Constructor Graphs as Useful Tools for the Classification of Hydrogen Bonded Solids: The Case Study of the Cationic (Dimethylphosphoryl)methanaminium (dpmaH+) Tecton
Abstract
:1. Introduction
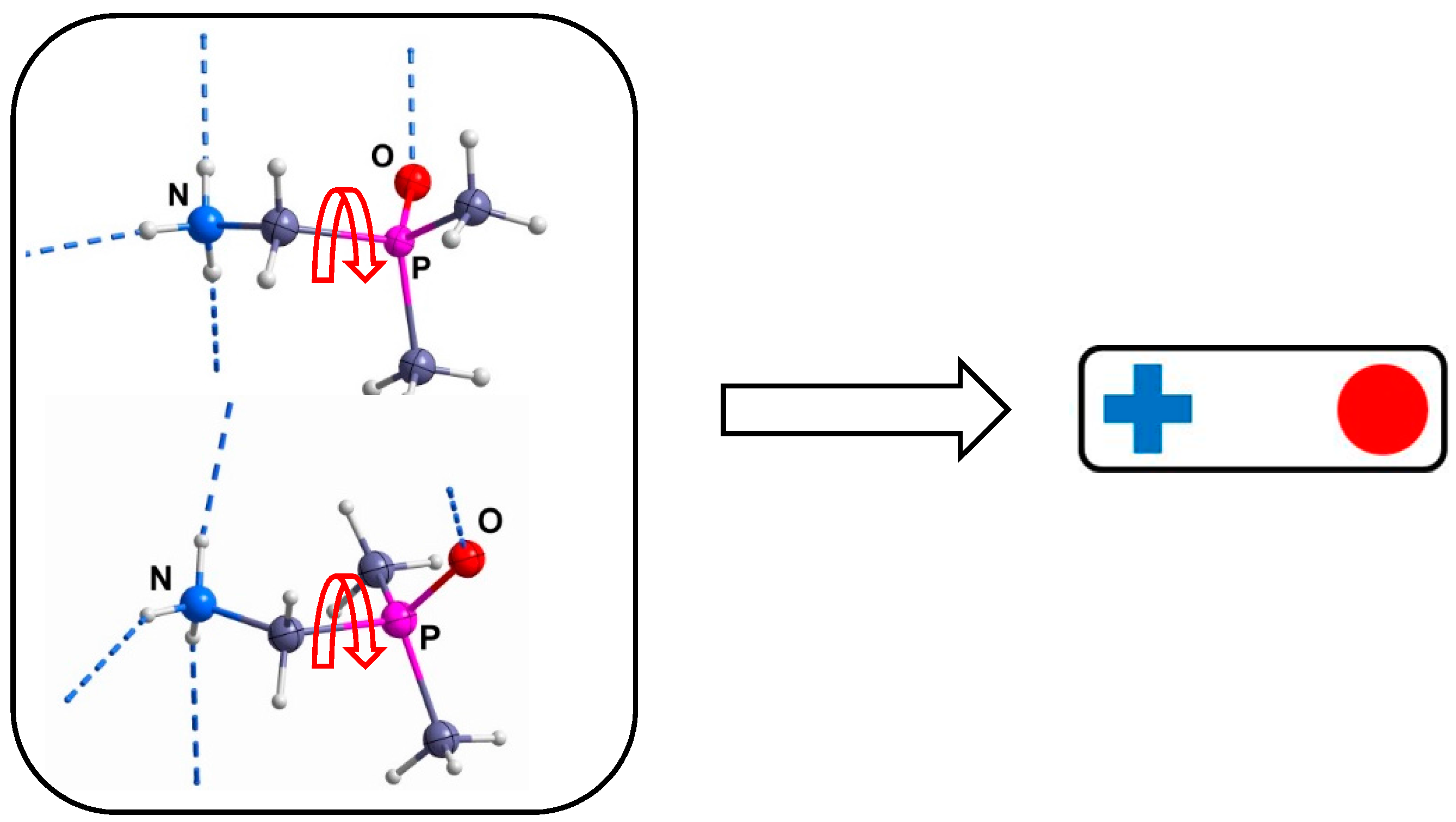
2. Results and Discussion
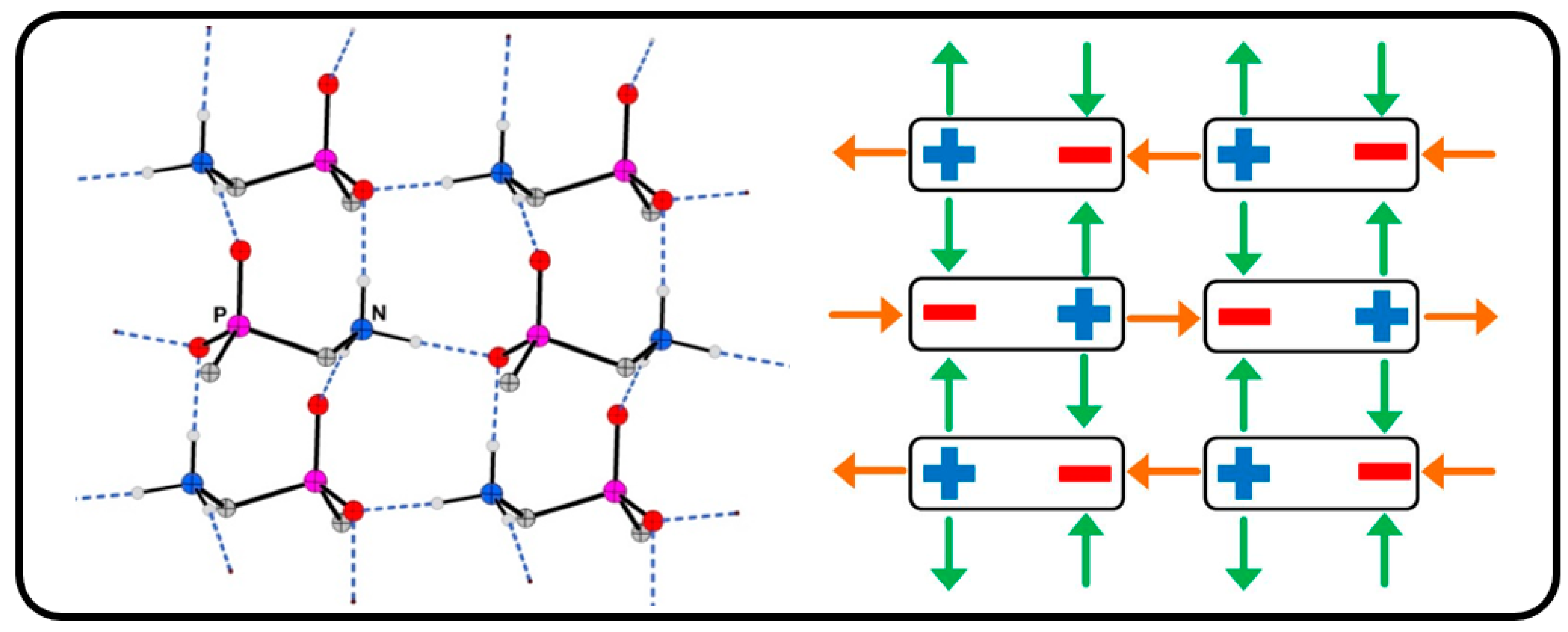
2.1. Salt Structures Based on Head-to-Tail Connected Hydrogen Bonded Polymers: (dpmaH+)n
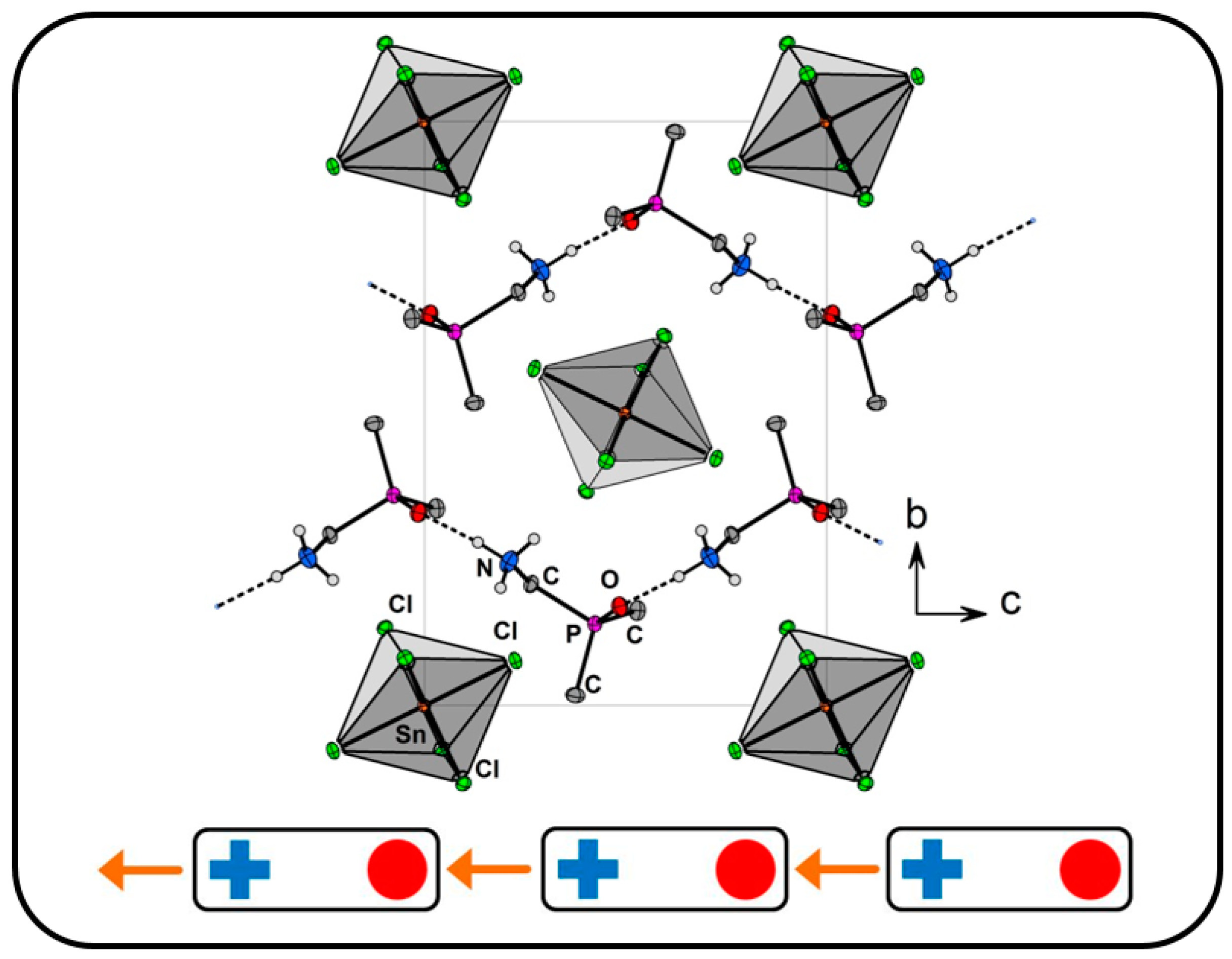
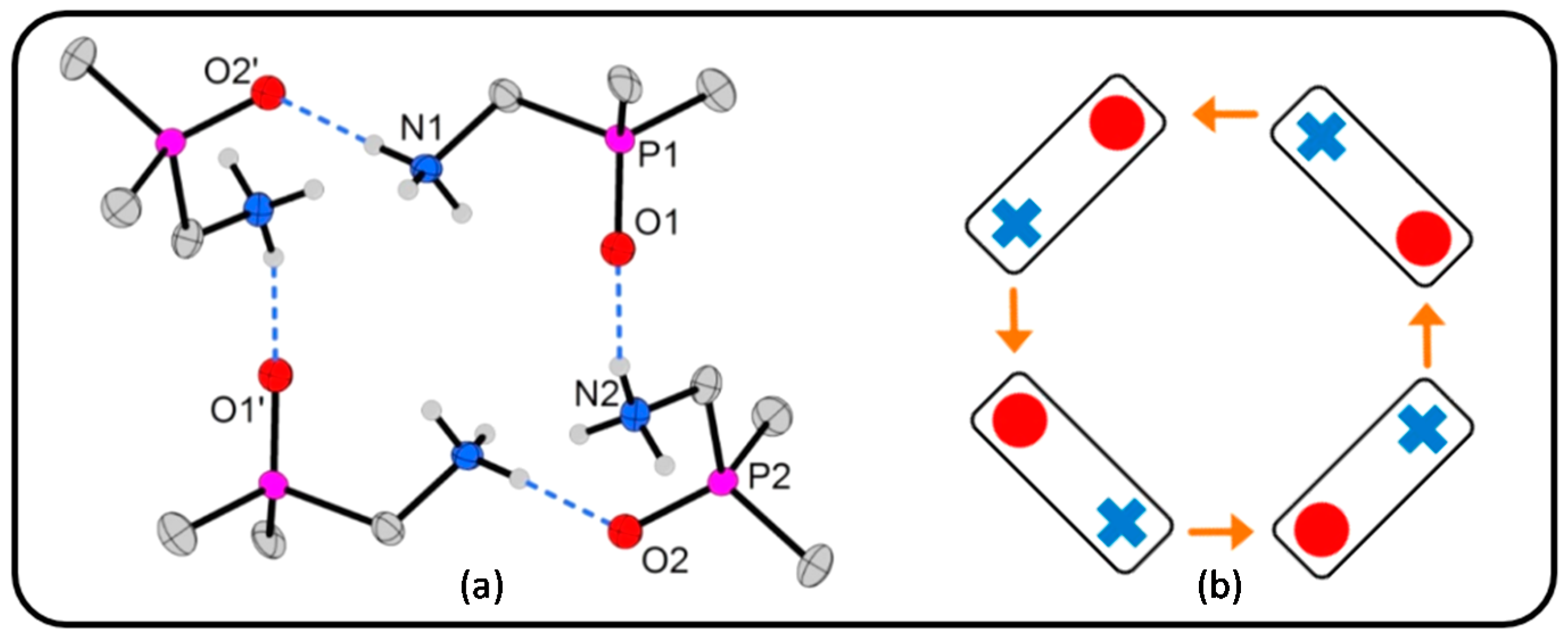
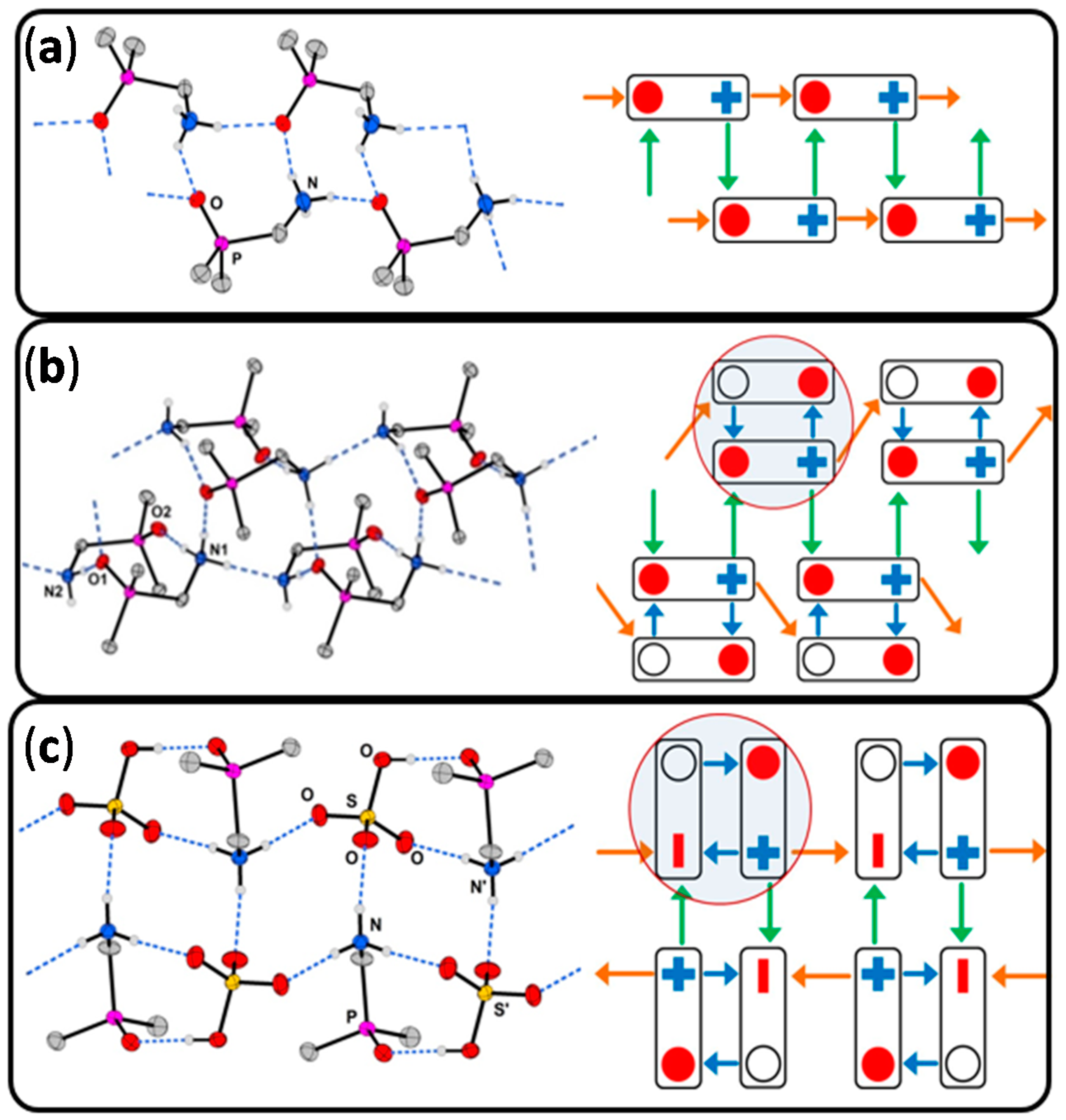
- A comparison of the chain-type and strand-type structures discussed before has been simplified by the use of the modified constructor graphs.
- The intuitive dissection of these complex hydrogen bonded structures into subunits (tectons) that look like as they were taken from a “chemical toolbox”, unquestionable supports the understanding of the individual structures and should support the prediction of structural features.
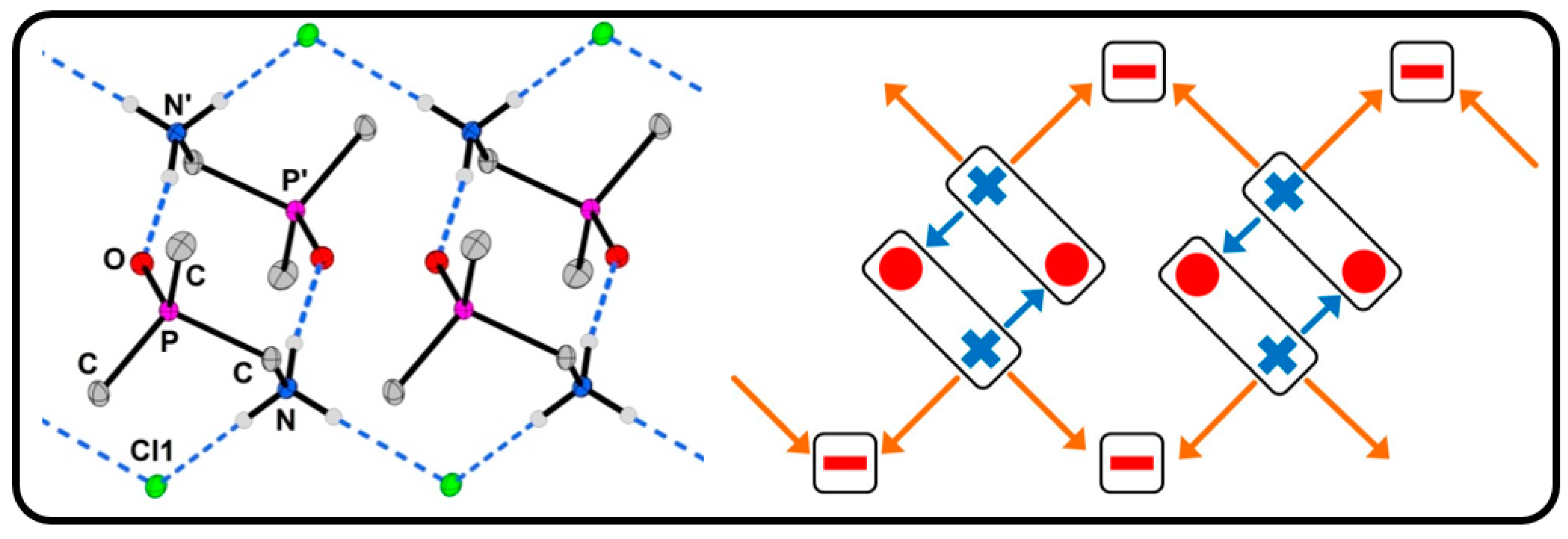
2.2. Salts Based on Hydrogen Bonded (dpmaH+)2 Dimers
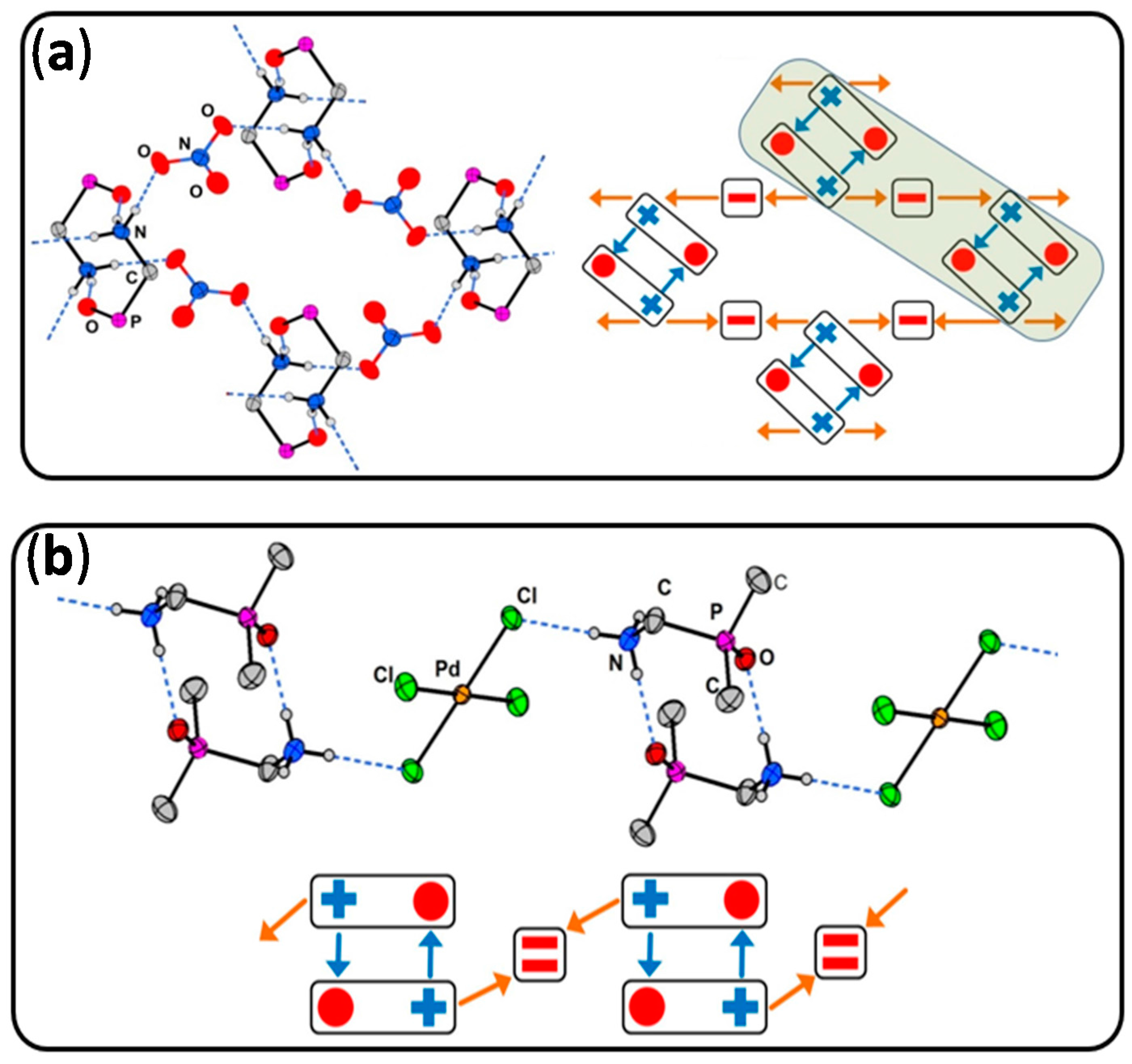
3. Limitations
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Tsvetkov, E.N.; Kron, T.E.; Kabachnik, M.I. Synthesis of chloromethylphosphine oxides. Izv. Akad. Nauk. Ser. Khim. 1980, 3, 669–672. [Google Scholar] [CrossRef]
- Varbanov, S.G.; Agopian, G.; Borisov, G. Polyurethane foams based on dimethylaminomethylphosphine oxides adducts with ethylene and propylene oxides. Eur. Polym. J. 1987, 23, 639–642. [Google Scholar] [CrossRef]
- Kochel, A. Synthesis and magnetic properties of the copper(II) complex derived from dimethylaminomethylphosphine oxide ligand. X-ray crystal structure of DMAO and [Cu(NO3)2(POC3H10N)2]. Inorg. Chim. Acta 2009, 362, 1379–1382. [Google Scholar] [CrossRef]
- Dodoff, N.; Macicek, J.; Angelova, O.; Varbanov, S.G.; Spassovska, N. Chromium(III), Cobalt(II), Nickel(II) and Copper(II) complexes of (dimethylphosphinyl)methanamine. Crystal structure of fac-tris{(dimethylphosphinyl)methanamine-N,O}nickel(II) chloride trihydrate. J. Coord. Chem. 1990, 22, 219–228. [Google Scholar] [CrossRef]
- Trendafilova, N.; Georgieva, I.; Bauer, G.; Varbanov, S.G.; Dodoff, N. IR and Raman study of Pt(II) and Pd(II) complexes of amino substituted phosphine oxides: Normal coordinate analysis. Spectrochim. Acta A 1997, 53, 819–828. [Google Scholar] [CrossRef]
- Vornholt, S.; Herrmann, R.; Reiss, G.J. Crystal structure of the trinuclear complex hexachlorido-1κ3Cl,3κ3Cl-bis(µ2-dimethylphosphorylmethanamine-1:2κ2N:O,3:2κ2N:O)-bis(dimethylphosphorylmethanamine-2κ2N,O)trizinc(II), C12H40Cl6N4O4P4Zn3. Z. Kristallogr. New Cryst. Struct. 2014, 229, 440–442. [Google Scholar] [CrossRef]
- Borisov, G.; Varbanov, S.G.; Venanzi, L.M.; Albinati, A.; Demartin, F. Coordination of dimethyl(aminomethyl)phosphine oxide with Zinc(II), Nickel(II), and Palladium(II). Inorg. Chem. 1994, 33, 5430–5437. [Google Scholar] [CrossRef]
- Richert, M.E.; Helmbrecht, C.; Reiss, G.J. Synthesis, Spectroscopy and Crystal Structure of a New Copper Complex Built up by Cationic (Dimethylphosphoryl)methanaminium Ligands. Mediterr. J. Chem. 2014, 3, 3847–3853. [Google Scholar] [CrossRef]
- Etter, M.C. Encoding and decoding hydrogen-bond patterns of organic compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Grell, J.; Bernstein, J.; Tinhofer, G. Investigation of Hydrogen Bond Patterns: A Review of Mathematical Tools For the Graph Set Approach. Crystallogr. Rev. 2002, 8, 1–56. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.-L. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew. Chem. Int. Ed. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Hursthouse, M.B.; Hughes, D.S.; Gelbrich, T.; Threlfall, T.L. Describing hydrogen-bonded structures; topology graphs, nodal symbols and connectivity tables, exemplified by five polymorphs of each of sulfathiazole and sulfapyridine. Chem. Cent. J. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Knop, O.; Cameron, T.S.; James, M.A.; Falk, M. Alkylammonium hexachlorostannates(IV), (RnNH4−n)2SnCl6: Crystal structure, infrared spectrum, and hydrogen bonding. Can. J. Chem. 1983, 61, 1620–1646. [Google Scholar] [CrossRef]
- Reiss, G.J. The pseudosymmetric structure of bis(diisopropylammonium) hexachloroiridate(IV) and its relationship to potassium hexachloroiridate(III). Acta Crystallogr. E 2002, 58, 47–50. [Google Scholar] [CrossRef]
- Bokach, N.A.; Pakhomova, T.B.; Kukushkin, V.Y.; Haukka, M.; Pombeiro, A.J.L. Hydrolytic metal-mediated coupling of dialkylcyanamides at a Pt(IV) center giving a new family of diimino ligands. Inorg. Chem. 2003, 42, 7560–7568. [Google Scholar] [CrossRef] [PubMed]
- Gillon, A.L.; Lewis, G.R.; Orpen, A.G.; Rotter, S.; Starbuck, J.; Wang, X.-M.; Rodriguez-Martin, Y.; Ruiz-Perez, C. Organic-inorganic hybrid solids: Control of perhalometallate solid state structures. J. Chem. Soc. Dalton Trans. 2000, 21, 3897–3905. [Google Scholar] [CrossRef]
- Coll, R.K.; Fergusson, J.E.; Penfold, B.R.; Rankin, D.A.; Robinson, W.T. The chloro and bromo complexes of iridium(III) and iridium(IV). III: The crystal structures of K3[IrCl6] and Cs2[IrCl5H2O] and interpretation of the N.Q.R. data of chloroiridates(III). Aust. J. Chem. 1987, 40, 2115–2122. [Google Scholar] [CrossRef]
- Reiss, G.J.; Helmbrecht, C. Bis(diisopropylammonium) hexachloridostannate(IV). Acta Crystallogr. E 2012, 68, 1402–1403. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Saraswatula, V.G.; Saha, B.K. Thermal Expansion in Alkane Diacids—Another Property Showing Alternation in an Odd–Even Series. Cryst. Growth Des. 2013, 13, 3651–3656. [Google Scholar] [CrossRef]
- Pienack, N.; Möller, K.; Näther, C.; Bensch, W. (1,4-dabH)2MnSnS4: The first thiostannate with integrated Mn2+ ions in an anionic chain structure. Solid State Sci. 2007, 9, 1110–1114. [Google Scholar] [CrossRef]
- Ratajczak-Sitarz, M.; Katrusiak, A.; Dega-Szafran, Z.; Stefański, G. Systematics in NH+···N-Bonded Monosalts of 4,4′-Bipyridine with Mineral Acids. Cryst. Growth Des. 2013, 13, 4378–4384. [Google Scholar] [CrossRef]
- Olejniczak, A.; Katrusiak, A.; Szafrański, M. Ten Polymorphs of NH+···N Hydrogen-Bonded 1,4-Diazabicyclo[2.2.2]octane Complexes: Supramolecular Origin of Giant Anisotropic Dielectric Response in Polymorph V. Cryst. Growth Des. 2010, 10, 3537–3546. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar]
- Buhl, D.; Gün, H.; Jablonka, A.; Reiss, G.J. Synthesis, Structure and Spectroscopy of Two Structurally Related Hydrogen Bonded Compounds in the dpma/HClO4 System; dpma = (dimethylphosphoryl)methanamine. Crystals 2013, 3, 350–362. [Google Scholar] [CrossRef]
- Reiss, G.J. (Dimethylphosphoryl)methanaminium iodide—(dimethylphosphoryl)methanamine (1:1). Acta Crystallogr. E 2013, 69, 1253–1254. [Google Scholar] [CrossRef] [PubMed]
- Czaikovsky, D.; Davidow, A.; Reiss, G.J. Crystal structure of (dimethylphosphoryl)methanaminium hydrogensulfate. Z. Kristallogr. New Cryst. Struct. 2014, 229, 29–30. [Google Scholar]
- Glowiak, T.; Sawka-Dobrowolska, W. The crystal and molecular structure of α-aminomethylmethylphosphinic acid. Acta Crystallogr. B 1977, 33, 1522–1525. [Google Scholar] [CrossRef]
- Reiss, G.J.; Jörgens, S. (Dimethylphosphoryl)methanaminium chloride. Acta Crystallogr. E 2012, 68, 2899–2900. [Google Scholar] [CrossRef] [PubMed]
- Bianga, C.M.; Eggeling, J.; Reiss, G.J. (Dimethylphosphoryl)methanaminium nitrate. Acta Crystallogr. E 2013, 69, 1639–1640. [Google Scholar] [CrossRef] [PubMed]
- Kahrovic, E.; Orioli, P.; Bruni, B.; di Vaira, M.; Messori, L. Crystallographic evidence for decomposition of dimethylformamide in the presence of ruthenium(III) chloride. Inorg. Chim. Acta 2003, 355, 420–423. [Google Scholar] [CrossRef]
- Xu, W.; Lin, J.-L. Tetrakis(dimethylammonium) hexachlorotungstate(III) chloride. Acta Crystallogr. E 2007, 63, 767–769. [Google Scholar] [CrossRef]
- Reiss, G.J. Bis((dimethylphosphoryl)methanaminium) tetrachloridopalladate(II). Acta Crystallogr. E 2013, 69, 614–615. [Google Scholar] [CrossRef] [PubMed]
- Guzei, I.A.; Spencer, L.C.; Ainooson, M.K.; Darkwa, J. Constructor graph description of the hydrogen-bonding supramolecular assembly in two ionic compounds: 2-(pyrazol-1-yl)ethylammonium chloride and diaquadichloridobis(2-hydroxyethylammonium)cobalt(II) dichloride. Acta Crystallogr. C 2010, 66, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Guzei, I.A.; Keter, F.K.; Spencer, L.C.; Darkwa, J. Constructor graph description of hydrogen bonding in a supramolecular assembly of (3,5-dimethyl-1H-pyrazol-4-ylmethyl)isopropylammonium chloride monohydrate. Acta Crystallogr. C 2007, 63, 481–483. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reiss, G.J. Constructor Graphs as Useful Tools for the Classification of Hydrogen Bonded Solids: The Case Study of the Cationic (Dimethylphosphoryl)methanaminium (dpmaH+) Tecton. Crystals 2016, 6, 6. https://doi.org/10.3390/cryst6010006
Reiss GJ. Constructor Graphs as Useful Tools for the Classification of Hydrogen Bonded Solids: The Case Study of the Cationic (Dimethylphosphoryl)methanaminium (dpmaH+) Tecton. Crystals. 2016; 6(1):6. https://doi.org/10.3390/cryst6010006
Chicago/Turabian StyleReiss, Guido J. 2016. "Constructor Graphs as Useful Tools for the Classification of Hydrogen Bonded Solids: The Case Study of the Cationic (Dimethylphosphoryl)methanaminium (dpmaH+) Tecton" Crystals 6, no. 1: 6. https://doi.org/10.3390/cryst6010006
APA StyleReiss, G. J. (2016). Constructor Graphs as Useful Tools for the Classification of Hydrogen Bonded Solids: The Case Study of the Cationic (Dimethylphosphoryl)methanaminium (dpmaH+) Tecton. Crystals, 6(1), 6. https://doi.org/10.3390/cryst6010006




