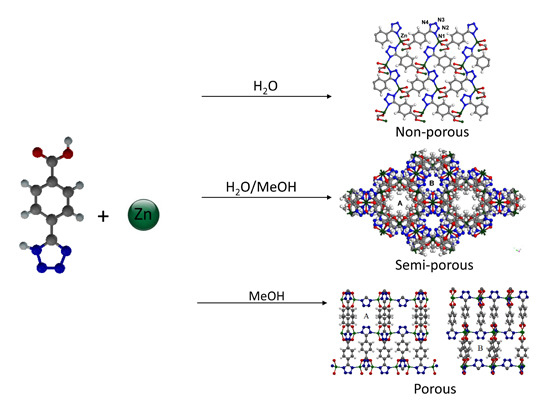
Synthesis of Framework Isomer MOFs Containing Zinc and 4-Tetrazolyl Benzenecarboxylic Acid via a Structure Directing Solvothermal Approach
Abstract
:1. Introduction
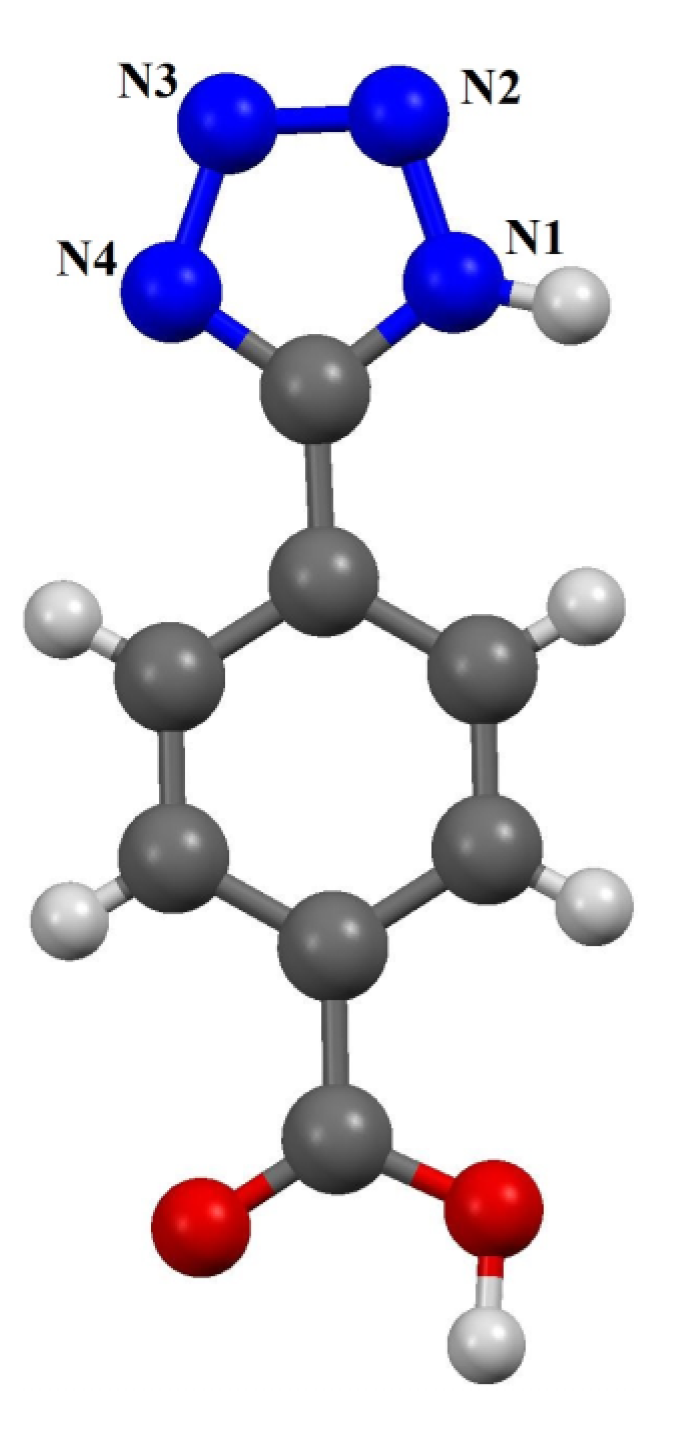
2. Results and Discussion
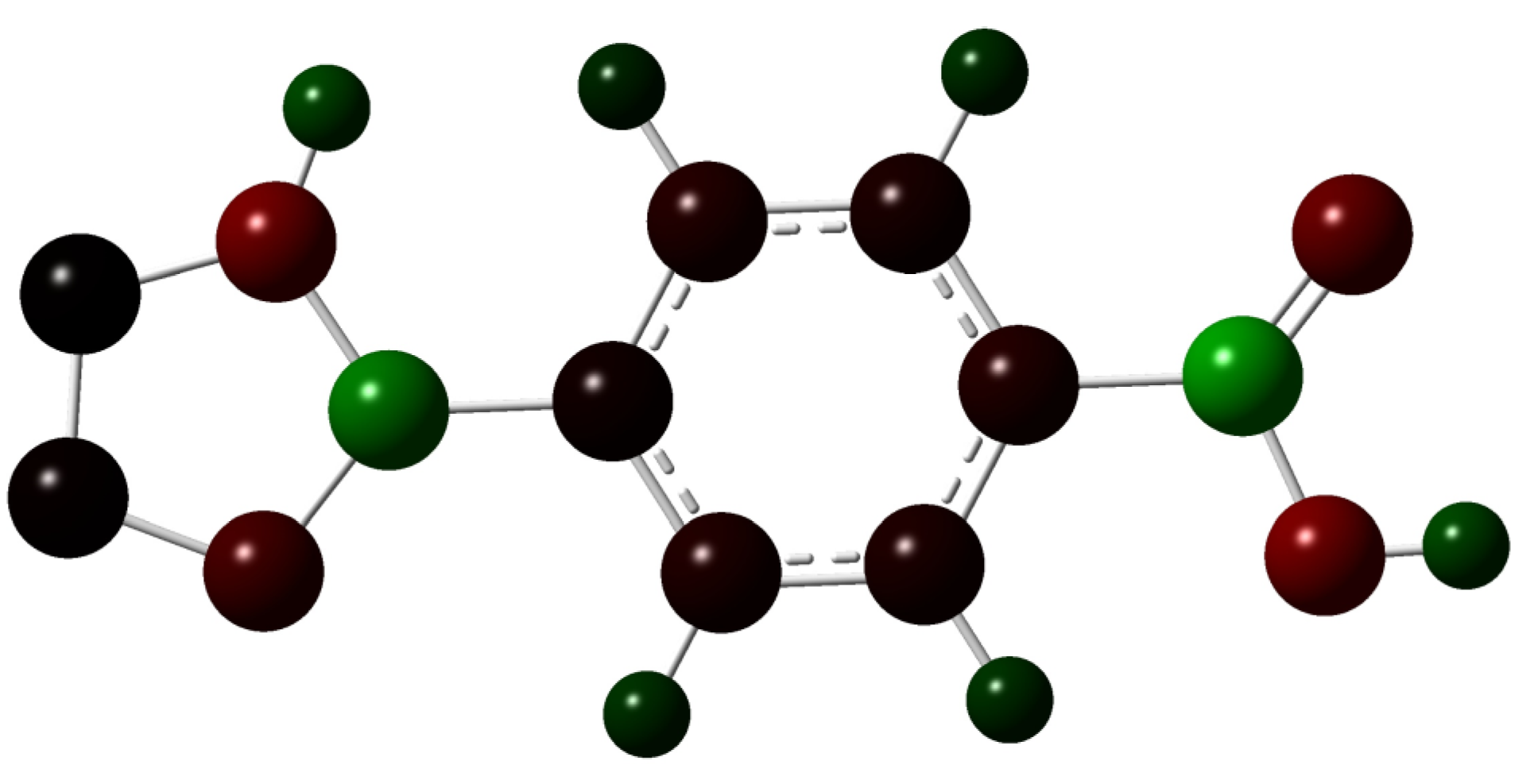
2.1. An “Imidazolate”-Like Coordination Mode for Tetrazolate in TBC
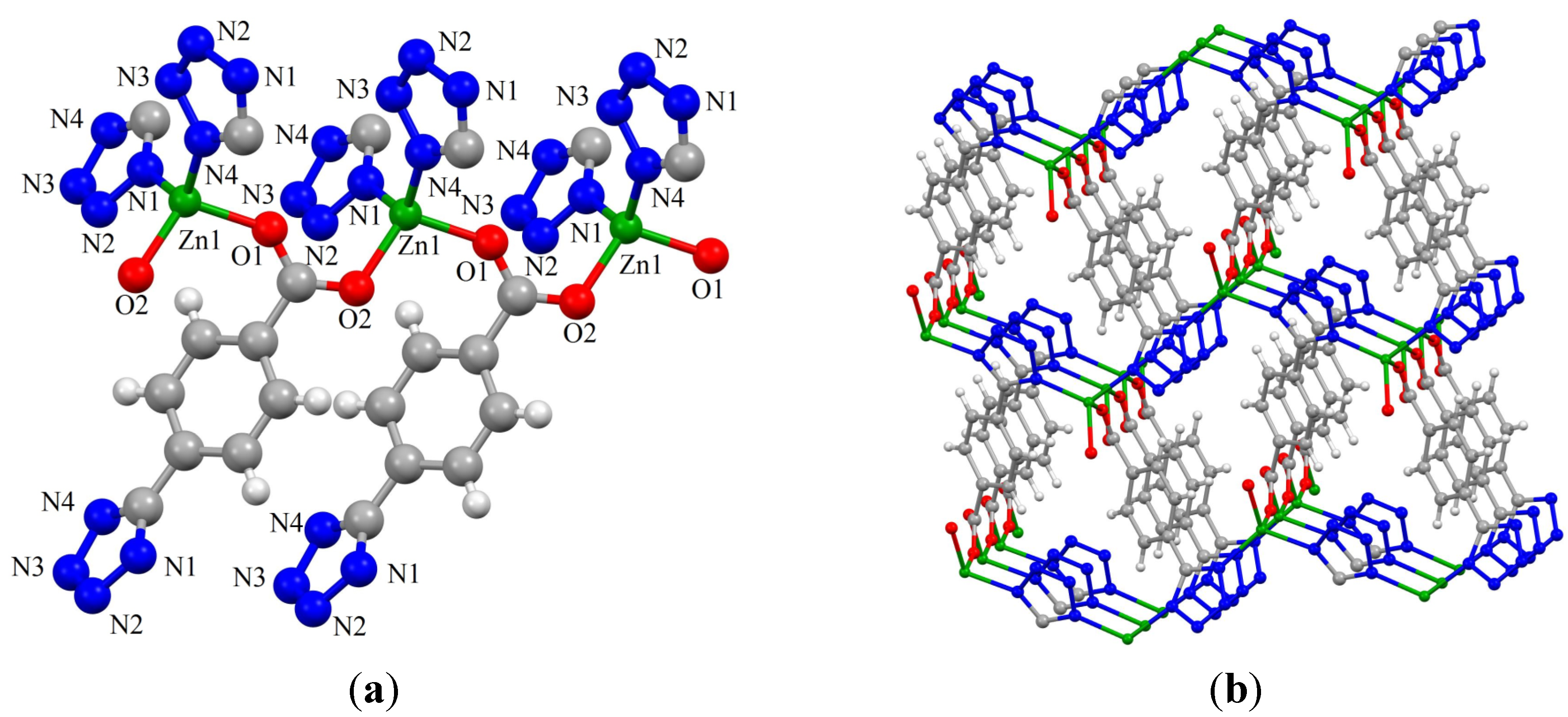
2.2. Synthesis and Characterization of ZnTBC, 2, Zn2(TBC)2(H2O), 3 and Zn2(TBC)2, 4
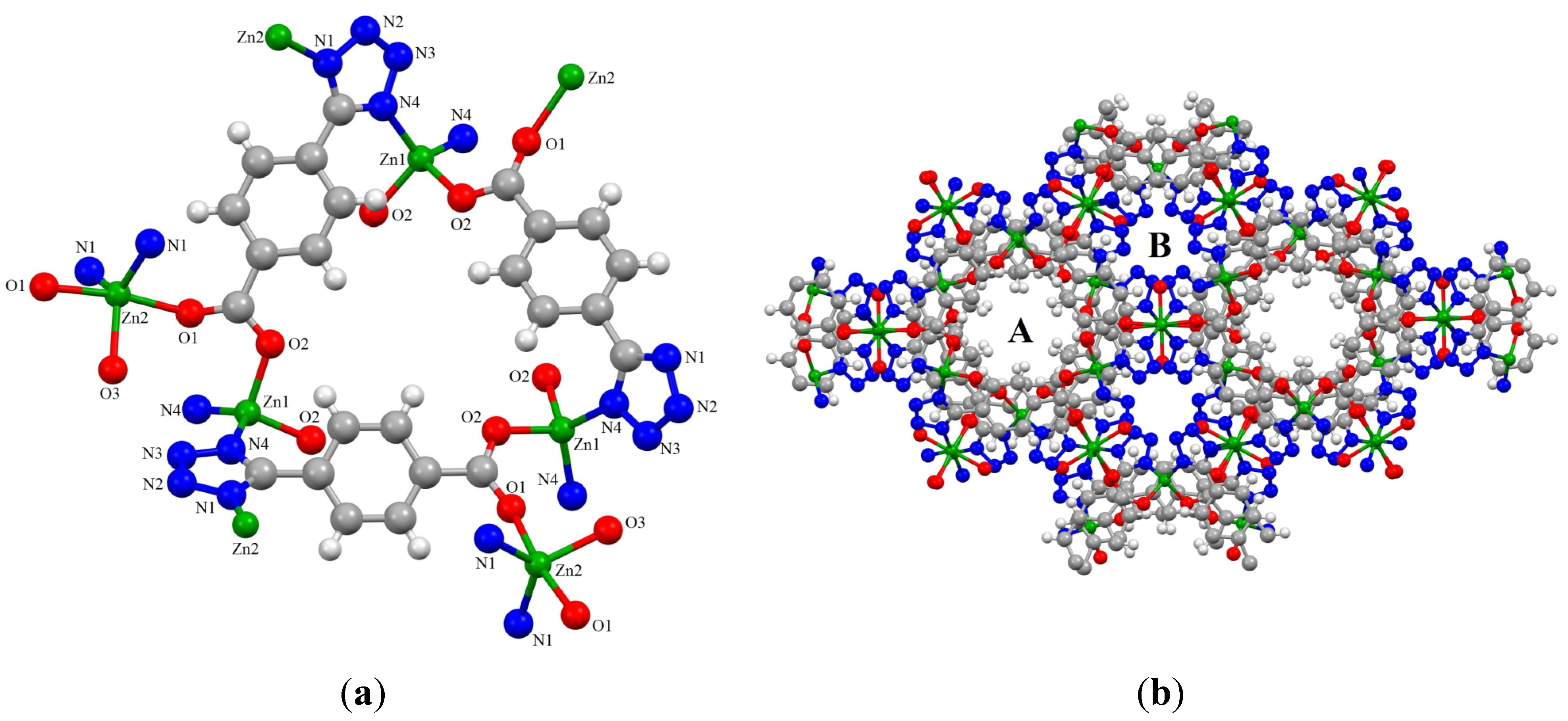
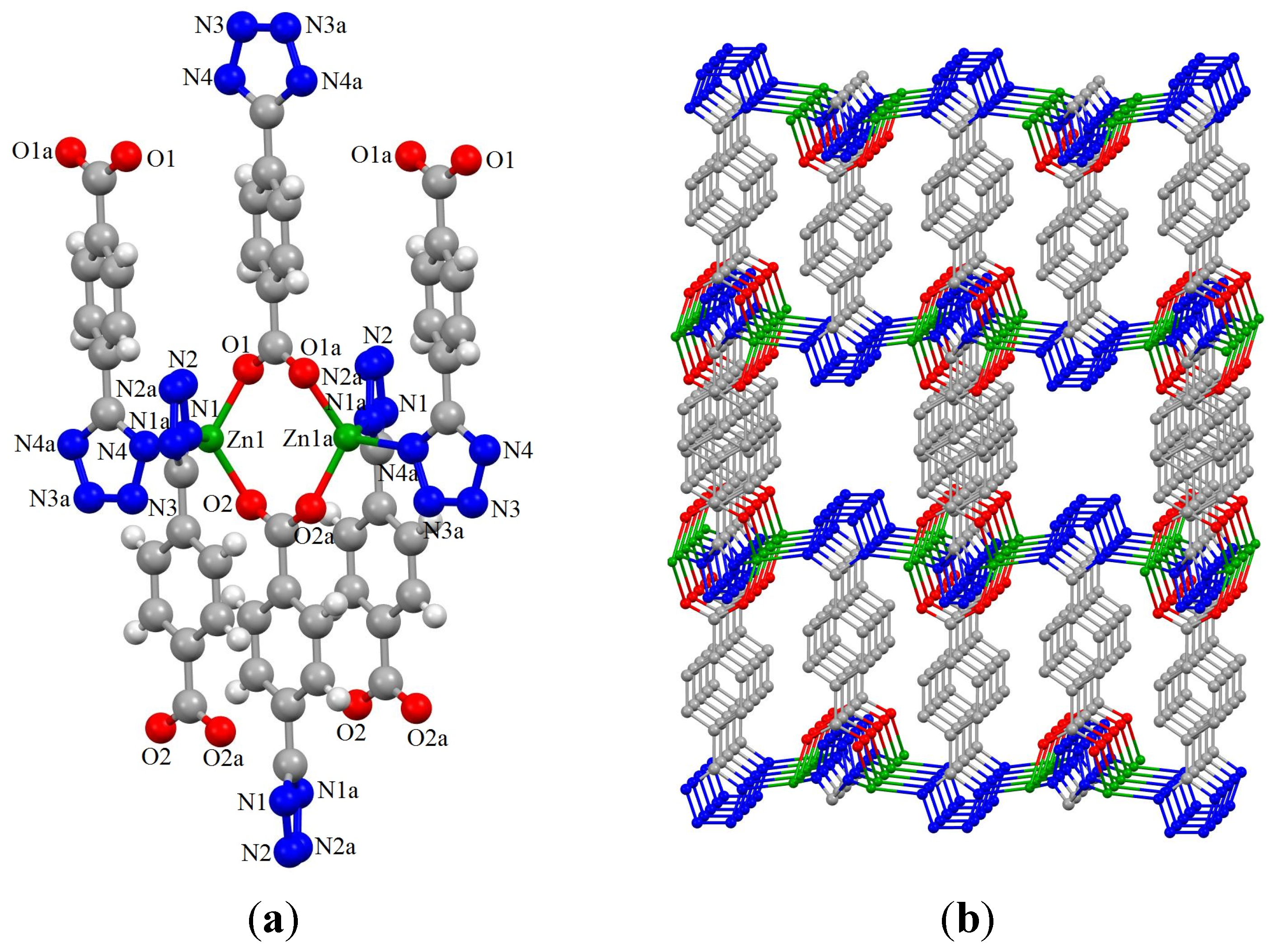
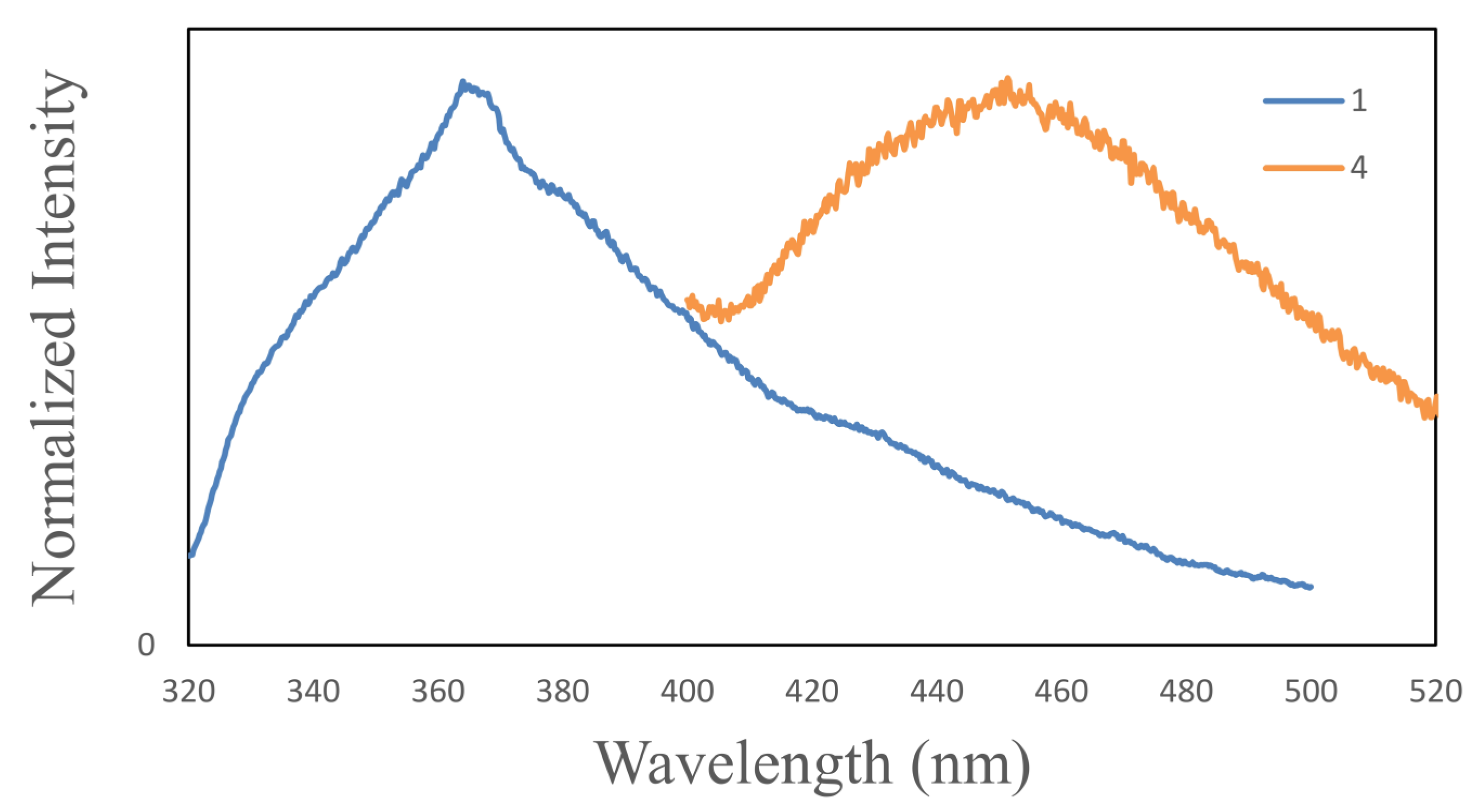
3. Experimental
3.1. Materials and General Methods
3.2. Synthesis of 4-Tetrazolyl Benzenecarboxylic Acid
3.3. Crystallization of 4-Tetrazolyl Benzenecarboxylic Acid, 1
3.4. Synthesis of Zn2(TBC)2, 2
3.5. Synthesis of Zn2(TBC)2(H2O), 3
3.6. Synthesis of [Zn2(TBC)2(MeOH)]0.5(H2O), 4
3.7. Computational Calculations
3.8. Crystal Structure Determination
4. Conclusions
| Complex # | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Empirical formula | C8H6N4O2·H2O | C8H4N4O2Zn | C16H9N8O5Zn2 | C8H6N4O2Zn |
| Formula weight (g/mol) | 208.18 | 253.52 | 524.05 | 255.54 |
| Crystal system | Monoclinic | Monoclinic | Hexagonal | Monoclinic |
| Space group | P21/n | Pc | R-3c | C2/c |
| a (Å) | 4.8650 (16) | 4.8086 (11) | 26.1408 (13) | 10.242 (4) |
| b (Å) | 5.1946 (17) | 8.2211 (19) | 26.1408 (13) | 34.136 (12) |
| c (Å) | 34.110 (11) | 10.578 (3) | 13.3926 (14) | 6.824 (2) |
| α (˚) | 90.00 | 90.00 | 90.00 | 90.00 |
| β (˚) | 90.592 (5) | 96.535 (3) | 90.00 | 115.334 (4) |
| γ (˚) | 90.00 | 90.00 | 120.00 | 90.00 |
| Volume (Å3) | 862.0 (5) | 415.44 (17) | 7925.6 (10) | 2156.4 (13) |
| Z | 4 | 2 | 18 | 8 |
| Density (Mg/m3) | 1.604 | 2.027 | 1.976 | 1.574 |
| μ(Mo Kα) (mm−1) | 0.127 | 2.936 | 2.777 | 2.263 |
| F (000) | 432 | 252 | 4698 | 1024 |
| Total reflections | 3179 | 4014 | 17689 | 19860 |
| Unique reflections | 684 | 1928 | 2433 | 2595 |
| R(int) | 0.0234 | 0.0231 | 0.0989 | 0.0718 |
| R1[I > 2σ(I)] | 0.0256 | 0.0257 | 0.0355 | 0.0619 |
| wR2[I > 2σ(I)] | 0.0781 | 0.0633 | 0.0649 | 0.1206 |
| R1 (all data) | 0.0281 | 0.0274 | 0.0747 | 0.0872 |
| wR2 (all data) | 0.0832 | 0.0642 | 0.0743 | 0.1302 |
| GOF on F2 | 0.800 | 1.047 | 0.916 | 1.176 |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Eddaoudi, M.; Moler, D.; Li, H.; Reineke, T.M.; OʼKeeffe, M.; Yaghi, O.M. Modular Chemistry: Secondary Building Units as a Basis for the Design of Highly Porous and Robust Metal-Organic Carboxylate Frameworks. Acc. Chem. Res. 2001, 34, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic design of pore size and functionality in isoreticular MOFs and their applications in methane storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Moulton, B.; Zaworotko, M.J. From Molecules to Crystal Engineering: Supramolecular Isomerism and Polymorphism in Network Solids. Chem. Rev. 2001, 101, 1629–1658. [Google Scholar] [CrossRef] [PubMed]
- James, S.L. Metal-organic frameworks. Chem. Soc. Rev. 2003, 32, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Sudik, A.C.; Millward, A.R.; Ockwig, N.W.; Côté, A.P.; Kim, J.; Yaghi, O.M. Design, Synthesis, Structure, and Gas (N2, Ar, CO2, CH4, and H2) Sorption Properties of Porous Metal-Organic Tetrahedral and Heterocuboidal Polyhedra. J. Am. Chem. Soc. 2005, 127, 7110–7118. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Liu, H.M.; Lei, X.G.; Huang, X.Y.; Olson, D.H.; Turro, N.J.; Li, J. A Recyclable Nanoporous Material Suitable for Ship-In-Bottle Synthesis and Large Hydrocarbon Sorption. Angew. Chem. Int. Ed. 2003, 42, 542–546. [Google Scholar] [CrossRef]
- Song, W.; Li, J.; Song, P.; Tao, Y.; Yu, Q.; Tong, X.; Bu, X. Tuning the Framework Topologies of CoII-Doped ZnII-Tetrazole-benzoate Coordination Polymers by Ligand Modifications: Structures and Spectral Studies. Inorg. Chem. 2009, 48, 3792–3799. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhong, X.; Zheng, F.; Wu, M.; Liu, Z.; Guo, G. A novel metal-organic framework with bifunctional tetrazolate-5-carboxylate ligand: Crystal structure and luminescent properties. Inorg. Chem. Commun. 2011, 14, 407–410. [Google Scholar] [CrossRef]
- Farha, O.K.; Hupp, J.T. Rational Design, Synthesis, Purification, and Activation of Metal-Organic Framework Materials. Acc. Chem. Res. 2010, 43, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-M.; Tong, M.-L. Solvothermal in Situ Metal/Ligand Reactions: A New Bridge between Coordination Chemistry and Organic Synthetic Chemistry. Acc. Chem. Res. 2007, 40, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Makal, T.A.; Yakovenko, A.A.; Zhou, H.-C. Isomerism in Metal-Organic Frameworks: “Framework Isomers”. J. Phys. Chem. Lett. 2011, 2, 1682–1689. [Google Scholar] [CrossRef]
- Li, Y.; Xu, G.; Zou, W.-Q.; Wang, M.-S.; Zheng, F.-K.; Wu, M.-F.; Zheng, H.-Y.; Guo, G.-C.; Hunag, J.-S. A Novel Metal-Organic Network with High Thermal Stability: Nonlinear Optical and Photoluminescent Properties. Inorg. Chem. Commun. 2008, 47, 7945–7947. [Google Scholar] [CrossRef]
- Wei, Q.; Yang, D.; Larson, T.E.; Kinnibrugh, T.; Zou, R.; Henson, N.; Timofeeva, T.; Xu, H. Kinetic hysteresis in gas adsorption behavior for a rigid MOF arising from zig-zag channel structures. J. Mater. Chem. 2012, 22, 10166–10171. [Google Scholar] [CrossRef]
- Wang, X.S.; Tang, Y.Z.; Huang, X.F.; Qu, Z.R.; Che, C.M.; Hong Chan, P.W.; Xiong, R.G. Syntheses, Crystal Structures, and Luminescent Properties of Three Novel Zinc Coordination Polymers with Tetrazolyl Ligands. Inorg. Chem. 2005, 44, 5278–5285. [Google Scholar] [CrossRef] [PubMed]
- Li, J.R.; Tao, Y.; Yu, Q.; Bu, X.H. A pcu-type metal-organic framework with spindle [Zn7(OH)8]6+ cluster as secondary building units. Chem. Commun. 2007, 15, 1527–1529. [Google Scholar] [CrossRef]
- Li, G.Q.; Wu, A.Q.; Li, Y.; Zheng, F.K.; Guo, G.C. 4-(1H-Tetrazol-5-yl)benzoic acid monohydrate. Acta Cryst. 2008, E64. [Google Scholar] [CrossRef]
- Jiang, T.; Zhao, Y.F.; Zhang, X.M. Blue-green photoluminescent 5- and 10-connected metal 5-(4′-carboxy-phenyl)tetrazolate coordination polymers. Inorg. Chem. Commun. 2007, 10, 1194–1197. [Google Scholar] [CrossRef]
- Bruker. SAINTP + for NT. Data Reduction and Correction Program v. 6.2; Bruker AXS: Madison, WI, USA, 2001. [Google Scholar]
- Sheldrick, G.M. SHELXTL NT v. 6.12, Structure Determination Software Suite; Bruker AXS: Madison, WI, USA, 2001. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ordonez, C.; Kinnibrugh, T.L.; Xu, H.; Lindline, J.; Timofeeva, T.; Wei, Q. Synthesis of Framework Isomer MOFs Containing Zinc and 4-Tetrazolyl Benzenecarboxylic Acid via a Structure Directing Solvothermal Approach. Crystals 2015, 5, 193-205. https://doi.org/10.3390/cryst5020193
Ordonez C, Kinnibrugh TL, Xu H, Lindline J, Timofeeva T, Wei Q. Synthesis of Framework Isomer MOFs Containing Zinc and 4-Tetrazolyl Benzenecarboxylic Acid via a Structure Directing Solvothermal Approach. Crystals. 2015; 5(2):193-205. https://doi.org/10.3390/cryst5020193
Chicago/Turabian StyleOrdonez, Carlos, Tiffany L. Kinnibrugh, Hongwu Xu, Jennifer Lindline, Tatiana Timofeeva, and Qiang Wei. 2015. "Synthesis of Framework Isomer MOFs Containing Zinc and 4-Tetrazolyl Benzenecarboxylic Acid via a Structure Directing Solvothermal Approach" Crystals 5, no. 2: 193-205. https://doi.org/10.3390/cryst5020193
APA StyleOrdonez, C., Kinnibrugh, T. L., Xu, H., Lindline, J., Timofeeva, T., & Wei, Q. (2015). Synthesis of Framework Isomer MOFs Containing Zinc and 4-Tetrazolyl Benzenecarboxylic Acid via a Structure Directing Solvothermal Approach. Crystals, 5(2), 193-205. https://doi.org/10.3390/cryst5020193