Sonocrystallization—Case Studies of Salicylamide Particle Size Reduction and Isoniazid Derivative Synthesis and Crystallization
Abstract
1. Introduction
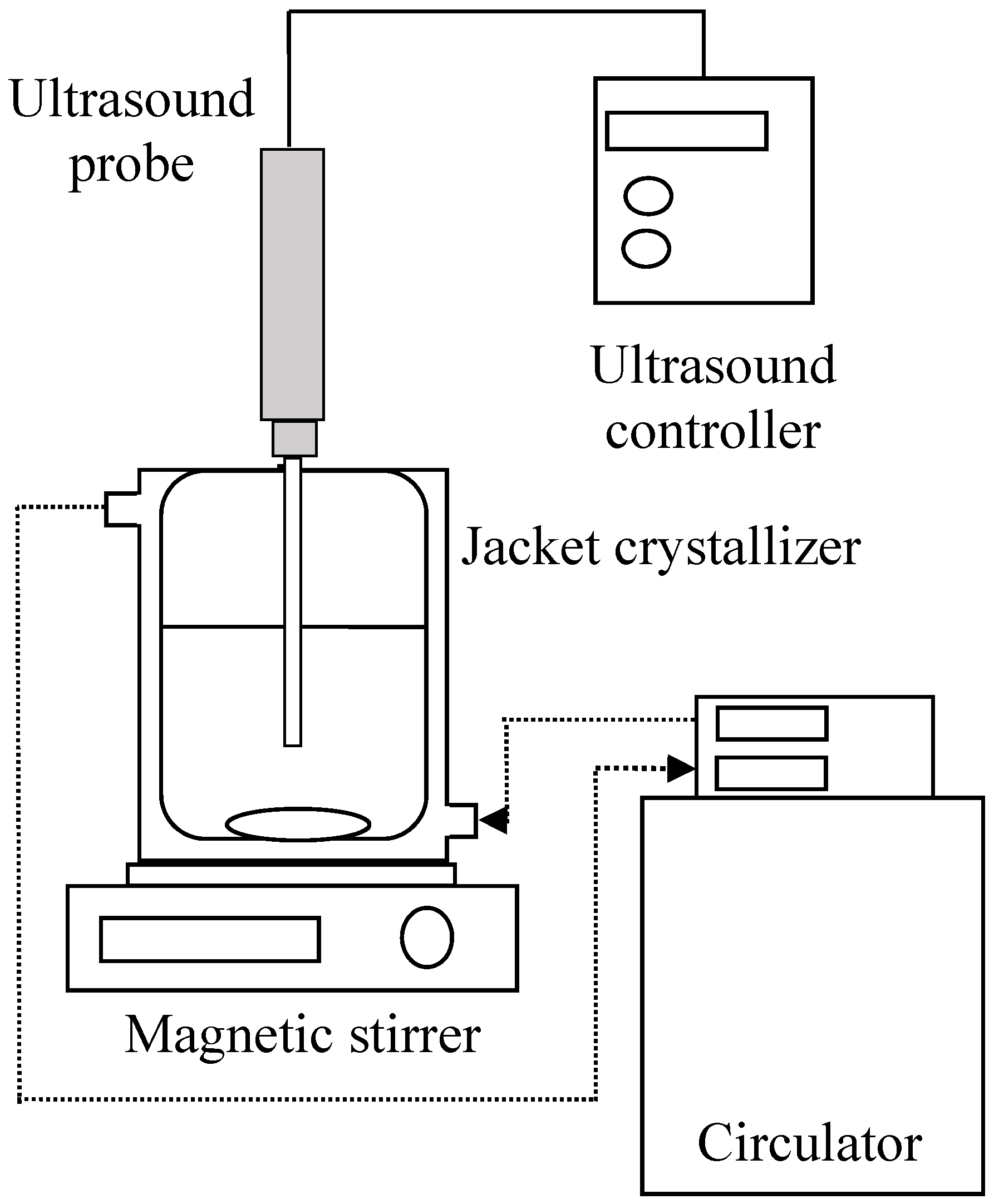
2. Materials and Method
3. Results and Discussion
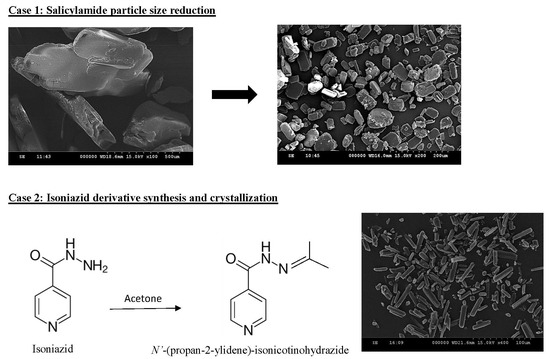
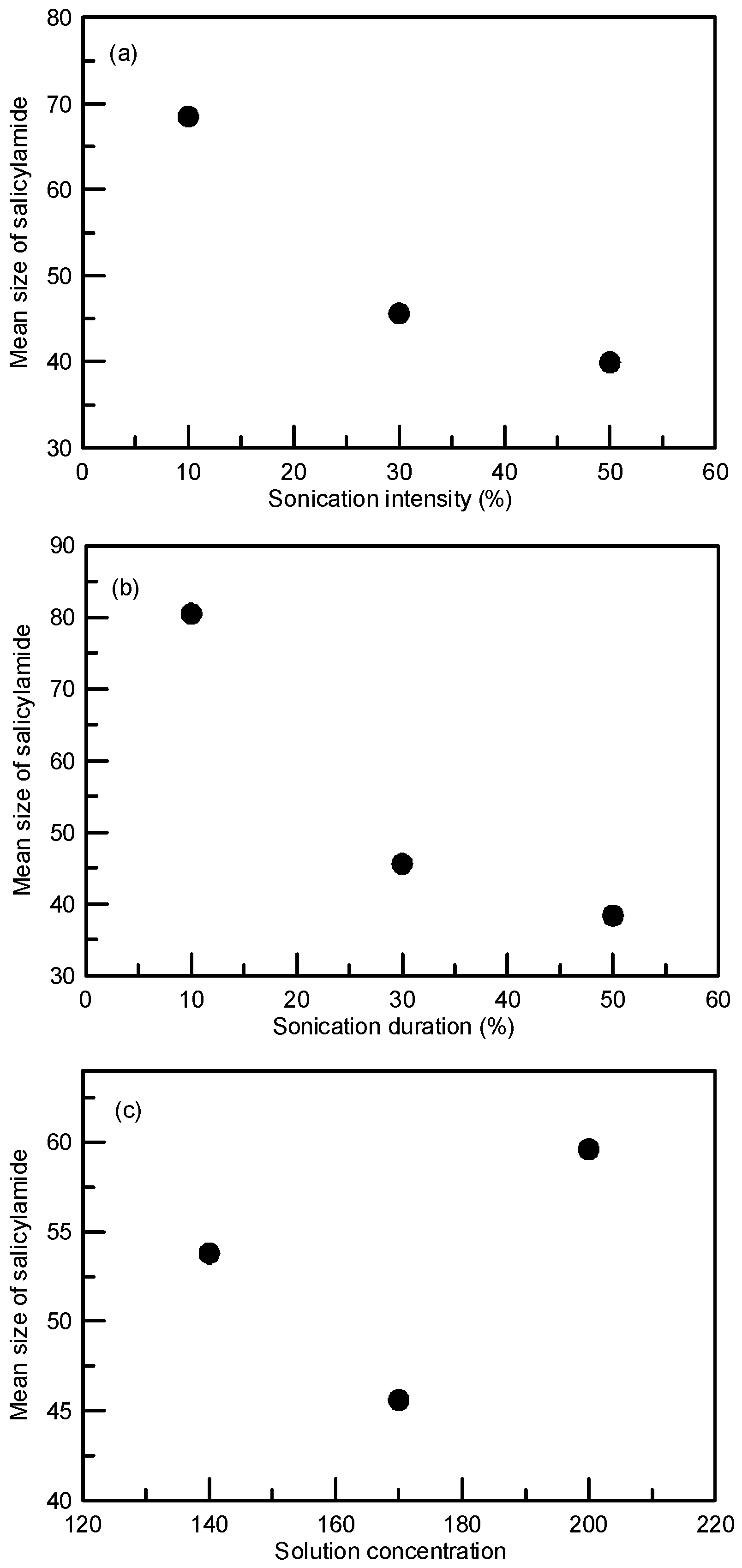
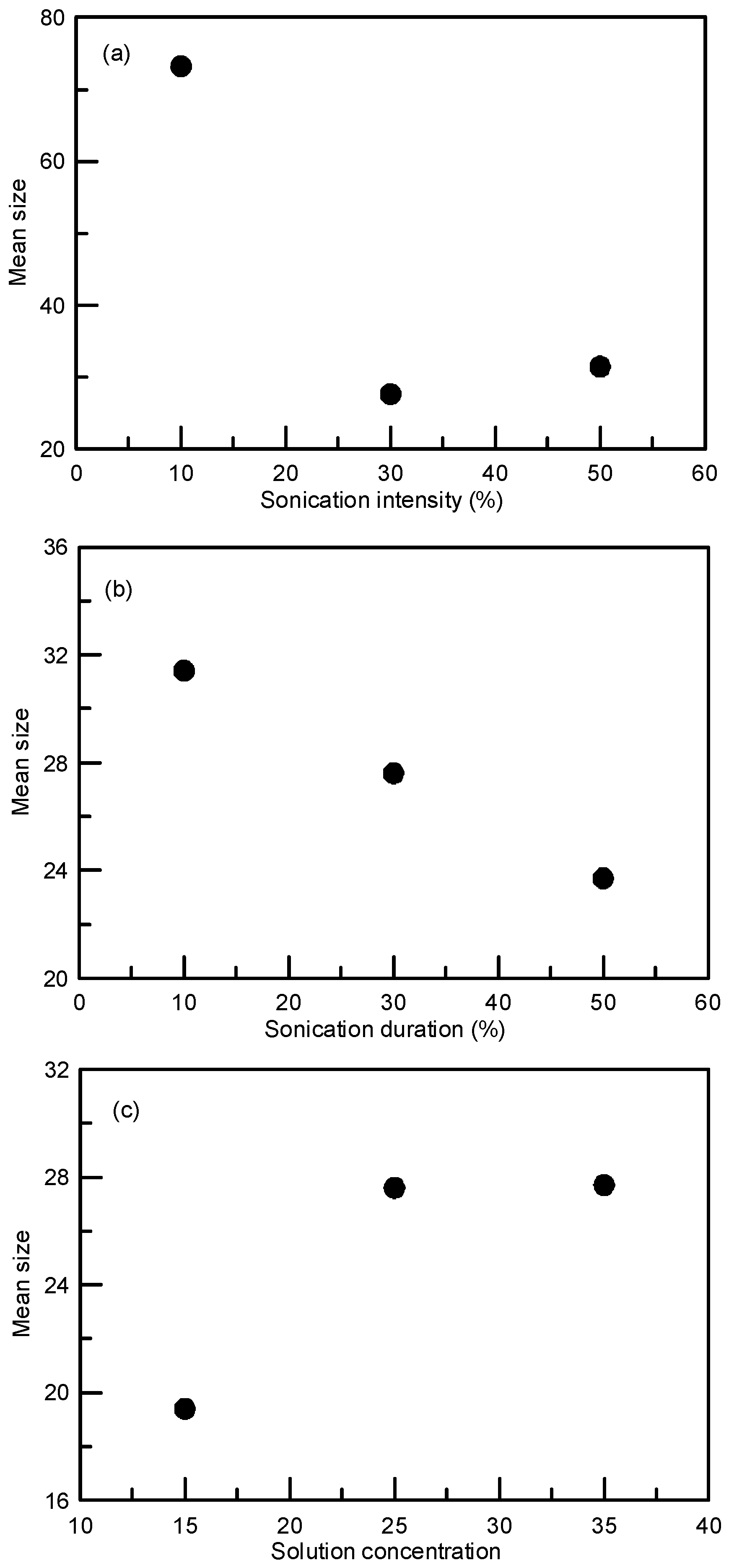
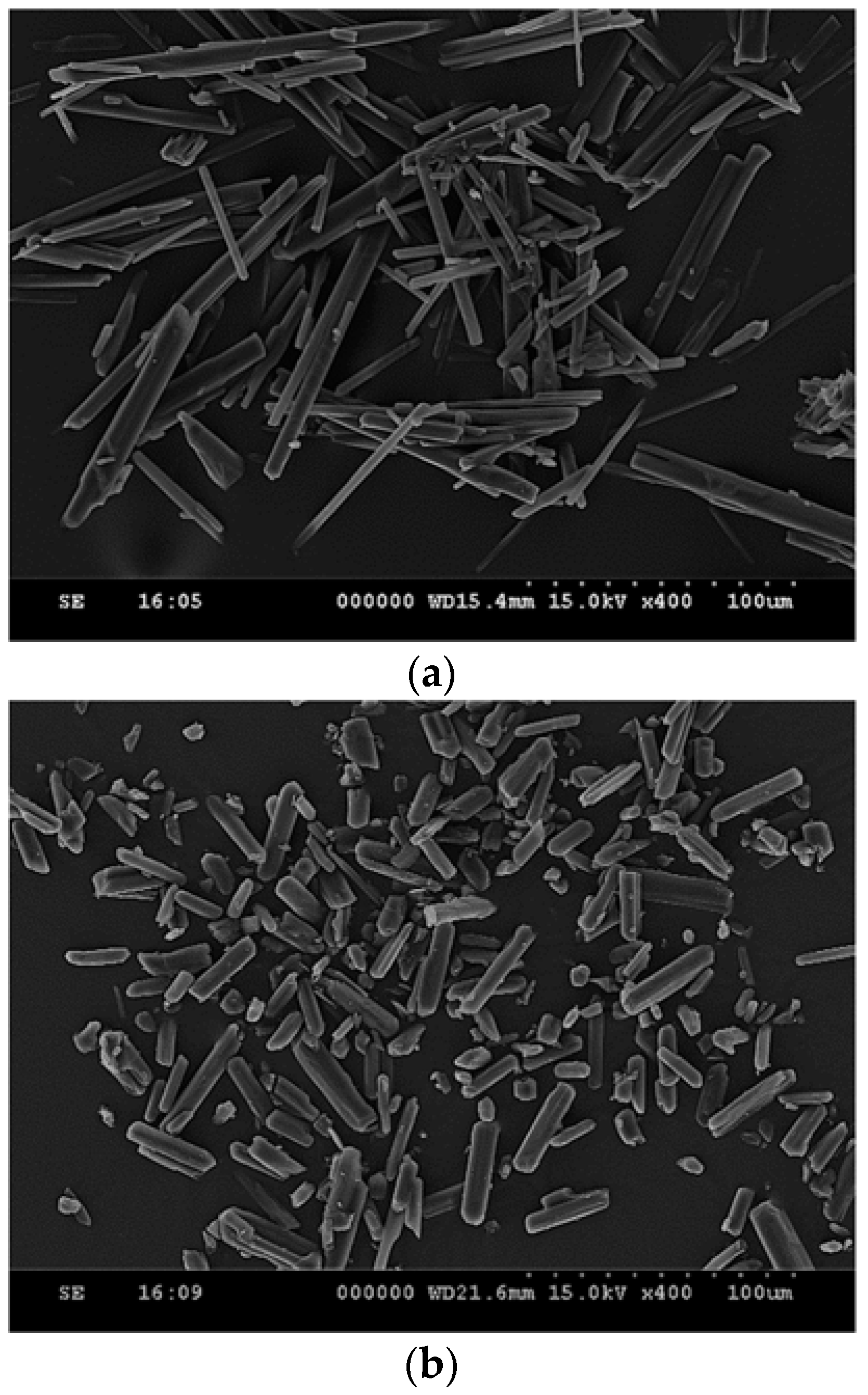
3.1. Particle Size Reduction of Salicylamide
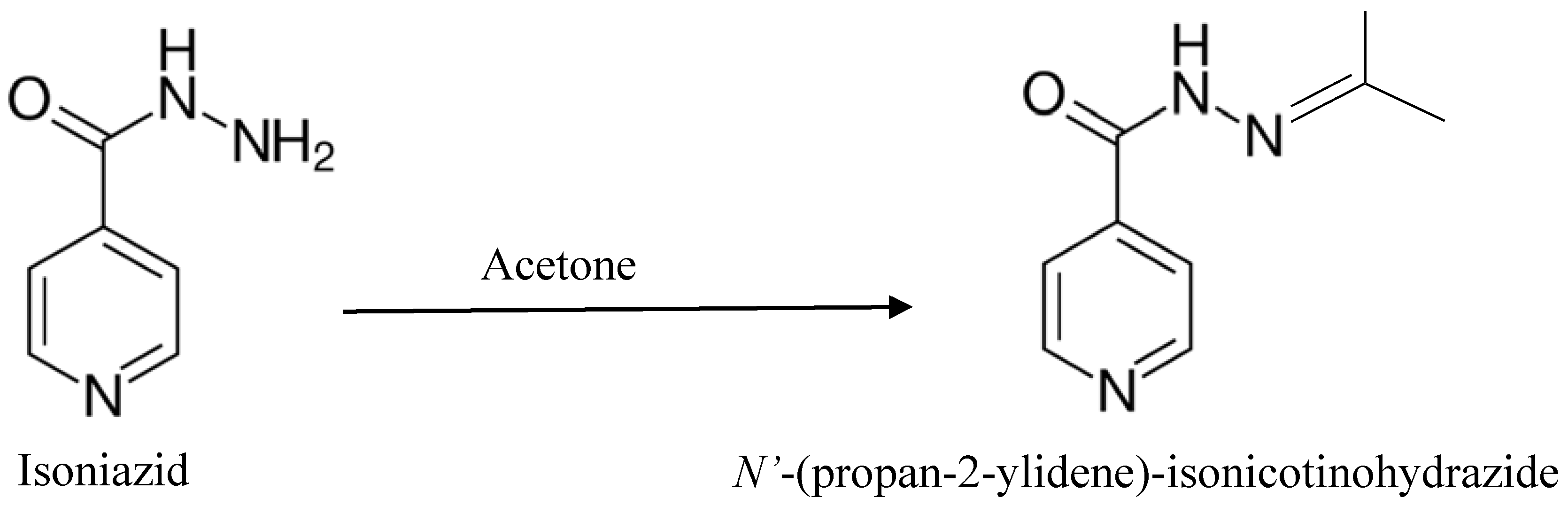
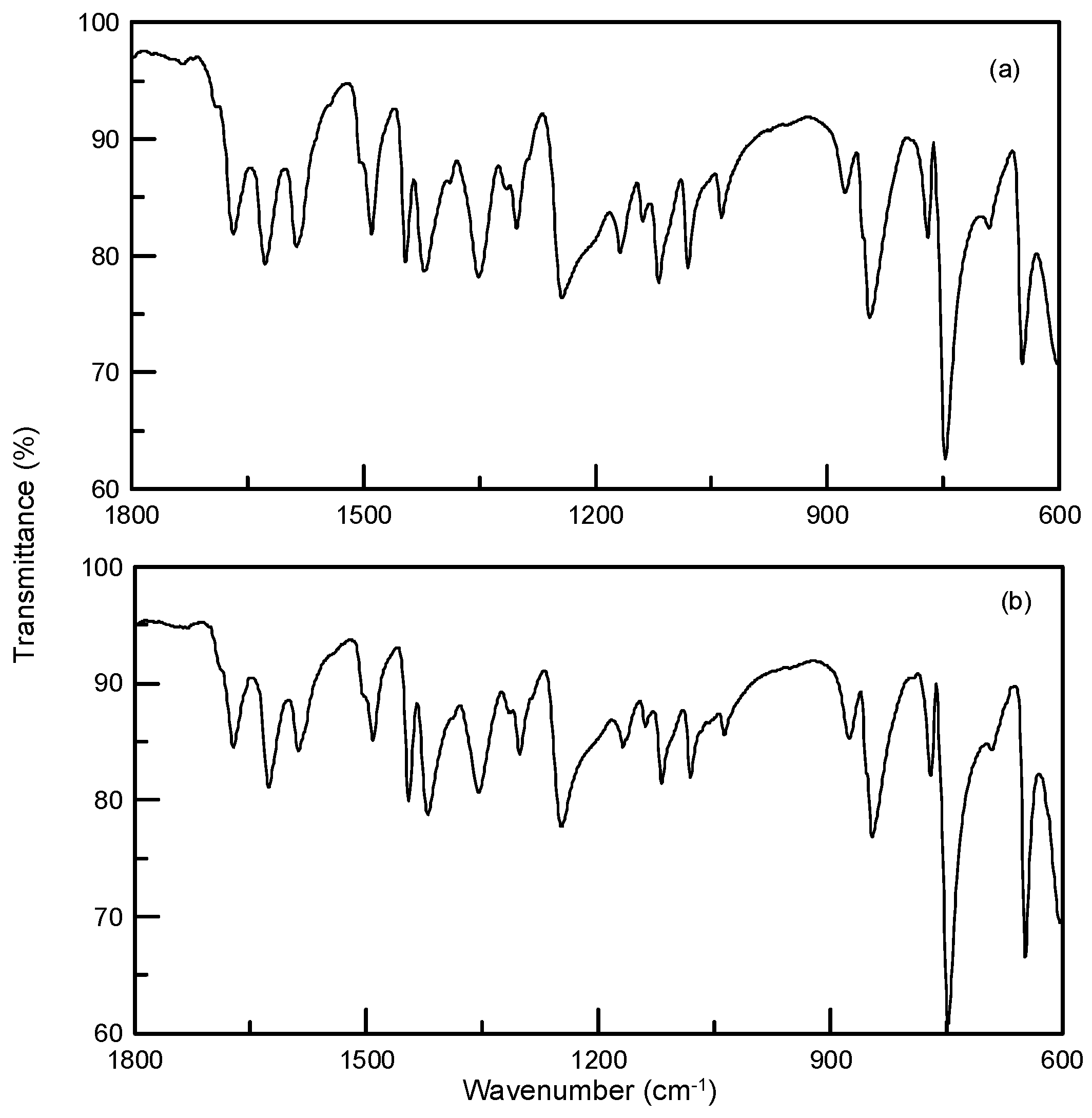
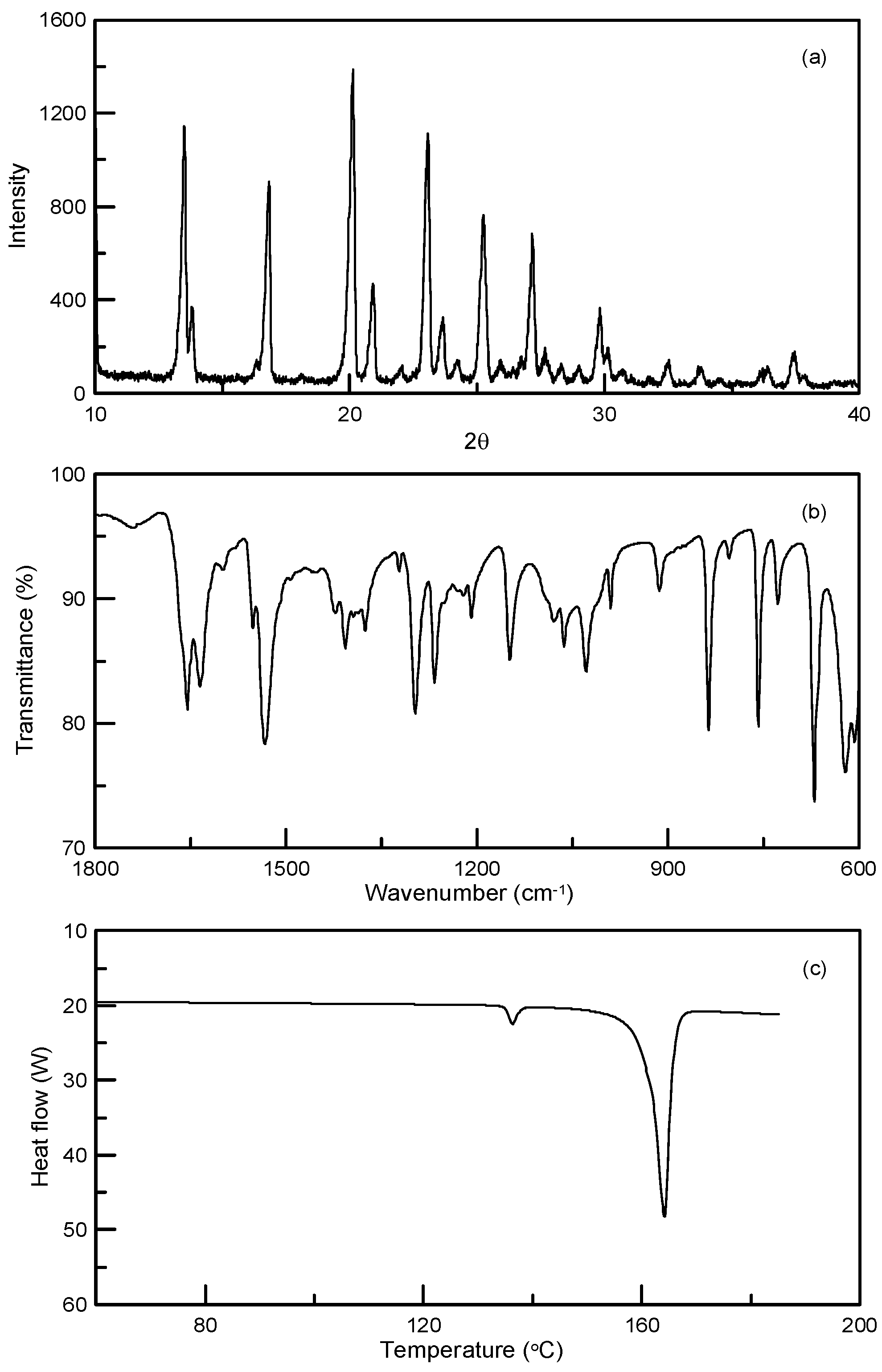
3.2. Synthesis and Crystallization of N′-(Propan-2-ylidene)-isonicotinohydrazide
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Variankaval, N.; Cote, A.S.; Doherty, M.F. From form to function: Crystallization of active pharmaceutical ingredients. AIChE J. 2008, 54, 1682–1688. [Google Scholar] [CrossRef]
- Chen, J.; Sarma, B.; Evans, J.M.B.; Myerson, A.S. Pharmaceutical crystallization. Cryst. Growth Des. 2011, 11, 887–895. [Google Scholar] [CrossRef]
- Wong, S.Y.; Chen, J.; Forte, L.E.; Myerson, A.S. Compact crystallization, filtration, and drying for the production of active pharmaceutical ingredients. Org. Process Res. Dev. 2013, 17, 684–692. [Google Scholar] [CrossRef]
- Tung, H.H. Industrial perspectives of pharmaceutical crystallization. Org. Process Res. Dev. 2013, 17, 445–454. [Google Scholar] [CrossRef]
- Shekunov, B.Y.; York, P. Crystallization processes in pharmaceutical technology and drug delivery design. J. Cryst. Growth 2000, 211, 122–136. [Google Scholar] [CrossRef]
- Pfund, L.Y.; Price, C.P.; Frick, J.J.; Matzger, A.J. Controlling pharmaceutical crystallization with designed polymeric heteronuclei. J. Am. Chem. Soc. 2015, 137, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Hezave, A.Z.; Esmaeilzadeh, F. Crystallization of micro particles of sulindac using rapid expansion of supercritical solution. J. Cryst. Growth 2010, 312, 3373–3383. [Google Scholar] [CrossRef]
- Li, Y.; Yang, D.J.; Zhou, W.; Chen, S.B.; Chen, S.L. Recrystallization of puerarin using the supercritical fluid antisolvent process. J. Cryst. Growth 2012, 340, 142–148. [Google Scholar] [CrossRef]
- Weber, C.C.; Kulkarni, S.A.; Kunov-Kruse, A.J.; Rogers, R.D.; Myerson, A.S. The use of cooling crystallization in an ionic liquid system for the purification of pharmaceuticals. Cryst. Growth Des. 2015, 15, 4946–4951. [Google Scholar] [CrossRef]
- Horstman, E.M.; Goyal, S.; Pawate, A.; Lee, G.; Zhang, G.G.Z.; Gong, Y.; Kenis, P.J.A. Crystallization optimization of pharmaceutical solid forms with X-ray compatible microfluidic platforms. Cryst. Growth Des. 2015, 15, 1201–1209. [Google Scholar] [CrossRef]
- Suslick, K.S. Sonochemistry. Science 1990, 23, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Sander, J.R.G.; Zeiger, B.W.; Suslick, K.S. Spray sonocrystallization. Cryst. Growth Des. 2015, 15, 1564–1567. [Google Scholar] [CrossRef]
- Sander, J.R.G.; Zeiger, B.W.; Suslick, K.S. Sonocrystallization and sonofragmentation. Ultrason. Sonochem. 2014, 21, 1908–1915. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, R.S.; Gogate, P.R. Intensified oxalic acid crystallization using ultrasonic reactors: Understanding effect of operating parameters and type of ultrasonic reactor. Ultrason. Sonochem. 2017, 39, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.H.; Khan, A.; Bruce, L.M.; Forbes, C.; O’Leary, R.L.; Price, C.J. The effect of ultrasound on the crystallization of paracetamol in the presence of structurally similar impurities. Crystals 2017, 7, 294. [Google Scholar] [CrossRef]
- Ike, Y.; Hirasawa, I. Polymorph control of L-ArgHCl on antisolvent crystallization by ultrasonic irradiation. Chem. Eng. Technol. 2017, 40, 1318–1322. [Google Scholar] [CrossRef]
- Gielen, B.; Jordens, J.; Thomassen, L.C.J.; Braeken, L.; Gerven, T.V. Agglomeration control during ultrasonic crystallization of an active pharmaceutical ingredient. Crystals 2017, 7, 40. [Google Scholar] [CrossRef]
- Gandhi, P.J.; Murthy, Z.V.P.; Pati, R.K. Optimization of process parameters by Taguchi robust design method for the development of nano-crystals of sirolimus using sonication based crystallization. Cryst. Res. Technol. 2012, 47, 53–72. [Google Scholar] [CrossRef]
- Li, J.; Bao, Y.; Wang, J. Effects of sonocrystallization on the crystal size distribution of cloxacillin benzathine crystals. Chem. Eng. Technol. 2013, 36, 1341–1346. [Google Scholar] [CrossRef]
- Crawford, D.E. Solvent-free sonochemistry: Sonochemical organic synthesis in the absence of a liquid medium. Beilstein J. Org. Chem. 2017, 13, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- Su, C.S.; Liao, C.Y.; Jheng, W.D. Particle size control and crystal habit modification of phenacetin using ultrasonic crystallization. Chem. Eng. Technol. 2015, 38, 181–186. [Google Scholar] [CrossRef]
- Su, C.S.; Wu, P.Y.; Jheng, W.D. Recrystallization of phenacetin and sulfathiazole using the sonocrystallization process. J. Taiwan Inst. Chem. Eng. 2016, 59, 106–112. [Google Scholar] [CrossRef]
- Kuo, P.H.; Zhang, B.C.; Su, C.S.; Liu, J.J.; Sheu, M.T. Application of two-level factorial design to investigate the effect of process parameters on the sonocrystallization of sulfathiazole. J. Cryst. Growth 2017, 471, 8–14. [Google Scholar] [CrossRef]
- Manin, A.N.; Voronin, A.P.; Manin, N.G.; Vener, M.V.; Shishkina, A.V.; Lermontov, A.S.; Perlovich, G.L. Salicylamide cocrystals: Screening, crystal structure, sublimation thermodynamics, dissolution, and solid-state DFT calculations. J. Phys. Chem. B 2014, 118, 6803–6814. [Google Scholar] [CrossRef] [PubMed]
- Su, C.S.; Chen, Y.P. Recrystallization of salicylamide using a batch supercritical antisolvent process. Chem. Eng. Technol. 2005, 28, 1177–1181. [Google Scholar] [CrossRef]
- Hu, Y.Q.; Zhang, S.; Zhao, F.; Gao, C.; Feng, L.S.; Lv, Z.S.; Xu, Z.; Wu, X. Isoniazid derivatives and their anti-tubercular activity. Eur. J. Med. Chem. 2017, 133, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Lemmerer, A.; Bernstein, J.; Kahlenberg, V. Covalent assistance in supramolecular synthesis: In situ modification and masking of the hydrogen bonding functionality of the supramolecular reagent isoniazid in co-crystals. CrystEngComm 2011, 13, 5692–5708. [Google Scholar] [CrossRef]
- Oruganti, M.; Khade, P.; Das, U.K.; Trivedi, D.R. The hierarchies of hydrogen bonds in salts/cocrystals of isoniazid and its schiff base—A case study. RSC Adv. 2016, 6, 15868–15876. [Google Scholar] [CrossRef]
- Nordström, F.L.; Rasmuson, Å.C. Solubility and melting properties of salicylamide. J. Chem. Eng. Data 2006, 51, 1775–1777. [Google Scholar] [CrossRef]
- Dhumal, R.S.; Biradar, S.V.; Paradkar, A.R.; York, P. Particle engineering using sonocrystallization: Salbutamol sulphate for pulmonary delivery. Int. J. Pharm. 2009, 368, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Narducci, O.; Jones, A.G.; Kougoulos, E. An assessment of the use of ultrasound in the particle engineering of micrometer-scale adipic acid crystals. Cryst. Growth Des. 2011, 11, 1742–1749. [Google Scholar] [CrossRef]
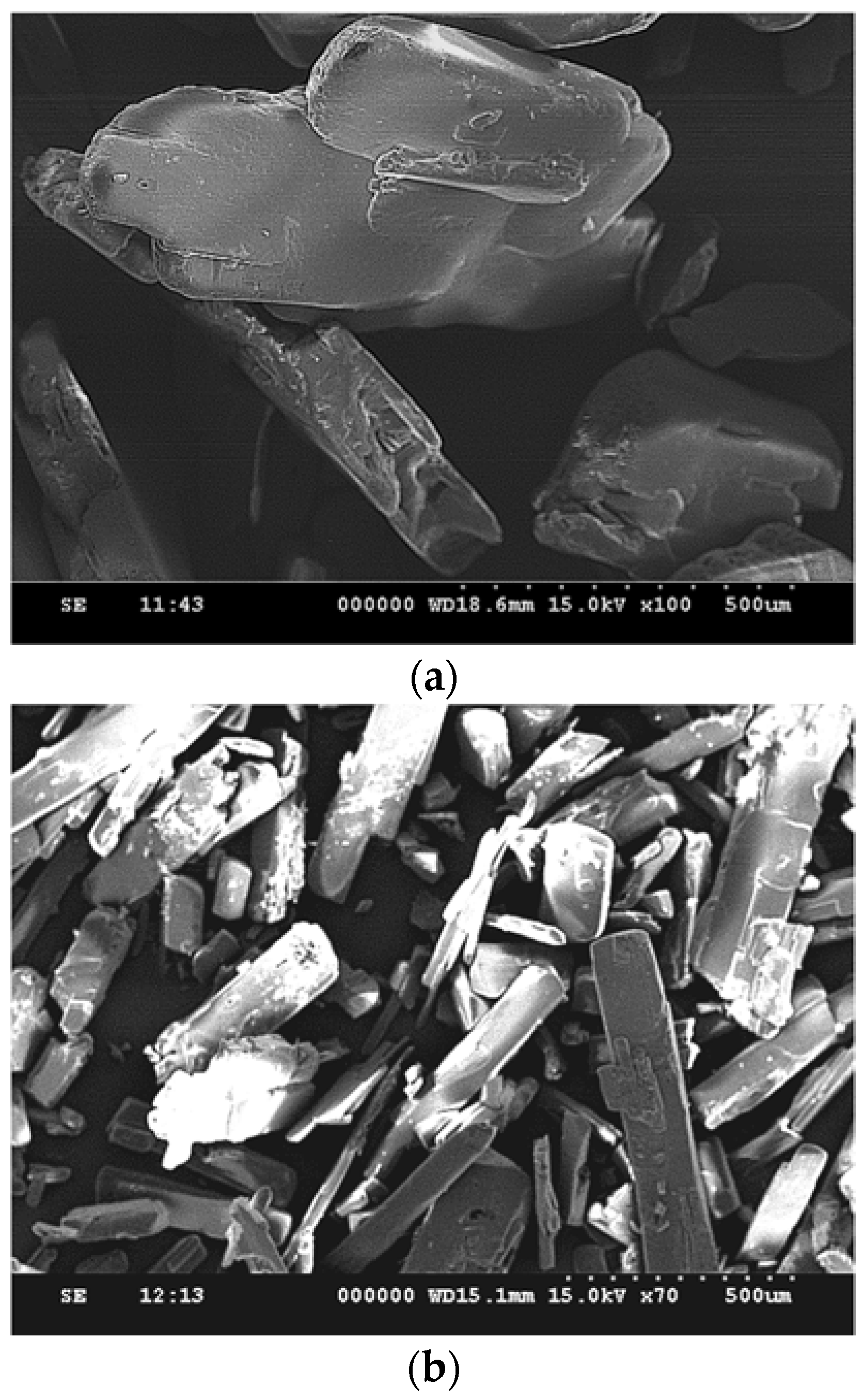
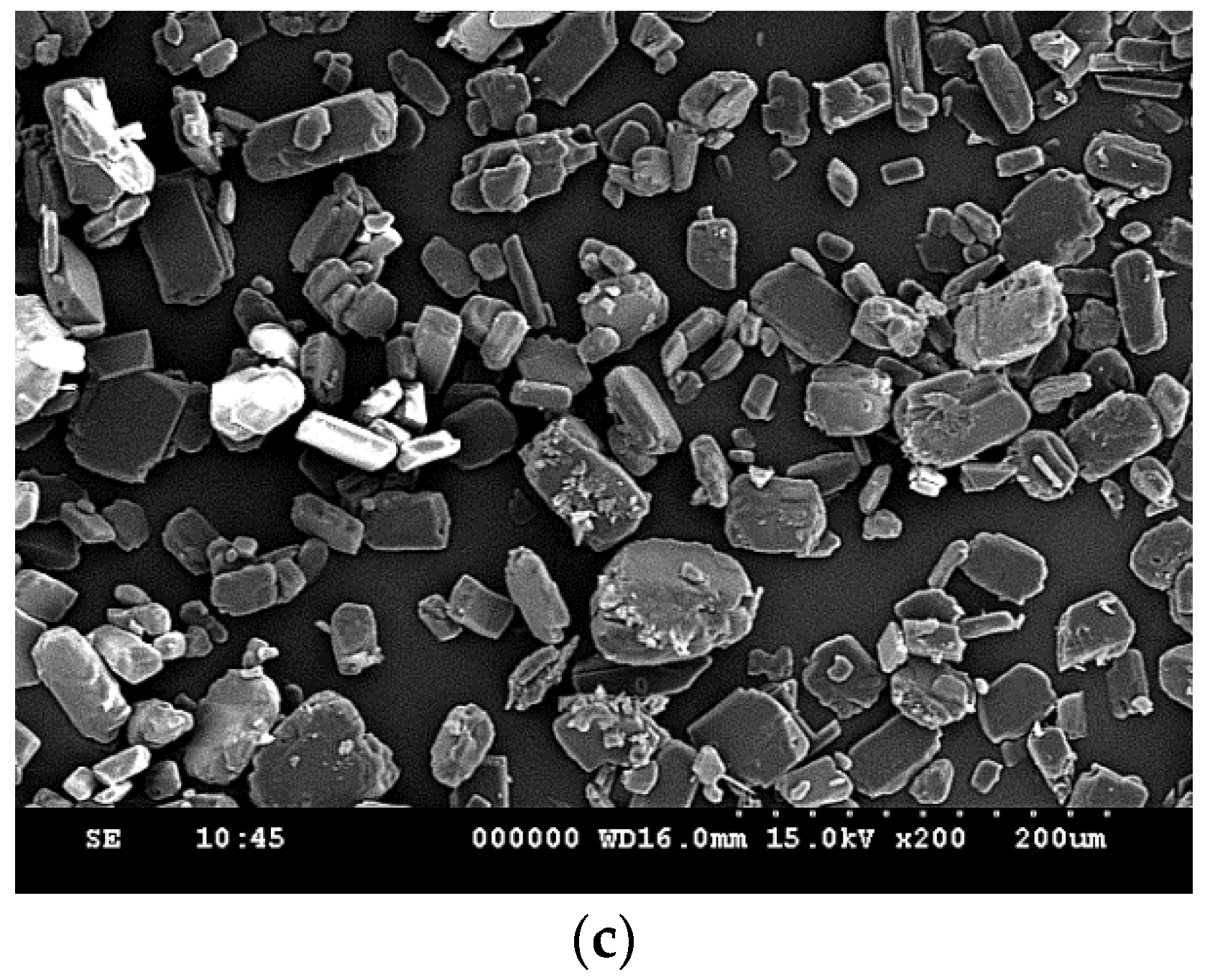
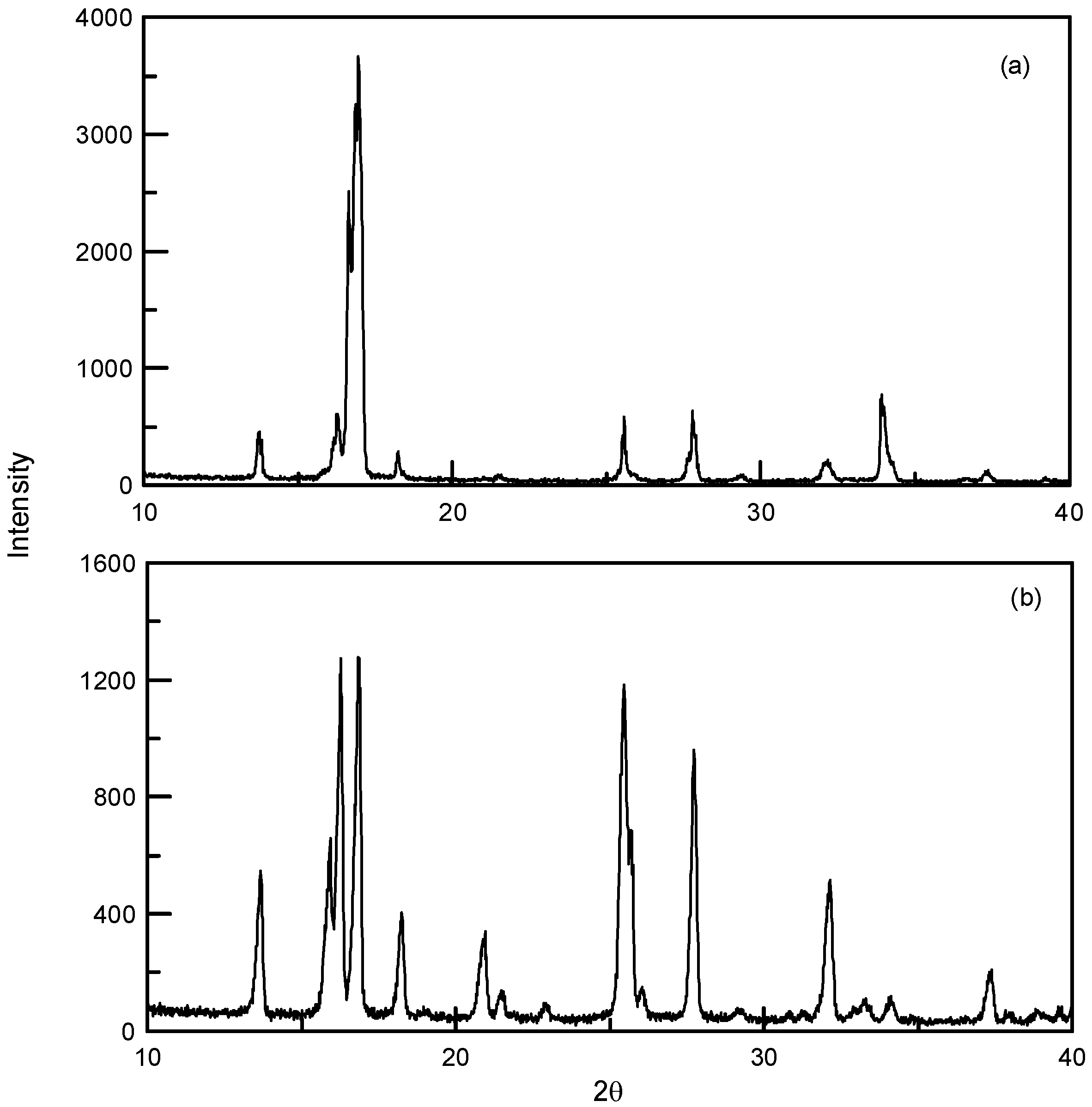
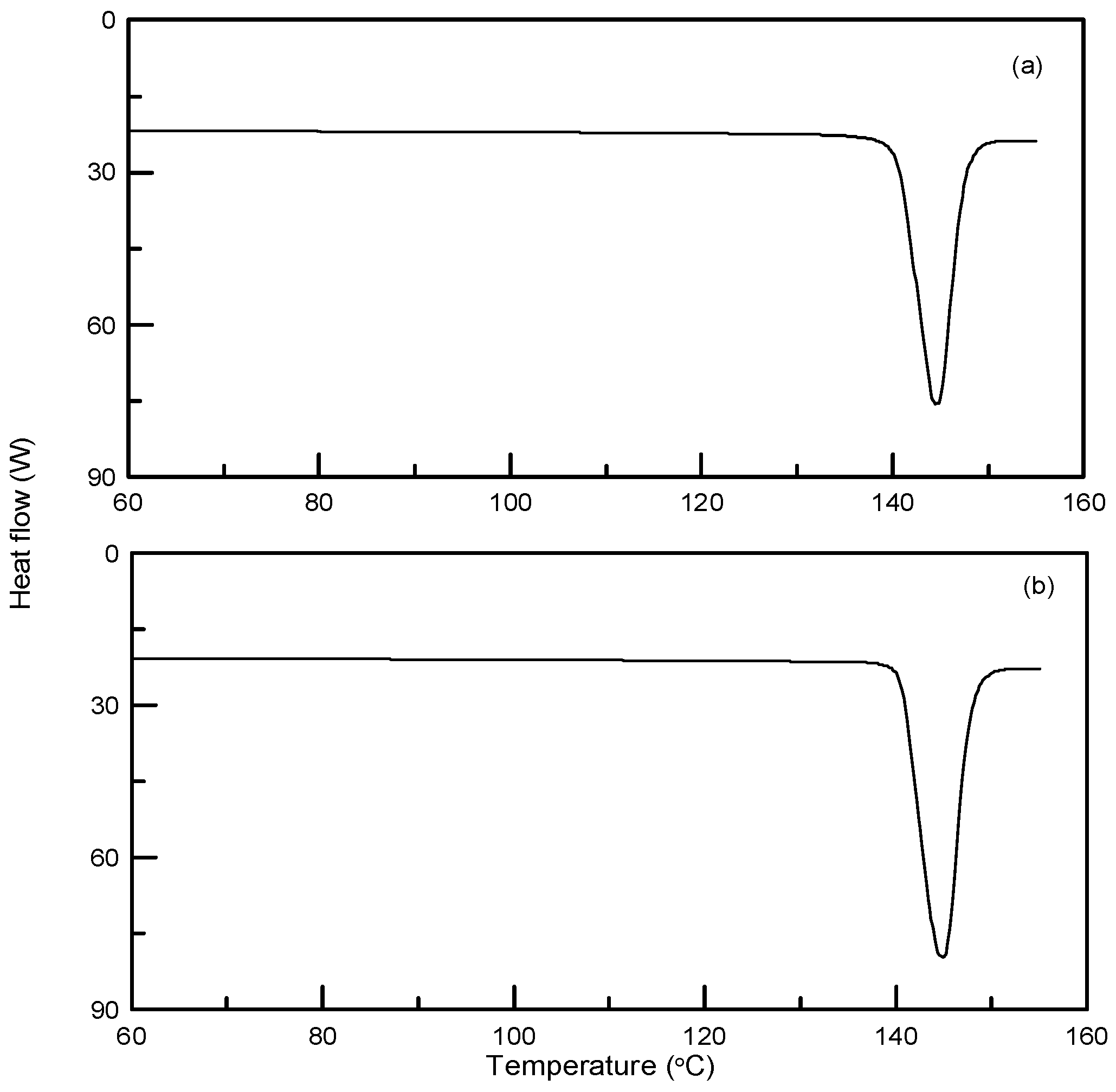
| Compound | CAS. No. | Formula | Mw (g/mol) | Supplier | Purity (%) |
|---|---|---|---|---|---|
| Acetone | 67-64-1 | C3H6O | 58.08 | Sigma-Aldrich | 99.8 |
| Isoniazid | 54-85-3 | C6H7N3O | 137.14 | Sigma-Aldrich | 99 |
| Methanol | 67-56-1 | CH3OH | 32.04 | Sigma-Aldrich | 99.8 |
| Salicylamide | 65-45-2 | C7H7NO2 | 137.14 | Sigma-Aldrich | 99 |
| Experiment No. | Intensity (%) | Duration (%) | Concentration (mg/mL) | Cooling Rate (°C/h) | Mean Size (μm) | S.D a (μm) |
|---|---|---|---|---|---|---|
| Ori | --- | --- | --- | --- | 595.0 | 178.4 |
| 1 | 30 | 30 | 170 | 20 | 45.6 | 18.2 |
| 2 | 10 | 30 | 170 | 20 | 68.5 | 30.4 |
| 3 | 50 | 30 | 170 | 20 | 39.9 | 13.3 |
| 4 | 30 | 10 | 170 | 20 | 80.5 | 26.7 |
| 5 | 30 | 50 | 170 | 20 | 38.4 | 13.7 |
| 6 | 30 | 30 | 140 | 20 | 53.8 | 17.0 |
| 7 | 30 | 30 | 200 | 20 | 59.6 | 22.3 |
| B1 b | 0 | 0 | 170 | 20 | 432.5 | 176.5 |
| Experiment No. | Intensity (%) | Duration (%) | Concentration (mg/mL) | Cooling Rate (°C/h) | Mean Size (μm) | S.D a (μm) |
|---|---|---|---|---|---|---|
| 8 | 30 | 30 | 25 | 20 | 27.6 | 8.7 |
| 9 | 10 | 30 | 25 | 20 | 73.2 | 43.1 |
| 10 | 50 | 30 | 25 | 20 | 31.4 | 10.2 |
| 11 | 30 | 10 | 25 | 20 | 31.4 | 12.3 |
| 12 | 30 | 50 | 25 | 20 | 23.7 | 8.1 |
| 13 | 30 | 30 | 35 | 20 | 27.7 | 10.3 |
| 14 | 30 | 30 | 15 | 20 | 19.4 | 7.9 |
| B2 b | 0 | 0 | 25 | 20 | 78.1 | 41.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.-Y.; Yen, S.-K.; Hu, W.-S.; Huang, Y.-Z.; Yang, T.-M.; Su, C.-S. Sonocrystallization—Case Studies of Salicylamide Particle Size Reduction and Isoniazid Derivative Synthesis and Crystallization. Crystals 2018, 8, 249. https://doi.org/10.3390/cryst8060249
Yang Z-Y, Yen S-K, Hu W-S, Huang Y-Z, Yang T-M, Su C-S. Sonocrystallization—Case Studies of Salicylamide Particle Size Reduction and Isoniazid Derivative Synthesis and Crystallization. Crystals. 2018; 8(6):249. https://doi.org/10.3390/cryst8060249
Chicago/Turabian StyleYang, Zhen-Yu, Shih-Kuo Yen, Wei-Syun Hu, Yu-Zhe Huang, Tsung-Mao Yang, and Chie-Shaan Su. 2018. "Sonocrystallization—Case Studies of Salicylamide Particle Size Reduction and Isoniazid Derivative Synthesis and Crystallization" Crystals 8, no. 6: 249. https://doi.org/10.3390/cryst8060249
APA StyleYang, Z.-Y., Yen, S.-K., Hu, W.-S., Huang, Y.-Z., Yang, T.-M., & Su, C.-S. (2018). Sonocrystallization—Case Studies of Salicylamide Particle Size Reduction and Isoniazid Derivative Synthesis and Crystallization. Crystals, 8(6), 249. https://doi.org/10.3390/cryst8060249