Abstract
Microbially induced calcite precipitation (MICP) through a ureolytic pathway is a process that promotes calcite precipitation as a result of the urease enzymatic activity of several microorganisms. It has been studied for different technological applications, such as soil bio-consolidation, bio-cementation, CO2 sequestration, among others. Recently, this process has been proposed as a possible process for removing heavy metals from contaminated soils. However, no research has been reported dealing with the MICP process for heavy metal removal from wastewater/waters. This (re)view proposes to consider to such possibility. The main characteristics of MICP are presented and discussed. The precipitation of heavy metals contained in wastewaters/waters via MICP is exanimated based on process characteristics. Moreover, challenges for its successful implementation are discussed, such as the heavy metal tolerance of inoculum, ammonium release as product of urea hydrolysis, and so on. A semi-continuous operation in two steps (cell growth and bio-precipitation) is proposed. Finally, the wastewater from some typical industries releasing heavy metals are examined, discussing the technical barriers and feasibility.
1. Heavy Metals and Environmental Problems
The contamination of watercourses by heavy metals is a serious environmental problem that has increased because of rapid industrial development. Indeed, several economic activities, such as metal plating, galvanization, the extraction and processing of minerals, tanning, battery production, paper manufacture, and pesticide synthesis, generate wastewaters that can contain pollutants [1]. Many of these metals are micronutrients, that is, they are essential for cell growth [2]. However, at high concentrations, they may turn toxic or carcinogenic, causing serious health problems. Moreover, when entering the food chain, these can accumulate in the human body [3]. In this sense, special attention has been paid to the most hazardous pollutants, such as zinc, copper, nickel, mercury, cadmium, lead, and chromium [4]. Their adverse effect on natural and human environments demands the development of efficient and cost-effective technologies in order to ensure the removal of these heavy metals from the environment.
2. Conventional Treatment of Wastewaters Containing Heavy Metals
Nowadays, several processes such as flotation, chemical precipitation, adsorption, ion exchange, membrane filtration, coagulation, and electrochemical deposition are available to treat metal-containing water [1,3]. Although these processes can remove metals efficiently (metal removal exceeding 90%) [1], their main constraints are associated with high energy requirements, the use of chemicals, and the production of toxic metal sludge. All of these characteristics contribute to increase overall costs [5,6]. In recent years, biological processes have been proposed and developed for metal removal from waters and wastewaters, for example bio-sorption, bio-accumulation, phytoremediation, bio-coagulation, bio-leaching, and application of sulfate-reducing bacteria (SRB) [6,7]. These biological processes have relevant advantages when compared with their conventional counterparts, such as reduced energy and material consumption, possibilities for metal recycling or recovery, and lower sludge production [8]. These characteristics transform them into potentially more eco-friendly alternatives. However, there are disadvantages mainly related to the final waste disposal, which is often a metal-containing biomass [9]. Moreover, the bacterial immobilization of metals may not constitute a long-term solution, when it is the result of changes in the redox state, as conditions in the environment may re-mobilize them [10].
3. Microbially Induced Calcite Precipitation (MICP) Process
3.1. Precipitation by Ureolytic MICP Process
MICP is a biological process in which calcite (CaCO3) formation is achieved as a result of the active metabolism of bacteria, which generates a favorable micro-environment for precipitation [11,12]. In calcite formation in the MICP through ureolytic pathway, bacteria catalyze urea hydrolysis into carbonate and ammonium. The latter produces an alkalization of the micro-environment, favoring the binding of calcium and carbonate, and furthermore, calcite precipitation [13,14]. Moreover, bacteria also provide nucleation sites in which the calcite precipitation takes place. Four steps can be identified for biological calcite precipitation, as illustrated in Figure 1.
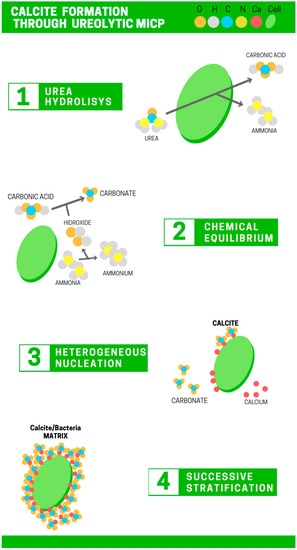
Figure 1.
Schematic illustration of calcite production through the microbially induced calcite precipitation (MICP) process: 1—urea hydrolysis; 2—chemical equilibrium; 3—heterogeneous nucleation; 4—successive stratification.
- (a)
- Urea hydrolysis: The urease enzyme hydrolyzes urea into carbamic acid and ammonia (Equation (1)). Furthermore, a spontaneous chemical equilibrium takes place and carbamic acid is converted into carbonic acid and ammonia (Equation (2)) [10,15].
- (b)
- Chemical equilibrium: Ammonia from the urea hydrolysis turns into ammonium, releasing hydroxide ions and increasing the micro-environmental pH, which generates favorable conditions for further precipitation [11] (Equation (3)). Hydroxide ions induce carbonate formation from carbonic acid (Equations (4) and (5)).
- (c)
- Heterogeneous nucleation: Calcium ions are bound to the external cell surface because of the negatively charged functional groups in the cell wall (Equation (6)). Then, calcite formation occurs in the cell surface, once the calcium ion activity is sufficient and the saturation conditions are favorable for CaCO3 precipitation (Equation (7)) [16].
- (d)
- Successive stratification: Successive calcite layers are developed on the external cell surface (stratification) [17]. The nutrients transfer is limited, and the cells get embedded by calcite crystals, provoking cellular death.
Calcite precipitation through MICP has been regarded as a promising technology for different applications, such as for the improvement of mechanical properties in soils (bio-consolidation) [18,19], bio-cementation [14,20], crack repair in concrete structures [21], CO2 sequestration [22], bio-composites [23], hydraulic control [10], and so on. For a detailed review about the engineering applications of the MICP process, the authors recommend viewing the following literature [10,19].
3.2. Factors Governing the Ureolytic MICP Process
Several factors govern the ureolytic MICP process, such as the bacteria type, urea and Ca2+ concentrations, nucleation sites, pH, and temperature. Several microorganisms presenting high levels of urease activity have been identified, such as Sporosarcina pasteurii, Bacillus spCR2, Lysinibacillus sphaericus CH5, Bacillus pasteurii NCIM 2477, Kocuria flava CR1, Bacillus megaterium SS3, Bacillus thuringiensis, and Halomonas ssp. [24]. S. pasteurii is a non-pathogenic bacterium that is able to tolerate extreme conditions [24], and is probably the most common species used for testing and studying the MICP process [13,25,26,27].
As already mentioned, the bacteria provide nucleation sites where the precipitation is enhanced. Specifically, the cell surface in the bacteria has negatively charged groups (negative zeta potential) [28], providing binding sites for the Ca2+ ions, where carbonate and calcium can react, forming calcite [24,29]. In this sense, the research carried out by Stocks-Fischer et al. (1999) showed the beneficial effect of bacteria as nucleation sites. They observed that the removal of calcium with chemical precipitation (adding carbonate) was a 34–54%, but this value was increased to 98% when MICP bacteria were used.
Another factor is the pH, which determines the acid-based chemical equilibria, and thus defines the presence of carbonate and the precipitation processes. Then, it is considered a key parameter, affecting ureolytic MICP. Moreover, the pH affects the activity of the urease enzyme [26]. It has been reported that the urease activity increases when the pH rises from 6 to 10, but decreases when the pH exceeds 10 [15]. As is the case with all enzymes, the urea catalysis is temperature-dependent. The optimal temperature is in the range of 20 to 37 °C [30,31]. Moreover, the temperature affects the chemical equilibrium and thus the solubility of the CaCO3 in the media.
4. The Ureolytic MICP Process as Treatment for Heavy Metal Removal from Wastewaters
4.1. How Can the Ureolytic MICP Process Remove Heavy Metals?
Heavy metals may be removed through direct precipitation, where metal carbonate is formed, or by co-precipitation, in which metals such Cu2+, Cd2+, Co2+, Ni2+, Zn2+, Pb2+, and Fe2+ can be incorporated in the lattice structure of calcite via the substitution of Ca2+ [32]. To date, research conducted on ureolyitic MICP for metal removal has been directed toward soil remediation. So far, the reported research has dealt with the isolation of bacteria, presenting important urease activity and metal resistance, the morphological evaluation of metal precipitates, and the removal efficiency of different metals. High removal efficiencies have been reported for different metals (Table 1), when working with species isolated from contaminated soils, as follows: 89–97% for copper, 98–100% for lead, 96–100% for cadmium, ca. 90% for nickel, 93–100% for zinc, and 90–94% for cobalt (Table 1). Unlike the high extent of metal removal mentioned above, the research carried out by Mugwar and Harbottle [28] showed that the metal removal for S. pasteurii decreased when the copper and zinc concentrations were increased to 0.5 and 2.0 mM, respectively, but the lead and cadmium removal was not affected by concentration (Table 1). Therefore, from the results summarized in Table 1, we can see that the ureolytic MICP process is more effective for cadmium and lead than for copper, which may be associated with the toxicity of copper. Moreover, the results in Table 1 confirm that the metal tolerance of a particular strain is a key parameter for an efficient ureolytic MICP process. Additionally, it is worth noting that previous research has dealt with the removal of heavy metals from soil by washing soil samples, and then applying MICP to the resulting aqueous solution. Thus, it is expected that the application of the ureolytic MICP to water/wastewater may be feasible from a technical point of view.

Table 1.
Summary of assay for heavy metal removal through the ureolytic microbially induced calcite precipitation (MICP) process.
4.2. Heavy Metals Precipitation through Ureolytic MICP Process for Wastewaters
To date, there is incipient research considering the ureolytic MICP for heavy metal removal from water/wastewater. However, researchers have tested this technology in aqueous solutions for removing radionuclides from groundwater [31], for the removal of ions Ca2+ and Mg2+ ions from wastewater [43], and for phosphate precipitation from anaerobic effluents [44]. Thus, the possibility of removing heavy metals through the ureolytic MICP process provides an interesting and less studied field of research [10,19,45]. Moreover, the ureolytic MICP process can provide an effective way to sequester metals as mineral precipitates for long periods [46]. Formed precipitates can resist an acid pH, reaching acid resistance values as low as 2 [27].
4.3. Key Aspects for the Application of Ureolytic MICP Process for Wastewater Treatment
Subsequently, it seems clear why the ureolytic MICP process may turn into an effective treatment alternative for heavy metal removal from water/wastewaters. However, its full-scale application will require taking several technical issues into consideration, which are discussed below. Potential solutions to overcome these challenges are proposed.
4.3.1. Inoculum (Soil/Wastewater)
Obviously, bacteria presenting relevant urease activity are required as inoculum for MICP. Moreover, inoculum should also show resistance to high concentrations of heavy metals. The isolation of bacteria strains from soil contaminated with the metal target for remediation has been the most common way to face this challenge. Several species have been isolated from contaminated soils in mining areas, showing tolerance to high concentrations of heavy metals, which may be useful when considering the remediation of contaminated sites [27,33,35,47,48,49]. In this sense, even though S. pasteurii is the most used strain for MICP applications, some soil isolated bacteria with lower urease activity have attained higher metal removal, as a result of their metal tolerance [27]. Moreover, the water/wastewater objective of the treatment could be an appropriated source for isolation.
An important factor for a potential operation of the MICP process will be the kind of culture that is used—mono or mixed culture are two possible strategies for operation. When (waste)water treatment is considered, the use of axenic cultures may not be possible, as a result of their scale. Then, the conditions for the proliferation of ureolytic bacteria could be considered. Furthermore, Kang et al. [50] reported that mixed cultures present advantages over mono cultures, such as a major metal tolerance and a higher degree of removal of heavy metals. Moreover, the presence of non-ureolytic bacteria in mono or mixed culture can contribute to MICP; even though no contribution for urea hydrolysis is expected, an increase in the nucleation sites may increase the precipitation rates. This was observed by Gat et al. [51], who cultivated S. pasteurii and N. subtilis (non-ureolytic bacteria) for CaCO3 precipitation. Thus, culture contamination may not be as inconvenient, as long as a minimum urease activity can be provided by the existing ureolytic bacteria.
4.3.2. Substrates
The ureolytic MICP process requires a source of urea and calcium. The application to wastewater treatment also imposes the condition that the substrate source should have a low cost or no cost at all. Then, the use of wastewater containing urea seems to be the simplest solution. Typical urea containing wastes can come from fertilizer plants, urea synthesis industry, or human activities (sewage) [52,53]. In relation to calcium, this substrate could be obtained from calcium-rich wastewater, such as from citric acid production, landfill leachates, paper recycling, and bone processing [54]. However, the presence of organic matter, recalcitrant compounds, inhibitors of bacterial activity, or other ions may potentially interfere with the bio-precipitation. Then, the characterization of wastewater is certainly relevant.
4.3.3. pH of Metal Containing Wastewaters
The ureolytic MICP process has an optimal pH range between 6 and 10 [15]. Thus, it is expected that bio-precipitation in acidic wastewaters may not be feasible, or may require a pH adjustment. However, it is important to highlight that, as a result of urease activity, the pH will increase, because of the ammonium release. Then, the eventual requirements of the pH adjustment will depend on the wastewater pH and on the alkalinity level. This will be certainly a key factor when considering MICP technology for metal removal from mining wastewaters, as most have a low pH, as is the case with acid mine drainage [55,56]. On the other hand, the treated wastewater will contain an alkaline pH, which have to be corrected in order to avoid any environmental impact in watercourses where it is being discharged. In this sense, an additional study and the evaluation of adequate neutralization treatment for a feasible solution will be necessary.
4.3.4. Bacterial Re-Use after Precipitation/Re-Inoculation
A key challenge arises when the ureolytic MICP process for metal treatment is considered—bacteria provide nucleation sites for precipitation, and once the bio-precipitation takes place, the bacteria are embedded in carbonate crystals. Then, the activity is reduced, as a result of mass transfer limitations [10]. This means that the ureolytic bacteria is not available for future precipitation, as they are trapped in the precipitates, and in order to promote additional precipitation in the process, bacterial re-inoculation will be required. In spite of this, the bacteria embedded in the precipitates would provide a free-biomass effluent, which means that no further treatment for biomass removal will be necessary.
Thus, the continuous operation of a MICP system based on a single reactor, where both cell growth and bio-precipitation occur, may not be feasible. Under this scenario, a MICP process in two steps would be needed. In the first step, the bacteria are grown, and in a second step, bio-precipitation takes place (Figure 2). Thus, the bacteria from the first step are supplied to the second step, where urea, calcium, and wastewater meet, providing the conditions for bio-precipitation.
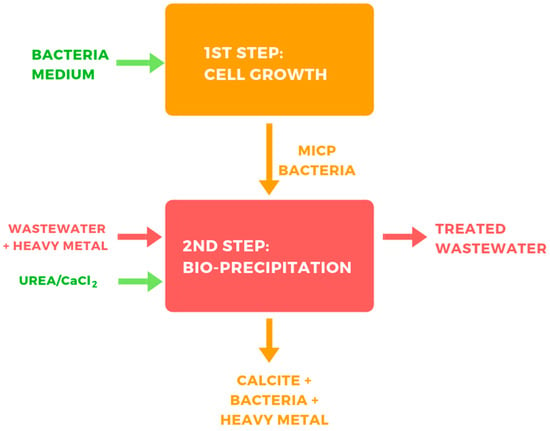
Figure 2.
Potential approach for treating heavy metal containing wastewater through the ureolytic MICP process.
4.3.5. Ammonium Release
When ureolytic MICP for heavy metal removal from wastewater is considered, probably one of the greatest challenges to be faced is ammonium release. Two moles of ammonium are released per one mole of hydrolyzed urea (Equations (1) and (2)). This will produce an ammonium-rich effluent, which must be managed. Moreover, if the pH rises over 8–9, ammonia volatilization may become relevant, depending on the gas/liquid mass transfer coefficient. Indeed, Gat et al. [57] reported that ammonia volatilization affected the long-term sustainability of the calcite precipitate, so that ammonia volatilization caused a pH decrease, which showed a 30% calcite dissolution. It is clear that the first step to approaching this issue must be the optimization of urea dosages, in order to minimize the ammonia release. One alternative to managing an ammonia-rich effluent, is to couple MICP to a process for the re-utilization of ammonium for urea synthesis, based on the Basarov reaction described by the authors of [58]. A second alternative is to use one of the processes for ammonia removal and for the conversion normally used in full-scale wastewater treatment, such as the anammox process [59].
5. Wastewaters That May Be Potentially Treated by the Ureolytic MICP Process
Several economic activities release wastewaters containing heavy metals that could be potentially treated through the MICP process. Some of them are the refining and mining of ores, pesticide industries, batteries production, paper industries, tanneries, fertilizer production facilities, solid wastes disposal including sewage sludge, and wastewater irrigation [60].
5.1. Tannery
Tannery and electroplating industries are the major sources of chromium contamination. Hexavalent chromium is genotoxic and carcinogenic, and the reaction of Cr(VI) with ascorbate and hydrogen peroxide results in the accumulation of hydroxyl radicals, thereby causing damage to the DNA [61]. MICP could be an alternative for chromium removal from tannery wastewaters, as calcium carbonate precipitates at pH levels where these effluents are normally present (neutral to alkaline). Moreover, calcium carbonate can be deposited around chromium (VI) compounds [10,62], making stable precipitates for long time periods [63]. Lead is another metal that can be present in tannery wastewater [64]. Because of its valence, Pb (II) could act in a similar way to calcium, and could bind carbonate ions to form stable lead carbonate that precipitates through the MICP process.
5.2. Mining and Metal Refinery
The main environmental impacts of the mining industry include pollution as a result of the discharge of liquid effluents into rivers, the infiltration of soils, the contamination of underground waters, residue disposal, and threats from tailings dams [65]. Processed tailings dam overflow, acid wastewaters, and acid mine drainage (AMD) are some of the most common liquid industrial residues of this sector [56,65].
AMD has pH levels under 4 [55], and they are usually around pH 2 [66]. At such a pH, the urease positive microbes cannot hydrolyze urea, because of the inactivation of this enzyme. Then, a previous step to neutralize the AMD would be necessary before a bio-precipitation treatment. A second option would be to find a urease positive bacterium adapted to these acidic conditions.
On the other hand, mine tailing in the active process has high pH levels [67]. Then, in such situations, the MICP process could be used as a treatment alternative for heavy metal precipitation, leading to recovering those metals or to their disposition in a safe way. The challenge with this wastewater is the high content of heavy metals. It would be necessary to work with bacteria with a high tolerance to those elements.
5.3. Electroplating
In general, wastewaters from electroplating contain a low concentration of organic matter, but are of high toxicity because of heavy metals. Rinse waters are continuously produced and contain relatively low concentrations of contaminants, depending on the electroplating process, such as cadmium, chromium, copper, nickel, lead, and zinc [68]. Bath solutions, in contrast, contain markedly higher concentrations of metals (of the order of hundreds of g/L) and are replaced every several weeks or months, depending on the process [68]. The treatment of wastewater and sludge containing heavy metals is one of the main ecological problems induced by the electroplating industry [69].
In general, solutions with a high content of chromium (VI) are acidic [68,70]. Therefore, the chromium (VI) bio-precipitation from these wastewaters will need a pH increase. However, the other metals, in the pH range, are suitable for the MICP process. On the other hand, bacteria with very high tolerance to heavy metal will be required, because of the high concentration of metal in these wastewaters.
6. Concluding Remarks
The ureolytic MICP is a promising process for the removal of heavy metals. Even though so far it has only been tested for soils, it is expected that it could also represent a potential tool for heavy metal removal from wastewaters. Several relevant economic activities produce wastewater containing heavy metals that could benefit from this process. However, some challenges need to be overcome, such as pH adjustment, ammonium release, and calcium/urea source. Then, future research should be focused on such challenges. Considering that the process requires the production of a biomass presenting urease activity, as well as its subsequent use for bio-precipitation, a potential process configuration is proposed, based on these two steps.
Funding
This publication was funded by CRHIAM center CONICYT FONDAP 1513001.
Acknowledgments
This publication was supported by CONICYT FONDECYT/POSTDOCTORADO 2018 3180648, CONICYT PAI/CONCURSO NACIONAL TESIS DE DOCTORADO EN EL SECTOR PRODUCTIVO, CONVOCATORIA 2017 T7817110005 and by CRHIAM center CONICYT FONDAP 1513001.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, S.; Nourbaksh, M.; Kilicarslan, S.; Ozdag, H. Removal of chromium, lead and copper ions from industrial waste waters by Staphylococcus saprophyticus. Turk. Electron. J. Biotechnol. 2004, 2, 50–57. [Google Scholar]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Zhao, M.; Xu, Y.; Zhang, C.; Rong, H.; Zeng, G. New trends in removing heavy metals from wastewater. Appl. Microbiol. Biotechnol. 2016, 100, 6509–6518. [Google Scholar] [CrossRef] [PubMed]
- Arbabi, M.; Hemati, S.; Amiri, M. Removal of lead ions from industrial wastewater: A review of Removal methods. Int. J. Epidemiol. Res. 2015, 2, 105–109. [Google Scholar]
- Ahluwalia, S.S.; Goyal, D. Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour. Technol. 2007, 98, 2243–2257. [Google Scholar] [CrossRef] [PubMed]
- Nancharaiah, Y.V.; Mohan, S.V.; Lens, P.N.L. Biological and Bioelectrochemical Recovery of Critical and Scarce Metals. Trends Biotechnol. 2016, 34, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Tsezos, M.; Hatzikioseyian, A.; Remoudaki, E. Biofilm Reactors in Mining and Metallurgical Effluent Treatment: Biosorption, Bioprecipitation, Bioreduction Processes. 2012. Available online: http://www.metal.ntua.gr/uploads (accessed on 2 September 2018).
- Lesmana, S.O.; Febriana, N.; Soetaredjo, F.E.; Sunarso, J.; Ismadji, S. Studies on potential applications of biomass for the separation of heavy metals from water and wastewater. Biochem. Eng. J. 2009, 44, 19–41. [Google Scholar] [CrossRef]
- Phillips, A.J.; Gerlach, R.; Lauchnor, E.; Mitchell, A.C.; Cunningham, A.B.; Spangler, L. Engineered applications of ureolytic biomineralization: A review. Biofouling 2013, 29, 715–733. [Google Scholar] [CrossRef] [PubMed]
- Hammes, F.; Verstraete, W. Key roles of pH and calcium metabolism in microbial carbonate precipitation. Rev. Environ. Sci. Biotechnol. 2002, 1, 3–7. [Google Scholar] [CrossRef]
- Seifan, M.; Samani, A.K.; Berenjian, A. New insights into the role of pH and aeration in the bacterial production of calcium carbonate (CaCO3). Appl. Microbiol. Biotechnol. 2017, 101, 3131–3142. [Google Scholar] [CrossRef] [PubMed]
- Whiffin, V.S.; van Paassen, L.A.; Harkes, M.P. Microbial Carbonate Precipitation as a Soil Improvement Technique. Geomicrobiol. J. 2007, 24, 417–423. [Google Scholar] [CrossRef]
- Seifan, M.; Samani, A.K.; Berenjian, A. Bioconcrete: Next generation of self-healing concrete. Appl. Microbiol. Biotechnol. 2016, 100, 2591–2602. [Google Scholar] [CrossRef] [PubMed]
- Stocks-Fischer, S.; Galinat, J.K.; Bang, S.S. Microbiological precipitation of CaCO3. Soil Biol. Biochem. 1999, 31, 1563–1571. [Google Scholar] [CrossRef]
- Kumari, D.; Qian, X.-Y.; Pan, X.; Achal, V.; Li, Q.; Gadd, G.M. Microbially-induced Carbonate Precipitation for Immobilization of Toxic Metals. Adv. Appl. Microbiol. 2016, 94, 79–108. [Google Scholar] [CrossRef] [PubMed]
- Castanier, S.; Le Métayer-Levrel, G.; Perthuisot, J.-P. Ca-carbonates precipitation and limestone genesis–The microbiogeologist point of view. Sediment. Geol. 1999, 126, 9–23. [Google Scholar] [CrossRef]
- DeJong, J.T.; Mortensen, B.M.; Martinez, B.C.; Nelson, D.C. Bio-mediated soil improvement. Ecol. Eng. 2010, 36, 197–210. [Google Scholar] [CrossRef]
- Dhami, N.K.; Reddy, M.S.; Mukherjee, M.S. Biomineralization of calcium carbonates and their engineered applications: A review. Front. Microbiol. 2013, 4, 314. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.K.; Ramakrishnan, V.; Bang, S.S. Remediation of concrete using micro-organisms. ACI Mater. J. 2001, 98, 3–9. [Google Scholar]
- Achal, V.; Mukherjee, A. A review of microbial precipitation for sustainable construction. Constr. Build. Mater. 2015. [Google Scholar] [CrossRef]
- Mitchell, A.C.; Dideriksen, K.; Spangler, L.H.; Cunningham, A.B.; Gerlach, R. Microbially enhanced carbon capture and storage by mineral-trapping and solubility-trapping. Environ. Sci. Technol. 2010, 44, 5270–5276. [Google Scholar] [CrossRef] [PubMed]
- Declet, A.; Reyes, E.; Suárez, O.M. Calcium Carbonate Precipitation: A Review of the Carbonate Crystallization Process and Applications in Bioinspired Composites. Rev. Adv. Mater. Sci. 2016, 44, 87–107. [Google Scholar]
- Anbu, P.; Kang, C.-H.; Shin, Y.-J.; So, J.-S. Formations of calcium carbonate minerals by bacteria and its multiple applications. Springerplus 2016, 5, 250. [Google Scholar] [CrossRef] [PubMed]
- Bhaduri, S.; Debnath, N.; Mitra, S.; Liu, Y.; Kumar, A. Microbiologically Induced Calcite Precipitation Mediated by Sporosarcina pasteurii. J. Vis. Exp. 2016, e53253. [Google Scholar] [CrossRef] [PubMed]
- Gorospe, C.M.; Han, S.H.; Kim, S.G.; Park, J.Y.; Kang, C.H.; Jeong, J.H.; So, J.S. Effects of different calcium salts on calcium carbonate crystal formation by Sporosarcina pasteurii KCTC 3558. Biotechnol. Bioprocess Eng. 2013, 18, 903–908. [Google Scholar] [CrossRef]
- Li, M.; Cheng, X.; Guo, H. Heavy metal removal by biomineralization of urease producing bacteria isolated from soil. Int. Biodeterior. Biodegrad. 2013, 76, 81–85. [Google Scholar] [CrossRef]
- Mugwar, A.J.; Harbottle, M.J. Toxicity effects on metal sequestration by microbially-induced carbonate precipitation. J. Hazard. Mater. 2016, 314, 237–248. [Google Scholar] [CrossRef] [PubMed]
- De Muynck, W.; De Belie, N.; Verstraete, W. Microbial carbonate precipitation in construction materials: A review. Ecol. Eng. 2010, 36, 118–136. [Google Scholar] [CrossRef]
- Okwadha, G.D.O.; Li, J. Optimum conditions for microbial carbonate precipitation. Chemosphere 2010, 81, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.C.; Ferris, F.G. The coprecipitation of Sr into calcite precipitates induced by bacterial ureolysis in artificial groundwater: Temperature and kinetic dependence. Geochim. Cosmochim. Acta 2005, 69, 4199–4210. [Google Scholar] [CrossRef]
- Krajewska, B. Urease-aided calcium carbonate mineralization for engineering applications: A review. J. Adv. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Achal, V.; Pan, X.; Zhang, D. Remediation of copper-contaminated soil by Kocuria flava CR1, based on microbially induced calcite precipitation. Ecol. Eng. 2011, 37, 1601–1605. [Google Scholar] [CrossRef]
- Li, M.; Fu, Q.L.; Zhang, Q.; Achal, V.; Kawasaki, S. Bio-grout based on microbially induced sand solidification by means of asparaginase activity. Sci. Rep. 2015, 5, 16128. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.-H.; Oh, S.J.; Shin, Y.; Han, S.-H.; Nam, I.-H.; So, J.-S. Bioremediation of lead by ureolytic bacteria isolated from soil at abandoned metal mines in South Korea. Ecol. Eng. 2015, 74, 402–407. [Google Scholar] [CrossRef]
- Mwandira, W.; Nakashima, K.; Kawasaki, S. Bioremediation of lead-contaminated mine waste by Pararhodobacter sp. based on the microbially induced calcium carbonate precipitation technique and its effects on strength of coarse and fine grained sand. Ecol. Eng. 2017, 109, 57–64. [Google Scholar] [CrossRef]
- Qian, X.; Fang, C.; Huang, M.; Achal, V. Characterization of fungal-mediated carbonate precipitation in the biomineralization of chromate and lead from an aqueous solution and soil. J. Clean. Prod. 2017, 164, 198–208. [Google Scholar] [CrossRef]
- Kang, C.H.; Han, S.H.; Shin, Y.; Oh, S.J.; So, J.S. Bioremediation of Cd by microbially induced calcite precipitation. Appl. Biochem. Biotechnol. 2014, 172, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Kumari, D.; Pan, X.; Lee, D.J.; Achal, V. Immobilization of cadmium in soil by microbially induced carbonate precipitation with Exiguobacterium undae at low temperature. Int. Biodeterior. Biodegrad. 2014, 94, 98–102. [Google Scholar] [CrossRef]
- Zhao, C.; Fu, Q.; Song, W.; Zhang, D.; Ahati, J.; Pan, X.; Al-Misned, F.A.; Mortuza, M.G. Calcifying cyanobacterium (Nostoc calcicola) reactor as a promising way to remove cadmium from water. Ecol. Eng. 2015, 81, 107–114. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X.; Fu, Q.; Zhang, D. Biomineralization based remediation of As(III) contaminated soil by Sporosarcina ginsengisoli. J. Hazard. Mater. 2012, 201–202, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Csetenyi, L.; Gadd, G.M. Biomineralization of metal carbonates by Neurospora crassa. Environ. Sci. Technol. 2014, 48, 14409–14416. [Google Scholar] [CrossRef] [PubMed]
- Hammes, F.; Seka, A.; Van Hege, K.; Van De Wiele, T.; Vanderdeelen, J.; Siciliano, S.D.; Verstraete, W. Calcium removal from industrial wastewater by bio-catalytic CaCO3 precipitation. J. Chem. Technol. Biotechnol. 2003, 78, 670–677. [Google Scholar] [CrossRef]
- Carballa, M.; Moerman, W.; De Windt, W.; Grootaerd, H.; Verstraete, W. Strategies to optimize phosphate removal from industrial anaerobic effluents by magnesium ammonium phosphate (MAP) production. J. Chem. Technol. Biotechnol. 2009, 84, 63–68. [Google Scholar] [CrossRef]
- Arias, D.; Cisternas, L.; Rivas, M. Biomineralization Mediated by Ureolytic Bacteria Applied to Water Treatment: A Review. Crystals 2017, 7, 345. [Google Scholar] [CrossRef]
- Fujita, Y.; Ferris, F.; Lawson, R.; Colwell, F.; Smith, R. Calcium Carbonate Precipitation by Ureolytic Subsurface Bacteria. Geomicrobiol. J. 2000, 17, 305–318. [Google Scholar] [CrossRef]
- Zhao, Y.; Yao, J.; Yuan, Z.; Wang, T.; Zhang, Y.; Wang, F. Bioremediation of Cd by strain GZ-22 isolated from mine soil based on biosorption and microbially induced carbonate precipitation. Environ. Sci. Pollut. Res. 2017, 24. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Pan, X.; Zhao, C.; Mou, S.; Achal, V.; Al-Misned, F.A.; Mortuza, M.G.; Gadd, G.M. Bioimmobilization of Heavy Metals in Acidic Copper Mine Tailings Soil. Geomicrobiol. J. 2016, 33, 261–266. [Google Scholar] [CrossRef]
- Arias, D.; Valdes, P.; Cisternas, L.A.; Rivas, M. Isolation and Selection of Halophilic Ureolytic Bacteria for Biocementation of Calcium and Magnesium from Seawater. Adv. Mater. Res. 2015, 1130, 489–492. [Google Scholar] [CrossRef]
- Kang, C.; Kwon, Y.; So, J. Bioremediation of heavy metals by using bacterial mixtures. Ecol. Eng. 2016, 89, 64–69. [Google Scholar] [CrossRef]
- Gat, D.; Tsesarsky, M.; Shamir, D.; Ronen, Z. Accelerated microbial-induced CaCO3 precipitation in a defined coculture of ureolytic and non-ureolytic bacteria. Biogeosciences 2014, 11, 2561–2569. [Google Scholar] [CrossRef]
- Matijašević, L.; Dejanović, I.; Lisac, H. Treatment of wastewater generated by urea production. Resour. Conserv. Recycl. 2010, 54, 149–154. [Google Scholar] [CrossRef]
- Barmaki, M.M.; Rahimpour, M.R.; Jahanmiri, A. Treatment of wastewater polluted with urea by counter-current thermal hydrolysis in an industrial urea plant. Sep. Purif. Technol. 2009, 66, 492–503. [Google Scholar] [CrossRef]
- Van Langerak, E.P.A.; Hamelers, H.V.M.; Lettinga, G. Influent calcium removal by crystallization reusing anaerobic effluent alkalinity. Water Sci. Technol. 1997, 36, 341–348. [Google Scholar] [CrossRef]
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Gaikwad, R.W.; Gupta, D.V. Review on Removal of Heavy Metals From Acid Mine Drainage. Appl. Ecol. Environ. Res. 2008, 6, 81–98. [Google Scholar] [CrossRef]
- Gat, D.; Ronen, Z.; Tsesarsky, M. Long-term sustainability of microbial-induced CaCO3 precipitation in aqueous media. Chemosphere 2017, 184, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Meessen, J.; Wo, F. Urea synthesis. 2014, 2180–2189. [Google Scholar] [CrossRef]
- Mao, N.; Ren, H.; Geng, J.; Ding, L.; Xu, K. Engineering application of anaerobic ammonium oxidation process in wastewater treatment. World J. Microbiol. Biotechnol. 2017, 33, 153. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2016, 182, 247–268. [Google Scholar] [CrossRef]
- Kalidhasan, S.; Kumar, A.S.K.; Rajesh, V.; Rajesh, N. The journey traversed in the remediation of hexavalent chromium and the road ahead toward greener alternatives—A perspective. Coord. Chem. Rev. 2016, 317, 157–166. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X.; Lee, D.J.; Kumari, D.; Zhang, D. Remediation of Cr(VI) from chromium slag by biocementation. Chemosphere 2013, 93. [Google Scholar] [CrossRef] [PubMed]
- Ferris, F.G.; Phoenix, V.; Fujita, Y.; Smith, R.W. Kinetics of calcite precipitation induced by ureolytic bacteria at 10 to 20 °C in artificial groundwater. Geochim. Cosmochim. Acta 2004, 68, 1701–1722. [Google Scholar] [CrossRef]
- Rehman, A.; Shakoori, F.R.; Shakoori, A.R. Heavy metal resistant freshwater ciliate, Euplotes mutabilis, isolated from industrial effluents has potential to decontaminate wastewater of toxic metals. Bioresour. Technol. 2008, 99, 3890–3895. [Google Scholar] [CrossRef] [PubMed]
- Castro, S.H.; Sa, M. Environmental viewpoint on small-scale copper, gold and silver mining in Chile. J. Clean. Prod. 2003, 11, 207–213. [Google Scholar] [CrossRef]
- Obreque-Contreras, J.; Pérez-Flores, D.; Gutiérrez, P.; Chávez-Crooker, P. Acid Mine Drainage in Chile: An Opportunity to Apply Bioremediation Technology. J. Forensic Res. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Wang, L.; Ji, B.; Hu, Y.; Liu, R.; Sun, W. A review on in situ phytoremediation of mine tailings. Chemosphere 2017, 184, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Martín-Lara, M.A.; Blázquez, G.; Trujillo, M.C.; Pérez, A.; Calero, M. New treatment of real electroplating wastewater containing heavy metal ions by adsorption onto olive stone. J. Clean. Prod. 2014, 81, 120–129. [Google Scholar] [CrossRef]
- Panayotova, T.; Dimova-Todorova, M.; Dobrevsky, I. Purification and reuse of heavy metals containing wastewaters from electroplating plants. Desalination 2007, 206, 135–140. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.H.; Babel, S. Physico-chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).