A Novel Approach to Quantitatively Assess the Uniformity of Binary Colloidal Crystal Assemblies
Abstract
:1. Introduction
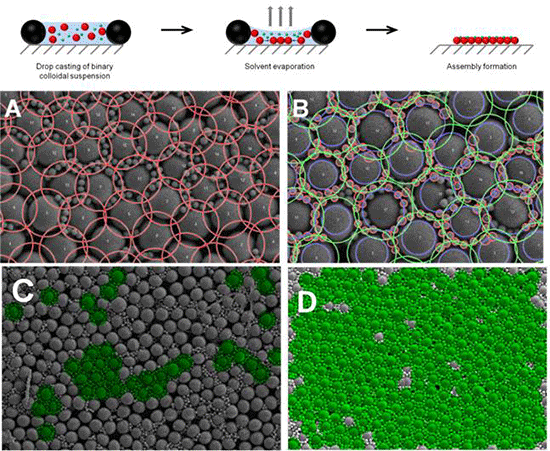
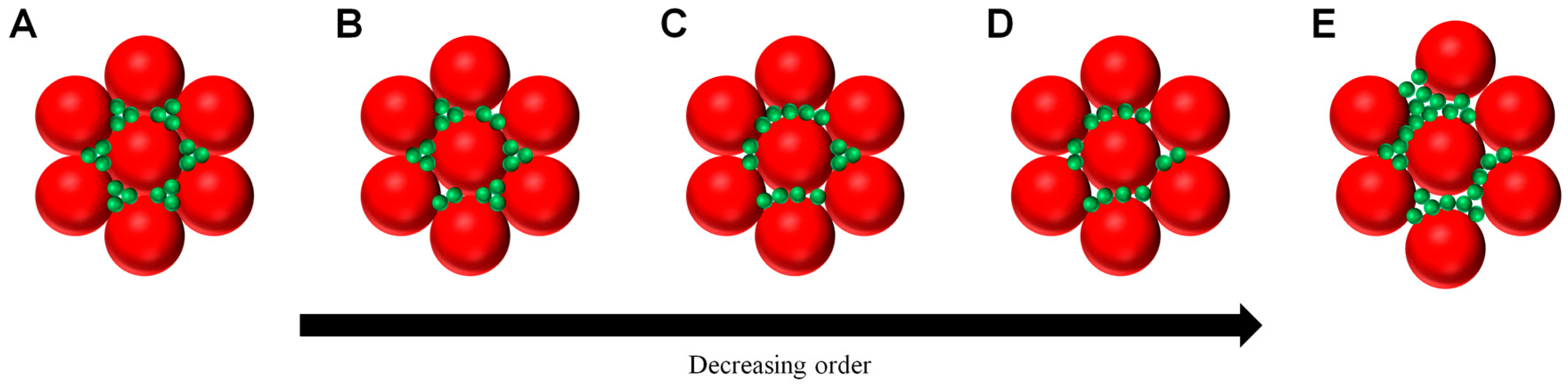
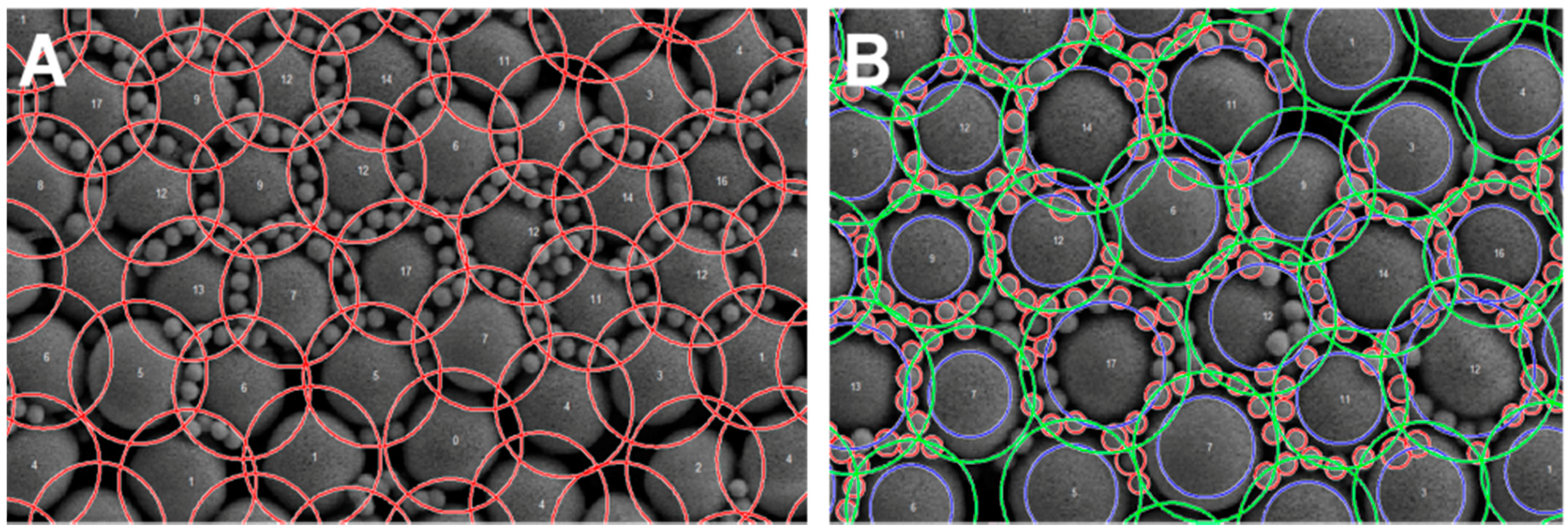
2. Results
3. Materials and Methods
3.1. Substrate Material
3.2. Particles
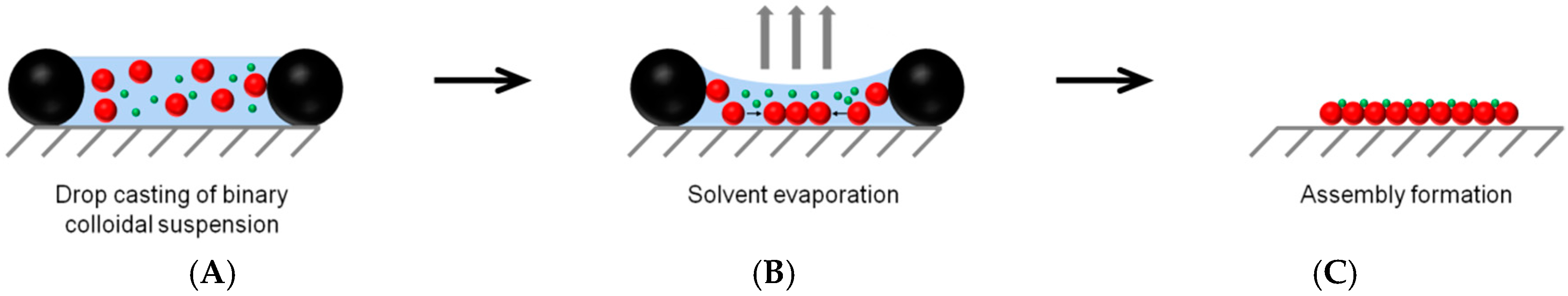
3.3. Formation of Binary Colloidal Assemblies (BCCs)
3.4. Scanning Electron Microscopy (SEM)
3.5. Software Development
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Koegler, P.; Clayton, A.; Thissen, H.; Santos, G.N.C.; Kingshott, P. The influence of nanostructured materials on biointerfacial interactions. Adv. Drug Deliv. Rev. 2012, 64, 1820–1839. [Google Scholar] [PubMed]
- Castner, D.G.; Ratner, B.D. Biomedical surface science: Foundations to frontiers. Surf. Sci. 2002, 500, 28–60. [Google Scholar] [CrossRef]
- Kingshott, P.; Thissen, H.; Griesser, H.J. Effects of cloud-point grafting, chain length, and density of PEG layers on competitive adsorption of ocular proteins. Biomaterials 2002, 23, 2043–2056. [Google Scholar] [CrossRef]
- Ameringer, T.; Fransen, P.; Bean, P.; Johnson, G.; Pereira, S.; Evans, R.A.; Thissen, H.; Meagher, L. Polymer coatings that display specific biological signals while preventing nonspecific interactions. J. Biomed. Mater. Res. A 2012, 100A, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Flavel, B.S.; Jasieniak, M.; Velleman, L.; Ciampia, S.; Luais, E.; Peterson, J.R.; Griesser, H.J. Grafting of poly(ethylene glycol) on click chemistry modified Si(100) surfaces. Langmuir 2013, 29, 8355–8362. [Google Scholar] [CrossRef] [PubMed]
- Koegler, P.; Pasic, P.; Gardiner, J.; Glattauer, V.; Kingshott, P.; Thissen, H. Polymerizable peptide copolymer coatings for the control of biointerfacial interactions. Biomacromolecules 2014, 15, 2265–2273. [Google Scholar] [CrossRef] [PubMed]
- Lilge, I.; Schoenherr, H. Covalently cross-linked poly(acrylamide) brushes on gold with tunable mechanical properties via surface-initiated atom transfer radical polymerization. Eur. Polym. J. 2013, 49, 1943–1951. [Google Scholar] [CrossRef]
- Koegler, P.; Pasic, P.; Johnson, G.; Bean, P.; Lorenz, G.; Meagher, L.; Thissen, H. Controlling cell-material interactions using coatings with advanced polymer architectures. Proc. SPIE 2011, 8204. [Google Scholar] [CrossRef]
- Rodriguez-Emmenegger, C.; Brynda, E.; Riedel, T.; Houska, M.; Subr, V.; Alles, A.B.; Hasan, E.; Gautrot, J.E.; Huck, W.T.S. Polymer brushes showing non-fouling in blood plasma challenge the currently accepted design of protein resistant surfaces. Macromol. Rapid Commun. 2011, 32, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Fairbanks, B.D.; Thissen, H.; Maurdev, G.; Pasic, P.; White, J.F.; Meagher, L. Inhibition of protein and cell attachment on materials generated from N-(2-hydroxypropyl)acrylamide. Biomacromolecules 2014, 15, 3259–3266. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.W.; Tsai, C.C.; Tsai, W.B.; Wang, M.J.; Kuo, W.H.; Wei, T.C.; Huang, S.T. Surface conjugation of zwitterionic polymers to inhibit cell adhesion and protein adsorption. Colloid Surf. B-Biointerfaces 2013, 107, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.K.; Kong, B.Y.; Choi, I.S. Highly efficient non-biofouling coating of zwitterionic polymers: Poly((3-(methacryloylamino)propyl)-dimethyl(3-sulfopropyl)ammonium hydroxide). Langmuir 2007, 23, 5678–5682. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.S.; Karthikeyan, C.V.; Eichelsdoerfer, D.J.; Micklefield, J.; Mirkin, C.A. A methodology for preparing nanostructured biomolecular interfaces with high enzymatic activity. Nanoscale 2012, 4, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Han, X.M.Y.; Sun, S.Q.; He, T. Preparation and photolithography of self-assembled monolayers of 10-mercaptodecanylphosphonic acid on glass mediated by zirconium for protein patterning. Colloid Surf. B-Biointerfaces 2013, 108, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, G.; Fenili, F.; Gianfelice, A.; Bongiorno, G.; Marchesi, D.; Scopelliti, P.E.; Borgonovo, A.; Podesta, A.; Inderieri, A.; Ranucci, E.; et al. Direct microfabrication of topographical and chemical cues for the guided growth of neural cell networks on polyamidoamine hydrogels. Macromol. Biosci. 2010, 10, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Tsimbouri, P.; Gadegaard, N.; Burgess, K.; White, K.; Reynolds, P.; Herzyk, P.; Oreffo, R.; Dalby, M.J. Nanotopographical effects on mesenchymal stem cell morphology and phenotype. J. Cell. Biochem. 2014, 115, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Garland, S.P.; McKee, C.T.; Chang, Y.-R.; Raghunathan, V.K.; Russell, P.; Murphy, C.J. A cell culture substrate with biologically relevant size-scale topography and compliance of the basement membrane. Langmuir 2014, 30, 2101–2108. [Google Scholar] [CrossRef] [PubMed]
- Vandrangi, P.; Gott, S.C.; Kozaka, R.; Rodgers, V.G.J.; Rao, M.P. Comparative endothelial cell response on topographically patterned titanium and silicon substrates with micrometer to sub-micrometer feature size. PLoS ONE 2014, 9, e11465. [Google Scholar] [CrossRef] [PubMed]
- Bettinger, C.J.; Langer, R.; Borenstein, J.T. Engineering substrate topography at the micro- and nanscale to control cell function. Angew. Chem. Int. Ed. 2009, 48, 5406–5415. [Google Scholar] [CrossRef] [PubMed]
- Luecker, P.B.; Javaherian, S.; Soleas, J.P.; Halverson, D.; Zandstra, P.W.; McGuigan, A.P. A microgroove patterned multiwall cell culture plate for high-throughput studies of cell alignment. Biotechnol. Bioeng. 2014, 111, 2537–2548. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Griesser, H.J.; Bremmell, K.; Kingshott, P. Highly ordered nanometer-scale chemical and protein patterns by binary colloidal crystal lithography combined with plasma polymerization. Adv. Funct. Mater. 2011, 21, 540–546. [Google Scholar] [CrossRef]
- Kristensen, S.H.; Pedersen, G.A.; Ogaki, R.; Bochenkov, V.; Nejsum, L.N.; Sutherland, D.S. Complex protein nanopatterns over large areas via colloidal lithography. Acta Biomater. 2013, 9, 6158–6168. [Google Scholar] [CrossRef] [PubMed]
- Ogaki, R.; Foss, M. Biofunctional surface patterns retaining activity after exposure to whole blood. Langmuir 2014, 30, 7014–7023. [Google Scholar] [CrossRef] [PubMed]
- Malainou, A.; Tsougeni, K.; Ellias, K.; Petrou, P.S.; Constantoudis, V.; Sarantopoulou, E.; Awsiuk, K.; Bernasik, A.; Budkowski, A.; Markou, A.; et al. Plasma-assisted nanoscale protein patterning on Si substrates via colloidal lithography. J. Phys. Chem. A 2013, 117, 13743–13751. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-Y.; Pingle, H.; Koegler, P.; Thissen, H.; Kingshott, P. Self-assembled binary colloidal crystal monolayers as cell culture substrates. J. Mater. Chem. B 2015, 3, 2545–2552. [Google Scholar] [CrossRef]
- Akerboom, S.; Appel, J.; Labonte, D.; Federle, W.; Sprakel, J.; Kamperman, M. Enhanced adhesion of bioinspired nanopatterned elastomers via colloidal surface assembly. J. R. Soc. Interface 2015, 12. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, L.M.; Wagner, J.; Stumpe, J.; Paulke, B.-R.; Gornitz, E. Ordered arrays of large latex particles organized by vertical deposition. Langmuir 2002, 18, 3319–3323. [Google Scholar] [CrossRef]
- Sun, S.; Huang, Y.; Zhao, B. Formation of silica colloidal crystals on soft hydrophobic vs. rigid hydrophilic surfaces. Colloid Surf. A-Physicochem. Eng. Asp. 2015, 467, 180–187. [Google Scholar] [CrossRef]
- Toolan, D.T.W.; Fujii, S.; Ebbens, S.J.; Nakamura, Y.; Howse, J.R. On the mechanisms of colloidal seld-assembly during spin-coating. Soft Matter 2014, 10, 8804–8812. [Google Scholar] [PubMed]
- Chen, J.; Dong, P.; Di, D.; Wang, C.; Wang, H.; Wang, J.; Wu, X. Controllable fabrication of 2D colloidal crystal films with polystyrene nanospheres of various diameters by spin-coating. Appl. Surf. Sci. 2013, 270, 6–16. [Google Scholar] [CrossRef]
- Kadiri, H.; Kostcheev, S.; Turover, D.; Salas-Montiel, R.; Nomenyo, K.; Gokarna, A.; Lerondel, G. Topology assisted self-organization of colloidal nanoparticles: Application to 2D large-scale mastering. Beilstein J. Nanotechnol. 2014, 5, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Pillai, S.; Arpanaei, A.; Kingshott, P. Multicomponent colloidal crystals that are tunable over large areas. Soft Matter 2011, 7, 3290–3294. [Google Scholar] [CrossRef]
- Singh, G.; Pillai, S.; Arpanaei, A.; Kingshott, P. Highly ordered mixed protein patterns over large areas from self-assembly of binary colloids. Adv. Mater. 2011, 23, 1519–1523. [Google Scholar] [CrossRef] [PubMed]
- Selhuber-Unkel, C.; Erdmann, T.; Lopez-Garcia, M.; Kessler, H.; Schwarz, U.S.; Spatz, J.P. Cell adhesion strength is controlled by intermolecular spaing of adhesion receptors. Biophys. J. 2010, 98, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.H.; Grater, S.V.; Corbellinl, F.; Rinck, S.; Bock, E.; Kemkemer, R.; Kessler, H.; Ding, J.D.; Spatz, J.P. Impact of order and disorder in RGD nanopatterns on cell adhesion. Nano Lett. 2009, 9, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
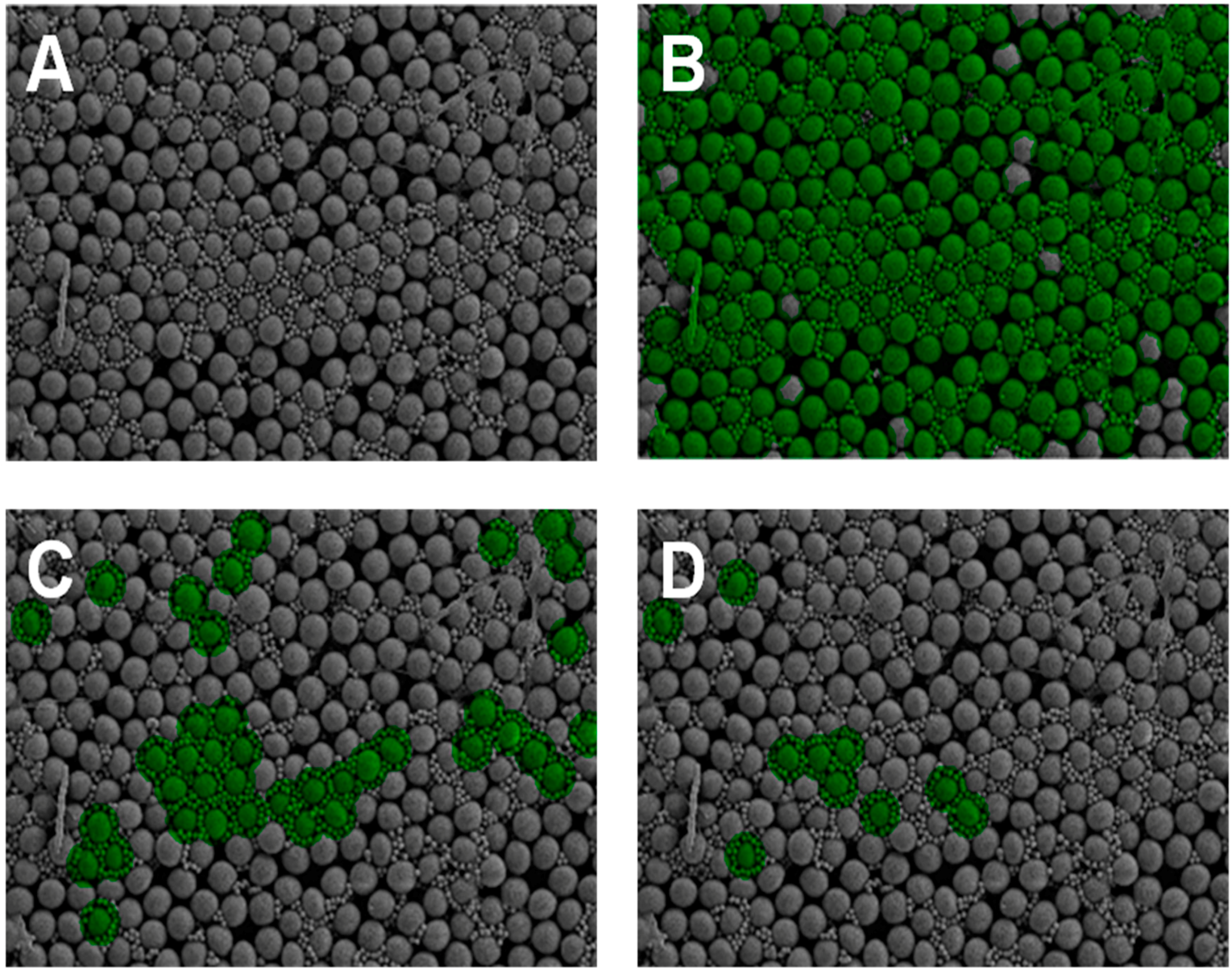

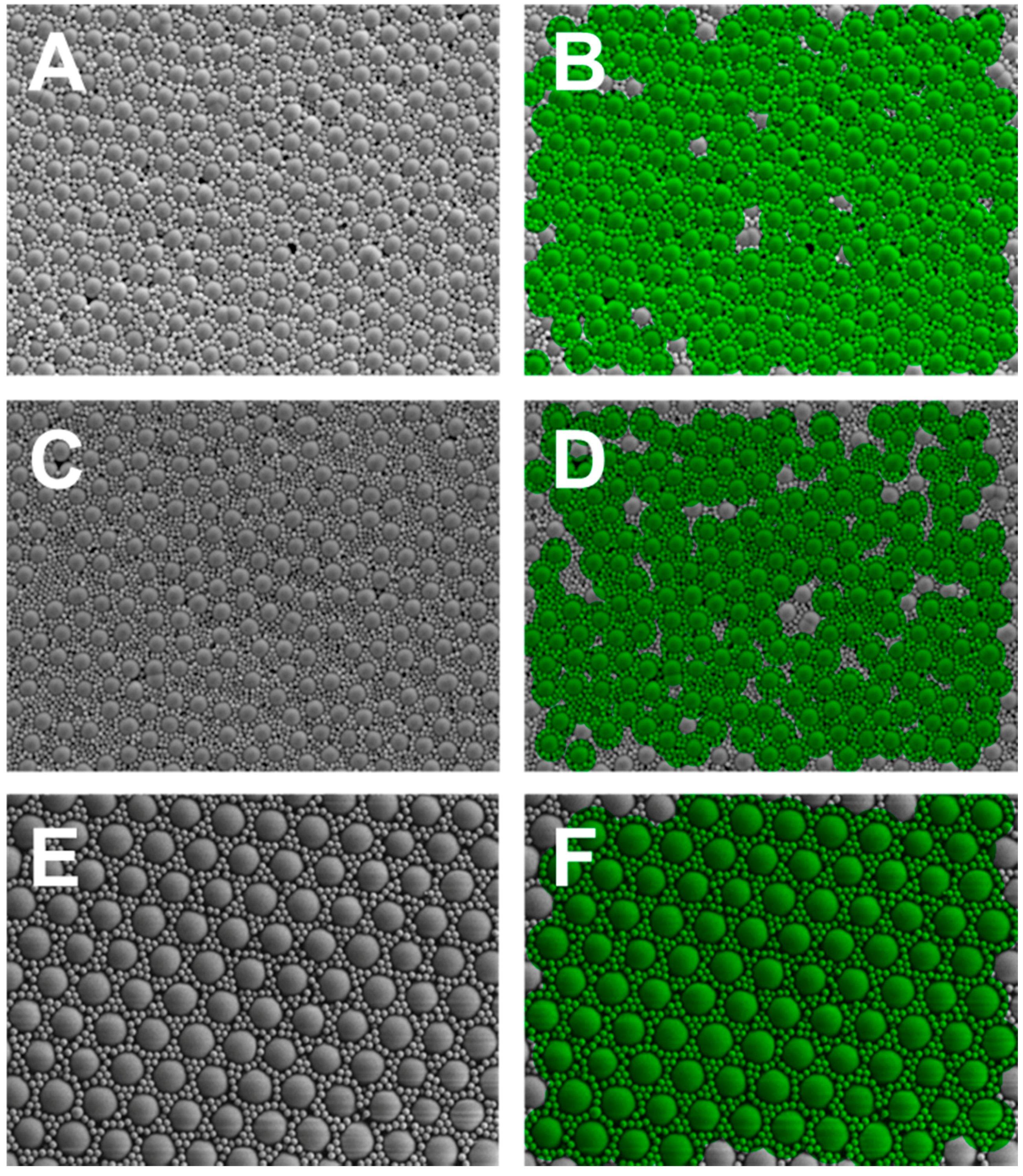
| Large Particle/Small Particle | Large Particle Size Range (pixels) | Small Particle Size Range (pixels) | Annulus Size Range (pixels) | Highly Uniform Threshold | Degree of Uniformity (%) |
|---|---|---|---|---|---|
| 2 µm SiO2/0.51 µm PS-COOH | 12–23 | 2–5 | 13–22 | >8 | 90 |
| 2 µm SiO2/0.4 µm PMMA | 10–24 | 2–5 | 13–22 | >12 | 78 |
| 2 µm PS-COOH/0.4 µm PMMA | 30–54 | 5–8 | 35–57 | >17 | 90 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koegler, P.; Dunn, M.; Wang, P.-Y.; Thissen, H.; Kingshott, P. A Novel Approach to Quantitatively Assess the Uniformity of Binary Colloidal Crystal Assemblies. Crystals 2016, 6, 84. https://doi.org/10.3390/cryst6080084
Koegler P, Dunn M, Wang P-Y, Thissen H, Kingshott P. A Novel Approach to Quantitatively Assess the Uniformity of Binary Colloidal Crystal Assemblies. Crystals. 2016; 6(8):84. https://doi.org/10.3390/cryst6080084
Chicago/Turabian StyleKoegler, Peter, Michelle Dunn, Peng-Yuan Wang, Helmut Thissen, and Peter Kingshott. 2016. "A Novel Approach to Quantitatively Assess the Uniformity of Binary Colloidal Crystal Assemblies" Crystals 6, no. 8: 84. https://doi.org/10.3390/cryst6080084
APA StyleKoegler, P., Dunn, M., Wang, P.-Y., Thissen, H., & Kingshott, P. (2016). A Novel Approach to Quantitatively Assess the Uniformity of Binary Colloidal Crystal Assemblies. Crystals, 6(8), 84. https://doi.org/10.3390/cryst6080084