1. Introduction
The world of today and future development are highly dependent on an adequate supply of minerals from the mining industry. Mining, like many other industries, is required to change towards more sustainable production methods. Today, the mining industry is facing problems of sustainable water supply, renewable energy sources, and depletion of minerals. Moreover, as the ore grades degrade, the higher the associated production costs become, including water and energy consumptions. At the same time, population increase, climate changes, and ongoing industrialization are also putting pressure on water, energy, and minerals. These resources are, moreover, limited and cannot be used without any concern. Fresh water resources are sufficient only in limited parts of the world. It is estimated that 50% of the world population will live in water stressed regions in 2025, which highlights the importance of adequate water management and treatment [
1]. For this reason, the mining industry is also forced not to deplete or contaminate the existing water resources, and at the same time not to risk the water supply of the local community. Water in mining is becoming a hot topic and some countries have restricted the mining industry to protect their own water. Energy consumption grew rapidly in the last decades and is projected to increase further in the following years [
2]. Furthermore, mineral deficiency is also a threat to future development. Therefore, the conventional mining industry has several constrains for a sustainable way of production.
Seawater can be an additional source for mineral extraction. The most part of the ions present in the periodic table might be recovered from seawater in the logic of “mining from the sea”. Historically, mining from the sea was considered during the oil crisis of the 1970. Yet, it never reached breakthrough due to several deficiencies, including high cost, low efficiency, lack of technological development,
etc. In reality, the proposed strategy of direct recovery from seawater is difficult and might still be an impossible task. However, it can be brought back to life in another context with respect to the 1970ties, and due to improved technological processes, higher risks of mineral depletion, requirements of sustainable water and energy sources. The problems that the mining industry is facing today, such as water, energy and minerals deficiency, can partly be solved by introducing membrane technology. Membrane engineering is aligned with sustainable development, which has become important for many industrial processes. However, lack of a precise definition of sustainable development has evolved specific guidelines, such as the process intensification strategy (PIS) helping to meet the requirements of sustainable development. Membrane engineering, through the process intensification strategy, can redesign conventional process engineering with applications in several industrial processes; e.g., wastewater treatment, desalination, and many other applications where separation is needed [
3]. Membrane engineering meets the goals of PIS for several reasons, including high selectivity and permeability for transport of specific components, ease of integration with other processes or other membrane operations, less energy intensive, high efficiency, low capital costs, small footprints, high safety, and operational simplicity and flexibility [
4,
5,
6,
7].
Membrane engineering can be an interesting outlook for the mining industry, for example, by associating mining and desalination. Not only can desalination contribute to mining by water production, but it can also contribute to energy production and minerals recovery. These kind of desalination systems are already being developed in large-scale desalination projects; e.g., Global MVP. Desalination is one of the industries that has changed from conventional processes to membrane technology, where reverse osmosis (RO) desalination today accounts for more than 60% of the capacity [
8]. There have been many developments over the last three decades which have contributed to a reduction in unit water cost of RO desalination, particularly: membrane performance and decrease of membrane cost, reduction in energy consumption, improvements in pretreatment processes, increases in plant capacity,
etc. [
9]. However, one of the limitations of RO desalination is the relatively low water recovery factor (~40%–60%). Due to the increase in salinity, and therefore the required applied pressure (driving force), it is not economically or technically feasible to go beyond this recovery factor. Other potential improvements for RO desalination include better exploitation of the RO brine and reduction in electrical energy consumptions. Several international large-scale desalination projects are renewing desalination to reduce the cost and increase efficiency. Some of these are: MEDINA (Membrane Based Desalination: An Integrated Approach 2006–2010, European project) [
10], SEAHERO (Seawater engineering & architecture of high efficiency reverse osmosis 2007–2012, 2013–2018, S. Korea) [
11], MEGATON (2009–2014, Japan) [
12,
13], Global MVP (2013–2018, Korea) [
13]. The large-scale projects are, for example, investigating the feasibility of novel membrane operations to increase water recovery, reduce brine disposal, and implement energy production. The novel membrane operations are membrane distillation (MD) for enhancing water recovery and pressure retarded osmosis (PRO), or reverse electrodialysis (RED) for energy production. The latest launched project, the Global MVP, aims to further develop the so-called 3
rd generation desalination plant by also introducing an additional step for valuable resource recovery. The Global MVP project emphasizes lithium and strontium recovery from the discharged RO brine [
13], but in fact, several other constituents might also be recovered from RO brine.
To recover minerals from highly concentrated solutions, a new and very interesting membrane technology—i.e., membrane crystallization (MCr)—has been developed within the last 30 years. MCr is able to treat solutions, which are difficult to treat for other unit operations in terms of costs, energy consumption, crystal quality and quantity, etc.
1.1. Membrane Crystallization (MCr)
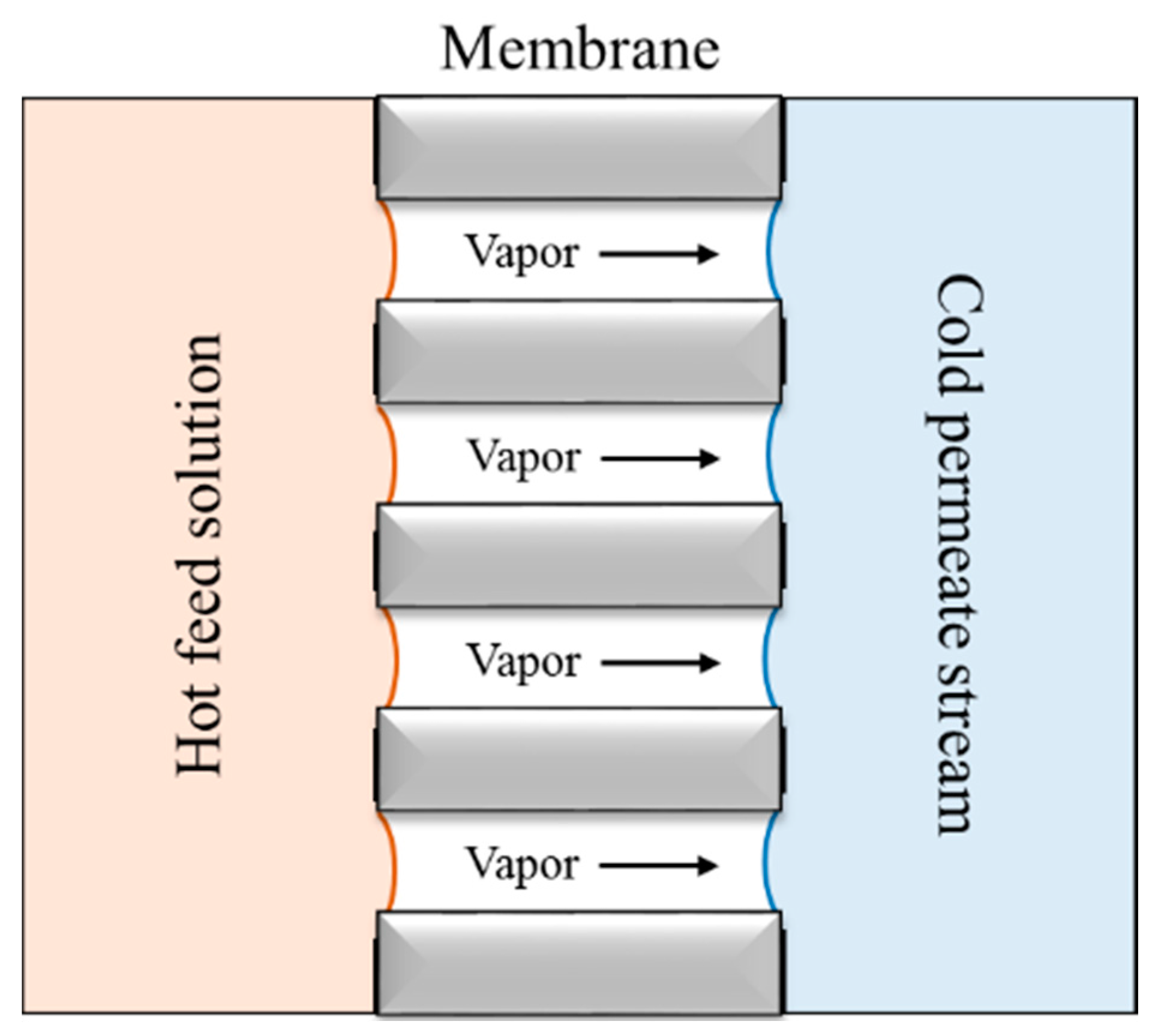
MCr is an extension of the MD concept based on mass transfer through a microporous hydrophobic membrane (
Figure 1). The driving force is normally a temperature gradient between the two membrane sides. The hydrophobic nature of the membrane prevents liquid intrusion into the pores. Therefore, only volatile components are transported through the membrane and are condensed on the permeate site. The mass transfer of volatile solvents allows concentration of feed solutions above their saturation limit, thus attaining a supersaturated environment where crystals may nucleate and grow. The advantages of using MD and MCr are the very low utilized temperatures and pressures, high permeate quality independent of feed characteristics (theoretical 100% rejection of non-volatile components), simple configuration, and the possibility to treat highly concentrated solutions [
14]. Unlike pressure driven membrane operations, the impact of concentration in MD and MCr is very small [
15]. Therefore, these processes are perfect to treat the brine leaving RO desalination. Furthermore, MCr has some important advantages with respect to traditional crystallization processes, such as well-controlled nucleation and growth kinetics, faster crystallization rates and reduced induction time, control of super-saturation level and rate. Therefore, it is possible to target the crystal polymorph form to obtain crystals with narrow size distribution and high purity [
16].
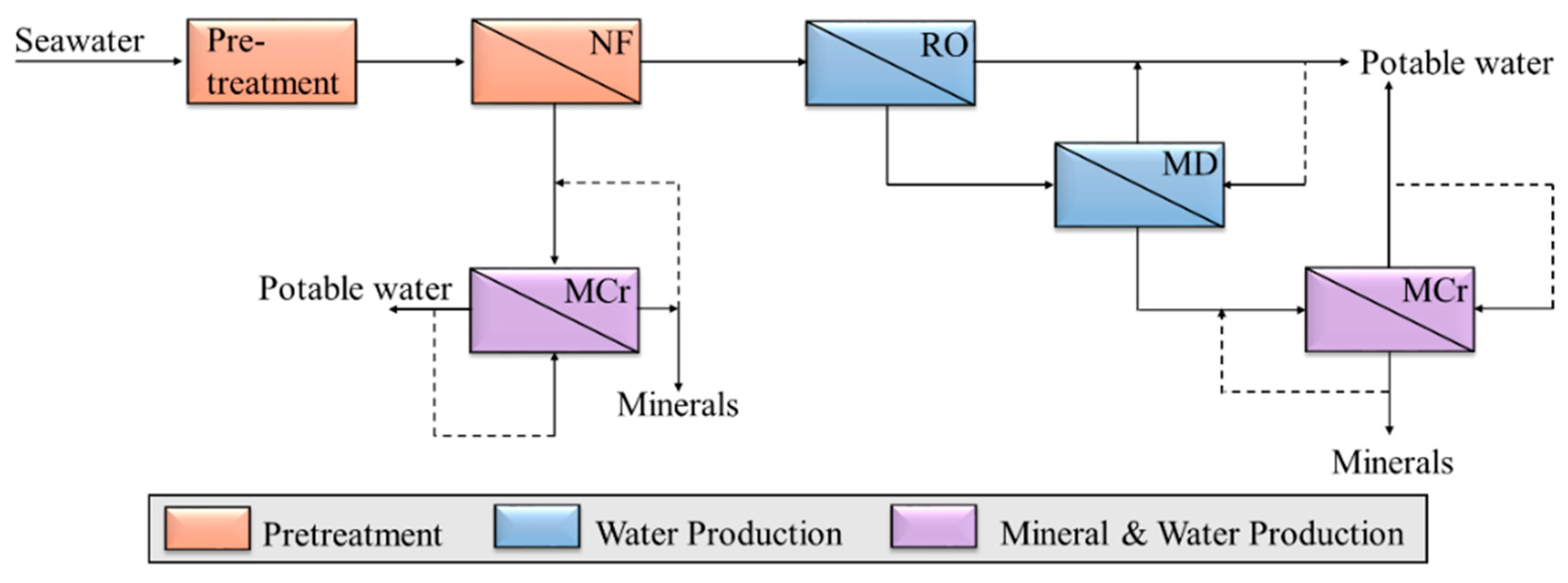
This study suggests a paradigm shift in the mining industry by combining minerals recovery and membrane desalination operations for water and mineral production, according to the scheme shown in
Figure 2. Smart integration of membrane operations in desalination can contribute to the conventional mining industry by recovering minerals from the brine. At the same time, it solves some of the drawbacks of the mining and the desalination industry. Membrane crystallizers are operating at low temperatures (normally below 60 °C) and at ambient pressure, which reduces the associated energy costs. The well-controlled nucleation and growth and the high purity also ensure less post-treatment of the minerals with respect to the mining industry. At the same time that minerals are being produced, MCr also produces a high-quality water stream. Therefore, the drawbacks of low water recovery factors and brine disposal in desalination can be minimized.
2. Methods
Minerals recovery has been considered through the integration of membrane operations according to the scheme illustrated in
Figure 2. Membrane distillation and membrane crystallization have been applied to NF and RO brine, respectively. In the theoretical evaluation of minerals recovery, a capacity of the RO unit of 923 m
3/d has been assumed (water recovery factor of 52%), and from this the volume of the various other streams have been estimated (
Table 1). Seawater composition and the corresponding ion rejection of the NF and RO membrane have been shown in
Table 2. In the simulation, no calcium or carbonate compounds are present, since the brine has been considered to be pre-treated with Na
2CO
3 to precipitate CaCO
3. CaCO
3 and CaSO
4 are considered to be high risk scaling components, and can therefore destroy or impede the MCr operation [
17]. For this reason, calcium and carbonate are not found in
Table 2.
Enrichment of the feed stream carried out by MD and MCr has been simulated through the geochemical software PHREEQC using the Pitzer specific-ion-interaction aqueous model [
18]. The PHREEQC software utilized is PHREEQC interactive-version 3. The Pitzer approach takes into account the interaction between the different ions and is therefore suitable for highly concentrated solutions. The existing database in PHREEQC has been updated to match all the ions considered in
Table 2. To simulate water removal, a so-called “REACTION” has been utilized to remove a specified amount of water in a given number of steps. The output of the software provides saturation indices and the amount of compounds which have precipitated,
etc. Temperature of RO brine and pH in the simulations has been assumed to 30 °C and 7, respectively. Clearly, the operative temperature can be adjusted to enhance or inhibit one compound to precipitate instead of another, which will be discussed in the following sections.
3. Results and Discussion
Today, some minerals are already being extracted from seawater, such as Na
+, Mg
2+ and K
+ [
19]. Several research activities have been carried out to extend the number of ions to be recovered from seawater [
19]. The ocean has, in general, a much greater content of mineral resources in comparison to on land [
19,
20]. Although, one can argue that the resources in the ocean are present in lower concentrations, which reduces the possibility of recovery. Nevertheless, mineral recovery might be too expensive and energy intensive with respect to extraction directly from seawater. Instead, it might be more economically feasible to recover minerals from NF retentate and RO brine and hereby turn waste streams into resources. In the following sections, the potential of minerals recovery from NF retentate and RO brine is highlighted.
3.1. Minerals Recovery from NF Retentate
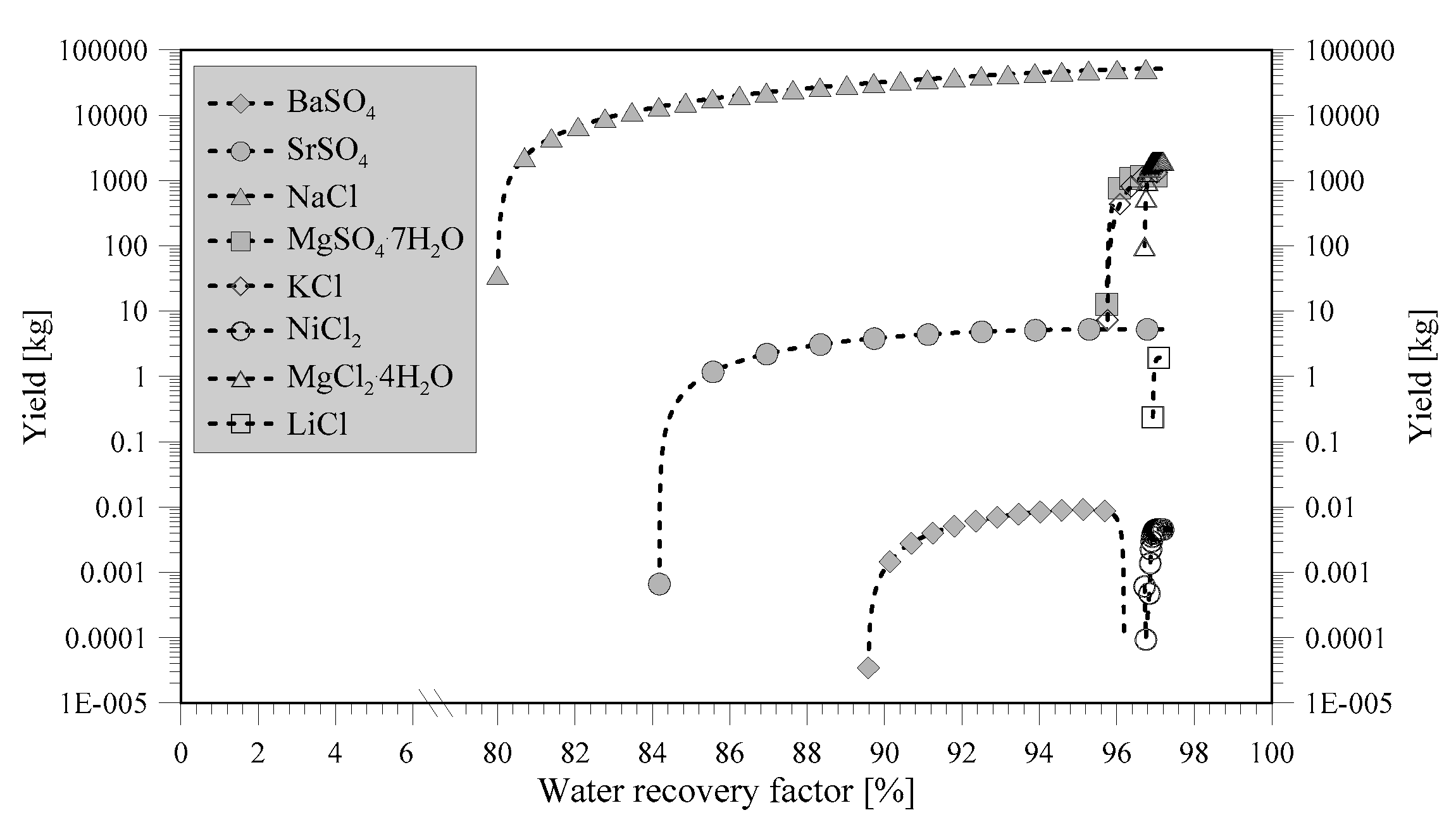
The first compounds to precipitate from NF retentate (
Figure 3) are BaSO
4 and SrSO
4 (Barite and Celestite, respectively). These compounds can be interesting to recover due to their utilization in the oil and gas industry as weighing material or stabilizer in drilling mud [
21]. According to U.S. Geological Survey, the average price of barium and strontium imported to USA in 2013 was 115 $/ton and 50 $/ton, respectively [
22]. Simulations have proven that from 631 m
3 of NF brine (
Table 1) it is possible to recover around 0.07 kg of barium and 40 kg of strontium. These might seem to be relatively low amounts, nevertheless, it has to be taken into consideration that at the industrial level, RO desalination plants have capacities ranging from 100,000 to 500,000 m
3/d, and even higher capacities are projected. For example, from a seawater reverse osmosis (SWRO) plant with a capacity of 100,000 m
3/d, it is potentially possible to recover 11.1 kg/d of barium and 6339 kg/d of strontium. Therefore, the future recovery potential is much higher than the one considered in this study. The larger amount of strontium can make this compound more feasible to separate from the RO brine with respect to barium [
23]. However, due to the higher amount of NaCl (starting to precipitate above water recovery factor (WRF) of 86%), it has to be recovered before NaCl precipitation to avoid incorporation of strontium into the NaCl lattice [
17]. After NaCl crystallization, magnesium sulfate, in the form of MgSO
4·7H
2O (Epsomite), starts to precipitate at a WRF of 93% when the temperature is kept at 30 °C. Other polymorphs of magnesium sulfate and magnesium chlorides can also precipitate. However, experimental results carried out in earlier studies have shown that, by properly tuning the operative conditions, epsomite can be produced via MCr [
24,
25]. This highlights one of the very important advantages of MCr with respect to conventional crystallizer: in MCr it is easy to tune the polymorph structure by changing operative conditions [
26,
27]. From the NF retentate considered in this study, it is possible to recover approximately 25.6 kg/m
3 of epsomite at a recovery factor of 98%. Minerals recovery found in this study is relatively higher with respect to experimental studies [
24,
28], but this is according to a higher WRF.
Figure 3 indicates that NiCl
2 can precipitate above a WRF of 97%, but the simulation also shows that the precipitated amount starts to decrease soon after, which specifies that NiCl
2 can re-dissolve. The content of nickel in the brine is much lower with respect to other chloride compounds, such as NaCl, KCl, and MgCl
2. In fact, it can be observed from
Figure 3 that KCl and MgCl
2 start to precipitate at similar WRF. Therefore, the saturation index of NiCl
2 decreases if chloride precipitates as KCl and MgCl
2. On the other hand, if the kinetics favors all three compounds, they might co-precipitate. However, it can be possible to inhibit the precipitation of chlorides other than NiCl
2 by controlling temperature and crystallization kinetics. Control of crystallization kinetics is a difficult task in common crystallization techniques, but due to the easy control of flux through the microporous membrane by changing operative conditions such as flow rate and temperature and hereby the saturation gradient, it is easier to tune/control the kinetics in MCr. The advantage of controlled growth and shape has been observed in several studies on MCr, such as in the crystallization of NaCl, MgSO
4·7H
2O, lysozyme, paracetamol, glutamic acid,
etc. [
16,
17,
24,
26,
27]. Yet, nickel precipitation from NF retentate requires experimental verification.
3.2. Minerals Recovery from RO Brine
The minerals that can be recovered from RO brine have been illustrated in
Figure 4. Most of the minerals precipitating from RO brine are also found to precipitate from NF retentate (
Figure 3). It should be noticed that, the fractionation of bivalent and monovalent ions, due to the NF membrane, changes the route of precipitation. Hence, NaCl is the first salt to crystallize from RO brine. The bivalent ions, such as strontium, barium, and magnesium, are harder to precipitate from RO brine, which correspond to experimental literature data stating that magnesium cannot be recovered from RO brine [
10,
25].
Lithium can precipitate as LiCl at a WRF of 97%. Lithium is of particular interest in the future due to the potential increase of lithium-ion batteries in electrical vehicles. Several studies discuss whether lithium resources on land suffice the increasing demand for lithium ion batteries [
29,
30,
31,
32]. In this logic, recovery from seawater can be an interesting contribution to the conventional mining industry. In fact, if all the disposed RO brine of today was utilized for lithium production, this could subsidize 13% of that obtained from mining [
23]. Recently, lithium has been recovered from single salt LiCl solutions by means of membrane crystallizers [
33]. However, it has been observed that lithium cannot be recovered by the normally used direct contact membrane distillation (DCMD) configuration of MCr (
Figure 1). The reason is the high solubility of LiCl, and therefore the driving force (vapor pressure difference) becomes negative prior to LiCl saturation. Instead of utilizing DCMD, the configuration has been changed to vacuum membrane distillation (VMD), where vacuum is present at the permeate side. In this configuration, the osmotic phenomenon has been removed and lithium can be recovered.
3.2.1. Experiments on Salts Recovery from RO Brine
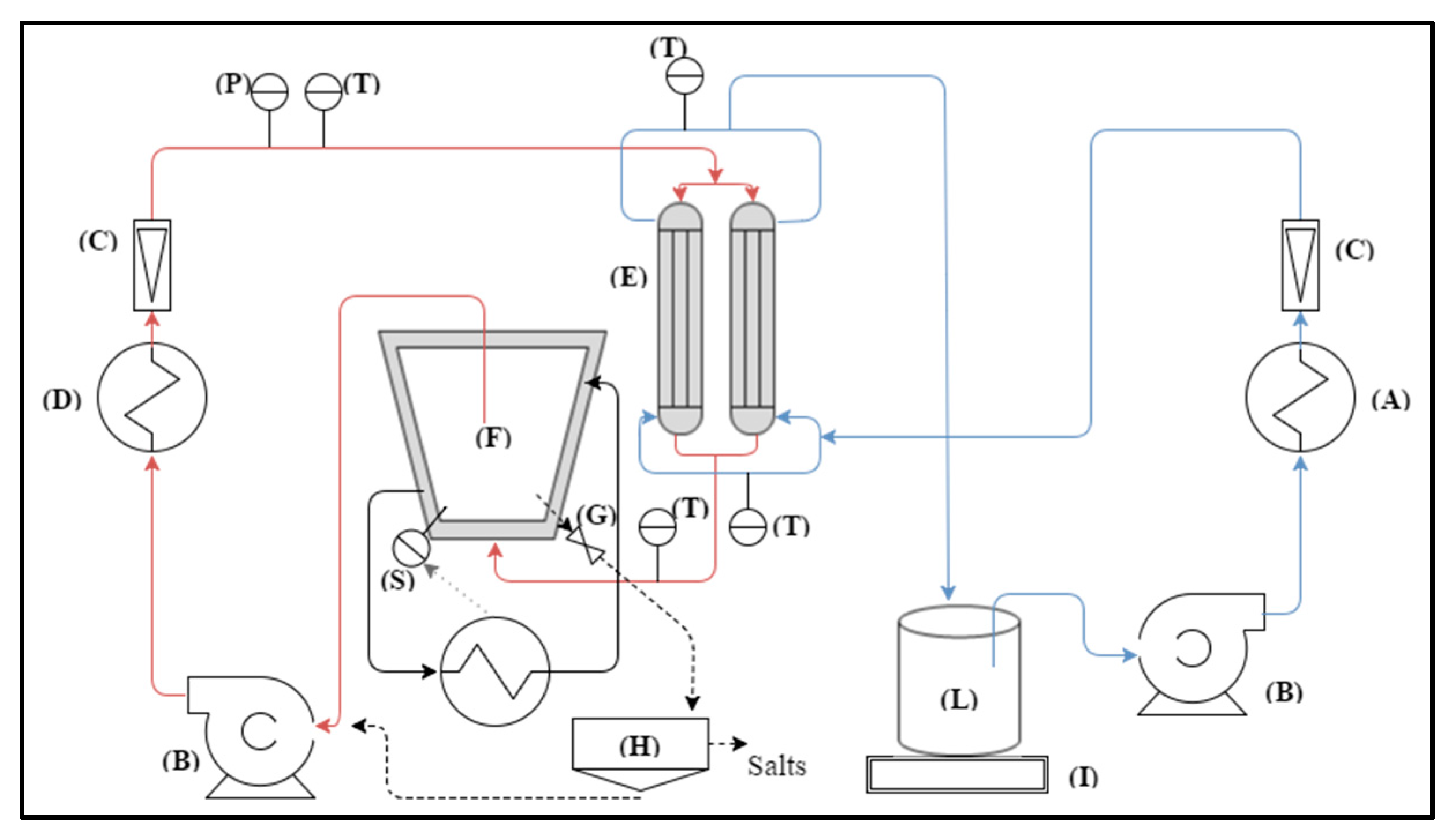
To verify the suitability of the simulation study presented above as a useful tool for a preliminary analysis of the types and amount of salts to be extracted from the RO brine of desalination plants, and to effectively prove the capability of MCr to produce high quality crystals, some experimental measurements were carried out. Experiments were performed by using the experimental set up described elsewhere [
25] and shown in
Figure 5.
The RO brine generated from a desalination plant was fed to the MCr semi pilot scale plant. The plant employed two polypropylene (PP) hollow fibers membrane modules (E) for 0.2 m2 of total membrane area. The plant was supplied with centrifugal pumps (B) and with the necessary tools for the control of the most significant parameters of the system: flow rate and temperature. The estimation of the trans-membrane flux occurred by evaluating weight variations in the distillate tank (L) with the balance (I). The amount of ions in the crystallizing solution and in the distillate were measured through a conductivity meter. Crystals, when formed, were removed from the plant through the crystals separation system (H). The achieved particles were visually examined with an optic microscope in order to determine crystal size, growth rate, and coefficient of variation (CV).
The experimental device operates in such a way that crystallization takes place in the crystallizer tank and not in the membrane module. This can be done with an appropriate control of temperature and flow rate.
Table 3 summarizes the operating conditions used in the crystallization experiments.
The composition of the components present in higher amounts in the RO brine considered in the experiments is reported in
Table 4. In order to well compare the experimental results with the simulations, the latter were repeated, considering the composition reported in
Table 4.
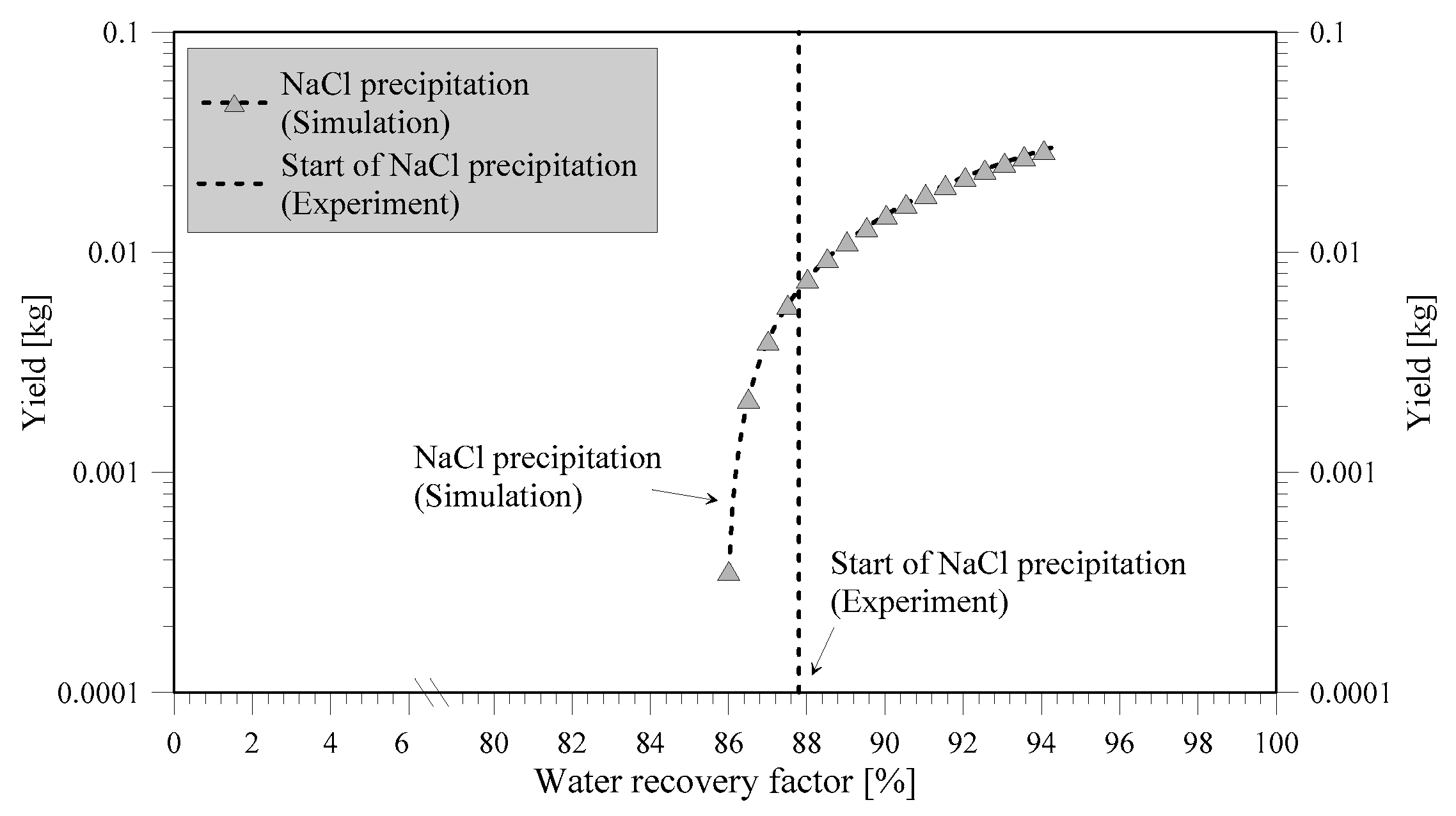
NaCl crystals were obtained at WRF of 87.8%, with CVs less than 38.6%. The produced crystals showed the characteristic cubic block-like form in accordance with the expected geometry of the NaCl crystals when examined visually with optic microscope (
Figure 6). Low CV, like that achieved in MCr, is characteristic of a good product [
25].
Good agreement between experimental tests and model (
Figure 7), with deviations of only 2.09%, can be observed. The reason for the slightly later start of precipitation in the experiments with respect to simulation is due to induction time. The comparison of simulation and experiments confirms the validity of the simulation study done and its suitability for a preliminary screening of the potentialities offered by membrane crystallization in the mining industry.
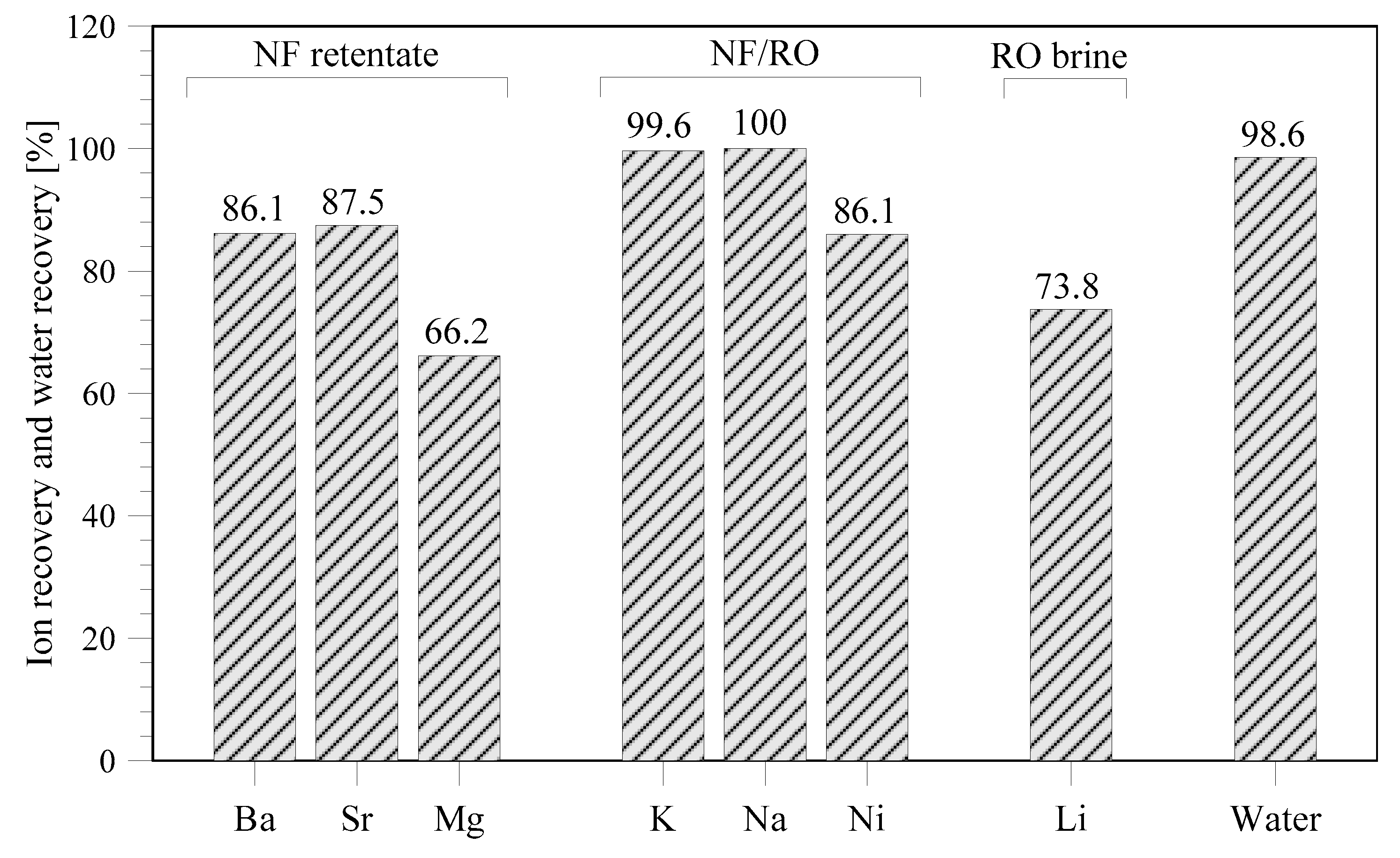
3.3. Recovery from RO Brine
The overall efficiency of an integrated membrane desalination plant with MCr treating the NF retentate and RO brine is reported in
Figure 8. Considering both the present thermodynamic simulation and the experimental results from earlier studies [
17,
23,
25,
28], it is assumed that some ions (including barium, strontium, and magnesium) can be more easily recovered from NF retentate. In case magnesium will also be recovered from RO brine, its recovery will be higher than the 66.2% (
Figure 8). Since sodium can be recovered from NF retentate and RO brine, its removal percentage from seawater is near to 100%. NaCl has been extensively studied for recovery by membrane crystallization from NF retentate and RO brine, and therefore the separation has been experimentally verified (in the previous section and in literature [
17,
23,
25,
28]). Simulations indicate that potassium and nickel can also be recovered from NF and RO brine with a high percentage of recovery. However, these results require experimental validation. The higher amount of monovalent ions in the RO brine with respect to NF retentate indicates that 73.8% of lithium can be recovered. However, it should be noticed that, since the curve of lithium precipitation (
Figure 4) has not reached steady state but is still increasing at the final studied water recovery factor, the amount of lithium recovered will be higher at increasing water recovery.
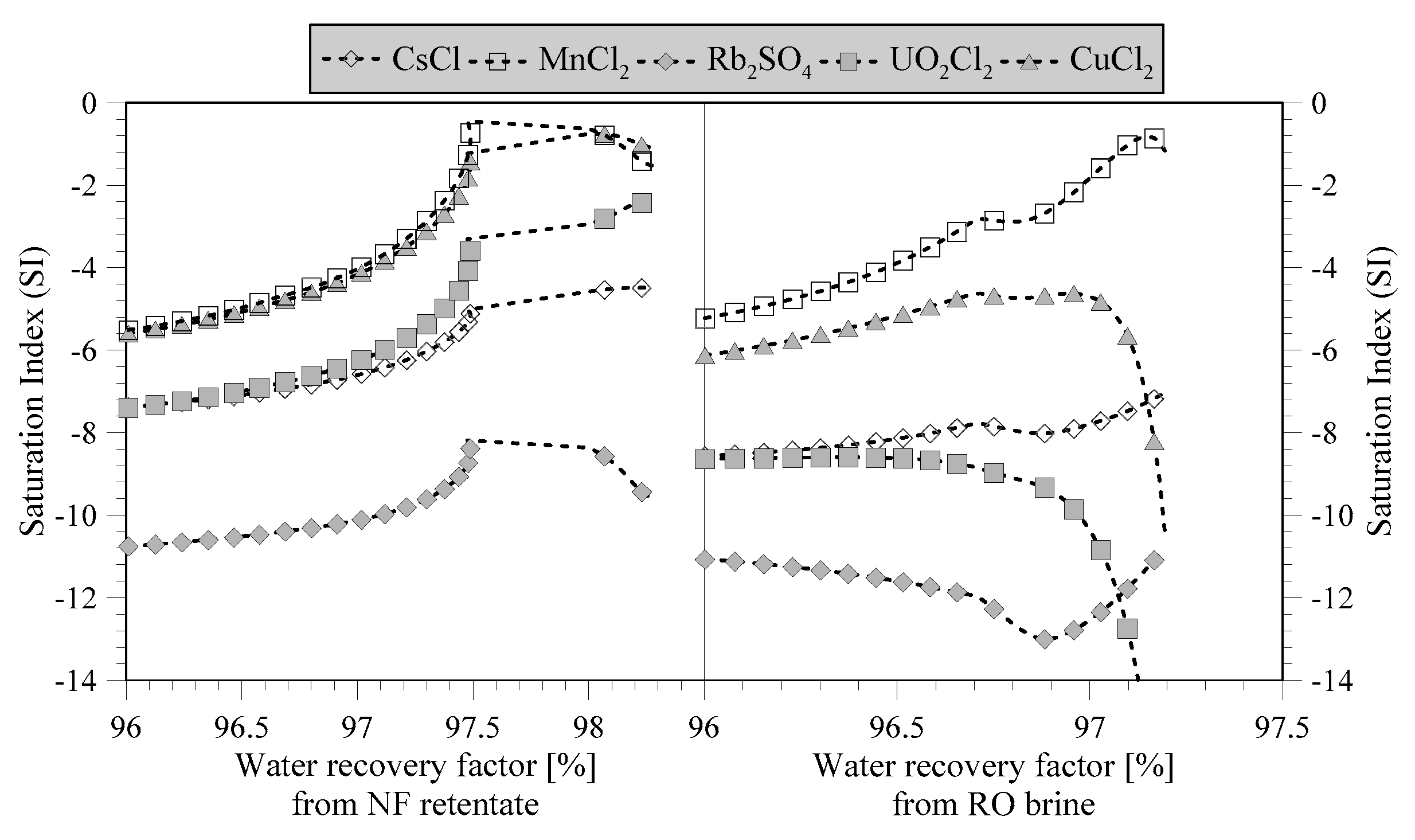
Some of the ions shown in
Table 2 have not been precipitated at an overall WRF of 98.6%. However, by evaluating the saturation index (SI), an improved understanding of the missing precipitation can be achieved. If the saturation index is negative, the minerals remain dissolved, whereas if it becomes positive, the minerals precipitate. For example, manganese and copper are near precipitation from NF retentate (
Figure 9). Due to the higher concentration of chlorides, it is more likely to precipitate MnCl
2 and CuCl
2 with respect to sulfate compounds, for example. However, above a WRF of 97.5%, the SI starts to decrease, which can be related to the precipitation of other chloride compounds (
Figure 3). In the treatment of RO brine, manganese is again close to precipitation. Eventually, membrane crystallization can be integrated with other unit operations (such as supported liquid membranes,
etc.) in order to address the separation and recovery of these minerals from the highly concentrated solutions produced via membrane crystallizer.
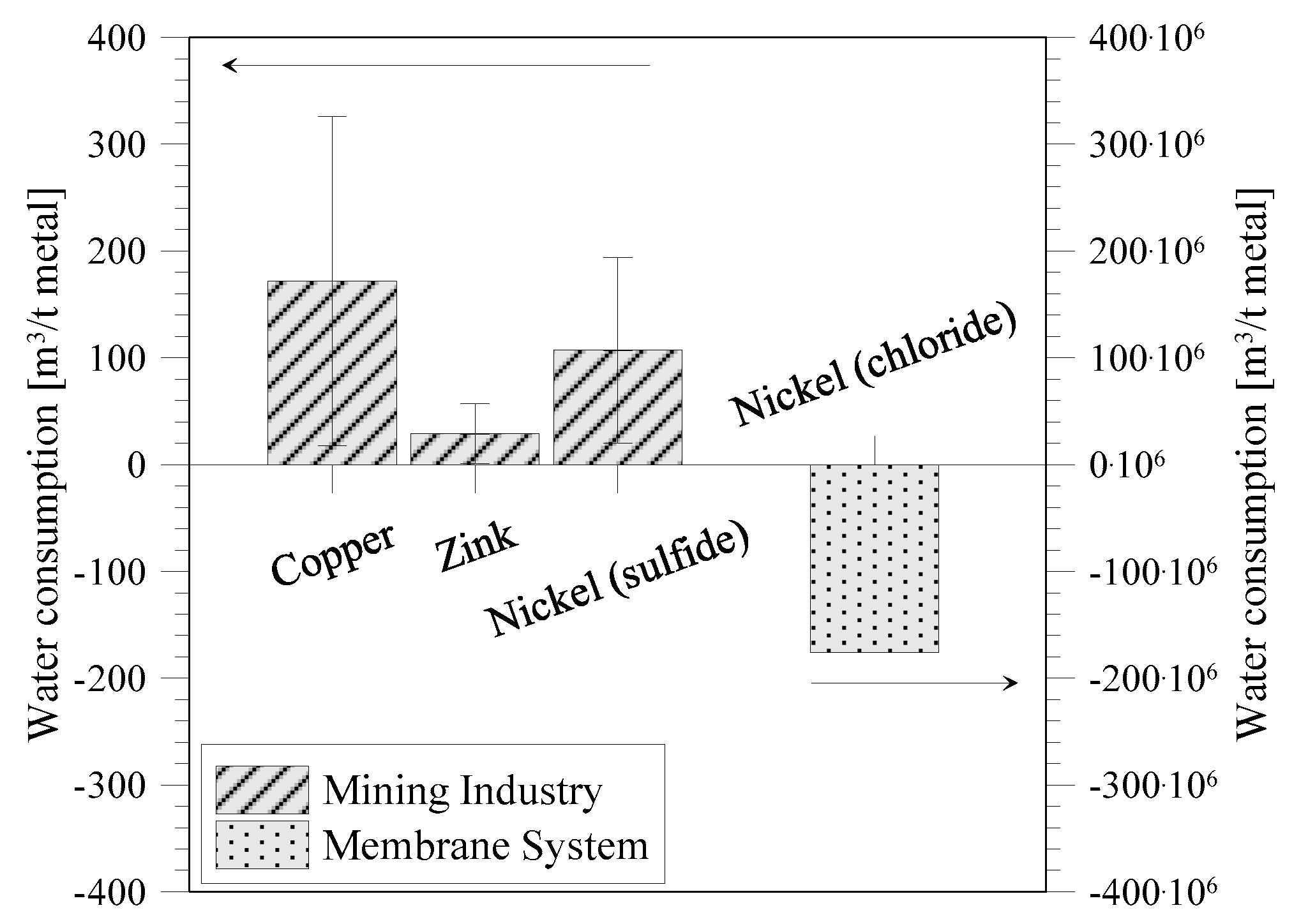
Figure 10 shows the comparison of water consumption for minerals recovery through (1) the conventional mining industry and (2) integrated membrane systems. Water in mining is used in a wide range of activities, such as mineral processing, dust suppression, slurry transport,
etc. [
34]. Mudd [
35] made a preliminary study on embodied water for different materials coming from the mining industry with data available from the industry itself. The available data show that the embodied water is very high and with high standard deviations (as can be observed in the case of nickel). The high standard deviation is associated with the different mining sites and the difference in ore grade, open cut mining or underground mining, mill configuration and design, water quality, project age, climate, and length of slurry pipelines, which all affect the embodied water [
35]. Although the mining industry has made many efforts and improved their water management significantly by closed loop water circuits, the water requirements are still very high. On the contrary, minerals recovery from the sea through integrated membrane systems has the great advantage that water is produced and not consumed. In particular, the amount of water produced in an integrated NF/RO/MCr system is much higher than the amount consumed by the conventional mining industry to recover one ton of nickel. However, high water production can only be obtained because of the low impact of concentration on MCr process performance, and that it is possible to avoid scaling. Depending on stream composition, proper pre-treatment and management of the operative conditions allow the control and minimization of scaling and fouling, to promote crystals formation in the precipitation tank and to avoid crystals growth on membrane surface [
17,
23,
25,
28,
36]. From the precipitation tank, the crystals can be filtered and recovered. This kind of crystals recovery system has been developed in the study by Macedonio
et al. [
36]. Macedonio
et al. [
28] have previously highlighted the economic perspective of introducing MCr in desalination. In fact, due to the potential sale of salts (in this study NaCl and MgSO
4·7H
2O), the overall water production cost can be negative. Depending on if waste heat and energy recovery devices are available or not, the water production costs are found to be in the range of −0.49 to −0.71 $/m
3 [
28]. This emphasizes that MCr can prospectively be a very interesting technology for minerals recovery from desalination brine. Moreover, extending the number of salts to recover might also further enhance the economic benefits.
Minerals recovery from the conventional mining industry and integrated membrane desalination systems could be compared also from an energetic point of view. Direct comparison of energy consumption in mining and integrated membrane systems is out of the scope of this paper. Nevertheless, it has to be mentioned that MCr, due to the low temperature, can utilize waste heat or other low-grade heat sources. This minimizes energy consumption.
Integrated membrane-based desalination processes could have, in future, another advantage in addition to water and minerals production. In fact, many large-scale desalination projects are emphasizing on energy utilization by exploiting the mixing energy of high concentration and low concentration solutions, e.g., seawater and brine. From this perspective, future desalination plants can produce water, minerals, and optimize the energy demand, thus further reducing the actual negative impacts of mining and desalination industry.
4. Conclusions
This study shows the potential of redesigning the mining industry through the adoption of integrated membrane systems. Clearly, today mining cannot be replaced completely with recovery from desalination brine. However, due to many limits of the mining industry also in the future, including water consumption, renewable energy sources, and mineral depletion, the recourse to membrane technology can offer an interesting perspective. By means of MCr, it is possible to recover many different ions from seawater. Thanks to the fractionation of ions by the NF membrane, barium, strontium, and magnesium are easier to recover from NF retentate with respect to RO brine. In this study, only one NF membrane has been considered, but different ion rejection of other membranes can fractionate the ions further. Besides NaCl, it can also be possible to recover KCl and NiCl2 from the NF retentate, but also from RO brine. On the other hand, lithium can only be recovered from RO brine. Considering water consumption in the mining industry, an integrated membrane system will produce a larger amount of water during the production of a similar amount of minerals. For example, the conventional mining industry consumes around 107 m3 of water to recover one ton of nickel (metal). In the integrated membrane system, it is possible to produce 174 × 106·m3 water per ton nickel (metal) obtained (when nickel is recovered both from NF retentate and RO brine), highlighting the advantages of combining mineral and water production. Furthermore, in the future, energy production might also be introduced in desalination, making minerals recovery even more economical and sustainably feasible.