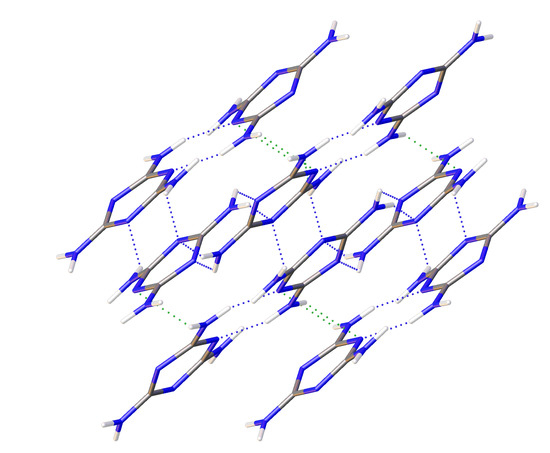
Evolution of Interatomic and Intermolecular Interactions and Polymorphism of Melamine at High Pressure
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. X-ray Diffraction
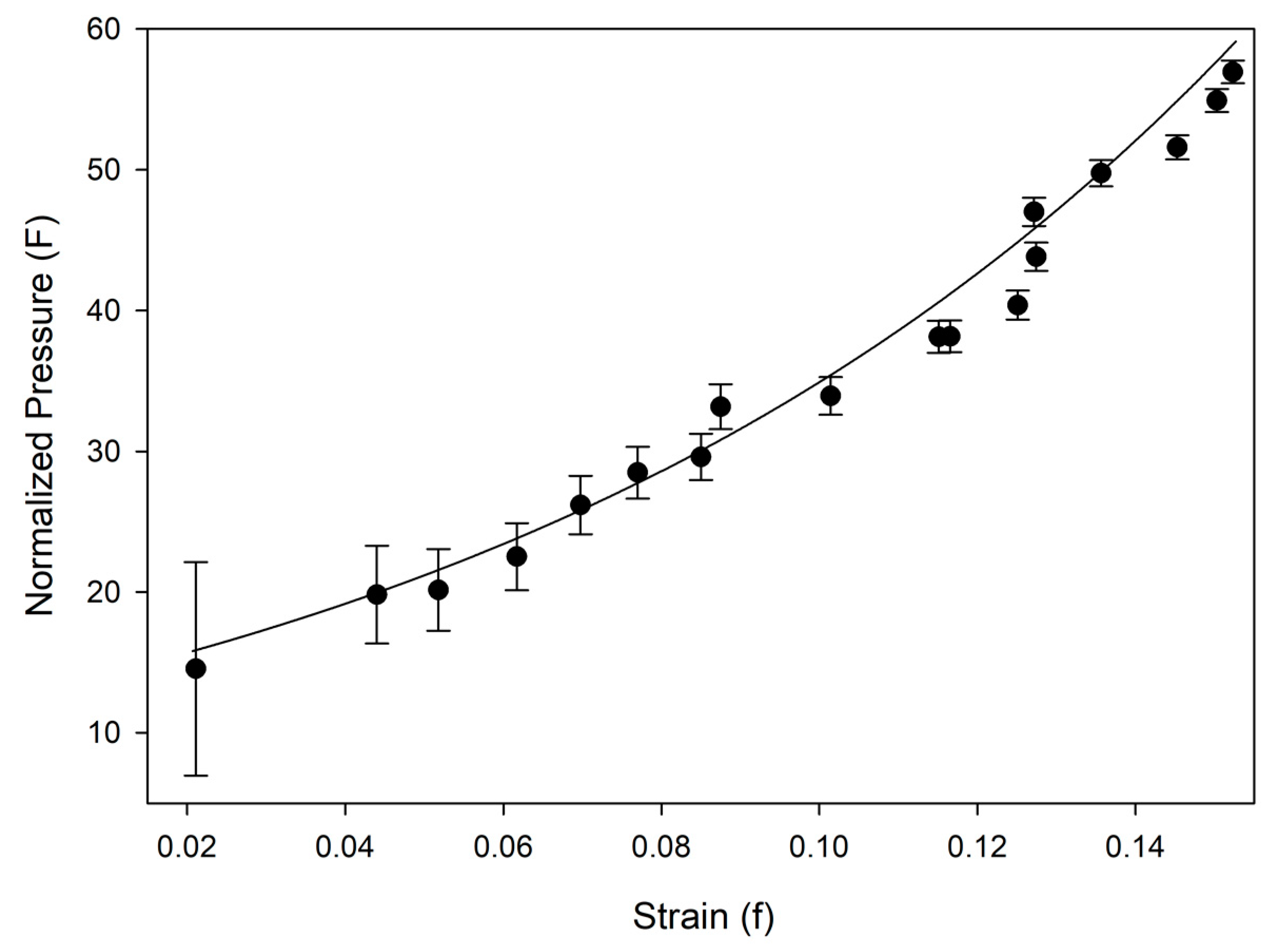
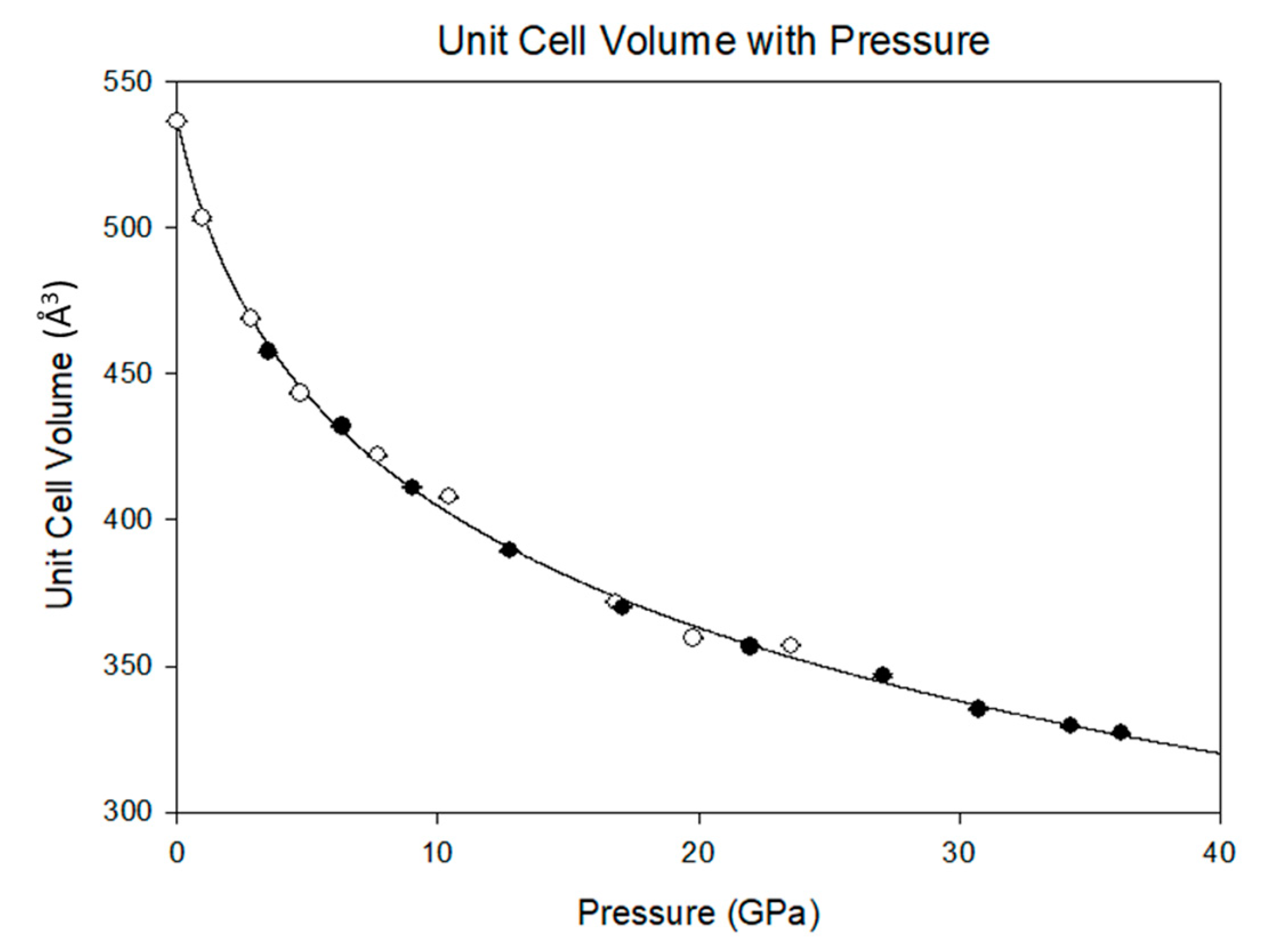
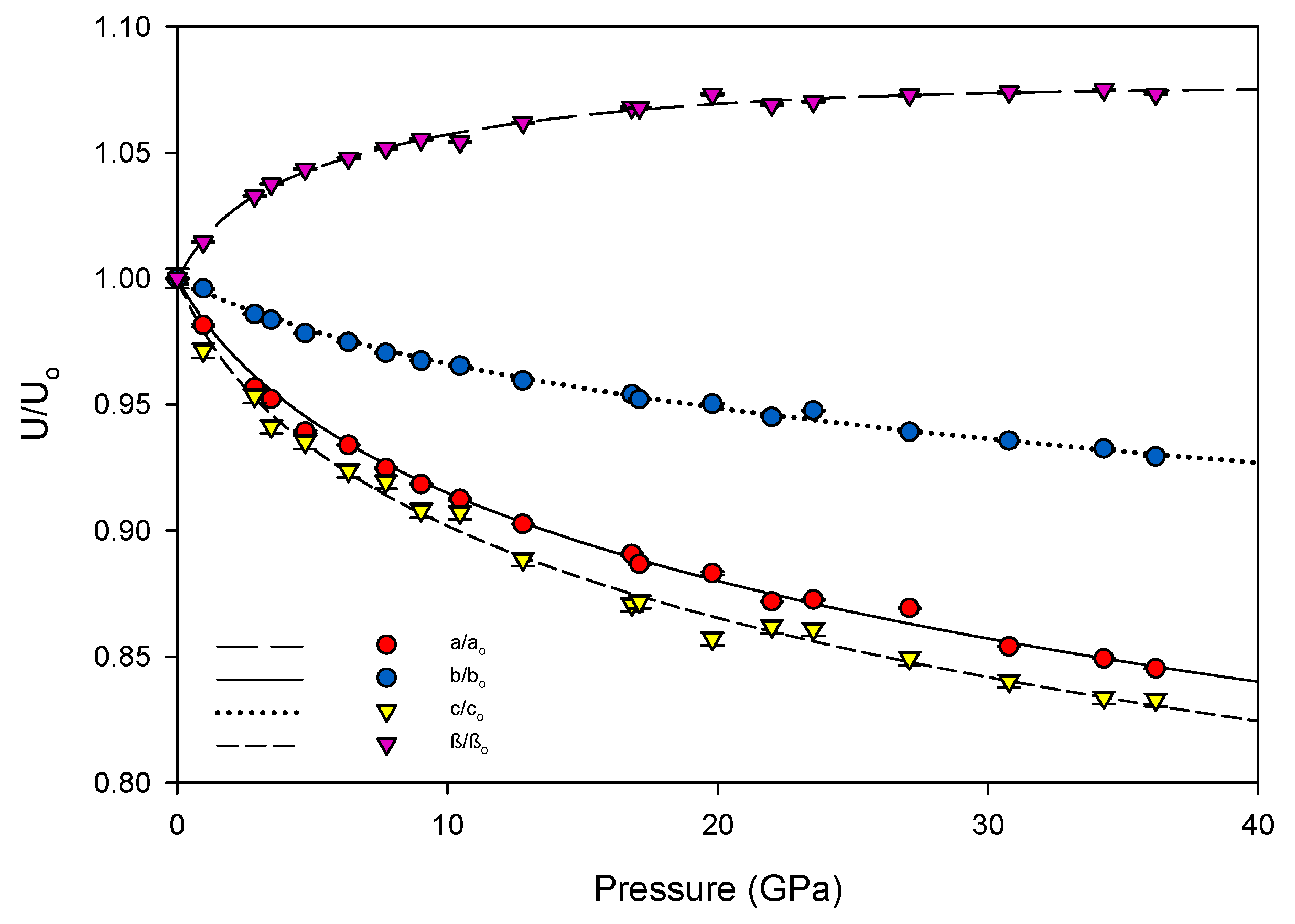
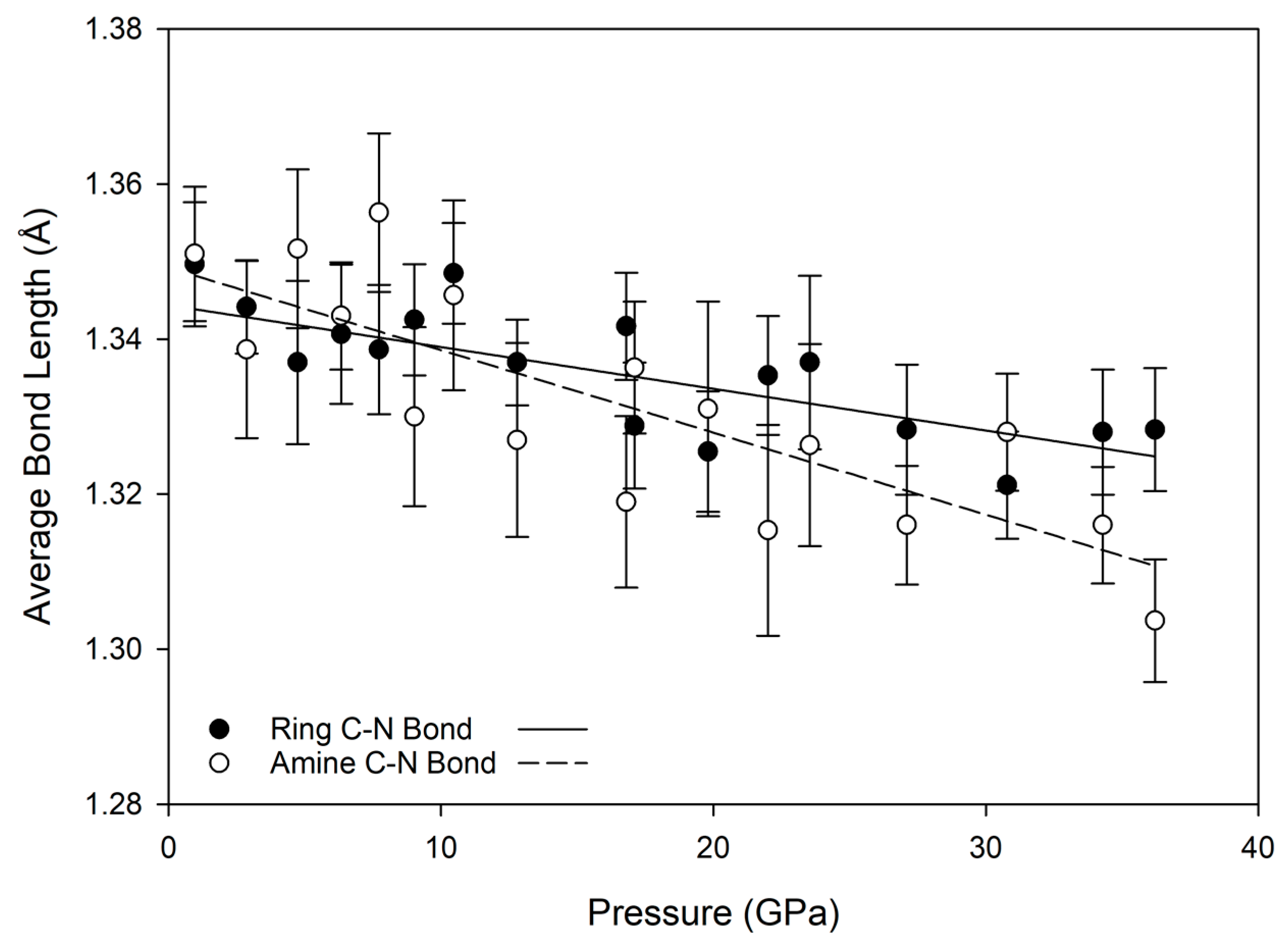
3.2. Equation of State and Bond Compressibility
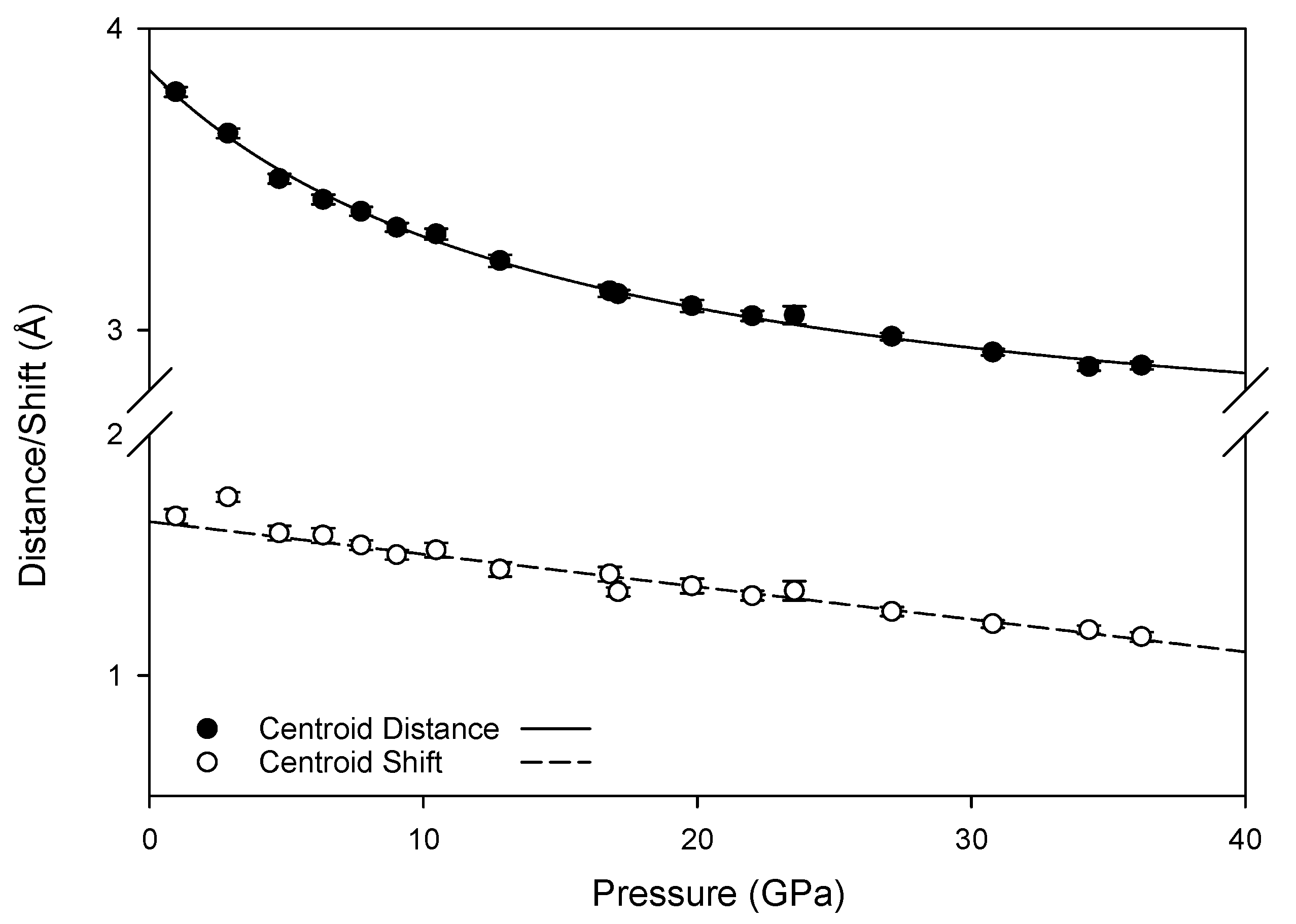
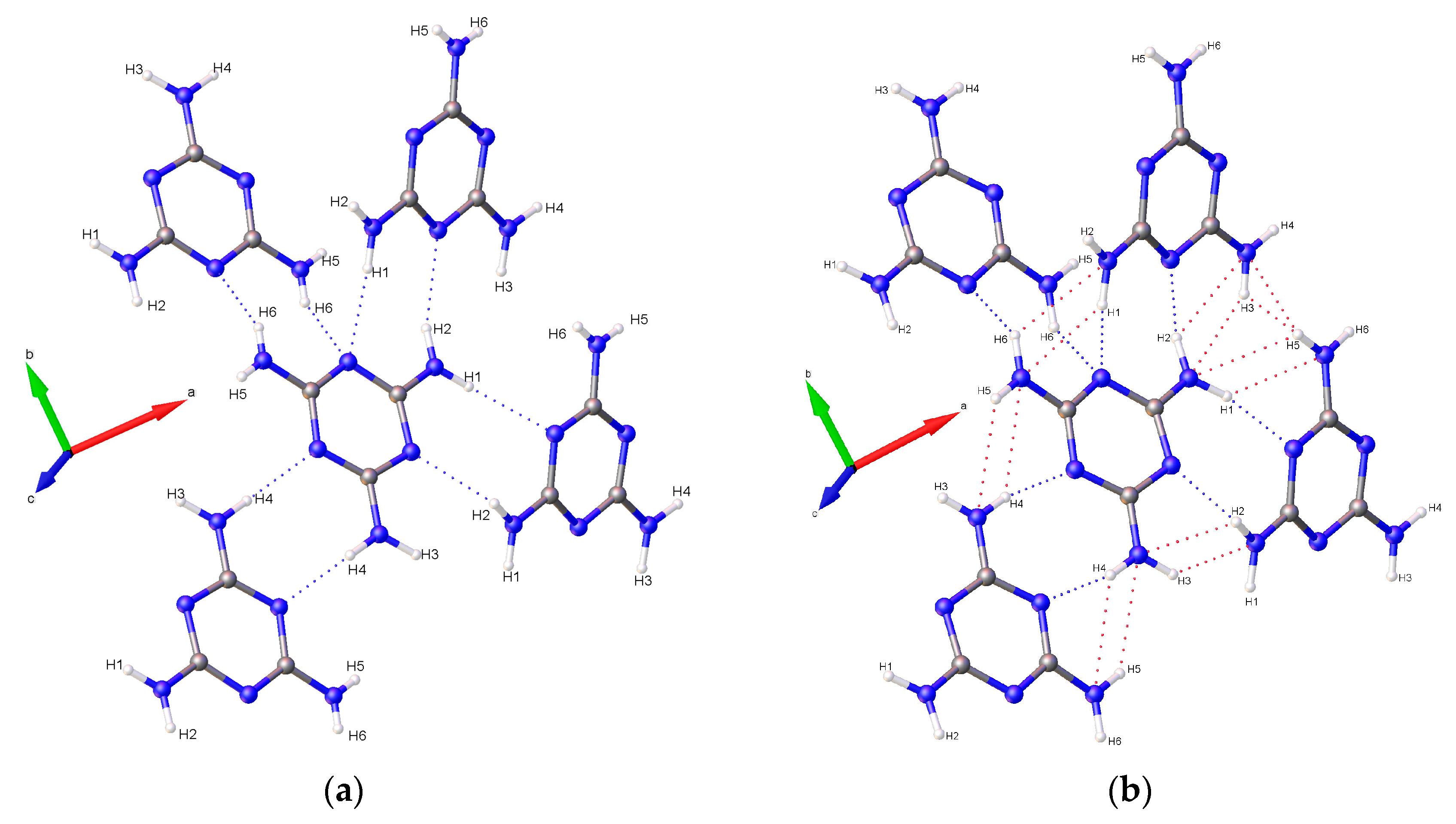
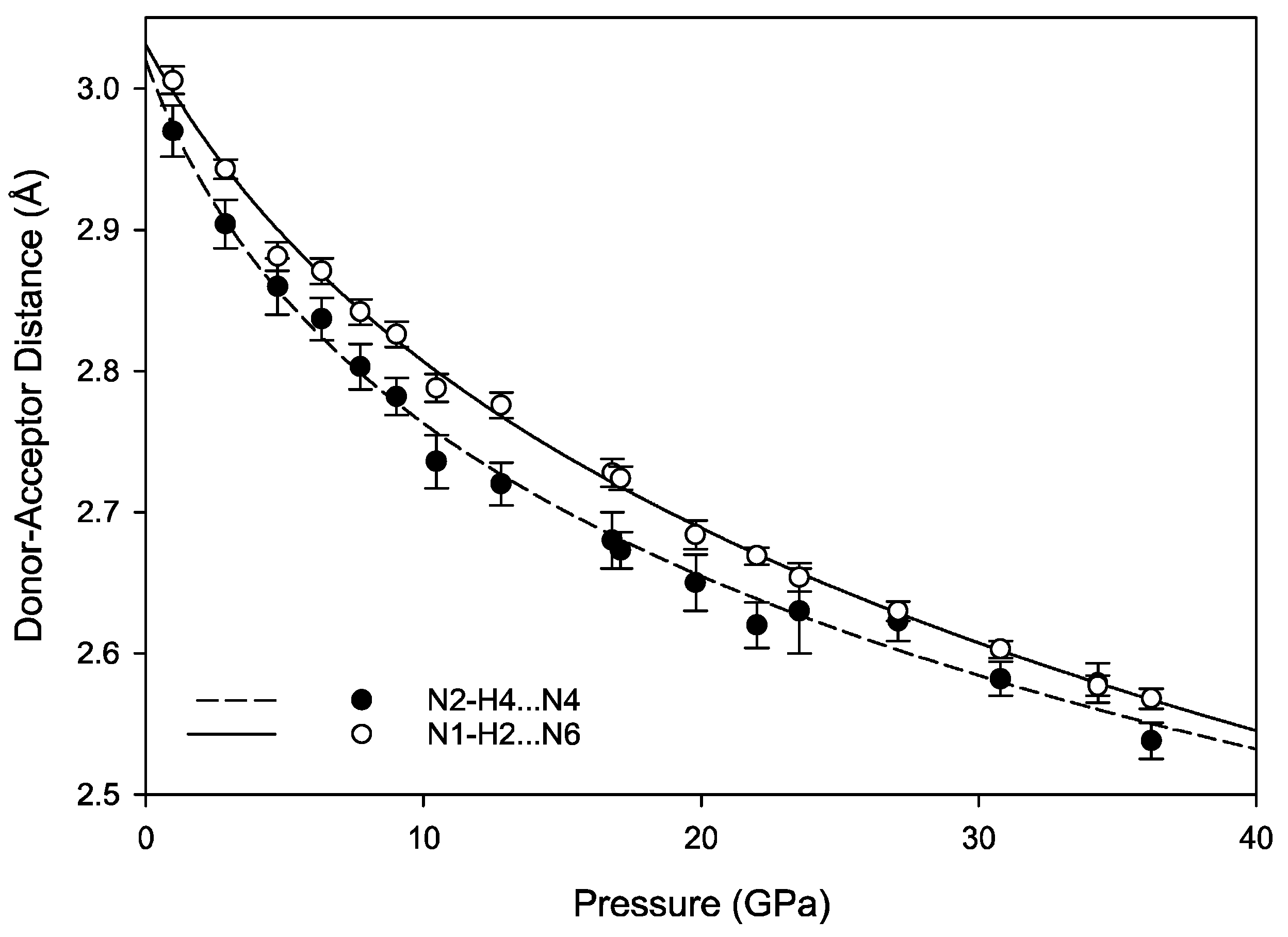
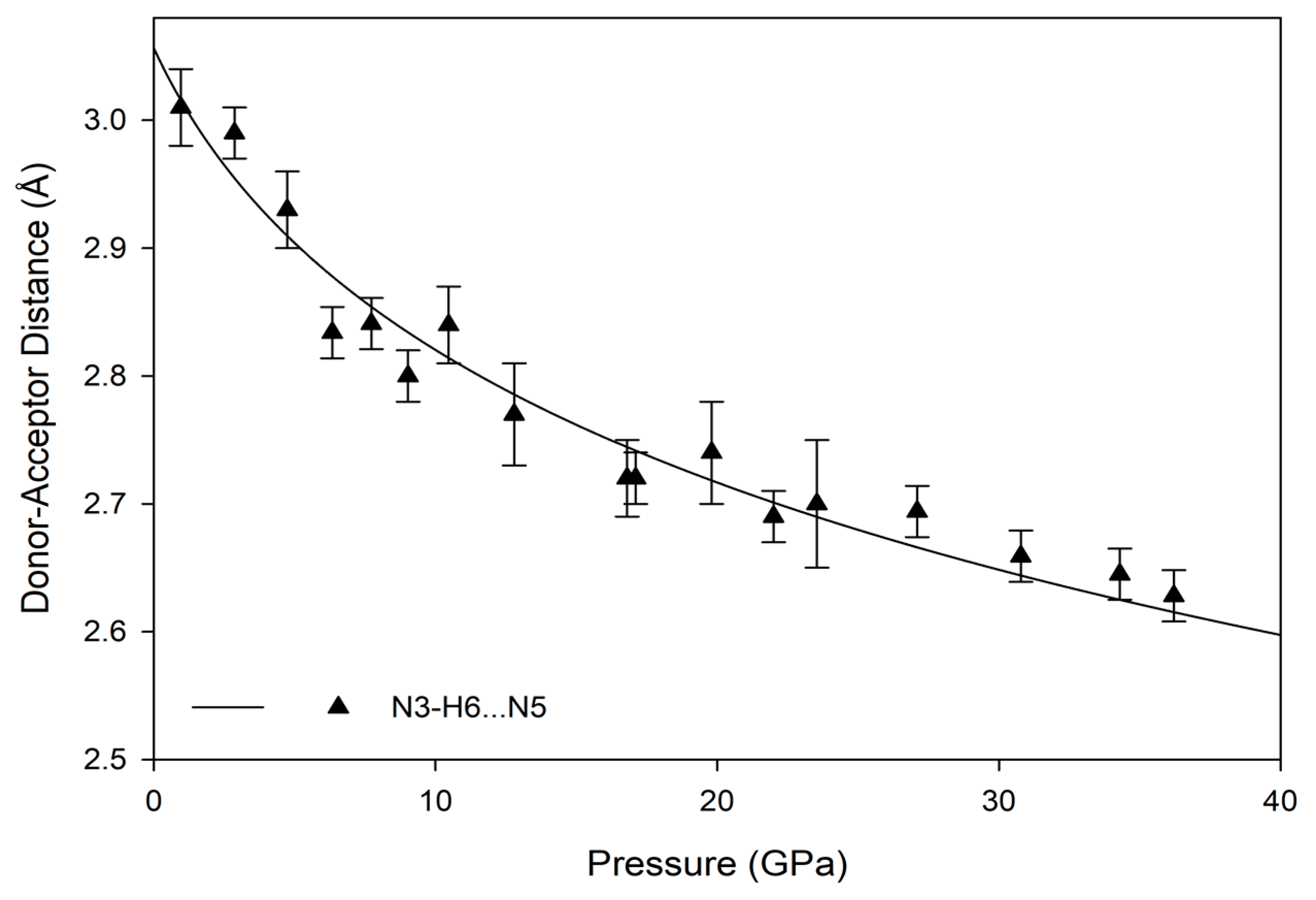
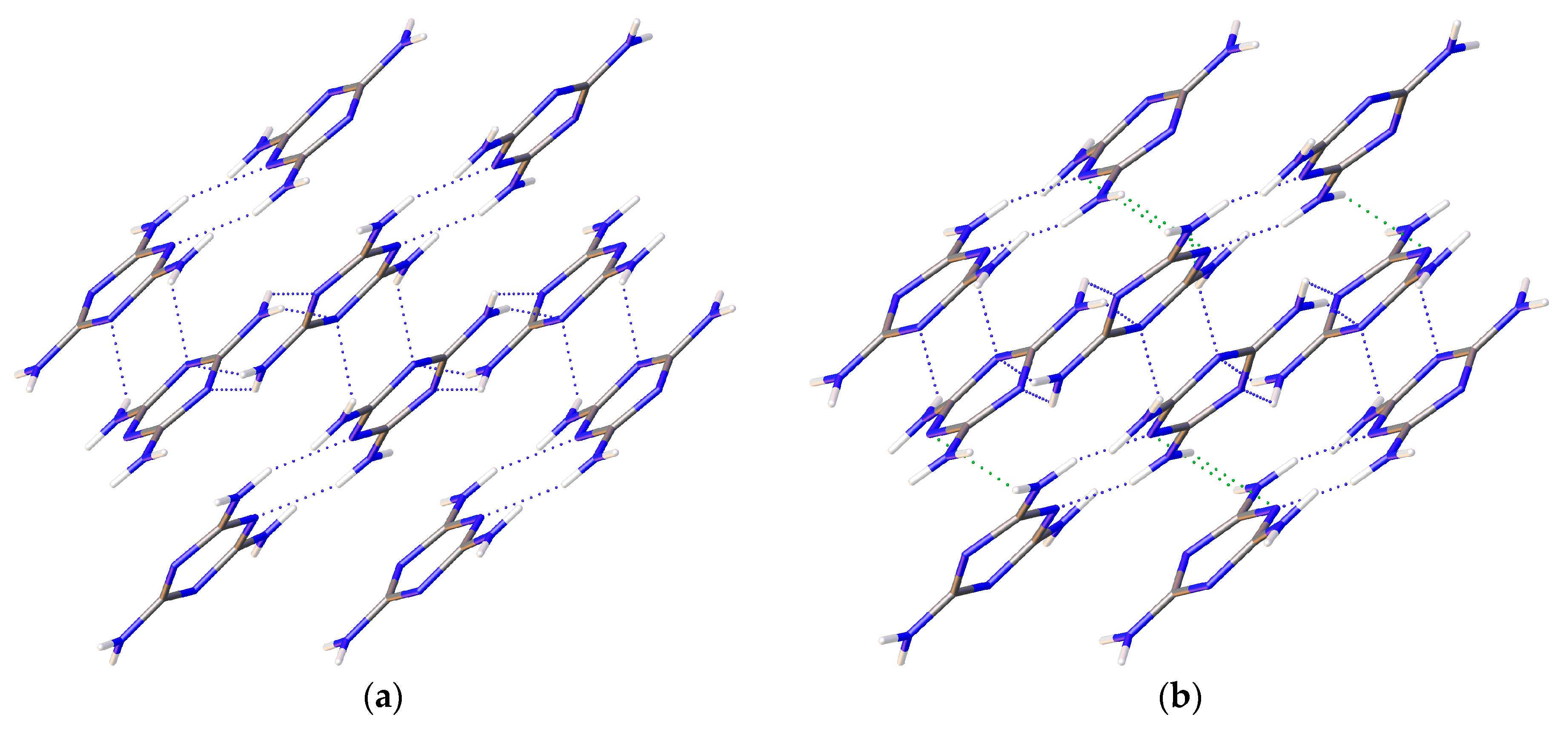
3.3. Hydrogen Bonding Behavior with Pressure
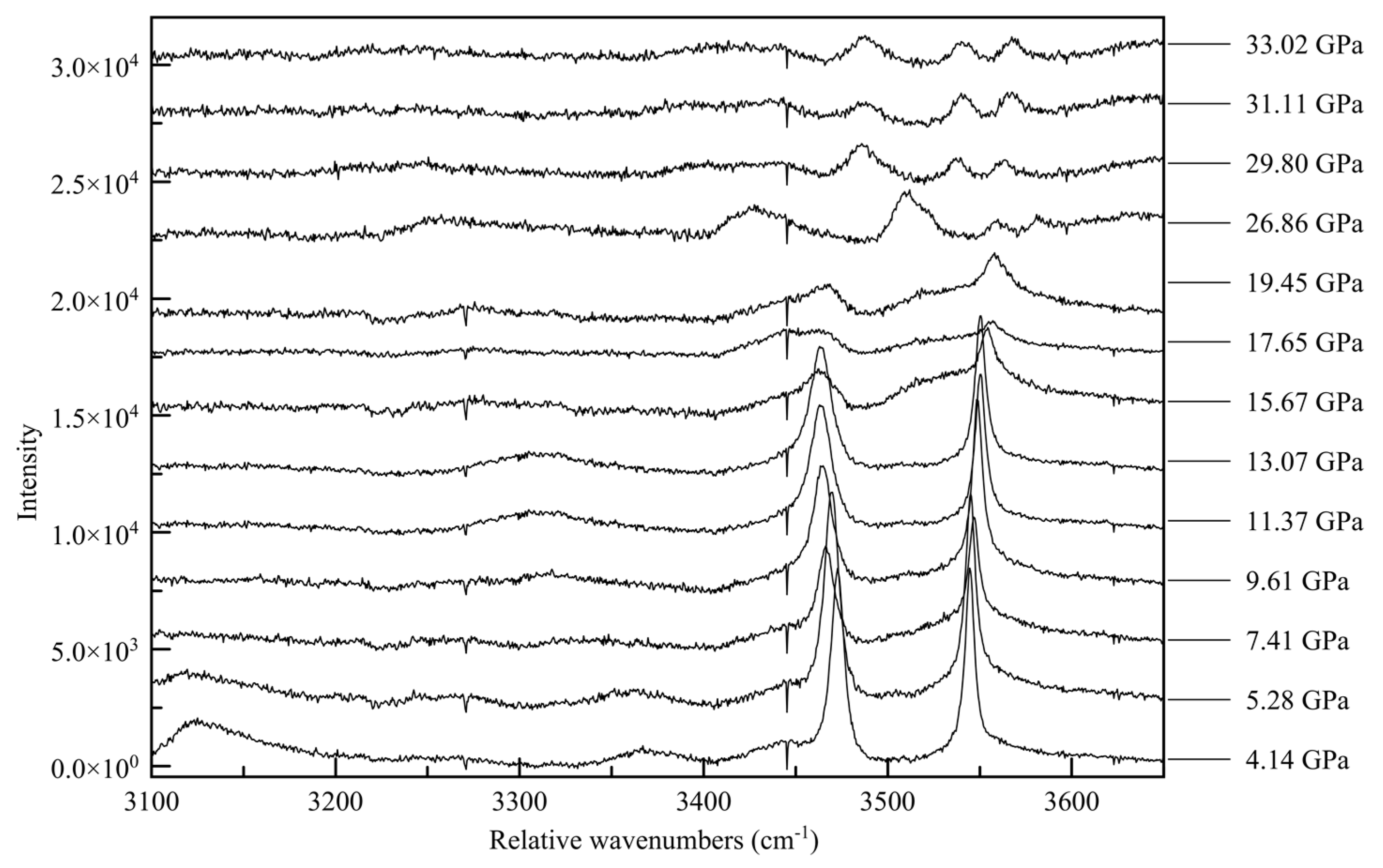
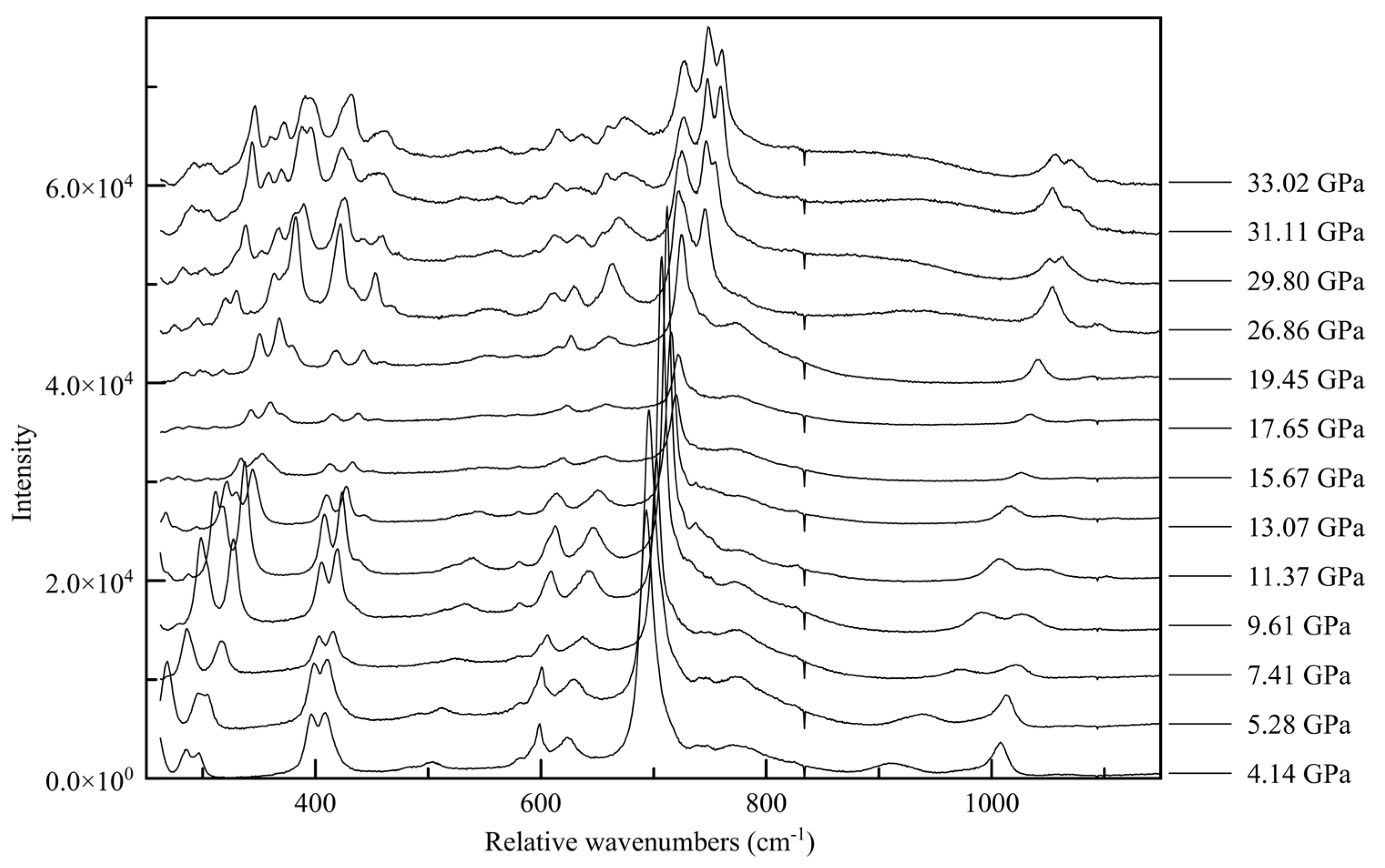
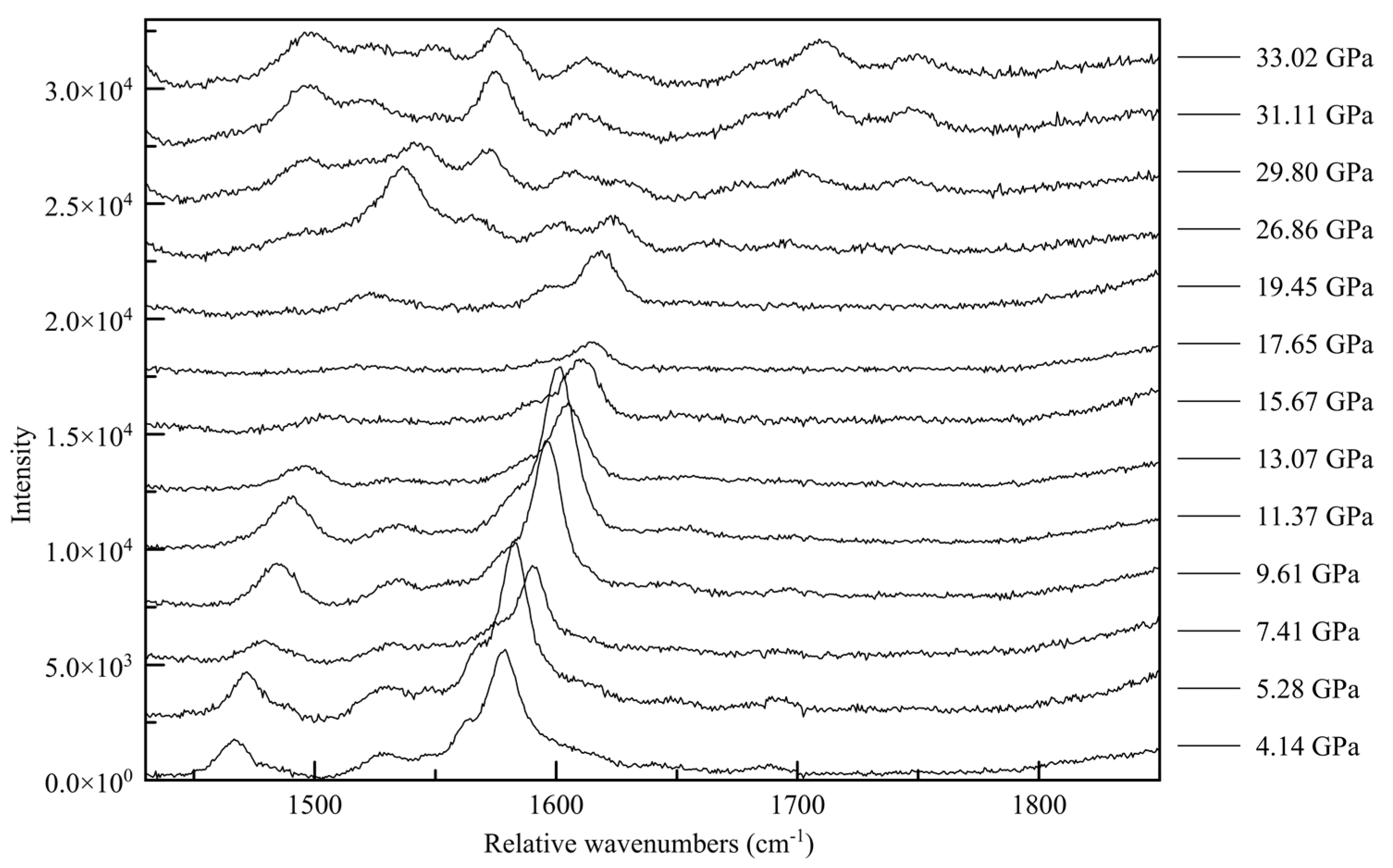
3.4. Raman Spectroscopy
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Bonded Atoms | Length (Å) | Atoms in Angle | Angle (°) |
|---|---|---|---|
| C(1)-N(1) | 1.26(2) | N(1)-C(1)-N(6) | 121.5(6) |
| C(1)-N(6) | 1.345(17) | N(1)-C(1)-N(5) | 118.1(12) |
| C(1)-N(5) | 1.361(8) | N(6)-C(1)-N(5) | 120.4(14) |
| C(2)-N(2) | 1.302(14) | N(2)-C(2)-N(6) | 120.1(6) |
| C(2)-N(6) | 1.316(17) | N(2)-C(2)-N(4) | 115.8(11) |
| C(2)-N(4) | 1.345(8) | N(6)-C(2)-N(4) | 124.1(10) |
| C(3)-N(5) | 1.30(2) | N(5)-C(3)-N(4) | 125.8(8) |
| C(3)-N(4) | 1.317(15) | N(5)-C(3)-N(3) | 117.3(10) |
| C(3)-N(3) | 1.335(10) | N(4)-C(3)-N(3) | 116.9(14) |
| Atom | X | Y | Z | Ueq |
|---|---|---|---|---|
| C(1) | 1771(16) | 6196(8) | 290(40) | 11(1) |
| C(2) | 1307(16) | 4725(7) | 3230(40) | 9(1) |
| C(3) | 634(16) | 7829(8) | 2330(40) | 11(1) |
| N(1) | 2234(14) | 6180(7) | 1340(40) | 10(1) |
| N(2) | 1280(12) | 3193(7) | 4440(30) | 7(1) |
| N(3) | 252(13) | 9514(7) | 2970(30) | 10(1) |
| N(4) | 586(15) | 6305(7) | 3570(40) | 11(1) |
| N(5) | 1098(13) | 7850(7) | 610(30) | 10(1) |
| N(6) | 2005(14) | 4661(7) | 1780(40) | 11(1) |
| Atom | Symmetry Operators | ||
|---|---|---|---|
| 1 | x, | y, | z − 1 |
| 2 | −x + 1/2, | y − 1/2, | −z |
| 3 | −x + 1/2, | y + 1/2, | −z |
| 4 | x, | y − 1, | z |
| 5 | −x + 1/2, | y − 1/2, | −z + 1 |
| 6 | −x, | −y + 1, | −z + 1 |
| 7 | x | Y + 1 | z |
| 8 | −x | −y + 2 | −z |
| 9 | −x | −y + 2 | −z + 1 |
| D-H··A Symmetry | d(D-H) | d(H··A) | <DHA | d(D··A) |
|---|---|---|---|---|
| N1-H1···N5_$2 | 0.935 | 1.792 | 155.72 | 2.672(8) |
| N1-H2···N6_$3 | 0.935 | 1.821 | 134.85 | 2.568(7) |
| N2-H3···N4_$5 | 0.865 | 2.16 | 126.5 | 2.763(12) |
| N2-H3···N6_$5 | 0.865 | 2.688 | 117.55 | 3.182(16) |
| N3-H6···N5_$8 | 0.886 | 1.89 | 139.51 | 2.628(18) |
| N2-H4···N4_$6 | 0.865 | 1.703 | 161.53 | 2.538(13) |
| N1-H1···N2_$1 | 0.935 | 2.481 | 120.73 | 3.067(18) |
| N1-H1···N3_$2 | 0.935 | 2.379 | 134.58 | 3.108(10) |
| N1-H2···N2_$3 | 0.935 | 2.477 | 127.01 | 3.131(14) |
| N2-H3···N1_$2 | 0.865 | 2.49 | 131.52 | 3.131(14) |
| N2-H3···N3_$4 | 0.865 | 2.152 | 122.47 | 2.717(10) |
| N2-H4···N3_$6 | 0.865 | 2.561 | 129.4 | 3.182(12) |
| N3-H6···N1_$3 | 0.886 | 2.546 | 121.95 | 3.108(10) |
| N3-H6···N2_$7 | 0.886 | 2.158 | 120.4 | 2.717(10) |
| N3-H5···N2_$6 | 0.886 | 2.565 | 127.35 | 3.182(12) |
| N3-H5···N3_$9 | 0.886 | 1.96 | 159.3 | 2.81(3) |
References
- Hiskey, M.A.; Chavez, D.E.; Naud, D.L. Insensitive High-Nitrogen Compounds; NTIS No: DE-2001-776133; Los Alamos National Lab.: Los Alamos, NM, USA, 2001. [Google Scholar]
- Xu, D.; Lu, H.; Huang, Q.; Deng, B.; Li, L. Flame-retardant effect and mechanism of melamine phosphate on silicone thermoplastic elastomer. RSC Adv. 2018, 8, 5034–5041. [Google Scholar] [CrossRef]
- Liu, A.Y.; Cohen, M.L. Prediction of new low compressibility solids. Science 1989, 245, 841–842. [Google Scholar] [CrossRef] [PubMed]
- Li, J. An ab initio theoretical study of 2,4,6-trinitro-1,3,5-triazine, 3,6-dinitro-1,2,4,5-tetrazine, and 2,5,8-trinitro-tri-s-triazine. Propellants Explos. Pyrotech. 2008, 33, 443–447. [Google Scholar] [CrossRef]
- Coburn, M.D.; Hayden, H.H.; Coon, C.L.; Mitchell, A.R. Synthesis of poly (S, S-dimethylsulfilimino) heterocycles. Synthesis 1986, 6, 490–492. [Google Scholar] [CrossRef]
- Hartman, G.D.; Schwering, J.E.; Hartman, R.D. Dimethyl sulfide ditriflate: A new reagent for the conversion of amino heterocycles to iminosulfuranes. Tetrahedron Lett. 1983, 24, 1011–1014. [Google Scholar] [CrossRef]
- Hejny, C.; Minkov, V.S. High-pressure crystallography of periodic and aperiodic crystals. IUCrJ 2015, 2, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.A.; Jia, X.P.; Chen, L.X.; Zhu, P.W.; Guo, W.L.; Guo, X.B.; Wang, Y.D.; Li, S.Q.; Zou, G.T.; Zhang, G.; et al. High-pressure pyrolysis study of C3N6H6: A route to preparing bulk C3N4. J. Phys. Condens. Matter 2002, 14, 11269–11273. [Google Scholar] [CrossRef]
- Montigaud, H.; Tanguy, B.; Demazeau, G.; Alves, I.; Courjault, S. C3N4: Dream or reality? Solvothermal synthesis as macroscopic samples of the C3N4 graphitic form. J. Mater. Sci. 2000, 35, 2547–2552. [Google Scholar] [CrossRef]
- Yao, L.D.; Li, F.Y.; Li, J.X.; Jin, C.Q.; Yu, R.C. Study of the products of melamine (C3N6H6) treated at high pressure and high temperature. Phys. Status Solidi 2005, 202, 2679–2685. [Google Scholar] [CrossRef]
- Wu, X.; Tao, Y.; Lu, Y.; Dong, L.; Hu, Z. High-pressure pyrolysis of melamine route to nitrogen-doped conical hollow and bamboo-like carbon nanotubes. Diam. Relat. Mater. 2006, 16, 164–170. [Google Scholar] [CrossRef]
- Yu, D.L.; He, J.L.; Liu, Z.Y.; Xu, B.; Li, D.C.; Tian, Y.J. Phase transformation of melamine at high pressure and temperature. J. Mater. Sci. 2008, 43, 689–695. [Google Scholar] [CrossRef]
- Hamann, S.D.; Linton, M. The influence of pressure on the infrared spectra of hydrogen bonded solids. III compounds with N-H…X bonds. Aust. J. Chem. 1976, 29, 1641–1647. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Z.; Cui, Q.; Liu, Z.; Li, D.; Zou, G. High pressure raman spectra of melamine (C3H6N6) and pressure induced transition. High Press. Res. 1990, 3, 233–235. [Google Scholar]
- Ma, H.A.; Jia, X.; Cui, Q.L.; Pan, Y.W.; Zhu, P.W.; Liu, B.B.; Liu, H.J.; Wang, X.C.; Liu, J.; Zou, G.T. Crystal structures of C3N6H6 under high pressure. Chem. Phys. Lett. 2003, 368, 668–672. [Google Scholar] [CrossRef]
- Liu, X.R.; Zinin, P.V.; Ming, L.C.; Acosta, T.; Sharma, S.K.; Misra, A.K.; Hong, S.M. Raman spectroscopy of melamine at high pressures. J. Phys. Conf. Ser. 2010, 215, 012045. [Google Scholar] [CrossRef]
- Pravica, M.; Kim, E.; Tkatchev, S.; Chow, P.; Xiao, Y. High-pressure studies of melamine. High Press. Res. 2010, 30, 65–71. [Google Scholar] [CrossRef]
- Rivers, M.L.; Prakapenka, V.B.; Kubo, A.; Pullins, C.; Hall, C.M.; Jacobsen, S.D. The compres/gsecars gas loading system for diamond anvil cells at the advanced photon source. High Press. Res. 2008, 28, 273–292. [Google Scholar] [CrossRef]
- Boehler, R.; De Hantsetters, K. New anvil designs in diamond-cells. High Press. Res. 2004, 24, 391–396. [Google Scholar] [CrossRef]
- Mao, H.K.; Xu, J.; Bell, P.M. Calibration of the ruby pressure gauge to 800 kbar under quasi-hydrostatic conditions. J. Geophys. Res. Solid Earth 1986, 91, 4673–4676. [Google Scholar] [CrossRef]
- Dera, P.; Zhuravlev, K.; Prakapenka, V.; Rivers, M.L.; Finkelstein, G.J.; Grubor-Urosevic, O.; Tschauner, O.; Clark, S.M.; Downs, R.T. High pressure single-crystal micro x-ray diffraction analysis with gse_ada/rsv software. High Press. Res. 2013, 33, 466–484. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Cell_Now; Version 2008/4; Georg-August-Universität Göttingen: Göttingen, Germany, 2008. [Google Scholar]
- Sheldrick, G. A short history of shelx. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Platas, J.; Alvaro, M.; Nestola, F.; Angel, R. EosFit7-GUI: A new graphical user interface for equation of state calculations, analyses and teaching. J. Appl. Crystallogr. 2016, 49, 1377–1382. [Google Scholar] [CrossRef]
- Vinet, P.; Smith, J.R.; Ferrante, J.; Rose, J.H. Temperature effects on the universal equation of state of solids. Phys. Rev. B 1987, 35, 1945–1953. [Google Scholar] [CrossRef]
- Jeanloz, R. Universal equation of state. Phys. Rev. B 1988, 38, 805–807. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. Olex2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Turner, M.J.; Jayatilaka, D.; Spackman, M.A. Crystalexplorer (Version 3.1); University of Western Australia: Crawley, Australia, 2012. [Google Scholar]
- Menges, F. Spectragryph—Optical Spectroscopy Software, Version 1.2.8; Spectroscopy Ninja: Oberstdorf, Germany, 2018. [Google Scholar]
- Varghese, J.N.; O’Connell, A.M.; Maslen, E.N. The x-ray and neutron crystal structure of 2,4,6-triamino-1,3,5-triazine (melamine). Acta Cryst. 1977, B33, 2102–2108. [Google Scholar] [CrossRef]
- Hazen, R.M.; Downs, R.T. High-temperature and high-pressure crystal chemistry. In Reviews in Mineralogy and Geochemistry; Hazen, R.M., Downs, R.T., Eds.; Mineralogical Society of America: Washington, DC, USA, 2000; Volume 41. [Google Scholar]
- Ciabini, L.; Gorelli, F.A.; Santoro, M.; Bini, R.; Schettino, V.; Mezouar, M. High-pressure and high-temperature equation of state and phase diagram of solid benzene. Phys. Rev. B 2005, 72, 094108. [Google Scholar] [CrossRef]
- Nobrega, M.M.; Temperini, M.L.A.; Bini, R. Probing the chemical stability of aniline under high pressure. J. Phys. Chem. C 2017, 121, 7495–7501. [Google Scholar] [CrossRef]
- Hanfland, M.; Brister, K.; Syassen, K. Graphite under pressure: Equation of state and first-order raman modes. Phys. Rev. B 1989, 39, 12598–12603. [Google Scholar] [CrossRef]
- Funnell, N.P.; Dawson, A.; Marshall, W.G.; Parsons, S. Destabilisation of hydrogen bonding and the phase stability of aniline at high pressure. CrystEngComm 2013, 15, 1047–1060. [Google Scholar] [CrossRef]
- Wen, X.D.; Hoffmann, R.; Ashcroft, N.W. Benzene under high pressure: A story of molecular crystals transforming to saturated networks, with a possible intermediate metallic phase. J. Am. Chem. Soc. 2011, 133, 9023–9035. [Google Scholar] [CrossRef] [PubMed]
- Peiris, S.M.; Piermarini, G.J. Static Compression of Energetic Materials; Springer: Berlin, Germany, 2008; pp. 108–110. [Google Scholar]
- Yoo, C.S.; Cynn, H.; Howard, W.M.; Holmes, N. Equations of State of Unreacted High Explosives at High Pressures 11th International Detonation Symposium, Snowmass Village, CO, USA, 31 August–4 September 1998; Lawrence Livermore National Laboratory: Snowmass Village, CO, USA, 1998. [Google Scholar]
- Stevens, L.L.; Velisavljevic, N.; Hooks, D.E.; Dattelbaum, D.M. Hydrostatic compression curve for triamino-trinitrobenzene determined to 13.0 GPa with powder x-ray diffraction. Propellants Explos. Pyrotech. 2008, 33, 286–295. [Google Scholar] [CrossRef]
- Gump, J.C.; Peiris, S.M. Isothermal equations of state of beta octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine at high temperatures. J. Appl. Phys. 2005, 97, 053513. [Google Scholar] [CrossRef]
- Plisson, T.; Pineau, N.; Weck, G.; Bruneton, E.; Guignot, N.; Loubeyre, P. Equation of state of 1,3,5-triamino-2,4,6-trinitrobenzene up to 66 gpa. J. Appl. Phys. 2017, 122, 235901. [Google Scholar] [CrossRef]
- Millar, D.I.A. Energetic Materials at Extreme Conditions; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Guo, F.; Zhang, H.; Hu, H.-Q.; Cheng, X.-L. Effects of hydrogen bonds on solid state tatb, rdx, and datb under high pressures. Chin. Phys. B 2014, 23, 046501. [Google Scholar] [CrossRef]
- Davidson, A.J.; Dias, R.P.; Dattelbaum, D.M.; Yoo, C.-S. “Stubborn” triaminotrinitrobenzene: Unusually high chemical stability of a molecular solid to 150 GPa. J. Chem. Phys. 2011, 135, 174507. [Google Scholar] [CrossRef] [PubMed]
- Dove, M.T.; Trachenko, K.O.; Tucker, M.G.; Keen, D.A. Rigid unit modes in framework structures: Theory, experiment and applications. Rev. Mineral. Geochem. 2000, 39, 1–33. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with hirshfeld surfaces. Chem. Commun. 2007, 37, 3814–3816. [Google Scholar] [CrossRef]
- Gilli, G.; Gilli, P. The Nature of the Hydrogen Bond: Outline of a Comprehensive Hydrogen Bond Theory; Oxford University Press: Oxford, UK, 2009; pp. 36–61. [Google Scholar]
- Mooibroek, T.J.; Gamez, P. The s-triazine ring, a remarkable unit to generate supramolecular interactions. Inorg. Chim. Acta 2007, 360, 381–404. [Google Scholar] [CrossRef]
- Martinez, C.R.; Iverson, B.L. Rethinking the term “pi-stacking”. Chem. Sci. 2012, 3, 2191–2201. [Google Scholar] [CrossRef]
- Mishra, B.K.; Arey, J.S.; Sathyamurthy, N. Stacking and spreading interaction in n-heteroaromatic systems. J. Phys. Chem. A 2010, 114, 9606–9616. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.A.; Sanders, J.K.M. The nature of π-π interactions. J. Am. Chem. Soc. 1990, 112, 5525–5534. [Google Scholar] [CrossRef]
- Fanetti, S.; Citroni, M.; Bini, R. Tuning the aromaticity of s-triazine in the crystal phase by pressure. J. Phys. Chem. C 2014, 118, 13764–13768. [Google Scholar] [CrossRef]
- Fanetti, S.; Citroni, M.; Dziubek, K.; Nobrega, M.M.; Bini, R. The role of h-bond in the high-pressure chemistry of model molecules. J. Phys. Condens. Matter 2018, 30, 094001. [Google Scholar] [CrossRef] [PubMed]
- Thiery, M.M.; Kobashi, K.; Spain, I.L. Raman spectra of solid benzene under high pressure. Solid State Commun. 1985, 54, 95–97. [Google Scholar] [CrossRef]
- Schneider, J.R.; Schrader, B. Measurement and calculation of the infrared and raman active molecular and lattice vibrations of the crystalline melamine (1,3,5-triamino-s-triazine). J. Mol. Struct. 1975, 29, 1–14. [Google Scholar] [CrossRef]
- Larkin, P.J.; Makowski, M.P.; Colthup, N.B. The form of the normal modes of s-triazine: Infrared and raman spectral analysis and ab initio force field calculations. Spectrochim. Acta A 1999, 55, 1011–1020. [Google Scholar] [CrossRef]
- Marchewka, M.K. Infrared and raman spectra of the new melaminium salt: 2,4,6-triamino-1,3,5-triazin-1-ium hydrogenphthalate. Mater. Lett. 2004, 58, 843–848. [Google Scholar] [CrossRef]
- Marchewka, M.K. Infrared and raman spectra of melaminium chloride hemihydrate. Mater. Sci. Eng. 2002, B95, 214–221. [Google Scholar] [CrossRef]
- Goncharov, A.; Gregoryanz, E. Chapter 8—Solid nitrogen at extreme conditions of high pressure and temperature. In Chemistry at Extreme Conditions; Manaa, M.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 241–267. [Google Scholar]
- Song, Y.; Hemley, R.J.; Mao, H.-K.; Herschbach, D.R. Chapter 6—Nitrogen-containing molecular systems at high pressures and temperature. In Chemistry at Extreme Conditions; Manaa, M.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 189–222. [Google Scholar]
- Li, W.; Huang, X.; Bao, K.; Zhao, Z.; Huang, Y.; Wang, L.; Wu, G.; Zhou, B.; Duan, D.; Li, F.; et al. A novel high-density phase and amorphization of nitrogen-rich 1h-tetrazole (ch2n4) under high pressure. Sci. Rep. 2017, 7, 39249. [Google Scholar] [CrossRef] [PubMed]
- Shishkina, S.V.; Konovalova, I.S.; Shishkin, O.V.; Boyko, A.N. Acceptor properties of amino groups in aminobenzene crystals: Study from the energetic viewpoint. CrystEngComm 2017, 19, 6274–6288. [Google Scholar] [CrossRef]
- Szatyłowicz, H. Structural aspects of the intermolecular hydrogen bond strength: H-bonded complexes of aniline, phenol and pyridine derivatives. J. Phys. Org. Chem. 2008, 21, 897–914. [Google Scholar] [CrossRef]
- Citroni, M.; Fanetti, S.; Bazzicalupi, C.; Dziubek, K.; Pagliai, M.; Nobrega, M.M.; Mezouar, M.; Bini, R. Structural and electronic competing mechanisms in the formation of amorphous carbon nitride by compressing s-triazine. J. Phys. Org. Chem. C 2015, 119, 28560–28569. [Google Scholar] [CrossRef]
- Citroni, M.; Fanetti, S.; Bini, R. Pressure and laser-induced reactivity in crystalline s-triazine. J. Phys. Org. Chem. C 2014, 118, 10284–10290. [Google Scholar] [CrossRef]
- Li, S.; Li, Q.; Xiong, L.; Li, X.; Li, W.; Cui, W.; Liu, R.; Liu, J.; Yang, K.; Liu, B.; et al. Effect of pressure on heterocyclic compounds: Pyrimidine and s-triazine. J. Phys. Org. Chem. 2014, 141, 114902. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Duan, D.; Huang, X.; Jin, X.; Yang, X.; Li, S.; Jiang, S.; Huang, Y.; Li, F.; Cui, Q.; et al. Pressure-induced diversity of π-stacking motifs and amorphous polymerization in pyrrole. J. Phys. Org. Chem. C 2014, 118, 12420–12427. [Google Scholar] [CrossRef]
- Zhuravlev, K.K.; Traikov, K.; Dong, Z.; Xie, S.; Song, Y.; Liu, Z. Raman and infrared spectroscopy of pyridine under high pressure. Phys. Rev. B 2010, 82, 064116. [Google Scholar] [CrossRef]
- Wang, K.; Duan, D.; Wang, R.; Lin, A.; Cui, Q.; Liu, B.; Cui, T.; Zou, B.; Zhang, X.; Hu, J.; et al. Stability of hydrogen-bonded supramolecular architecture under high pressure conditions: Pressure-induced amorphization in melamine−boric acid adduct. Langmuir 2009, 25, 4787–4791. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Duan, D.; Wang, R.; Liu, D.; Tang, L.; Cui, T.; Liu, B.; Cui, Q.; Liu, J.; Zou, B.; et al. Pressure-induced phase transition in hydrogen-bonded supramolecular adduct formed by cyanuric acid and melamine. J. Phys. Org. Chem. B 2009, 113, 14719–14724. [Google Scholar] [CrossRef] [PubMed]
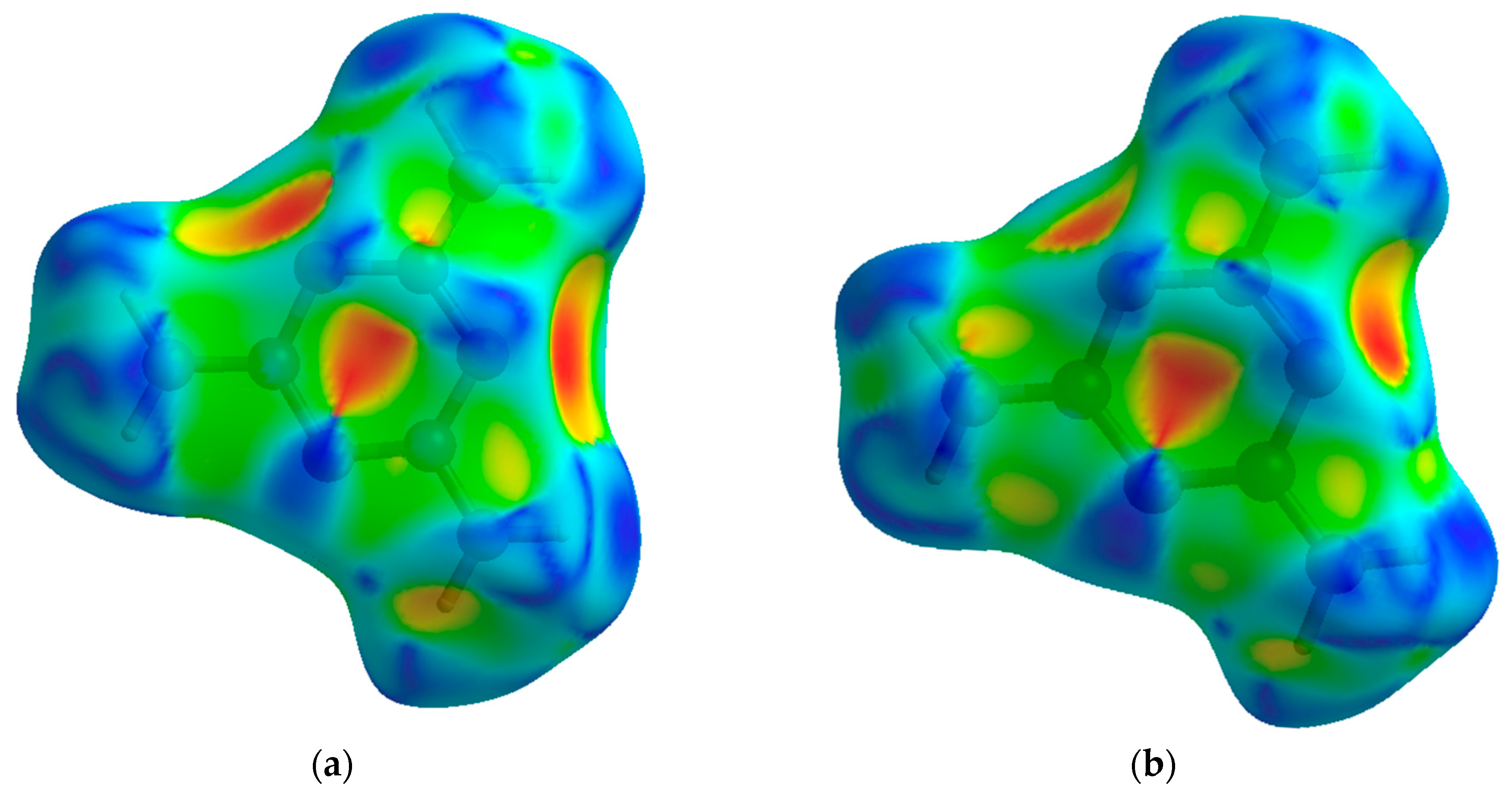
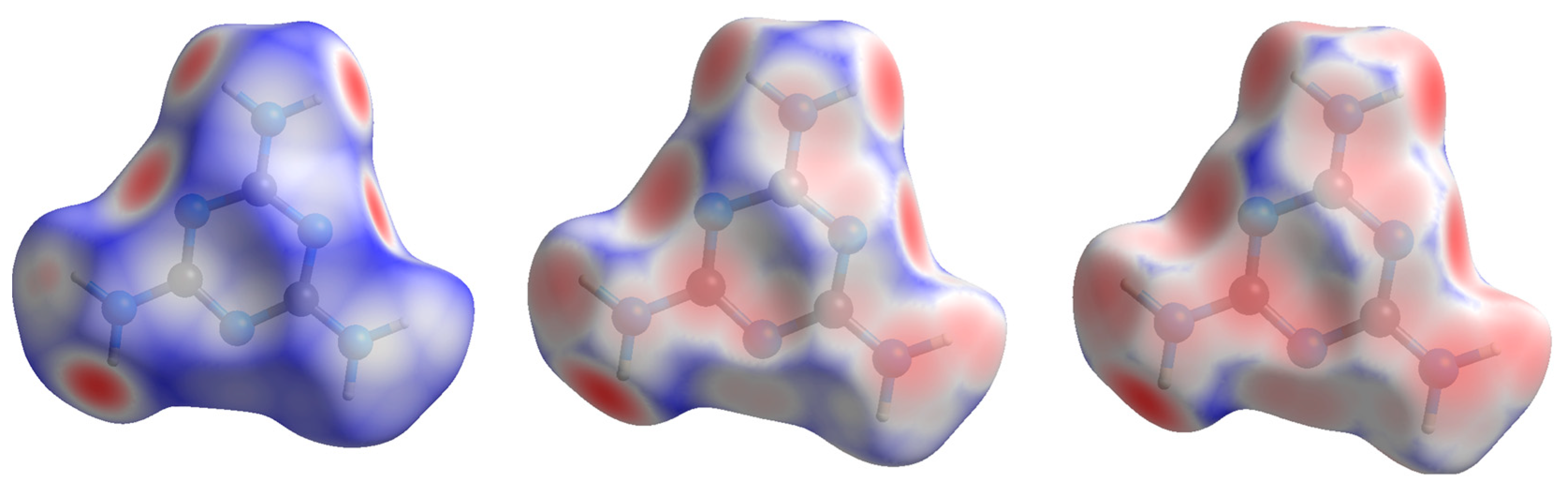

| 2010_1 (0.96(5) GPa) | Parameters | 2015_10 (36.21(5) GPa) | Parameters |
|---|---|---|---|
| No. of reflections collected | 453 | No. of reflections collected | 654 |
| No. of independent reflections | 186 | No. of independent reflections | 221 |
| Rint | 0.1272 | Rint | 0.0944 |
| R[F2 > 4σ(F2)] | 0.0611 | R[F2 > 4σ(F2)] | 0.0700 |
| wR(F2) | 0.1396 | wR(F2) | 0.1652 |
| Goodness-of-fit | 1.114 | Goodness-of-fit | 1.231 |
| No. of parameters refined | 40 | No. of parameters refined | 40 |
| No. of restraints used | 0 | No. of restraints used | 0 |
| Data Set | P (GPa) | a (Å) | b (Å) | c (Å) | β (°) | V (Å3) |
|---|---|---|---|---|---|---|
| 2010 | 10−4 | 10.606(1) | 7.495(1) | 7.295(2) | 112.26(2) | 536.7(2) |
| 2010 | 0.96(5) | 10.410(6) | 7.465(2) | 7.086(6) | 113.90(4) | 503.5(6) |
| 2010 | 2.9(1) | 10.148(6) | 7.389(2) | 6.954(6) | 115.93(4) | 468.9(5) |
| 2015 | 3.5(1) | 10.099(1) | 7.372(1) | 6.866(1) | 116.47(1) | 457.6(6) |
| 2010 | 4.7(1) | 9.962(6) | 7.333(2) | 6.821(6) | 117.14(4) | 443.4(5) |
| 2015 | 6.3(1) | 9.906(1) | 7.306(1) | 6.737(6) | 117.62(3) | 432.0(4) |
| 2010 | 7.7(1) | 9.807(4) | 7.274(1) | 6.705(4) | 118.07(3) | 422.0(4) |
| 2015 | 9.0(1) | 9.740(1) | 7.250(1) | 6.623(7) | 118.47(4) | 411.1(5) |
| 2010 | 10.5(1) | 9.678(6) | 7.235(1) | 6.616(5) | 118.34(4) | 407.8(4) |
| 2015 | 12.8(1) | 9.572(1) | 7.191(1) | 6.481(6) | 119.21(3) | 389.4(4) |
| 2010 | 16.8(1) | 9.446(6) | 7.151(2) | 6.350(5) | 119.89(4) | 371.9(4) |
| 2015 | 17.1(1) | 9.404(1) | 7.136(1) | 6.359(6) | 119.86(4) | 370.1(4) |
| 2010 | 19.8(1) | 9.366(7) | 7.122(7) | 6.252(6) | 120.47(5) | 359.4(5) |
| 2015 | 22.0(1) | 9.246(1) | 7.083(1) | 6.287(7) | 120.00(4) | 356.6(4) |
| 2010 | 23.5(1) | 9.254(4) | 7.103(4) | 6.279(4) | 120.14(3) | 356.9(3) |
| 2015 | 27.1(1) | 9.219(1) | 7.039(1) | 6.195(7) | 120.44(5) | 346.6(4) |
| 2015 | 30.8(1) | 9.057(2) | 7.013(1) | 6.128(7) | 120.57(5) | 335.1(4) |
| 2015 | 34.3(1) | 9.006(1) | 6.990(1) | 6.081(7) | 120.67(4) | 329.3(4) |
| 2015 | 36.2(1) | 8.965(1) | 6.966(1) | 6.075(7) | 120.48(5) | 326.9(4) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shelton, H.; Dera, P.; Tkachev, S. Evolution of Interatomic and Intermolecular Interactions and Polymorphism of Melamine at High Pressure. Crystals 2018, 8, 265. https://doi.org/10.3390/cryst8070265
Shelton H, Dera P, Tkachev S. Evolution of Interatomic and Intermolecular Interactions and Polymorphism of Melamine at High Pressure. Crystals. 2018; 8(7):265. https://doi.org/10.3390/cryst8070265
Chicago/Turabian StyleShelton, Hannah, Przemyslaw Dera, and Sergey Tkachev. 2018. "Evolution of Interatomic and Intermolecular Interactions and Polymorphism of Melamine at High Pressure" Crystals 8, no. 7: 265. https://doi.org/10.3390/cryst8070265
APA StyleShelton, H., Dera, P., & Tkachev, S. (2018). Evolution of Interatomic and Intermolecular Interactions and Polymorphism of Melamine at High Pressure. Crystals, 8(7), 265. https://doi.org/10.3390/cryst8070265