Incidental Polymorphism, Non-Isomorphic and Isomorphic Substitution in Calcium-Valine Coordination Polymers
Abstract
:1. Introduction
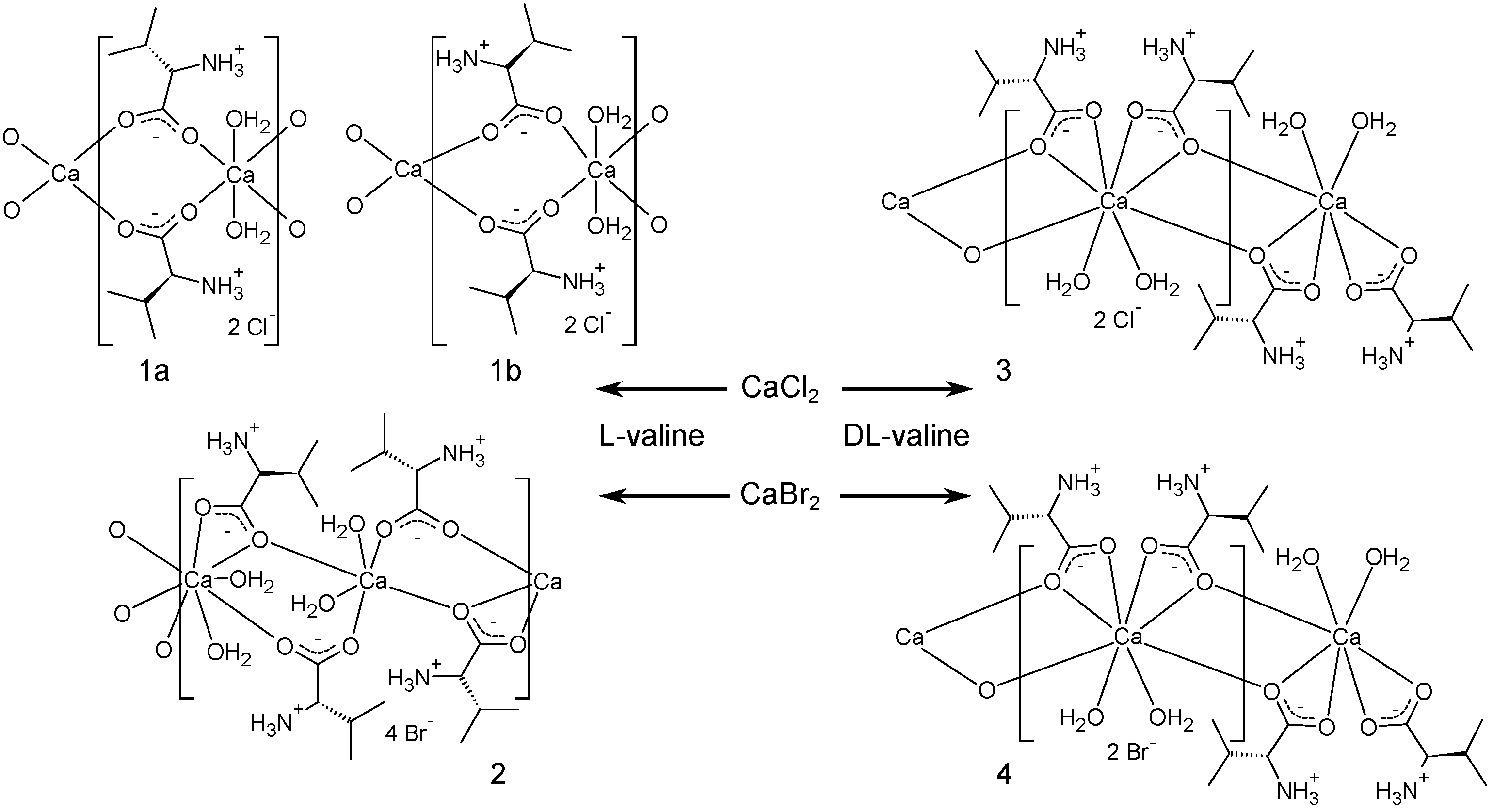
2. Results and Discussion
| Compound/CCDC | 1a/1061342 | 1b/1061343 | 2/1061344 | 3/1061345 | 4/1061346 |
|---|---|---|---|---|---|
| Number [13] | |||||
| Crystal Data | |||||
| Chemical formula | C10H26CaN2O6Cl2 | C10H26CaN2O6Cl2 | C10H26CaN2O6Br2 | C10H26CaN2O6Cl2 | C10H26CaN2O6Br2 |
| Mr | 381.31 | 381.31 | 470.23 | 381.31 | 470.23 |
| Crystal system | Triclinic | Orthorhombic | Monoclinic | Orthorhombic | Orthorhombic |
| Space group | P1 | P21212 | C2 | Pcca | Pcca |
| a (Å) | 4.9856 (18) | 17.420 (3) | 13.879 (8) | 24.180 (7) | 24.657 (7) |
| b (Å) | 9.542 (4) | 20.468 (4) | 20.293 (12) | 9.861 (3) | 10.010 (3) |
| c (Å) | 10.303 (4) | 5.0236 (9) | 7.841 (5) | 7.666 (2) | 7.710 (2) |
| α (°) | 77.743 (6) | ||||
| β (°) | 76.975 (7) | 117.282 (10) | |||
| γ (°) | 83.919 (7) | ||||
| V (Å3) | 465.8 (3) | 1791.2 (6) | 1963 (2) | 1827.9 (9) | 1903.0 (9) |
| Z | 1 | 4 | 4 | 4 | 4 |
| μ (mm−1) | 0.65 | 0.67 | 4.41 | 0.66 | 4.55 |
| Crystal size (mm) | 0.21 × 0.08 × 0.03 | 0.25 × 0.05 × 0.05 | 0.25 × 0.05 × 0.04 | 0.34 × 0.12 × 0.11 | 0.19 × 0.05 × 0.03 |
| Data collection | |||||
| Tmin, Tmax | 0.600, 0.746 | 0.678, 0.745 | 0.550, 0.745 | 0.630, 0.745 | 0.524, 0.745 |
| Rint | 0.043 | 0.113 | 0.08 | 0.096 | 0.145 |
| (sinθ/λ)max (Å−1) | 0.719 | 0.626 | 0.613 | 0.61 | 0.61 |
| Measured, independent and observed [I > 2σ(I)] reflections | 7257, 5245, 4554 | 21760, 3677, 3113 | 11174, 3754, 3239 | 18905, 1737, 1460 | 17812, 1815, 1325 |
| Refinement | |||||
| R[F2 > 2σ (F2)], wR(F2) | 0.071, 0.174 | 0.047, 0.104 | 0.046, 0.095 | 0.034, 0.086 | 0.043, 0.095 |
| S | 1.03 | 1.03 | 1.00 | 1.09 | 1.05 |
| No. of reflections | 5245 | 3677 | 3754 | 1737 | 1815 |
| No. of parameters | 224 | 234 | 225 | 113 | 113 |
| No. of restraints | 24 | 21 | 23 | 4 | 4 |
| Δρmax, Δρmin (e Å−3) | 0.76, −0.58 | 0.44, −0.48 | 0.63, −0.43 | 0.33, −0.24 | 0.60, −0.51 |
| Absolute structure parameter | 0.02 (8) | −0.02 (4) | −0.006 (18) | − | − |
| Number of quotients | 1774 | 1108 | 1316 | − | − |
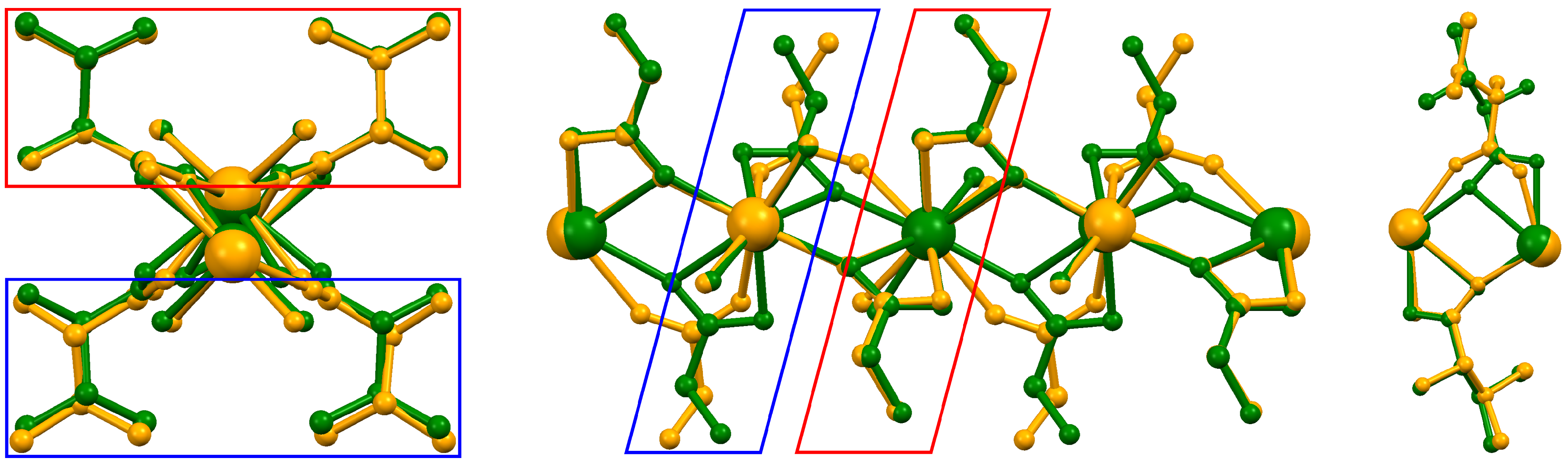
2.1. Structural Details of 1a and 1b

| Compound | 1a | 1b | 2 | 3 | 4 | ||
|---|---|---|---|---|---|---|---|
| Ca1–O1 (Å) | 2.390(5) | 2.386(3) | Ca1–O1 (Å) | 2.660(7) | Ca1–O1 (Å) | 2.3484(16) | 2.357(4) |
| Ca1–O2 a (Å) | 2.319(6) | 2.272(3) | Ca1–O3 b (Å) | 2.373(7) | Ca1–O1 c (Å) | 2.6276(17) | 2.639(4) |
| Ca1–O3 (Å) | 2.386(6) | 2.275(3) | Ca1–O5 (Å) | 2.521(7) | Ca1–O2 c (Å) | 2.4403(17) | 2.440(4) |
| Ca1–O4 a (Å) | 2.300(5) | 2.400(3) | Ca1–O6 (Å) | 2.385(8) | Ca1–O3 (Å) | 2.3802(17) | 2.376(4) |
| Ca1–O5 (Å) | 2.360(5) | 2.366(4) | Ca2–O1 (Å) | 2.279(6) | |||
| Ca1–O6 (Å) | 2.363(5) | 2.331(3) | Ca2–O2 (Å) | 2.320(8) | |||
| Ca2–O4 (Å) | 2.372(4) |
| 1a | D· · · A (Å) | D–H (Å) | H· · · A (Å) | DH· · · A (°) | 1b | D· · · A (Å) | D–H (Å) | H· · · A (Å) | DH· · · A (°) |
|---|---|---|---|---|---|---|---|---|---|
| N1–H1A· · · O1 a | 2.814(8) | 0.93(8) | 1.93(8) | 159(7) | N1–H1C· · · O1 f | 2.826(5) | 0.93(4) | 1.92(4) | 166(3) |
| N1–H1C· · · Cl1 b | 3.137(5) | 0.93(7) | 2.25(6) | 161(7) | N1–H1A· · · Cl1A | 3.023(5) | 0.95(3) | 2.10(4) | 164(4) |
| N1–H1B· · · Cl2 c | 3.167(6) | 0.93(4) | 2.26(5) | 164(6) | N1–H1B· · · Cl2 | 3.184(4) | 0.93(3) | 2.27(3) | 167(3) |
| N2–H2A· · · O3 a | 2.813(7) | 0.92(5) | 1.90(6) | 172(7) | N2–H2C· · · O4 c | 2.870(5) | 0.93(4) | 1.94(4) | 174(4) |
| N2–H2B· · · Cl1 | 3.156(6) | 0.92(8) | 2.26(6) | 158(7) | N2–H2A· · · Cl1B g | 2.935(6) | 0.94(3) | 2.05(4) | 174(4) |
| N2–H2C· · · Cl2 | 3.145(6) | 0.92(8) | 2.27(8) | 158(7) | N2–H2B· · · Cl2 h | 3.197(4) | 0.94(4) | 2.30(4) | 158(4) |
| O5–H5E· · · Cl2 c | 3.247(6) | 0.82(6) | 2.47(6) | 157(8) | O5–H5D· · · Cl1A h | 3.182(5) | 0.82(5) | 2.40(5) | 160(5) |
| O5–H5D· · · Cl2 e | 3.124(7) | 0.83(9) | 2.32(9) | 164(8) | O5–H5E· · · Cl1B | 3.037(5) | 0.82(4) | 2.28(5) | 152(5) |
| O6–H6B· · · Cl1 | 3.210(6) | 0.83(9) | 2.42(8) | 159(8) | O6–H6A· · · Cl2 i | 3.170(4) | 0.82(4) | 2.37(4) | 167(4) |
| O6–H6A· · · Cl1 d | 3.136(5) | 0.83(6) | 2.32(6) | 166(7) | O6–H6B· · · Cl2 j | 3.127(4) | 0.83(4) | 2.32(4) | 168(4) |

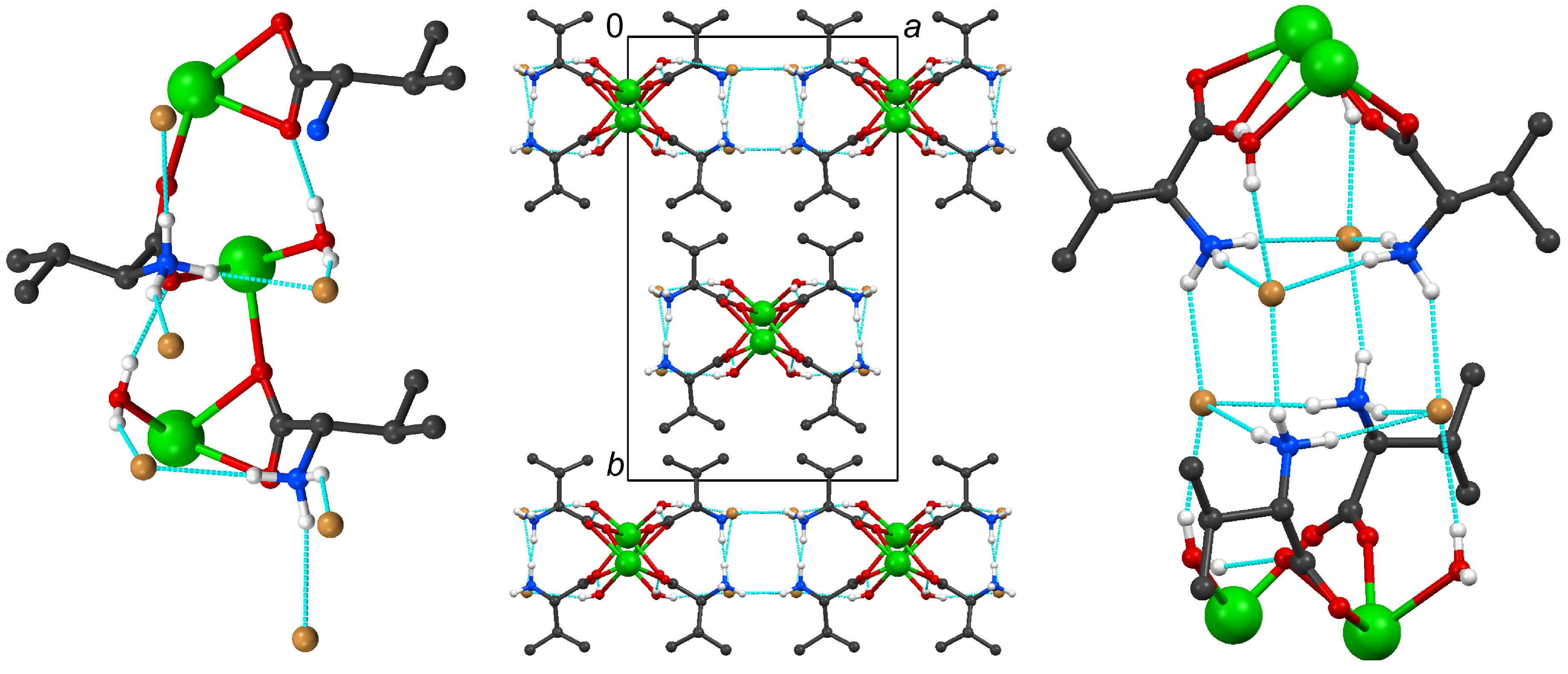
2.2. Structural Details of 2
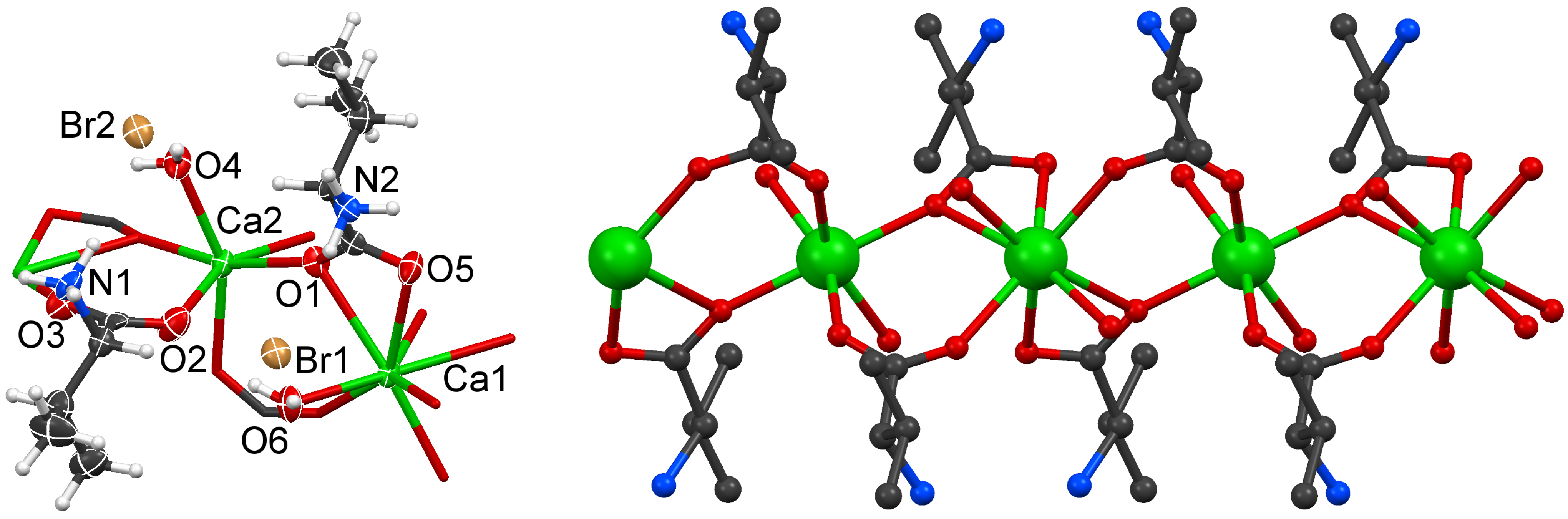
| 2 | D· · · A (Å) | D–H (Å) | H· · · A (Å) | DH· · · A (°) |
|---|---|---|---|---|
| N1–H2· · · Br2 | 3.356(8) | 0.95(5) | 2.42(4) | 168(7) |
| N1–H1· · · Br1 a | 3.361(9) | 0.93(6) | 2.46(7) | 163(7) |
| N1–H3· · · Br1 b | 3.331(7) | 0.94(6) | 2.48(6) | 150(7) |
| N2–H14· · · Br1 | 3.353(8) | 0.93(4) | 2.42(4) | 173(7) |
| N2–H16· · · Br2 c | 3.449(9) | 0.93(7) | 2.53(6) | 169(7) |
| N2–H15· · · Br2 b | 3.302(8) | 0.95(9) | 2.51(6) | 141(7) |
| O4–H13· · · O5 a | 2.755(10) | 0.83(8) | 1.96(9) | 161(10) |
| O4–H12· · · Br2 | 3.242(7) | 0.82(5) | 2.45(6) | 161(7) |
| O6–H26· · · O2 | 2.878(10) | 0.82(8) | 2.07(9) | 170(9) |
| O6–H25· · · Br1 | 3.334(6) | 0.83(5) | 2.56(5) | 157(8) |
2.3. The Isomorphous Structures 3 and 4
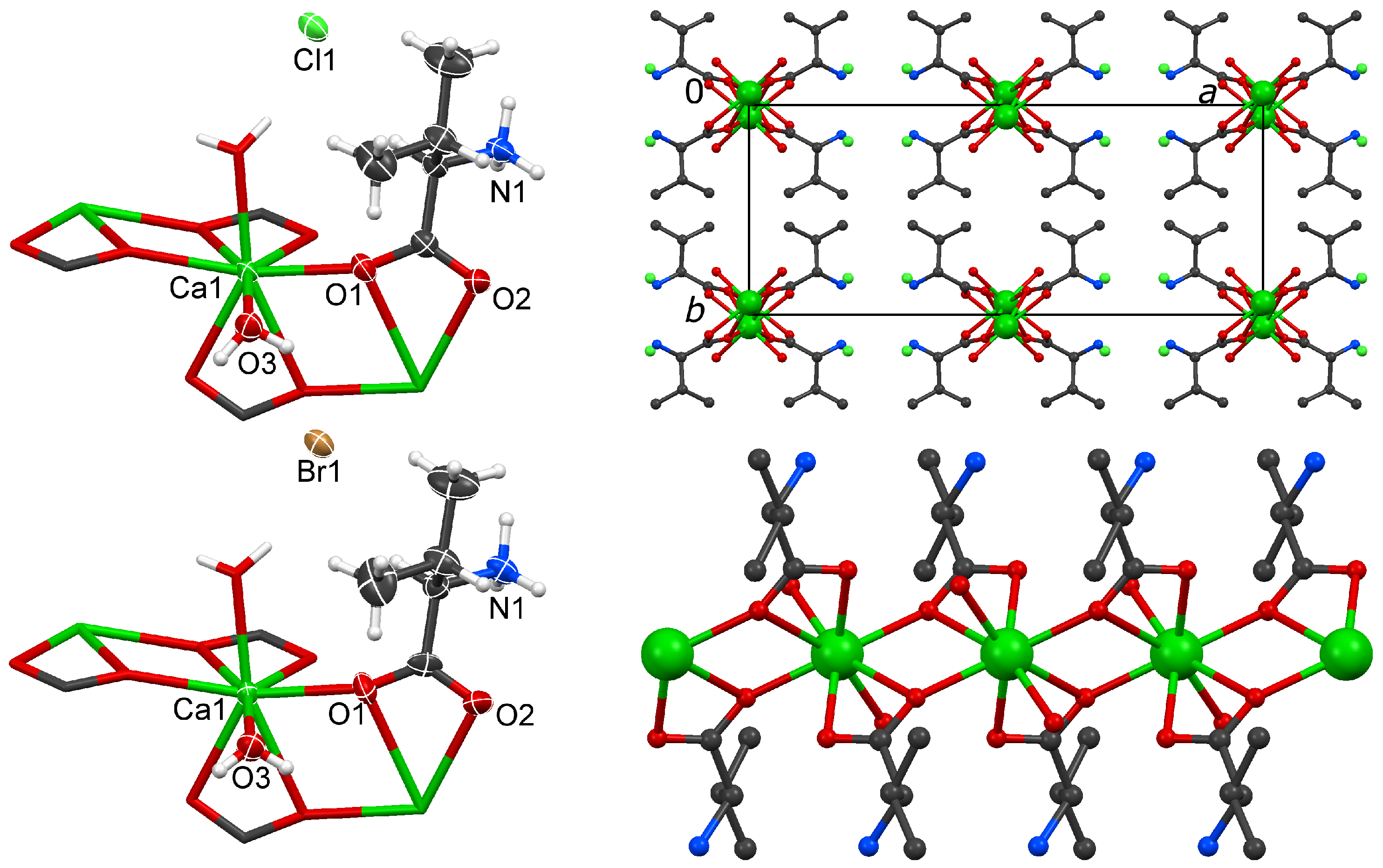
| 3 | D· · · A (Å) | D–H (Å) | H· · · A (Å) | DH· · · A (°) | 4 | D· · · A (Å) | D–H (Å) | H· · · A (Å) | DH· · · A (°) |
|---|---|---|---|---|---|---|---|---|---|
| N1–H2· · · Cl1 a | 3.288(3) | 0.90(2) | 2.41(2) | 163(2) | N1–H2· · · Br1 a | 3.405(5) | 0.97(3) | 2.51(3) | 154(3) |
| N1–H3· · · Cl1 b | 3.185(2) | 0.92(2) | 2.27(2) | 178(2) | N1–H3· · · Br1 b | 3.312(4) | 0.96(3) | 2.36(3) | 173(3) |
| N1–H1· · · Cl1 c | 3.166(2) | 0.90(2) | 2.33(2) | 154(2) | N1–H1· · · Br1 c | 3.296(4) | 0.96(4) | 2.42(4) | 151(4) |
| O3–H12· · · O2 d | 2.775(2) | 0.83(2) | 2.00(2) | 157(2) | O3–H12· · · O2 d | 2.776(6) | 0.79(4) | 2.02(4) | 161(5) |
| O3–H13· · · Cl1 e | 3.130(2) | 0.84(2) | 2.32(2) | 162(2) | O3–H13· · · Br1 e | 3.257(4) | 0.79(4) | 2.53(4) | 152(4) |
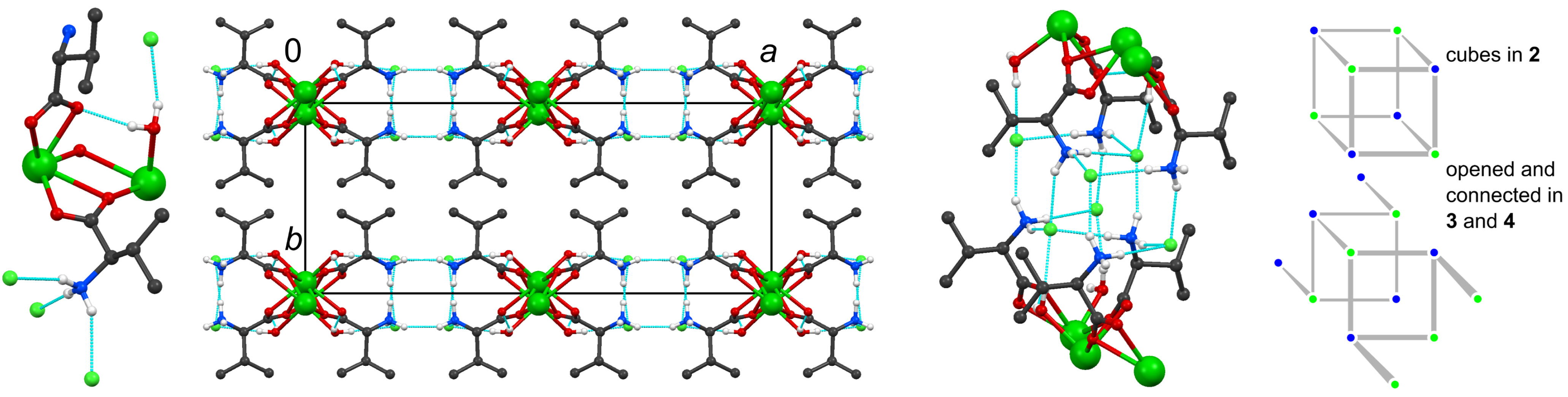
3. Conclusions
4. Experimental Section
4.1. Instrumentation
4.2. Syntheses

4.3. Data Collection and Refinement
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Lamberts, K.; Englert, U. Structures from MnX2 and proline: Isomorphous racemic compounds and a series of chiral non-isomorphous chain polymers. Acta Crystallogr. 2012, 68, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Lamberts, K.; Möller, A.; Englert, U. Enantiopure and racemic alanine as bridging ligands in Ca and Mn chain polymers. Acta Crystallogr. 2014, 70, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Lamberts, K.; Porsche, S.; Hentschel, B.; Kuhlen, T.; Englert, U. An unusual linker and an unexpected node: CaCl2 dumbbells linked by proline to from square lattice networks. CrystEngComm 2014, 16, 3305–3311. [Google Scholar] [CrossRef]
- Lamberts, K.; Şerb, M.-D.; Englert, U. One- and two-dimensional polymers from proline and calcium bromide. Acta Crystallogr. 2015, C71, 311–317. [Google Scholar]
- Fleck, M.; Petrosyan, A.M. Salts of Amino Acids, Crystallization, Structure and Properties; Springer: Berlin, Germany, 2014. [Google Scholar]
- Ye, B.-H.; Tong, M.-L.; Chen, X.-M. Metal-organic molecular architectures with 2,2’-bipyridyl-like and carboxylate ligands. Coord. Chem. Rev. 2005, 249, 545–565. [Google Scholar] [CrossRef]
- Einspahr, H.; Bugg, C. E. The Geometry of Calcium-Carboxylate Interactions in Crystalline Complexes. Acta Crystallogr. 1981, 37, 1044–1052. [Google Scholar] [CrossRef]
- Kaufman Katz, A.; Glusker, J.P.; Beebe, S.A.; Bock, C.W. Calcium Ion Coordination: A Comparison with That of Beryllium, Magnesium, and Zinc. J. Am. Chem. Soc. 1996, 118, 5752–5763. [Google Scholar] [CrossRef]
- Burgess, K.M.N.; Xu, Y.; Leclerc, M.C.; Bryce, D.L. Alkaline-Earth Metal Carboxylates Characterized by 43Ca and 87Sr Solid-State NMR: Impact of Metal-Amine Bonding. Inorg. Chem. 2014, 53, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Glowiak, T.; Ciunik, Z. The crystal structure of bis(DL-valine) calcium chloride dihydrate and bis(DL-2-aminobutyric acid) calcium chloride dihydrate. Bull. Acad. Pol. Sci. Ser. Sci. Chim. 1978, 26, 43–51. [Google Scholar]
- Batsanov, A.S.; Howard, J.A.K.; Wu, N.; Yang, Z.; Marder, T.B. An irreversible phase transition in 1-n-butylindeno[2,1-c]pyran-3,9-dione. Acta Crystallogr. 2012, 68, o413–o416. [Google Scholar] [CrossRef] [PubMed]
- Fábry, J.; Fridrichová, M.; Dušek, M.; Fejfarová, K.; Krupková, R. Two polymorphs of bis(2-carbamoylguanidinium) fluorophosphonate dihydrate. Acta Crystallogr. 2012, 68, o71–o75. [Google Scholar] [CrossRef] [PubMed]
- CCDC 1061342–1061346 Contain the Supplementary Crystallographic Data for This Paper. These Data can be Obtained Free of Charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44-1223-336-033; E-Mail: deposit@ccdc.cam.ac.uk).
- Parsons, S.; Flack, H. Precise absolute-structure determination in light-atom crystals. Acta Crystallogr. 2004, 60. [Google Scholar] [CrossRef]
- SAINT-Plus and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2008.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamberts, K.; Englert, U. Incidental Polymorphism, Non-Isomorphic and Isomorphic Substitution in Calcium-Valine Coordination Polymers. Crystals 2015, 5, 261-272. https://doi.org/10.3390/cryst5020261
Lamberts K, Englert U. Incidental Polymorphism, Non-Isomorphic and Isomorphic Substitution in Calcium-Valine Coordination Polymers. Crystals. 2015; 5(2):261-272. https://doi.org/10.3390/cryst5020261
Chicago/Turabian StyleLamberts, Kevin, and Ulli Englert. 2015. "Incidental Polymorphism, Non-Isomorphic and Isomorphic Substitution in Calcium-Valine Coordination Polymers" Crystals 5, no. 2: 261-272. https://doi.org/10.3390/cryst5020261
APA StyleLamberts, K., & Englert, U. (2015). Incidental Polymorphism, Non-Isomorphic and Isomorphic Substitution in Calcium-Valine Coordination Polymers. Crystals, 5(2), 261-272. https://doi.org/10.3390/cryst5020261







