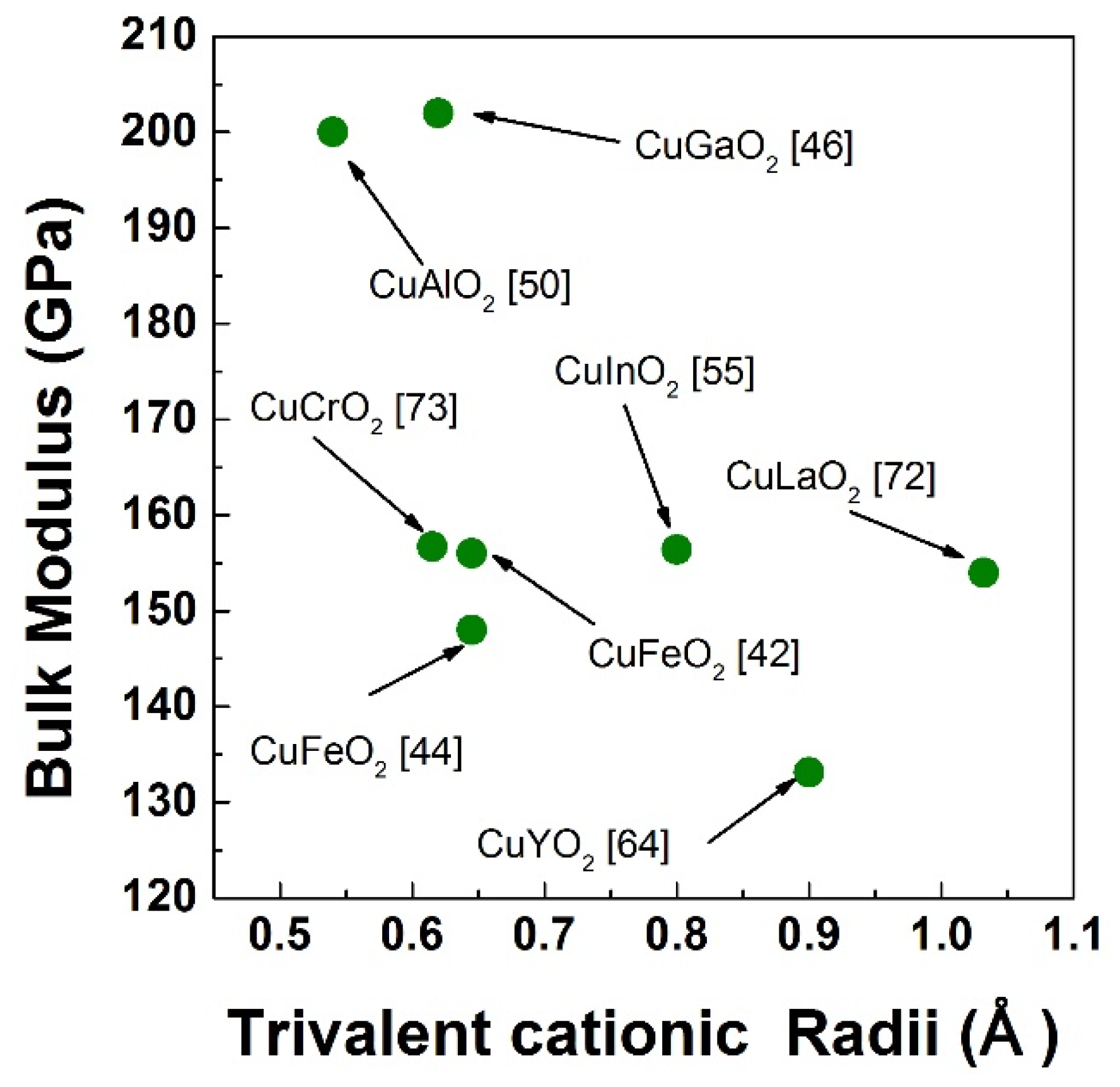
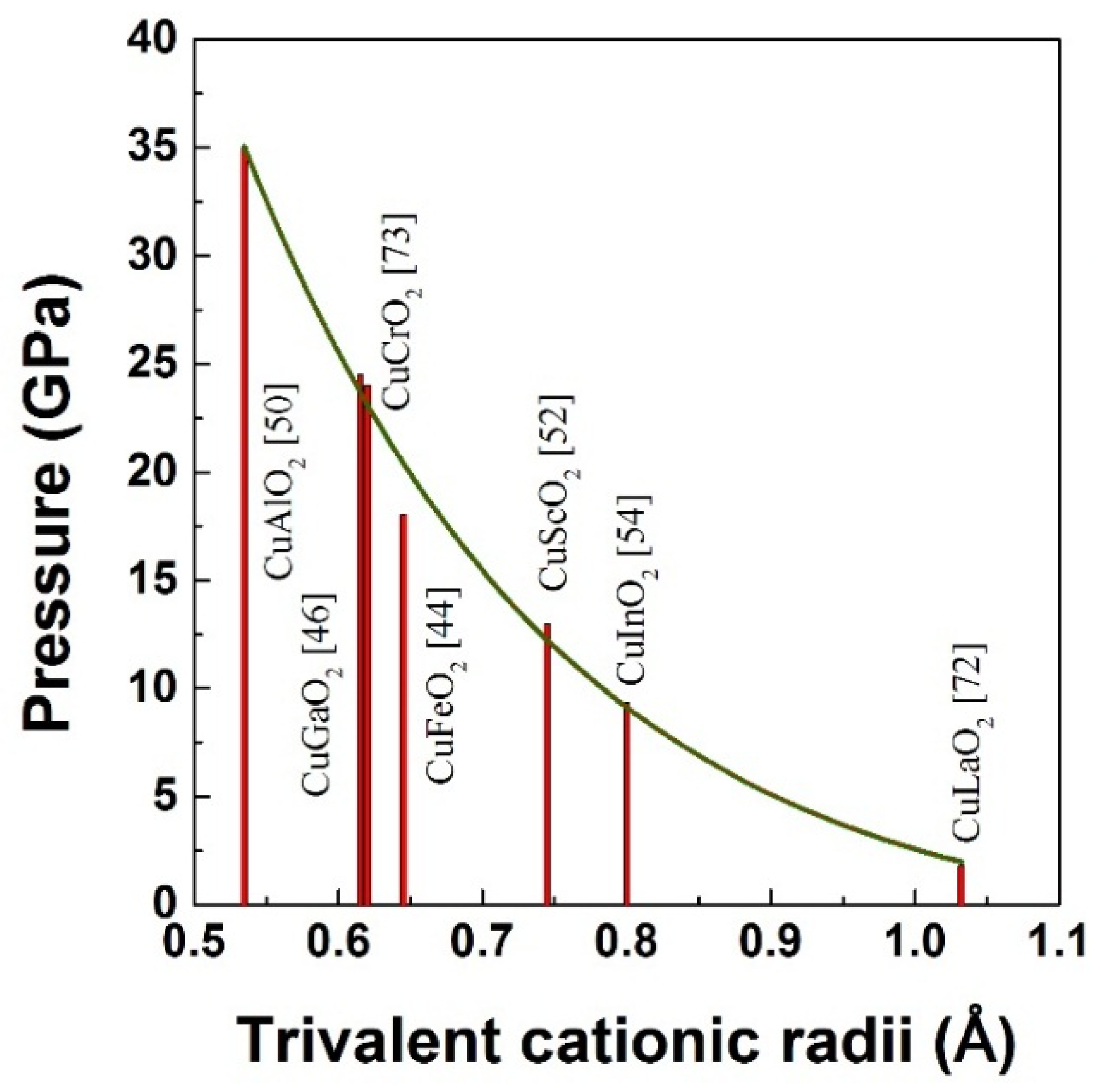
3. High Pressure Studies
4. Summary
Conflicts of Interest
References
- Shannon, R.D.; Rogers, D.B.; Prewitt, C.T. Chemistry of noble metal oxides I. Syntheses and properties of ABO2 delafossite compounds. Inorg. Chem. 1971, 10, 713–718. [Google Scholar] [CrossRef]
- Dordor, P.; Chaminade, J.P.; Wichainchai, A.; Marquestaut, E.; Doumerc, J.P.; Pouchard, M.; Hagenmuller, P. Crystal growth and electrical properties of CuFeO2 single crystals. J. Solid State Chem. 1988, 75, 105–112. [Google Scholar] [CrossRef]
- Marquardt, M.A.; Ashmore, N.A.; Cann, D.P. Crystal chemistry and electrical properties of the delafossite structure. Thin Solid Films 2006, 496, 146–156. [Google Scholar] [CrossRef]
- Kawazoe, H.; Yasukawa, M.; Hyodo, H.; Kurita, M.; Yanagi, H.; Hosono, H. P-type electrical conduction in transparent thin films of CuAlO2. Nature 1997, 389, 939–942. [Google Scholar] [CrossRef]
- Mackenzie, A.P. The Properties of ultrapure delafossite metals. Rep. Prog. Phys. 2017, 80, 032501–032519. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.N.; Chattopadhyay, K.K. Recent developments in the emerging field of crystalline p-type transparent conducting oxide thin films. Prog. Cryst. Growth Charact. Mater. 2005, 50, 52–105. [Google Scholar] [CrossRef]
- Walsh, A.; Da Silva, J.L.F.; Wei, S.-H. Multi-component transparent conducting oxides: Progress in materials modelling. J. Phys. Condens. Matter 2011, 23, 334210. [Google Scholar] [CrossRef] [PubMed]
- Sheng, S.; Fang, G.; Li, C.; Xu, S.; Zhao, X. P-type transparent conducting oxides. Phys. Status Solidi A 2006, 203, 1891–1900. [Google Scholar] [CrossRef]
- King, P.D.C.; Veal, T.D. Conductivity in transparent oxide semiconductors. J. Phys. Condens. Matter 2011, 23, 334214. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Natu, G.; Ji, Z.; Wu, Y. P-type dye-sensitized solar cells based on delafossite CuGaO2 nanoplates with saturation photovoltages exceeding 460 mv. J. Phys. Chem. Lett. 2012, 3, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
- Chae, G.J. A modified transparent conducting oxide for flat panel displays only. J. Appl. Phys. 2001, 40, 1282–1286. [Google Scholar] [CrossRef]
- Granqvist, C.G.; Azens, A.; Hjelm, A.; Kullman, L.; Niklasson, G.A.; Ronnow, D.; Mattsson, M.S.; Veszele, M.; Vaiva, G. Recent advances in electrochromics for smart windows applications. Sol. Energy 1998, 63, 199–276. [Google Scholar] [CrossRef]
- Diaz-Garcia, A.K.; Lana-Villarreal, T.; Gomez, R. Sol–gel copper chromium delafossite thin films as stable oxide photocathodes for water splitting. J. Mater. Chem. A 2015, 3, 19683–19687. [Google Scholar] [CrossRef]
- Gurunathan, K.; Baeg, J.O.; Lee, S.M.; Subramanian, E.; Moon, S.J.; Kong, K.J. Visible light assisted highly efficient hydrogen production from H2S decomposition by CuGaO2 and CuGa1−xInxO2 delafossite oxides bearing nanostructured co-catalysts. Catal. Commun. 2008, 9, 395–402. [Google Scholar] [CrossRef]
- Kykyneshi, R.; Nielsen, B.C.; Tate, J.; Li, J.; Sleight, A.W. Structural and transport properties of CuSc1−xMgxO2+ydelafossites. J. Appl. Phys. 2004, 96, 6188–6194. [Google Scholar] [CrossRef]
- Mazumder, N.; Sen, D.; Ghorai, U.K.; Roy, R.; Saha, S.; Das, N.S.; Chattopadhyay, K.K. Realizing direct gap, polytype, group IIIA delafossite: Ab initio forecast and experimental validation considering prototype CuAlO2. J. Phys. Chem. Lett. 2013, 4, 3539–3543. [Google Scholar] [CrossRef]
- Qiu, X.; Liu, M.; Sunada, K.; Miyauchi, M.; Hashimoto, K. A facile one-step hydrothermal synthesis of rhombohedral CuFeO2 crystals with antivirus property. Chem. Commun. 2012, 48, 7365–7367. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Cao, C.; Chui, Y.S.; Zapien, J.A. Facile hydrothermal synthesis of CuFeO2 hexagonal platelets/rings and graphene composites as anode materials for lithium ion batteries. Chem. Commun. 2014, 50, 10151–10154. [Google Scholar] [CrossRef] [PubMed]
- Patzsch, J.; Balog, I.; Krau, P.; Lehmann, C.W.; Schneider, J.J. Synthesis, characterization and p–n type gas sensing behaviour of CuFeO2delafossite type inorganic wires using Fe and Cu complexes as single source molecular precursors. RSC Adv. 2014, 4, 15348–15355. [Google Scholar] [CrossRef]
- Amrute, A.P.; Larrazabal, G.O.; Mondelli, C.; Perez-Ramirez, J. CuCrO2Delafossite: A stable copper catalyst for chlorine production. Angew. Chem. Int. Ed. 2013, 52, 9772–9775. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.I.; Dalba, G.; Fornasini, P.; Vaccari, M.; Rocca, F.; Sanson, A.; Li, J.; Sleight, A.W. Negative thermal expansion in crystals with the delafossite structure: An extended X-ray absorption fine structure study of CuScO2 and CuLaO2. Phys. Rev. B 2009, 79, 104302. [Google Scholar] [CrossRef]
- Seki, S.; Onose, Y.; Tokura, Y. Spin-driven ferroelectricity in triangular lattice antiferromagnets ACrO2 (A = Cu, Ag, Li, or Na). Phys. Rev. Lett. 2008, 101, 067204. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Cao, H.; Fang, J.; Jiang, X.; Ji, X.; Dong, Z. Spin-lattice coupling and helical-spin driven ferroelectric polarization in multiferroic CuFeO2. Appl. Phys. Lett. 2010, 97, 094103. [Google Scholar] [CrossRef]
- Omata, T.; Nagatani, H.; Suzuki, I.; Kita, M.; Yanagi, H.; Ohashi, N. A new direct and narrow band gap oxide semiconductor applicable as a solar cell absorber. J. Am. Chem. Soc. 2014, 136, 3378–3381. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gong, Y.; Mellott, N.P.; Wang, B.; Ye, H.; Wu, Y. Luminescence of delafossite-type CuAlO2 fibers with Eu substitution for Al cations. Sci. Technol. Adv. Mater. 2016, 17, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wang, F.; Wang, Y.; Wang, D.; Zhao, B.; Zhang, L.; Zhao, D.; Shen, D. Photoluminescence and photocatalytic properties of rhombohedral CuGaO2 nanoplates. Sci. Rep. 2016, 6, 21135. [Google Scholar] [CrossRef] [PubMed]
- Yassin, O.A.; Alamri, S.N.; Joraid, A.A. Effect of particle size and laser power on the Raman spectra of CuAlO2 delafossite nanoparticles. J. Phys. D Appl. Phys. 2013, 46, 235301. [Google Scholar] [CrossRef]
- Ahmed, J.; Mao, Y. Delafossite CuAlO2 nanoparticles with electrocatalytic activity toward oxygen and hydrogen evolution reactions. In Nanomaterials for Sustainable Energy; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2015; Chapter 4; Volume 1213, pp. 57–72. [Google Scholar]
- Harada, T.; Fujiwara, K.; Tsukazaki, A. Highly conductive PdCoO2 ultrathin films for transparent electrodes. APL Mater. 2018, 6, 046107. [Google Scholar] [CrossRef]
- Deng, Z.; Fang, X.; Wu, S.; Dong, W.; Shao, J.; Wang, S.; Lei, M. The morphologies and optoelectronic properties of delafossite CuFeO2 thin films prepared by PEG assisted. J. Sol-Gel Sci. Technol. 2014, 71, 297–302. [Google Scholar] [CrossRef]
- Sinnarasa, I.; Thimont, Y.; Presmanes, L.; Barnabé, A.; Tailhades, P. Thermoelectric and transport properties of delafossite CuCrO2:Mg thin films prepared by RF magnetron sputtering. Nanomaterials 2017, 7, 157. [Google Scholar] [CrossRef] [PubMed]
- Barnabe, A.; Thimont, Y.; Lalanne, M.; Presmanes, L.; Tailhades, P. P-type conducting transparent characteristics of delafossite Mg-doped CuCrO2 thin films prepared by RF-sputtering. J. Mater. Chem. C 2015, 3, 6012–6024. [Google Scholar] [CrossRef]
- Errandonea, D. Exploring the properties of MTO4 compounds using high-pressure powder X-ray diffraction. Cryst. Res. Technol. 2015, 50, 729–736. [Google Scholar] [CrossRef]
- Errandonea, D.; Ruiz-Fuertes, A. Brief review of the effects of pressure on wolframite-type oxides. Crystals 2018, 8, 71. [Google Scholar] [CrossRef]
- Hasegawa, M.; Tanaka, M.; Yagi, T.; Takei, H.; Inoue, A. Compression behavior of the delafossite-type metallic oxide PdCoO2 below 10 GPa. Solid State Commun. 2003, 128, 303–307. [Google Scholar] [CrossRef]
- Sheets, W.C.; Mugnier, E.; Barnabe, A.; Marks, T.J.; Poeppelmeier, K.R. Hydrothermal synthesis of delafossite-type oxides. Chem. Mater. 2006, 18, 7–20. [Google Scholar] [CrossRef]
- Kumar, S.; Miclau, M.; Christine, M. Hydrothermal synthesis of AgCrO2 delafossite in supercritical water: A new single-step process. Chem. Mater. 2013, 25, 2083–2088. [Google Scholar] [CrossRef]
- Jin, Y.; Chuamanov, G. Solution synthesis of pure 2H CuFeO2 at low temperatures. RSC Adv. 2016, 6, 26392–26397. [Google Scholar] [CrossRef]
- Effenberger, H. Structure of Hexagonal Copper(I) Ferrite. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1991, 47, 2644–2646. [Google Scholar] [CrossRef]
- Godinho, K.G.; Morgan, B.J.; Allen, J.P.; Scanlon, D.O.; Watson, G.W. Chemical bonding in copper-based transparent conducting oxides: CuMO2 (M = In, Ga, Sc). J. Phys. Condens. Matter 2011, 23, 334201. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, D.L.; Bauman, R.P.; Porto, S.P.S. Normal mode determination in crystals. J. Raman Spectrosc. 1981, 10, 253–290. [Google Scholar] [CrossRef]
- Zhao, T.R. X-ray diffraction study of copper iron oxide [CuFeO2] under pressures up to 10 GPa. Mater. Res. Bull. 1997, 32, 151–157. [Google Scholar] [CrossRef]
- Birch, F. Finite strain isotherm and velocities for single-crystal and polycrystalline NaCl at high pressures and 300° K. J. Geophys. Res. Solid Earth 1978, 83, 1257–1268. [Google Scholar] [CrossRef]
- Xu, W.M.; Rozenberg, G.K.; Pasternak, M.P.; Kertzer, M.; Kurnosov, A.; Dubrovinsky, L.S.; Pascarelli, S.; Munoz, M.; Vaccari, M.; Hanfland, M.; et al. Pressure-induced Fe-Cu cationic valence exchange and its structural consequences: High-pressure studies of delafossite CuFeO2. Phys. Rev. B 2010, 81, 104110. [Google Scholar] [CrossRef]
- Terada, N.; Osakabe, T.; Kitazawa, H. High-pressure suppression of long range magnetic order in the triangular lattice antiferromagnet CuFeO2. Phys. Rev. B 2010, 83, 020403. [Google Scholar] [CrossRef]
- Pellicer-Porres, J.; Segura, A.; Ferrer-Roca, C.; MartıiNez-Garcıi, A.D.; Sans, J.A.; MartıiNez, E.; Itie, J.P.; Polian, A.; Baudelet, F.; Munoz, A.; et al. Structural evolution of the CuGaO2 delafossite under high pressure. Phys. Rev. B 2004, 69, 024109. [Google Scholar] [CrossRef]
- Pellicer-Porres, J.; Segura, A.; Martínez, E.; Saitta, A.M.; Polian, A.; Chervin, J.C.; Canny, B. Vibrational properties of delafossite CuGaO2 at ambient and high pressure. Phys. Rev. B 2005, 72, 064301. [Google Scholar] [CrossRef]
- Pellicer-Porres, J.; Martínez-García, D.; Segura, A.; Rodríguez-Hernández, P.; Muñoz, A.; Chervin, J.C.; Garro, N.; Kim, D. Pressure and temperature dependence of the lattice dynamics of CuAlO2 investigated by Raman scattering experiments and ab initiocalculations. Phys. Rev. B 2006, 74, 184301. [Google Scholar] [CrossRef]
- Liu, Q.J.; Liu, Z.T.; Feng, L.P.; Tian, H.; Liu, W.T.; Yan, F. Density functional theory study of 3r–and 2h–CuAlO2 under pressure. Appl. Phys. Lett. 2010, 97, 141917. [Google Scholar] [CrossRef]
- Pellicer-Porres, J.; Segura, A.; Ferrer-Roca, C.; Polian, A.; Munsch, P.; Kim, D. XRD and XAS structural study of CuAlO2 under high pressure. J. Phys. Condens. Matter 2013, 25, 115406. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, A.; Katayama-Yoshida, H. Pressure-induced structural transition and enhancement of energy gap of CuAlO2. J. Phys. Soc. Jpn. 2011, 80, 024706. [Google Scholar] [CrossRef]
- Gilliland, S.; Pellicer-Porres, J.; Segura, A.; Muñoz, A.; Rodríguez-Hernández, P.; Kim, D.; Lee, M.S.; Kim, T.Y. Electronic structure of CuAlO2 and CuScO2delafossites under pressure. Phys. Status Solidi B 2007, 244, 309–314. [Google Scholar] [CrossRef]
- Nie, X.; Su-Huai, W.; Zhang, S.B. Bipolar doping and band-gap anomalies in delafossite transparent conductive oxides. Phys. Rev. Lett. 2002, 88, 066405. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, Q.; Liu, Z.-T. First principles studies of structural, mechanical, electronic, optical properties and pressure-induced phase transition of CuInO2 polymorph. Physica B 2012, 407, 4665–4670. [Google Scholar] [CrossRef]
- Jayalakshmi, V.; Murugan, R.; Palanivel, B. Electronic and structural properties of CuMO2 (M = Al, Ga, In). J. Alloy. Compd. 2005, 388, 19–22. [Google Scholar] [CrossRef]
- Aoyama, T.; Miyake, A.; Kagayama, T.; Shimizu, K.; Tsuyoshi, K. Pressure effects on the magnetoelectric properties of a multiferroic triangular-lattice antiferromagnet CuCrO2. Phys. Rev. B 2013, 87, 094401. [Google Scholar] [CrossRef]
- Piermarini, G.J.; Block, S.; Barnett, J.D. Hydrostatic limits in liquids and solids to 100 kbar. J. Appl. Phys. 1973, 44, 5377–5382. [Google Scholar] [CrossRef]
- Carter, W.T.; Marsh, S.P.; Fritz, J.N.; McQueen, R.G. Accurate Characterization of the High Pressure Environment; Lloyd, E.C., Ed.; NBS Special Pub.: Washington, DC, USA, 1971; Volume 326, p. 147. [Google Scholar]
- Larson, A.C.; Von Dreele, R.B. GSAS: General Structure Analysis System; Report LAUR 86-748; Los Alamos National Laboratory: Los Alamos, NM, USA, 2000. [Google Scholar]
- Petrenko, O.A.; Balakrishnan, G.; Lees, M.R.; Paul, D.M.; Hoser, A. High-magnetic-field behavior of the triangular-lattice antiferromagnet CuFeO2. Phys. Rev. B 2000, 62, 8983. [Google Scholar] [CrossRef]
- Aktas, O.; Truong, K.D.; Otani, T.; Balakrishnan, G.; Clouter, M.J.; Kimura, T.; Quirion, G. Raman scattering study of delafossite magnetoelectric multiferroic compounds: CuFeO2 and CuCrO2. J. Phys. Condens. Matter. 2012, 24, 036003. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Miyasaka, N.; Doi, Y.; Hinatsu, Y. Synthesis and magnetic properties of ALnO2 (A =Cu or Ag; Ln = rare earths) with the delafossite structure. J. Solid State Chem. 2009, 182, 2104–2110. [Google Scholar] [CrossRef]
- Cheng, C.; Lv, Z.L.; Cheng, Y.; Ji, G.F. Structural, elastic and electronic properties of CuYO2 from first-principles study. J. Alloy. Compd. 2014, 603, 183–189. [Google Scholar] [CrossRef]
- Shimode, M.; Sasaki, M.; Mukaida, K. Synthesis of the delafossite-type CuInO2. J. Solid State Chem. 2000, 151, 16–20. [Google Scholar] [CrossRef]
- Li, J.; Yokochi, A.F.T.; Sleight, A.W. Oxygen intercalation of two polymorphs of CuScO2. Solid State Sci. 2004, 6, 831–839. [Google Scholar] [CrossRef]
- Poienar, M.; Hardy, V.; Kundys, B.; Singh, K.; Maignan, A.; Damay, F.; Martin, C. Revisiting the properties of delafossite CuCrO2: A single crystal study. J. Solid State Chem. 2012, 185, 56–61. [Google Scholar] [CrossRef]
- Elkhouni, T.; Amami, M.; Hlil, E.K.; Salah, A.B. The structural, anisotropic magnetization, and spectroscopic study of delafossite CuCr1−xMxO2 systems. J. Supercond. Nov. Magn. 2015, 28, 1895–1903. [Google Scholar] [CrossRef]
- Elkhouni, T.; Amami, M.; Strobel, P.; Salah, A.B. Structural and magnetic properties of substituted delafossite-type oxides CuCr1−xScxO2. World J. Condens. Matter Phys. 2013, 3, 1–8. [Google Scholar] [CrossRef]
- Elkhoun, T.; Amami, M.; Hlil, E.K.; Salah, A.B. Effect of Spin dilution on the magnetic state of delafossite CuFeO2 with an S = 5/2 antiferromagnetic triangular sublattice. J. Supercond. Novel Magn. 2015, 28, 1439–1447. [Google Scholar] [CrossRef]
- Pavunny, S.P.; Kumar, A.; Katiyar, R.S. Raman spectroscopy and field emission characterization of delafossite CuFeO2. J. Appl. Phys. 2010, 107, 013522. [Google Scholar] [CrossRef]
- Salke, N.P.; Garg, A.B.; Rao, R.; Achary, S.N.; Gupta, M.K.; Mittal, R.; Tyagi, A.K. Phase transitions in delafossite CuLaO2 at high pressures. J. Appl. Phys. 2014, 115, 133507. [Google Scholar] [CrossRef]
- Garg, A.B.; Mishra, A.K.; Pandey, K.K.; Sharma, S.M. Multiferroic CuCrO2under high pressure: In situ X-ray diffraction and Raman spectroscopic studies. J. Appl. Phys. 2014, 116, 133514. [Google Scholar] [CrossRef]
- Inaba, M.; Iriyama, Y.; Ogumi, Z.; Todzuka, Y.; Tasaka, A. Raman study of layered rock-salt LiCoO2 and its electrochemical lithium deintercalation. J. Raman Spectrosc. 1997, 28, 613–617. [Google Scholar] [CrossRef]
- Gomis, O.; Lavina, B.; Rodríguez-Hernández, P.; Muñoz, A.; Errandonea, R.; Errandonea, D.; Bettinelli, M. High-pressure structural, elastic, and thermodynamic properties of zircon-type HoPO4 and TmPO4. J. Phys. Condens. Matter 2017, 29, 095401. [Google Scholar] [CrossRef] [PubMed]
- Salke, N.P.; Kamali, K.; Ravindran, T.R.; Balakrishnan, G.; Rao, R. Raman spectroscopic studies of CuFeO2 at high pressures. Vib. Spectrosc. 2015, 81, 112–118. [Google Scholar] [CrossRef]
- Mota, D.A.; Almeida, A.; Rodrigues, V.H.; Costa, M.M.R.; Tavares, P.; Bouvier, P.; Guennou, M.; Kreisel, J.; Moreira, J.A. Dynamic and structural properties of orthorhombic rare-earth manganites under high pressure. Phys. Rev. B 2014, 90, 054104. [Google Scholar] [CrossRef]
- Garg, A.B.; Errandonea, D.; Rodríguez-Hernández, P.; Muñoz, A. ScVO4 under non-hydrostatic compression: A new metastable polymorph. J. Phys. Condens. Matter 2017, 29, 055401. [Google Scholar] [CrossRef] [PubMed]
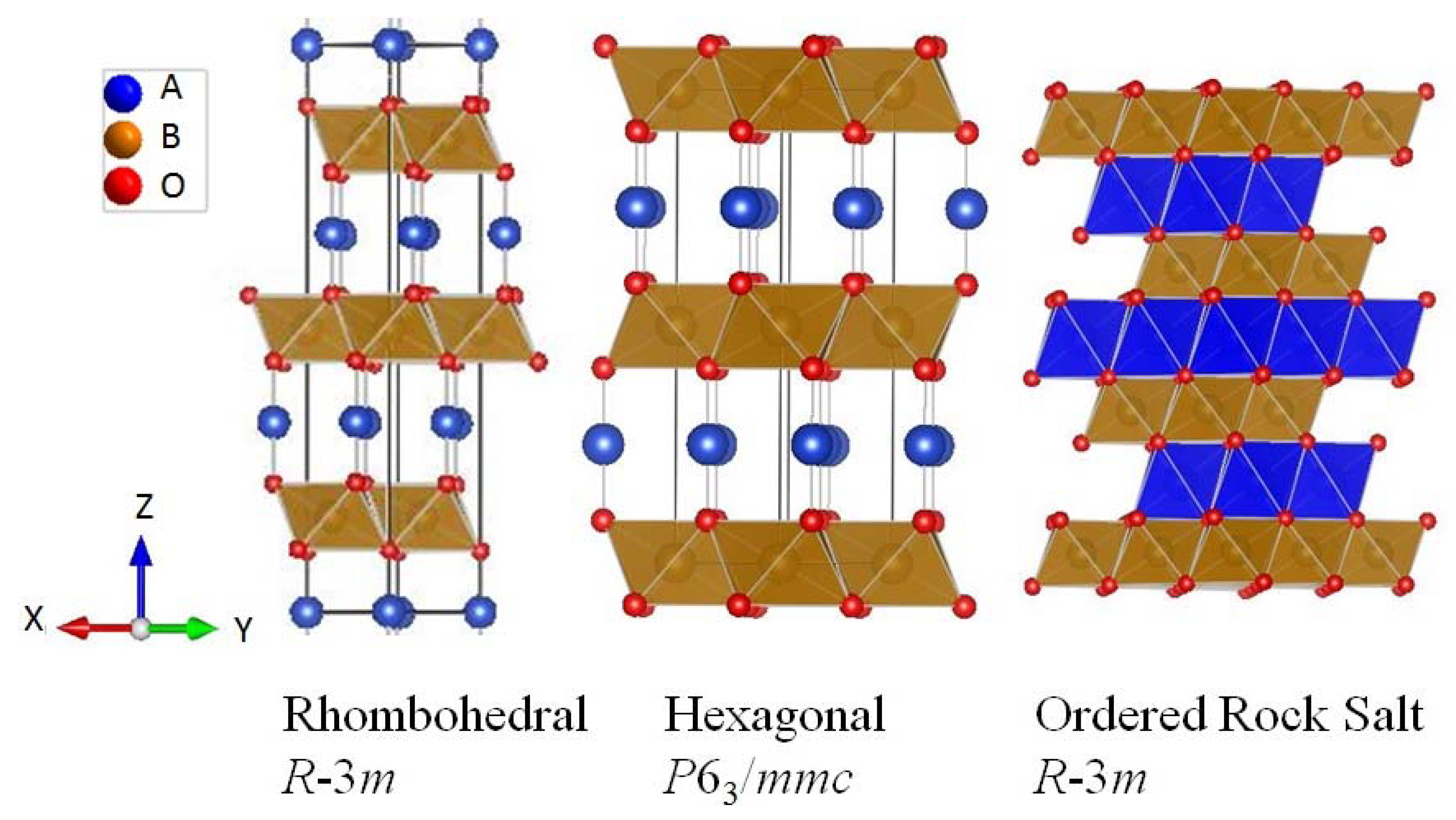
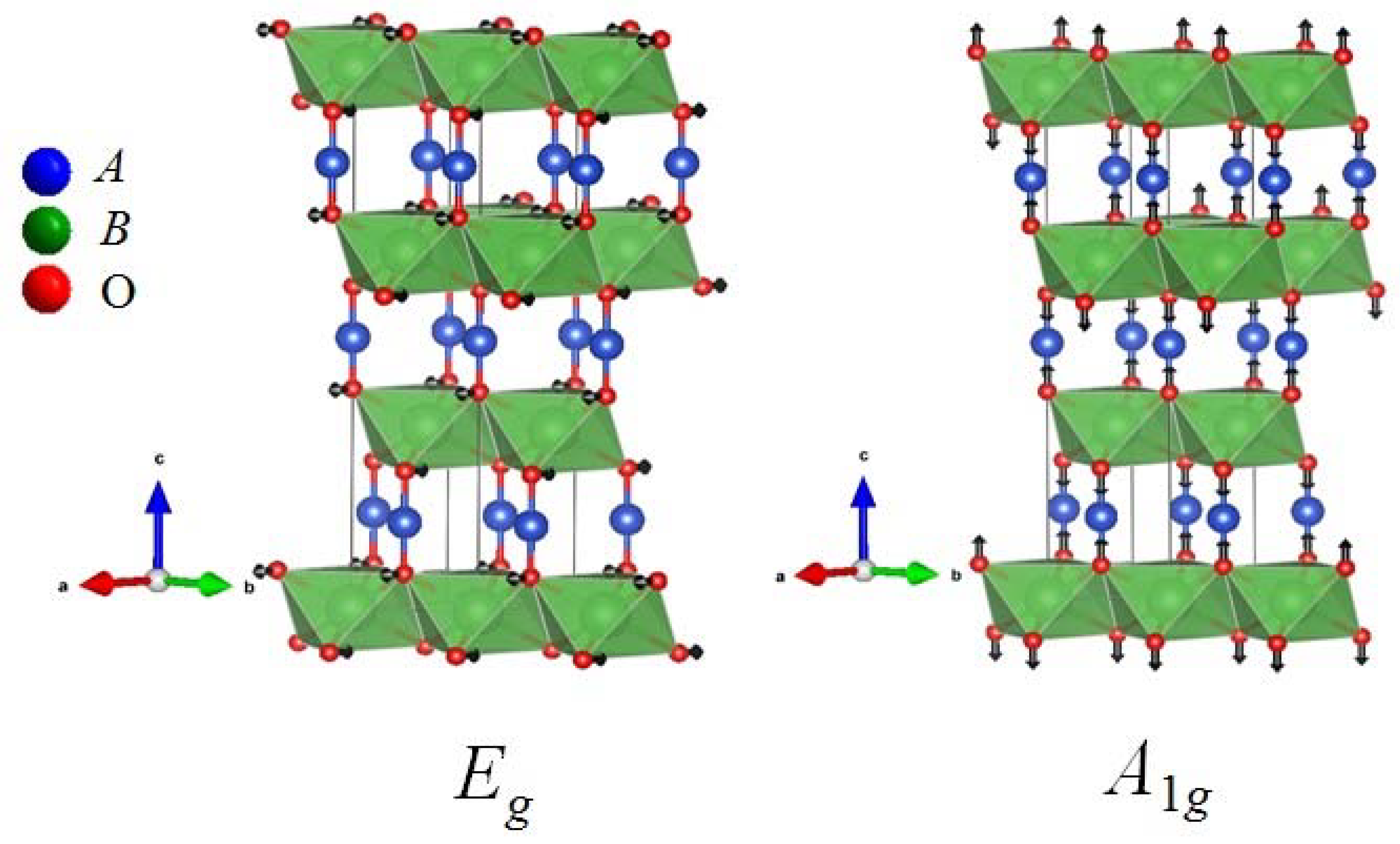
| Hexagonal P63/mmc, Z = 2 | Atomic coordinates | Vibrations at the zone centre | |||||||||
| Wyckoff position | x | y | z | A1g | E1g | E2g | B1g | A2u | E1u | E2u | B2u |
| Monovalent cation A at 2c | 1/3 | 2/3 | 1/4 | - | - | 1 | 1 | 1 | 1 | - | - |
| Trivalent cation B at 2a | 0 | 0 | 0 | - | - | - | - | 1 | 1 | 1 | 1 |
| Oxygen at 4f | 1/3 | 2/3 | 0.0892 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Rhombohedral R-3m, Z = 1 | Atomic co-ordinate | Vibrations at the zone centre | |||||||||
| Wyckoff position | x | y | z | A1g | Eg | A2u | Eu | ||||
| Monovalent cation A at 3b | 0 | 0 | 0 | - | - | 1 | 1 | ||||
| Trivalent cation B at 3a | 0 | 0 | 1/2 | - | - | 1 | 1 | ||||
| Oxygen at 6c | 0 | 0 | 0.108 | 1 | 1 | 1 | 1 | ||||
| Ordered rock salt R-3m, Z = 1 | Atomic co-ordinate | Vibrations at the zone centre | |||||||||
| Wyckoff position | x | y | z | A1g | Eg | A2u | Eu | ||||
| Monovalent cation A at 3a | 0 | 0 | 1/2 | - | - | 1 | 1 | ||||
| Trivalent cation B at 3b | 0 | 0 | 0 | - | - | 1 | 1 | ||||
| Oxygen at 6c | 0 | 0 | 0.743 | 1 | 1 | 1 | 1 | ||||
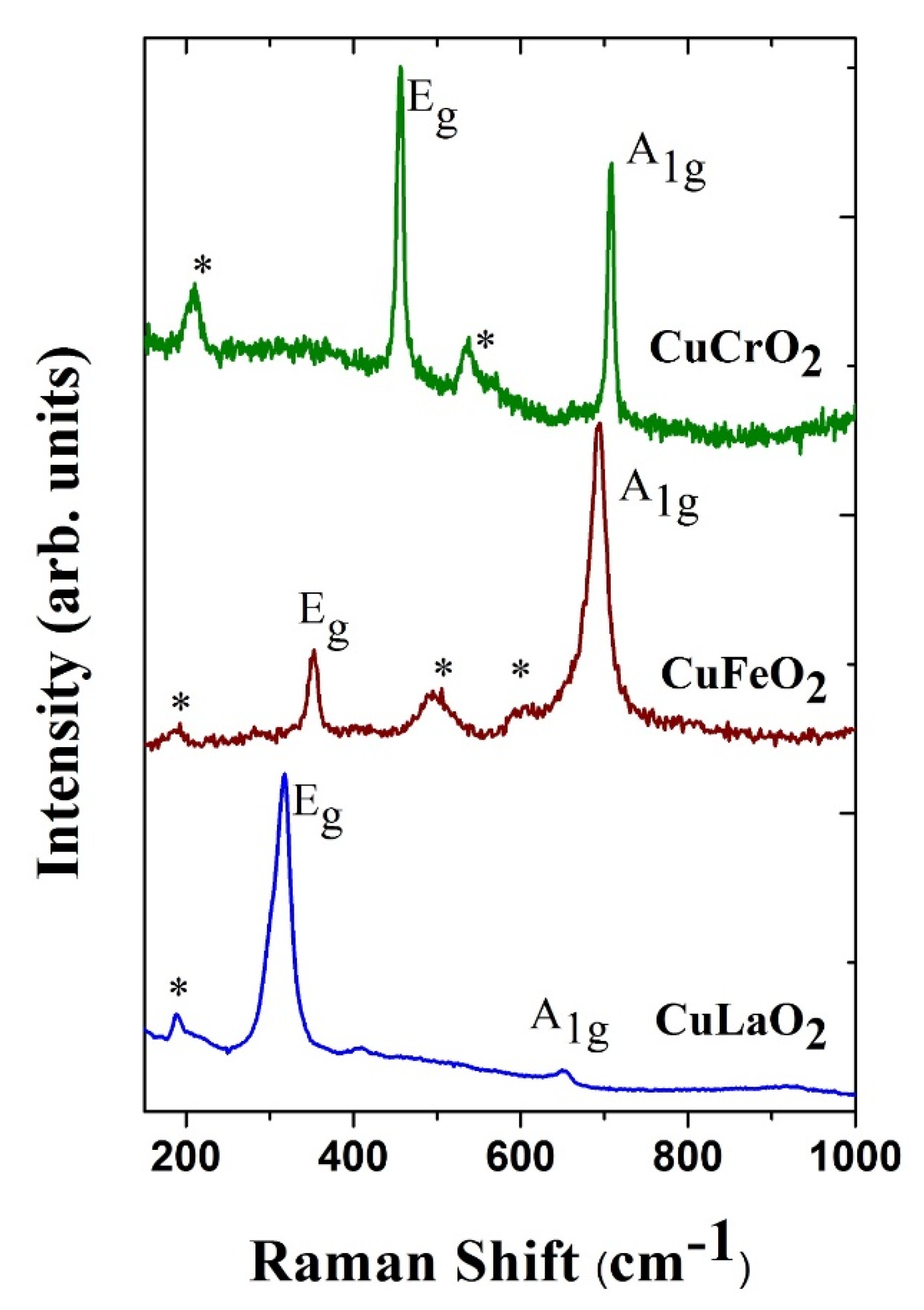
| Delafossite | Ionic Radii of Trivalent Cation (Å) [62] | Raman Mode Frequency | Lattice Parameter | Bond-Length | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Eg (cm−1) | A1g (cm−1) | a (Å) | c (Å) | Cu-O (Å) | M-O (Å) | |||
| CuLaO2 | 1.032 | 318 | 652 | 3.8326 | 17.092 | 1.760 | 2.466 | [63] |
| CuPrO2 | 0.99 | 3.7518 | 17.086 | 1.789 | 2.411 | [63] | ||
| CuNdO2 | 0.983 | 3.7119 | 17.085 | 1.836 | 2.370 | [63] | ||
| CuSmO2 | 0.958 | 3.6628 | 17.078 | 1.880 | 2.325 | [63] | ||
| CuEuO2 | 0.947 | 3.6316 | 17.074 | 1.895 | 2.302 | [63] | ||
| CuYO2 | 0.90 | 3.5330 | 17.136 | 1.827 | 2.285 | [64] | ||
| CuInO2 | 0.8 | 378 | 678 | 3.2922 | 17.388 | 1.845 | 2.172 | [65] |
| CuScO2 | 0.745 | 3.2204 | 17.099 | 1.831 | 2.121 | [66] | ||
| CuFeO2 | 0.645 | 352 | 692 | 3.0351 | 17.166 | 1.835 | 2.033 | [61] |
| CuGaO2 | 0.62 | 368 | 729 | 2.9770 | 17.171 | 1.848 | 1.996 | [46] |
| CuCrO2 | 0.615 | 454 | 703 | 2.9767 | 17.111 | 1.8455 | 1.989 | [67] |
| CuAlO2 | 0.535 | 418 | 767 | 2.8584 | 16.958 | 1.8617 | 1.912 | [50] |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garg, A.B.; Rao, R. Copper Delafossites under High Pressure—A Brief Review of XRD and Raman Spectroscopic Studies. Crystals 2018, 8, 255. https://doi.org/10.3390/cryst8060255
Garg AB, Rao R. Copper Delafossites under High Pressure—A Brief Review of XRD and Raman Spectroscopic Studies. Crystals. 2018; 8(6):255. https://doi.org/10.3390/cryst8060255
Chicago/Turabian StyleGarg, Alka B., and Rekha Rao. 2018. "Copper Delafossites under High Pressure—A Brief Review of XRD and Raman Spectroscopic Studies" Crystals 8, no. 6: 255. https://doi.org/10.3390/cryst8060255
APA StyleGarg, A. B., & Rao, R. (2018). Copper Delafossites under High Pressure—A Brief Review of XRD and Raman Spectroscopic Studies. Crystals, 8(6), 255. https://doi.org/10.3390/cryst8060255




