Abstract
The dynamism of proteins is central to their function, and several proteins have been described as flexible, as consisting of multiple domains joined by flexible linkers, and even as intrinsically disordered. Several techniques exist to study protein structures, but small angle X-ray scattering (SAXS) has proven to be particularly powerful for the quantitative analysis of such flexible systems. In the present report, we have used SAXS in combination with X-ray crystallography to highlight their usefulness at characterizing flexible proteins, using as examples two proteins involved in different steps of ribosome biogenesis. The yeast BRCA2 and CDKN1A-interactig protein, Bcp1, is a chaperone for Rpl23 of unknown structure. We showed that it consists of a rigid, slightly elongated protein, with a secondary structure comprising a mixture of alpha helices and beta sheets. As an example of a flexible molecule, we studied the SBDS (Shwachman-Bodian-Diamond Syndrome) protein that is involved in the cytoplasmic maturation of the 60S subunit and constitutes the mutated target in the Shwachman-Diamond Syndrome. In solution, this protein coexists in an ensemble of three main conformations, with the N- and C-terminal ends adopting different orientations with respect to the central domain. The structure observed in the protein crystal corresponds to an average of those predicted by the SAXS flexibility analysis.
1. Introduction
Small Angle X-ray Scattering (SAXS) is a powerful technique that is used to obtain structural information of both ordered and disordered biological molecules at low resolution. It provides information about the size and shape of proteins and complexes, as well as about structural changes that occur at different experimental conditions [1]. SAXS requires small (milligram) amounts of purified and monodisperse samples. The experiment can be performed rapidly using the dedicated beamlines at synchrotron light sources [2]. Even data analysis, when the quality of the sample is good enough, can be fast, thanks to powerful specialized software [3]. SAXS is a particularly useful technique for the characterization of multidomain proteins [4], which consist of two or more domains connected by linkers determining their flexibility. In a typical SAXS experiment, a collimated monochromatic X-ray beam illuminates a solution of particles, and the intensity of the scattered X-rays is registered by a detector. The recorded scattering pattern is reduced to a radially-averaged one-dimensional scattering profile, which results in low structural resolution but can still provide important structural information. In contrast, Macromolecular Crystallography (MX) is an older and mature technique capable of revealing high-resolution details of biological macromolecules when good-sized and well-ordered crystals of such molecules are obtained. Information obtained from different biophysical experiments can be combined to obtain structural insights represented by molecular models. Combination of X-ray crystallography with SAXS data and other techniques including NMR chemical shift perturbation or FRET has proven very useful for obtaining detailed models of dynamic protein complexes such as the ESCRT membrane-protein trafficking system [5,6] or protein kinases and their regulatory partners [7].
In the present article, SAXS and MX were used to study the proteins Bcp1 and SBDS. These proteins participate at different stages of the cytoplasmic maturation of the large ribosomal subunit. Ribosomes are the cell machinery responsible for protein synthesis. They are composed of a small subunit that acts as decoding center of the genetic information and a large subunit where peptide bonds are formed. The production of ribosomes is a highly coordinated and extremely demanding process for the cell, known as ribosome biogenesis. In addition to the production of the four ribosomal RNA (rRNAs) and the approximately 79 ribosomal proteins, it requires the action of more than a hundred assembly factors located in different cellular compartments that act at different stages of this pathway. About 200 trans-acting factors are involved in rRNA processing, rRNA folding, protein loading, and export of the ribosomal subunits [8,9,10,11]. Bcp1 is an essential protein that functions as an escortin for Rpl23 (uL14), facilitating its dissociation from the karyopherins during reimport to the nucleus and subsequent loading into the nascent 60S subunits [12]. The function of the human Bcp1 homologue (BCCIP) is unclear and may not be equivalent to that of the yeast protein. The BCCIPβ isoform seems to have a role in ribosome biogenesis via its interaction with Rpl23, but its depletion does not impair the 60S synthesis pathway [13]. Rpl23 is also the major binding site of Tif6, an anti-association factor that prevents premature association of the not-fully mature 60S subunits with the 40S subunits [14]. Although both Bcp1 and Tif6 interact with Rpl23, they do not co-exist in the same particle, and Tif6 only interacts with Rpl23 on the 60S subunit. After export from the nucleus, the release of Tif6 from the pre-60S subunit occurs via joint action of the GTPase Efl1 and Sdo1 (yeast orthologue of the SBDS protein) [15]. Sdo1/SBDS functions as the guanine exchange factor for EFL1 [16,17] that, upon GTP hydrolysis, displaces Tif6 by competing for a partial overlapping site on the surface of the 60S ribosomal subunit [18]. This last step promotes joining of the mature 60S ribosomal subunits with the 40S subunits to produce translationally competent ribosomes.
The main goal of this paper is to show how the use of SAXS is pivotal in the determination of all likely conformations of a protein using as model the proteins Bcp1 and SBDS. This investigation demonstrates the inherent flexibility of SBDS that in turn will provide better ground for further investigations on the protein and its functionality.
2. Materials and Methods
2.1. Protein Expression and Purification
The proteins used in this study consisted of the Saccharomyces cerevisiae Bcp1 (UniProt Q06338) and the Archaeoglobus fulgidus SBDS (AfSBDS, UniProt O29759). Archaeal SBDS was expressed and purified as described in [19]. The expression vector for the Bcp1 protein used a modified pRSET-A vector (Invitrogen) coding for a N-terminal 6x-His tag, amino acids 1–85 of the Bacillus stearothermophillus dihydrolipoyl acetyl transferase (EC 2.1.12), and a TEV protease recognition site followed by the coding region of Bcp1 residues 46–283. All proteins used in this study were expressed in E. coli C41 after induction with 0.5 mM IPTG for 12 h at 20 °C. After expression, bacterial cells were lysed by sonication in 50 mM Phosphate buffer pH 7, 300 mM NaCl, and 25 mM imidazole. The crude extract was clarified by centrifugation at 15,000 rpm for 30 min and the soluble fraction initially purified using Ni2+-affinity chromatography using a 5 mL Ni-NTA superflow cartridge (QIAGEN). After this purification step, the AfSBDS protein was further purified by anionic exchange chromatography using a HiTrap SP FF column. The protein was 6-fold diluted with 50 mM Phosphate buffer pH 6.5, introduced onto the column, and eluted in one step with 50 mM Phosphate buffer pH 6.5, 1 M NaCl. Purified samples were dialyzed against 50 mM Phosphate buffer pH 6.5, 150 mM NaCl, and concentrated and stored at −80 °C until further use. On the other hand, the Bcp1 protein was digested with TEV protease to remove the histidine tag and the fusion protein while dialyzing against lysis buffer. The digested protein was reintroduced onto the Ni2+ column as described previously, and the non-bound material pooled together to finally purify it by size exclusion chromatography using a Superdex 75 HiLoad 26/600 (GE Healthcare) equilibrated with 50 mM Phosphate buffer pH 7.5, 150 mM NaCl. Finally, samples were concentrated and stored at −80 °C.
2.2. Circular Dichroism Spectroscopy (CD)
Circular dichroism wavelength-scan measurements were followed at 20 °C with a JASCO J-720 spectropolarimeter equipped with a Peltier temperature controller. CD spectra were recorded using a 1 mm cuvette and a protein concentration of 5 µM and 170 µM for the Bcp1 protein in the far-UV (260–190 nm) and near-UV (250–340 nm), respectively. Spectra were acquired in Phosphate buffered saline solution (PBS) with 1 mM dithioerythritol, at a 1 nm increase per step, an averaging time of 5 s, and a spectral resolution of 1 nm.
2.3. Macromolecular Crystallography (MX)
2.3.1. Crystallization
AfSBDS had been previously crystallized and solved in [19,20]. This provided us with the opportunity of obtaining a high-resolution structure of the protein of interest for the present study. Preliminary crystallization of AfSBDS was tested with an array of commercial screens. The best conditions were obtained from sitting drop vapour diffusion experiments in which 100 nL of protein (5 mg mL−1) was mixed with 100 nL of reservoir solution from the Morpheus screen [21]. Crystals grew at 20 °C in two days to a maximum size of 60 × 60 × 250 µm3 and were harvested directly in loops and cryo-cooled with liquid nitrogen without the addition of any cryoprotectant.
2.3.2. Data Collection, Data Processing, Model Building, and Refinement
Diffraction data were collected at the Diamond Light Source microfocus beamline I24, at an energy of 0.9686 Å in cryogenic conditions (100 K) [22]. In order to screen the best crystals and identify the strongest diffracting regions, the samples were rastered with a 10 × 10 μm2 beam in orthogonal grid scans (at φ = 0° and φ = 90°) [23]. The best crystals were obtained in condition D4 of the Morpheus Screen, and data were collected using fine φ-slicing to optimise reflection profiles [23] and at high speed (25 Hz) to mitigate the effects of radiation damage [24]. Data were reduced and integrated with the package XDS [25], and subsequently scaled and merged using AIMLESS [26] within the automated pipeline provided by Diamond Light Source.
The crystal producing the dataset used for structure resolution belonged to space group P1. Its unit cell dimensions were a = 33.81 Å, b = 44.52 Å, c = 54.71 Å, α = 75.08°, β = 84.52°, and γ = 69.72°. This dataset provided useful data up to a resolution of 1.73 Å. At resolution 1.73 Å I/σ corresponded to 1.73, while at 1.8 Å I/σ was 2.31. We considered the cutoff selected for data reduction by the automated pipeline provided by Diamond Light Source acceptable, on the basis of the I/σI values provided above. The CC1/2 value corresponding to the same resolution was 0.380, well above the 0.3 threshold adopted by AIMLESS to characterize data highest resolution. The structure was solved by molecular replacement using Phaser [27]. The final translation function had a value of TFZ = 10.2, well above the 8.0 value suggested for a unique solution. Rigid-body refinement followed using REFMAC [28]. The single A chain of the 1T95 PDB model was divided in 3 domains (residues 11–94, 95–171 and 172–240), which were used as TLS groups for improved restrained refinement. The molecular replacement model mostly fitted the electron density with a few exceptions. The initial model was analysed and completed using an alternation of visual inspection in COOT [29], as well as simulated annealing and restrained refinement with REFMAC and Phenix [30]. In the end, a final resolution of 1.9 Å was deemed satisfactory. Even though the data resolution used initially (1.73 Å) was helpful in the phasing and model building stage, refinement proved to be trickier at this resolution. The quality of maps was insufficient to refine completely the structure. The discordance between ideal geometry and the real values found at the early stages of structure solution suggested the exclusion of data below 1.9 Å. At this new resolution cutoff corresponding to 95% completeness and I/σI = 4, the final model fitted better the electron density, and the parameters describing geometrical quality were satisfactory (no Ramachandran or Cβ geometry outliers). The quality of the refinement was assessed with MolProbity [31] with 100% of the residues present in the favourable region of the Ramachandran plot. Final statistics for the structure are reported in Table 1. The final coordinates and structure factor files have been deposited in the PDB with coordinates 6FSW.

Table 1.
Archaeoglobus fulgidus SBDS crystallographic data collection and refinement statistics.
2.4. Small Angle X-ray Scattering (SAXS)
SAXS data were collected in the bioSAXS beamline B21, at Diamond Light Source, Harwell, United Kingdom. Bcp1 experiments were carried out in 50 mM Tris pH 7.5, 150 mM NaCl. Samples were loaded in an automated sample changer using PCR tubes with 4 µL delivered into a temperature controlled quartz capillary and exposed for 10 s at 20 °C. Data were collected for 54 frames using a PILATUS 2M (Dectris, Switzerland) detector at a distance of 4 m from the sample. For the AfSBDS, 45 µL samples with a concentration of 5 mg mL−1 were injected onto a Superdex 200 Increase size-exclusion column at 20 °C using 50 mM phosphate buffer pH 7.5, 150 mM NaCl as the running buffer. The output flow from the Agilent HPLC was directed through a 1.6 mm diameter quartz capillary cell held in vacuum. The flow rate was set to 0.05 mL min−1, and 491 frames (with 3 s exposure time) were collected using the same detector and at the same distance as for the Bcp1 protein. Collected two-dimensional images were corrected for variations in beam current, normalized for exposure time, and processed into one-dimensional scattering curves using GDA and the DAWN software (Diamond Light Source, UK). The background was manually subtracted using the program ScÅtter (http://www.bioisis.net/scatter). SAXS data collection and experimental parameters are summarized in Table 2.

Table 2.
SAXS data collection and experimental parameters for the proteins Bcp1 and Archaeoglobus fulgidus SBDS.
3. Results and Discussion
Yeast Bcp1 is the representative member of an unknown-structure protein family, with no recognizable domains. This family of proteins includes the BRCA2 and CDKN1A-interacting protein (BCCIP) and Bcp1. In contrast to the fungi Bcp1 involved in ribosome biogenesis, the human and mouse homologues seem to promote cell cycle arrest by enhancing the inhibition of CDK2 activity by CDKN1A/p21 [32] and may be required for DNA damage repair by homologous recombination together with BRCA2 [33]. An alignment of different members of this protein family showed that the N-terminus is not conserved with large differences in length and is predicted to be unstructured. Removal of the first 45 residues in the S. cerevisiae Bcp1 did not prevent folding of the protein as evidenced by its circular dichroism spectra (Figure 1). The near UV CD showed the presence of secondary structure consisting of a mixture of both α-helices and β-sheets corresponding to the signals observed at 222 and 208 nm, respectively. Additionally, the presence of five minima in the near UV CD spectra suggests that the aromatic residues, particularly the single tryptophan in Bcp1, are located within a folded protein core. With this in mind, we were initially interested in solving the structure of the yeast protein Bcp1. Its structure may not only render useful information regarding its function but also may be interesting in itself, as it is not possible to get information of possible domains or fold using programs that rely on its primary sequence. Initial attempts to crystallize the wild-type protein rendered no crystals despite trying several hundreds of crystallization conditions at different protein concentrations. This coincided with the “very difficult” crystallization prediction score assigned by the XtalPred Server [34] for the yeast Bcp1. Moreover, this unfavorable score extends to representative Bcp1 homologues comprising different Kingdoms such as Plantae, Animalia, Protist, and Fungi. We used SAXS as a technique that does not rely on crystals to obtain structural information of Bcp1 in solution. On the other hand, SBDS is the protein mutated in the Shwachman-Diamond Syndrome whose structure for different orthologs has already been described by means of MX and NMR spectroscopy. The currently available crystallographic PDB structures for SBDS correspond to those of Archaeoglobus fulgidus (1T95 [20] and 1P9Q [19]) and Methanothermobacter thermautotrophicus (2WBM [35]). Despite different crystal packing, the two orthologs show very similar structural features, which consist of three domains connected by short loops, with a RMSD of 3.02 Å. The major structural differences are found in the two internal loops, namely those between β3–β4 in domain 1, and connecting β7 and α8 in domain 3. Crystallization of the A. fulgidus protein showed a propensity to produce crystals in the presence of polyethylene glycols (PEG) and alcohols at different pH values. Alcohols prevented gemination (twinning of the crystals) that was evident in several conditions. PEG probably accounted for changes in the orientation of the side chain of surface-exposed amino acids such as those observed for E38 and E42. Alignment of the available structures with the one described in this study using the long α5 in domain 2 as backbone showed the classical three-domain arrangement with only minor changes. The analysis of B-factors defined a fairly compact domain 1 and two more flexible C-terminal domains. Inter-domain loops presented lower than average temperature factors, indicating a stable conformation for the protein in its crystallographic packing (Figure 2).
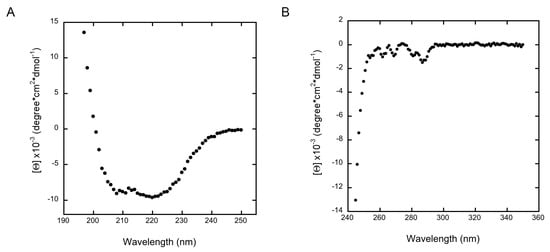
Figure 1.
Circular dichroism spectra of Bcp1 in the far- (A) and near-UV (B).
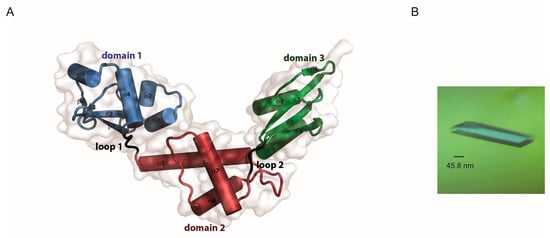
Figure 2.
Refined model of the AfSBDS (A) and the diffracting crystal (B).
Analysis of the one-dimensional SAXS experimental curves was initially performed to judge the quality of the data and obtain basic structural information related to the size and shape of the studied proteins (Figure 3A). One such structural parameter is the radius of gyration (Rg), which is calculated from the slope of the Guinier plot described as ln(Iq) vs. q2, in which q = 4πsin(θ/λ) is the scattering vector (2θ is the scattering angle and λ is the wavelength) [36]. For globular proteins, this plot is expected to be linear at low q, corresponding to values of q × Rg < 1.3 in the Guinier zone of 0–1/Rg. Linearity of the Guinier plot is considered a quality measurement of the data, but does not ensure ideality of the sample. SAXS curve analysis also provides an estimate on the molecular mass of a protein that in turn relates to its oligomeric state. The molecular mass of a protein approximately corresponds to half of its Porod volume (excluded volume of the hydrated protein particle). Porod analysis reflects the behavior of the scattering intensity at higher q range (Porod plot). Finally, the maximum size of a protein (Dmax) can be obtained from analysis of SAXS data by means of the Pair-Distance Distribution Function [P(r)], which corresponds to the distribution of distances between all the electrons within the protein. The pair-distance distribution function is obtained using the Indirect Fourier Transformation (Glatter et al. [37]) with a trial and error procedure, at the end of which the obtained Dmax corresponds to the smoothest and positive distribution. Differences in the Dmax of a protein relate to conformational changes. Additionally, it is possible to calculate the Rg from the Pair-Distance Distribution Function and to compare its value with that estimated from the Guinier plot.
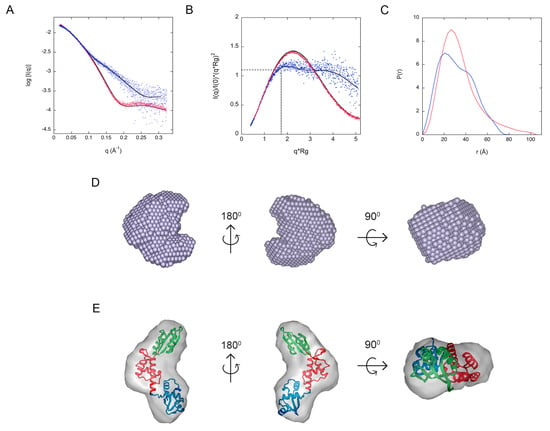
Figure 3.
SAXS data for Bcp1 (blue) and AfSBDS (red). (A) Fit of the simulated scattering curves compared to the observed scattering signal. (B) Dimensionless Kratky plot of the experimental data and simulated scattering shown in A. The intersection of the dotted black trace corresponds to the value for BSA. (C) Pair distribution function plot. (D) Ab initio molecular envelope of Bcp1. (E) Ab initio molecular envelope of AfSBDS showing the fitting of the crystallographic model.
SAXS parameters for Bcp1 and AfSBDS are listed in Table 2. Both proteins have similar Rg values (29.02 Å and 24.73 Å, respectively) but are different in size. According to their Dmax values, 102 Å for Bcp1 and 87 Å for AfSBDS, the former is a larger protein despite both having a similar number of residues in the recombinant constructs used for this study. Additionally, the Rg values calculated using the P(r) function (27.03 Å and 25.51 Å, respectively) are in good agreement with those estimated from the Guinier plot. The dimensionless Kratky plot was used to investigate the flexibility (and shape) of Bcp1 and AfSBDS (Figure 3B). The maximum value of 1.104 at (dashed black line) corresponds to a globular and compact protein that is similar to the bovine serum albumin (BSA) protein used as a standard in these experiments. In the case of Bcp1, this maximum is shifted towards a larger value of 1.4, suggesting a compact but asymmetric particle. For AfSBDS, the curve displays a hyperbolic-plateau behavior, typical of an intrinsically disordered protein. Interestingly, the curve reaches a plateau at intermediate q values and continues as a parallel straight line at higher q values. This behavior differs from that described by an asymptotically upward curve typical of unfolded proteins. This strongly suggests that AfSBDS is a very flexible protein. Inspection of the P(r) curves for Bcp1 confirmed an elongated shape, while that for AfSBDS was described by a bimodal graph, implying the existence of different (at least two) conformations in the solution (Figure 3C).
A low-resolution model of the protein can be reconstructed from the SAXS data using ab-initio algorithms such as DAMMIN [38], DAMMIF [39], MONSA [38], or by hybrid modeling using known atomic structures of domains with SASRES [40]. The ab-initio methods represent the particle as finite volume elements (or dummy atoms) and employ a simulated annealing to fit the experimental data. We used DAMMIF in combination with the DAMAVER program [41] to model the Bcp1 and AfSBDS proteins. The unicity of the SAXS models was calculated with AMBIMETER whose values of 0.0 and 2.9 for Bcp1 and AfSBDS, respectively, suggested a potentially unique model for Bcp1 and a highly ambiguous three-dimensional reconstruction for AfSBDS. Subsequently, we used DAMMIF to generate ten “dummy atoms” models, which were averaged with DAMAVER using the default mode. The models selected for averaging had a Normalized Spatial Discrepancy (NSD) value of 0.571 and a standard deviation (SD) of 0.022. Models with values larger than <NSD> + 2*SD were discarded. The calculated SAXS curves for the models were compared to the experimental ones, and good agreement was validated by the chi square values (χ2) between 2.08–2.67 for Bcp1 and 1.25–1.26 for AfSBDS. The averaged model obtained for Bcp1 (Figure 3D) confirmed the approximate globular structure predicted from the P(r) function. The correctness of the AfSBDS model obtained from the SAXS data was, at this point, also confirmed using the crystallographic model. Figure 3E shows the fitting of the latter into the SAXS envelope. For flexible macromolecules, specialized programs exist, designed to deal with ensemble-averaged data, like EOM 2.0 (Ensemble Optimization Method) [4,42]. This program assumes the coexistence of different conformations in solution for which the average scattering intensity fits the experimental SAXS curves. To this end, we used EOM to investigate the different conformations of AfSBDS. The protein was divided into its three domains according to the crystallographic structure; the inter-domain linkers were removed, and domain 2 was fixed, while the other two were left free to move randomly and cover the entire conformational space. A pool of 10,000 independent random coil models was initially generated. The theoretical scattering curve was automatically computed for each model in the pool by CRYSOL [43]. A genetic algorithm was used to select ensembles ranging from 2 to 50 conformers of different sizes. Their average theoretical profiles were calculated and fitted to the experimental SAXS data. The process was repeated 100 times, and the ensemble with the lowest discrepancy was reported as the best solution out of 100 final ensembles. This analysis revealed that in solution the final ensemble (averaged model) has an Rg of 25.73 Å, which is in good agreement with that of the crystal structure (25.65 Å). AfSBDS mainly populates three conformations: 46% exists in a “compact conformation” with a Rg of 22.75 Å and a Dmax of 69.36 Å, a second “stretched conformation” with 25.96 Å and 80.11 Å, respectively, and 27% abundance, and a third “relaxed conformation” with Rg = 26.79 Å and Dmax = 86.11 Å and 18% abundance. A population in a completely extended conformation (≈180°) was also found in 9% of the cases, with Rg = 35.27 Å and Dmax = 112.73. Figure 4 shows the three main conformations and the Rg and Dmax distribution of the initially generated population, and that selected at the end of the process. This distribution confirmed that AfSBDS is not a fully flexible molecule but a molecule with limited movement. The numerical parameter of Rflex for the ensemble corresponded to 78%, compared to the 89% value for the pool, suggesting that the former is less flexible (a value of 100% denotes a fully flexible system and 0% a fully rigid one). Additionally, the Rsigma parameter of 0.83 supported the hypothesis that the system is flexible (Rsigma values lower than 1 refer to systems with a high degree of flexibility).
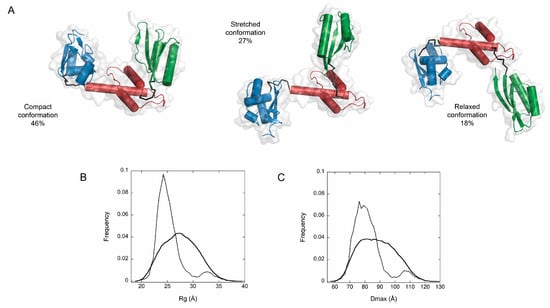
Figure 4.
Flexibility analysis of AfSBDS in solution. (A) Selected AfSBDS models and their contribution percentage estimated from the final population of EOM models. Domain 1—blue, domain 2—red, domain 3—green. Distribution of radii of gyration (Rg) (B) and intra-atomic distance maxima (Dmax) (C) in the initial pool of random AfSBDS structures (solid trace) overlapped to that of the final conformational sub-ensemble (dotted trace).
Comparison of the three conformations showed that both domains 1 and 3 undergo larger movements. Using the compact conformation as reference, domain 1 rotates 37° in the opposite direction of domain 3, which moves 80° in the stretched conformation. On the other hand, in the relaxed conformation these rotations correspond to 73° and 49° against domain 2, respectively. This conformational flexibility has already been observed for other members of the SBDS protein family. Crystallographic analysis of the archaeal M. thermautotrophicus SBDS showed that the protein adopts different conformations with rotations of ~13° for domain 1 and ~29° for domain 3, with respect to domain 2 [35]. This conformational flexibility is not restricted to the archaeal proteins; NMR relaxation experiments demonstrated that domain 1 of the human and yeast SBDS is also highly mobile with respect to domains 2–3 [44]. Thus flexibility seems inherent to the SBDS protein family and may facilitate its function. Human SBDS binds the 60S ribosomal subunit in a conformation similar to that described here as the “compact conformation”. Upon binding of EFL1, domain 3 of SBDS undergoes a rotational movement of almost 180° away from the ribosomal P-stalk [44], adopting a new conformation that resembles the “relaxed conformation” obtained from the flexibility analysis. It seems that SBDS has a propensity to transient along these three main functional conformations in solution, and binding to the cognate partner selects one such conformation.
4. Conclusions
SAXS is a solution technique that provides structural information on molecules such as proteins, and although the resolution is low, it is very useful for evaluating conformational changes in response to external conditions and conformational ensembles in flexible proteins. Most importantly, it does not rely on protein labeling or on the availability of high quality protein crystals, which in itself may be challenging and may result in the selection of the most stable conformation in the crystal. The AfSBDS is a flexible protein that coexists in at least three different conformations in solution with an averaged model represented by the crystallographic structure. This observation is in agreement with the low B-factors obtained for the inter-domain loops that result from attaining a stable conformation mediated by the crystal packing. In contrast, the other protein evaluated in this study, Bcp1, corresponds to a rigid protein present mostly in a unique fairly globular conformation in solution.
Acknowledgments
N.S.-P acknowledges the financial support from CONACyT project number 283909 and DGAPA-PAPIIT IN201615. D.S. acknowledges the financial support from projects “PGR2014-2017 Messico” with the contribution of the Ministero degli Affari Esteri e dalla Cooperazione Internazionale, Direzione Generale per la Promozione del Sistema Paese. The SAXS experiments were performed by proposal SM14029-1 and SM16970-3 beamline B21, Diamond Light Source. MX experiments were performed by proposal MX11690-13 at beamline I24, Diamond Light Source.
Author Contributions
N.S.-P. and D.S. designed and coordinated research. M.M., J.F., D.S., and D.A. performed crystallography experiments. M.M. and J.F. analyzed the crystallography experiments. A.M.-G. expressed and purified the proteins. D.S. and D.A. performed SAXS experiments. N.S.-P. and D.S. analyzed the SAXS experiments. All authors assisted the assembly and editing of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rambo, R.P.; Tainer, J.A. Super-resolution in solution X-ray scattering and its applications to structural systems biology. Annu. Rev. Biophys. 2013, 42, 415–441. [Google Scholar] [CrossRef] [PubMed]
- Blanchet, C.E.; Spilotros, A.; Schwemmer, F.; Graewert, M.A.; Kikhney, A.; Jeffries, C.M.; Franke, D.; Mark, D.; Zengerle, R.; Cipriani, F.; et al. Versatile sample environments and automation for biological solution X-ray scattering experiments at the P12 beamline (PETRA III, DESY). J. Appl. Crystallogr. 2015, 48, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Franke, D.; Petoukhov, M.V.; Konarev, P.V.; Panjkovich, A.; Tuukkanen, A.; Mertens, H.D.T.; Kikhney, A.G.; Hajizadeh, N.R.; Franklin, J.M.; Jeffries, C.M.; et al. ATSAS 2.8: A comprehensive data analysis suite for small-angle scattering from macromolecular solutions. J. Appl. Crystallogr. 2017, 50, 1212–1225. [Google Scholar] [CrossRef] [PubMed]
- Bernado, P.; Mylonas, E.; Petoukhov, M.V.; Blackledge, M.; Svergun, D.I. Structural characterization of flexible proteins using small-angle X-ray scattering. J. Am. Chem. Soc. 2007, 129, 5656–5664. [Google Scholar] [CrossRef] [PubMed]
- Boura, E.; Rozycki, B.; Herrick, D.Z.; Chung, H.S.; Vecer, J.; Eaton, W.A.; Cafiso, D.S.; Hummer, G.; Hurley, J.H. Solution structure of the ESCRT-I complex by small-angle X-ray scattering, EPR, and FRET spectroscopy. Proc. Natl. Acad. Sci. USA 2011, 108, 9437–9442. [Google Scholar] [CrossRef] [PubMed]
- Boura, E.; Rozycki, B.; Chung, H.S.; Herrick, D.Z.; Canagarajah, B.; Cafiso, D.S.; Eaton, W.A.; Hummer, G.; Hurley, J.H. Solution structure of the ESCRT-I and -II supercomplex: Implications for membrane budding and scission. Structure 2012, 20, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Chalupska, D.; Eisenreichova, A.; Rozycki, B.; Rezabkova, L.; Humpolickova, J.; Klima, M.; Boura, E. Structural analysis of phosphatidylinositol 4-kinase IIIbeta (PI4KB)—14-3-3 protein complex reveals internal flexibility and explains 14-3-3 mediated protection from degradation in vitro. J. Struct. Biol. 2017, 200, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Panse, V.G.; Johnson, A.W. Maturation of eukaryotic ribosomes: Acquisition of functionality. Trends. Biochem. Sci. 2010, 35, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Tschochner, H.; Hurt, E. Pre-ribosomes on the road from the nucleolus to the cytoplasm. Trends. Cell Biol. 2003, 13, 255–263. [Google Scholar] [CrossRef]
- Venema, J.; Tollervey, D. Ribosome synthesis in Saccharomyces cerevisiae. Annu. Rev. Genet. 1999, 33, 261–311. [Google Scholar] [CrossRef] [PubMed]
- Woolford, J.L., Jr.; Baserga, S.J. Ribosome biogenesis in the yeast Saccharomyces cerevisiae. Genetics 2013, 195, 643–681. [Google Scholar] [CrossRef] [PubMed]
- Ting, Y.H.; Lu, T.J.; Johnson, A.W.; Shie, J.T.; Chen, B.R.; Kumar, S.S.; Lo, K.Y. Bcp1 Is the Nuclear Chaperone of Rpl23 in Saccharomyces cerevisiae. J. Biol. Chem. 2017, 292, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Wyler, E.; Wandrey, F.; Badertscher, L.; Montellese, C.; Alper, D.; Kutay, U. The beta-isoform of the BRCA2 and CDKN1A(p21)-interacting protein (BCCIP) stabilizes nuclear RPL23/uL14. FEBS Lett. 2014, 588, 3685–3691. [Google Scholar] [CrossRef] [PubMed]
- Gartmann, M.; Blau, M.; Armache, J.P.; Mielke, T.; Topf, M.; Beckmann, R. Mechanism of eIF6-mediated inhibition of ribosomal subunit joining. J. Biol. Chem. 2010, 285, 14848–14851. [Google Scholar] [CrossRef] [PubMed]
- Menne, T.F.; Goyenechea, B.; Sanchez-Puig, N.; Wong, C.C.; Tonkin, L.M.; Ancliff, P.J.; Brost, R.L.; Costanzo, M.; Boone, C.; Warren, A.J. The Shwachman-Bodian-Diamond syndrome protein mediates translational activation of ribosomes in yeast. Nat. Genet. 2007, 39, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Marquez, A.; Gijsbers, A.; de la Mora, E.; Sanchez-Puig, N. Defective Guanine Nucleotide Exchange in the Elongation Factor-like 1 (EFL1) GTPase by Mutations in the Shwachman-Diamond Syndrome Protein. J. Biol. Chem. 2015, 290, 17669–17678. [Google Scholar] [CrossRef] [PubMed]
- Gijsbers, A.; Garcia-Marquez, A.; Luviano, A.; Sanchez-Puig, N. Guanine nucleotide exchange in the ribosomal GTPase EFL1 is modulated by the protein mutated in the Shwachman-Diamond Syndrome. Biochem. Biophys. Res. Commun. 2013, 437, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Weis, F.; Giudice, E.; Churcher, M.; Jin, L.; Hilcenko, C.; Wong, C.C.; Traynor, D.; Kay, R.R.; Warren, A.J. Mechanism of eIF6 release from the nascent 60S ribosomal subunit. Nat. Struct. Mol. Biol. 2015, 22, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Savchenko, A.; Krogan, N.; Cort, J.R.; Evdokimova, E.; Lew, J.M.; Yee, A.A.; Sanchez-Pulido, L.; Andrade, M.A.; Bochkarev, A.; Watson, J.D.; et al. The Shwachman-Bodian-Diamond syndrome protein family is involved in RNA metabolism. J. Biol. Chem. 2005, 280, 19213–19220. [Google Scholar] [CrossRef] [PubMed]
- Shammas, C.; Menne, T.F.; Hilcenko, C.; Michell, S.R.; Goyenechea, B.; Boocock, G.R.; Durie, P.R.; Rommens, J.M.; Warren, A.J. Structural and mutational analysis of the SBDS protein family. Insight into the leukemia-associated Shwachman-Diamond Syndrome. J. Biol. Chem. 2005, 280, 19221–19229. [Google Scholar] [CrossRef] [PubMed]
- Gorrec, F. The MORPHEUS protein crystallization screen. J. Appl. Crystallogr. 2009, 42, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Allan, D.R.; Collins, S.P.; Evans, G.; Hall, D.; McAuley, K.; Owen, R.L.; Sorensen, T.; Tang, C.C.; von Delft, F.; Wagner, A.; et al. Status of the crystallography beamlines at Diamond Light Source. Eur. Phys. J. Plus 2015, 130, 50. [Google Scholar] [CrossRef]
- Mueller, M.; Wang, M.; Schulze-Briese, C. Optimal fine phi-slicing for single-photon-counting pixel detectors. Acta Crystallogr. D Biol. Crystallogr. 2012, 68, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Krojer, T.; Pike, A.C.; von Delft, F. Squeezing the most from every crystal: The fine details of data collection. Acta Crystallogr. D Biol. Crystallogr. 2013, 69, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Evans, P. Scaling and assessment of data quality. Acta Crystallogr. D Biol. Crystallogr. 2006, 62, 72–82. [Google Scholar] [CrossRef] [PubMed]
- McCoy, A.J.; Grosse-Kunstleve, R.W.; Adams, P.D.; Winn, M.D.; Storoni, L.C.; Read, R.J. Phaser crystallographic software. J. Appl. Crystallogr. 2007, 40, 658–674. [Google Scholar] [CrossRef] [PubMed]
- Murshudov, G.N.; Skubak, P.; Lebedev, A.A.; Pannu, N.S.; Steiner, R.A.; Nicholls, R.A.; Winn, M.D.; Long, F.; Vagin, A.A. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. D Biol. Crystallogr. 2011, 67, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Afonine, P.V.; Grosse-Kunstleve, R.W.; Echols, N.; Headd, J.J.; Moriarty, N.W.; Mustyakimov, M.; Terwilliger, T.C.; Urzhumtsev, A.; Zwart, P.H.; Adams, P.D. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D 2012, 68, 352–367. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.B.; Arendall III, W.B.; Headd, J.J.; Keedy, D.A.; Immormino, R.M.; Kapral, G.J.; Murray, L.W.; Richardson, J.S.; Richardson, D.C. MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 2010, 66, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Liu, J.; Shen, Z. Inhibition of G1 to S cell cycle progression by BCCIP beta. Cell Cycle 2004, 3, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Guo, X.; Meng, X.; Liu, J.; Allen, C.; Wray, J.; Nickoloff, J.A.; Shen, Z. The BRCA2-interacting protein BCCIP functions in RAD51 and BRCA2 focus formation and homologous recombinational repair. Mol. Cell. Biol. 2005, 25, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Slabinski, L.; Jaroszewski, L.; Rychlewski, L.; Wilson, I.A.; Lesley, S.A.; Godzik, A. XtalPred: A web server for prediction of protein crystallizability. Bioinformatics 2007, 23, 3403–3405. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.L.; Waterman, D.G.; Koonin, E.V.; Walters, A.D.; Chong, J.P.; Isupov, M.N.; Lebedev, A.A.; Bunka, D.H.; Stockley, P.G.; Ortiz-Lombardia, M.; et al. Conformational flexibility and molecular interactions of an archaeal homologue of the Shwachman-Bodian-Diamond syndrome protein. BMC Struct. Biol. 2009, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Guinier, A. La diffraction des rayons X aux très petits angles: Application à l’étude de phénomènes ultramicroscopiques. Ann. Phys. 1939, 11, 161–237. [Google Scholar] [CrossRef]
- Glatter, O. A new method for the evaluation of small-angle scattering data. J. Appl. Cryst. 1977, 10, 415–421. [Google Scholar] [CrossRef]
- Svergun, D.I. Restoring low resolution structure of biological macromolecules from solution scattering using simulated annealing. Biophys. J. 1999, 76, 2879–2886. [Google Scholar] [CrossRef]
- Franke, D.; Svergun, D.I. DAMMIF, a program for rapid ab-initio shape determination in small-angle scattering. J. Appl. Crystallogr. 2009, 42, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Tuukkanen, A.T.; Kleywegt, G.J.; Svergun, D.I. Resolution of ab initio shapes determined from small-angle scattering. IUCrJ 2016, 3, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Volkov, V.V.; Svergun, D.I. Uniqueness of ab initio shape determination in small-angle scattering. J. Appl. Crystallogr. 2003, 36, 860–864. [Google Scholar] [CrossRef]
- Tria, G.; Mertens, H.D.; Kachala, M.; Svergun, D.I. Advanced ensemble modelling of flexible macromolecules using X-ray solution scattering. IUCrJ 2015, 2, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Svergun, D.I.; Barberato, C.; Koch, M.H.J. CRYSOL—A program to evaluate X-ray solution scattering of biological macromolecules from atomic coordinates. J. Appl. Cryst. 1995, 28, 768–773. [Google Scholar] [CrossRef]
- Finch, A.J.; Hilcenko, C.; Basse, N.; Drynan, L.F.; Goyenechea, B.; Menne, T.F.; Gonzalez Fernandez, A.; Simpson, P.; D’Santos, C.S.; Arends, M.J.; et al. Uncoupling of GTP hydrolysis from eIF6 release on the ribosome causes Shwachman-Diamond syndrome. Genes Dev. 2011, 25, 917–929. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).