Synthesis and Properties of 2-Alkylidene-1,3-dithiolo[4,5-d]-4,5-ethylenediselenotetrathiafulvalene Derivatives and Crystal Structures of Their Cation Radical Salts
Abstract
:1. Introduction
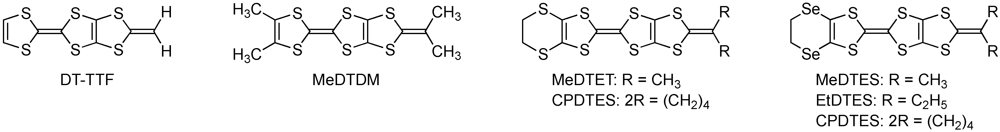
2. Results and Discussion
2.1. Synthesis and Property of MeDTES, EtDTES, and CPDTES
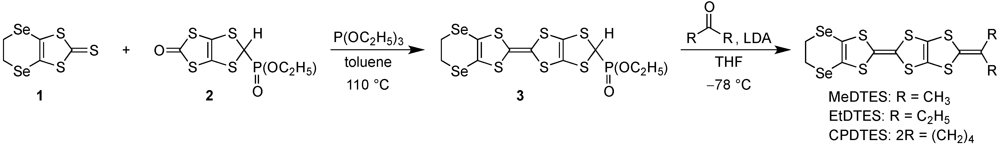
| Donor | E1 | E2 | E3 b | ΔE (=E2 − E1) |
|---|---|---|---|---|
| MeDTES | 0.04 | 0.34 | 1.00 | 0.30 |
| EtDTES | 0.05 | 0.35 | 1.01 | 0.30 |
| CPDTES | 0.04 | 0.35 | 1.00 | 0.31 |
| MeDTET | 0.06 | 0.37 | 0.99 | 0.31 |
| BEDT-TTF c | 0.06 | 0.38 | – | 0.32 |
2.2. Preparation, Structures of Cation Radical Salts
2.2.1. Preparation of Cation Radical Salts
| Salt | Donor (/ mg) | Electrolyte (/ mg) b | Solvent, 18 mL c | Current/μA | Period/day |
|---|---|---|---|---|---|
| 4 | MeDTES (2.0) | TBAAu(CN)4 (39.1) | THF | 0.5 | 2 |
| 5 | MeDTES (2.0) | TBAReO4 (35.5) | THF | 0.5 | 2 |
| 6 | MeDTES (2.0) | TBAI3 (44.9) | DCE (6% EtOH) | 0.5 | 2 |
| 7 | CPDTES (2.0) | TBAI3 (44.9) | PhCl (6% EtOH) | 0.5 | 2 |
| Compound | 4 | 5 | 6 | 7 |
|---|---|---|---|---|
| CCDC no. | 879812 | 879813 | 879814 | 879815 |
| Chemical formula | C12H10S6Se2·AuC4N4 | C12H10S6Se2·ReO4·(H2O)0.5 | (C12H10S6Se2)2·(I6)·(C4H4Cl2)0.5 | C14H12S6Se2·I3 |
| Formula weight | 805.53 | 763.69 | 1819.84 | 911.22 |
| Crystal size (mm3) | 0.05 × 0.04 × 0.01 | 0.04 × 0.02 × 0.01 | 0.06 × 0.02 × 0.01 | 0.08 × 0.06 × 0.03 |
| Crystal system | Orthorhombic | Triclinic | Triclinic | Triclinic |
| Space group | Pnma | P  | P  | P  |
| a/Å | 42.054(4) | 8.532(6) | 7.893(2) | 8.443(2) |
| b/Å | 12.3735(9) | 9.750(7) | 8.594(3) | 8.859(2) |
| c/Å | 4.2760(3) | 13.597(10) | 34.646(10) | 16.686(5) |
| α/° | 90 | 94.949(17) | 89.481(8) | 84.118(11) |
| β/° | 90 | 94.723(14) | 84.178(6) | 89.712(13) |
| γ/° | 90 | 93.783(13) | 77.788(4) | 68.365(9) |
| V/Å3 | 2225.0(3) | 1120.0(14) | 2285.0(12) | 1153.2(5) |
| Z | 4 | 2 | 2 | 2 |
| Dcalc/mg m−3 | 2.405 | 2.264 | 2.645 | 2.624 |
| μ/mm−1 | 10.459 | 9.250 | 7.894 | 7.764 |
| Temperature/K | 273 | 120 | 273 | 273 |
| Measured reflections | 19893 | 13612 | 23602 | 9519 |
| Independent reflections (Rint) | 2660 (0.0660) | 5066 (0.0800) | 10260 (0.0856) | 5193 (0.0705) |
| Observed reflections [I ≥ 2σ (I)] | 2464 | 3763 | 6592 | 2485 |
| Restraints/parameters | 0/149 | 0/206 | 0/437 | 0/229 |
| R1/wR2 [I ≥ 2σ (I)] | 0.0535, 0.1123 | 0.0835, 0.2044 | 0.0654, 0.1591 | 0.0455, 0.0993 |
| R1/wR2 (all data) | 0.0593, 0.1154 | 0.1084, 0.2303 | 0.1006, 0.1818 | 0.0972, 0.1235 |
| Goodness of fit | 1.214 | 1.070 | 1.093 | 0.902 |
| σrt/S cm−1 | 4.4 × 10−3 | 1.3 × 10−2 | 5.3 × 10−2 | 6.1 × 10−4 |
| Ea/eV | 0.15 | 0.058 | 0.051 | 0.17 |
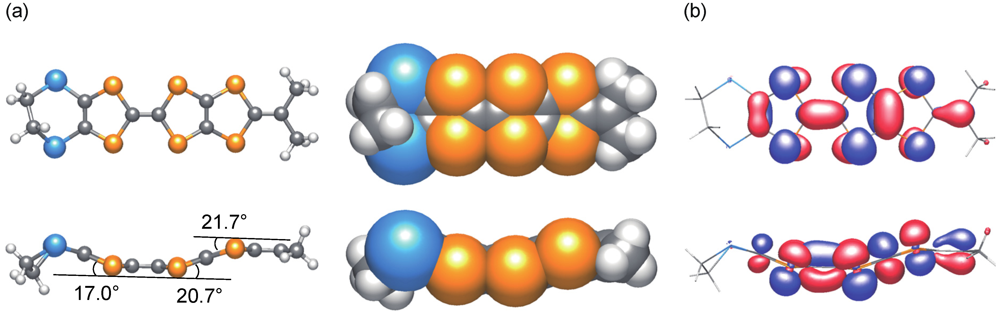
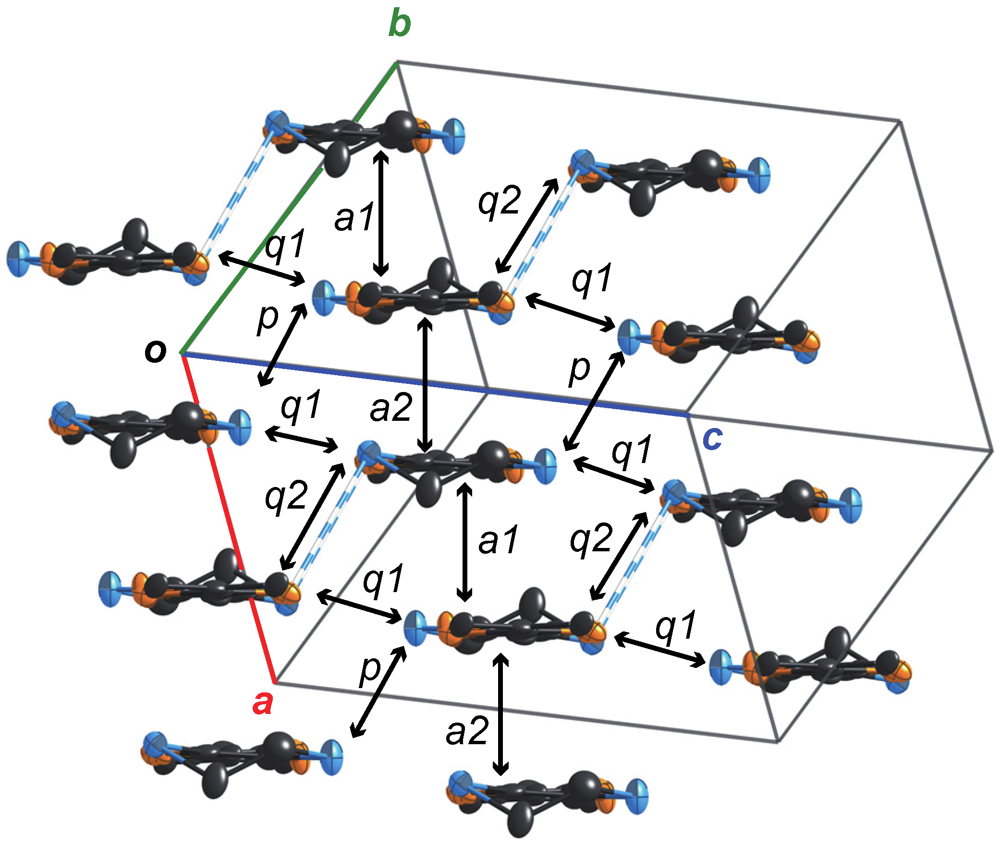
2.2.2. Structures and Electrical Properties of (MeDTES)[Au(CN)4] (4)
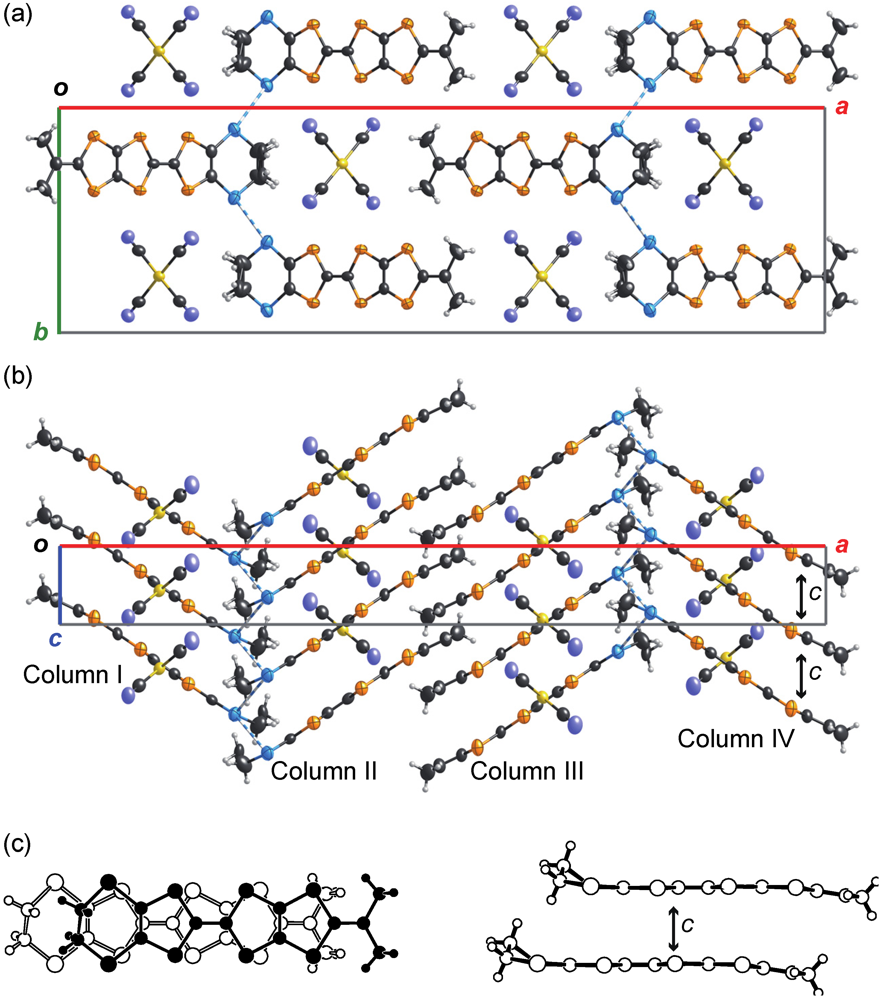

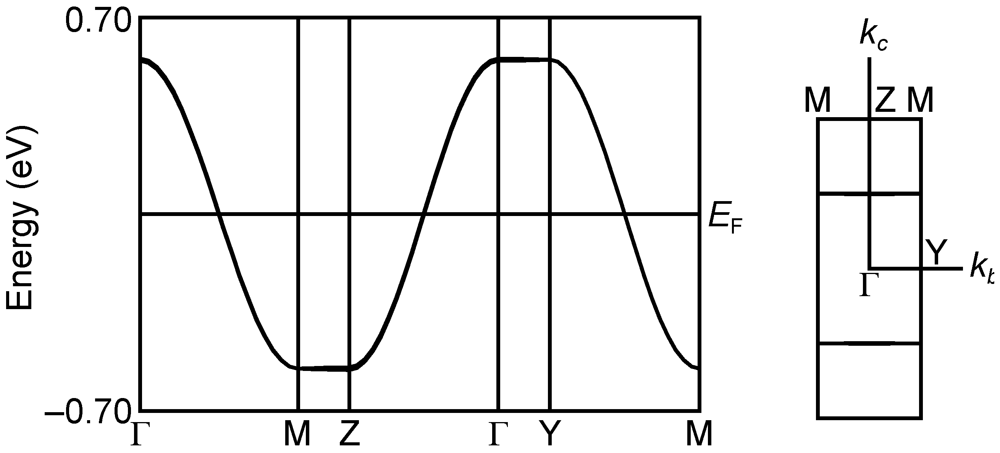
2.2.3. Structures and Electrical Properties of (MeDTES)(ReO4)(H2O)0.5 (5)
 . The molecular structure of the donor molecule is shown in Figure 8. The MeDTES molecule in this salt shows high planarity except for the terminal ethylene bridge. One donor molecule and one ReO4− ion are crystallographically independent and they are located in the general position, giving the 1:1 donor−anion ratio. An oxygen atom is found around the center of inversion with a half occupancy. Since the origin of the oxygen is not clear, we assumed it as a part of the solvent, tetrahydrofuran (THF). However, the refinement did not converge to give the THF molecule. Therefore we conclude that the oxygen atom is either water (H2O) or of oxonium ion (H3O+) origin, which might explain the conducting property of the salt.
. The molecular structure of the donor molecule is shown in Figure 8. The MeDTES molecule in this salt shows high planarity except for the terminal ethylene bridge. One donor molecule and one ReO4− ion are crystallographically independent and they are located in the general position, giving the 1:1 donor−anion ratio. An oxygen atom is found around the center of inversion with a half occupancy. Since the origin of the oxygen is not clear, we assumed it as a part of the solvent, tetrahydrofuran (THF). However, the refinement did not converge to give the THF molecule. Therefore we conclude that the oxygen atom is either water (H2O) or of oxonium ion (H3O+) origin, which might explain the conducting property of the salt. 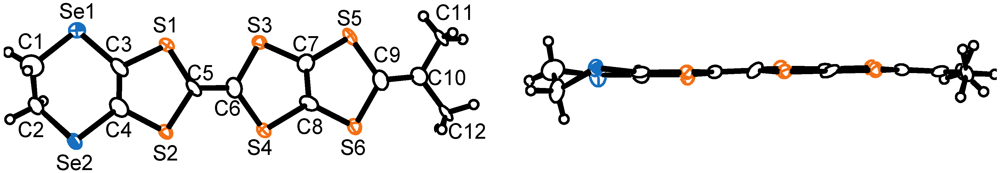

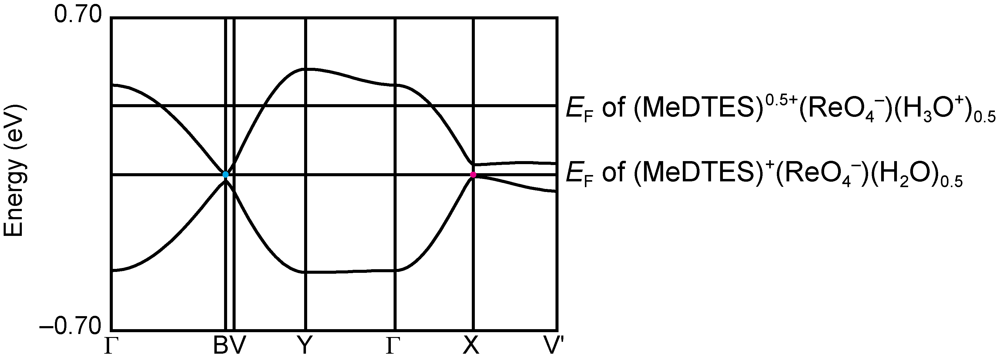
2.2.4. Structures and Electrical Properties of (MeDTES)(I3)(DCE)0.25 (6) (DCE = 1,2-Dichloroethane)
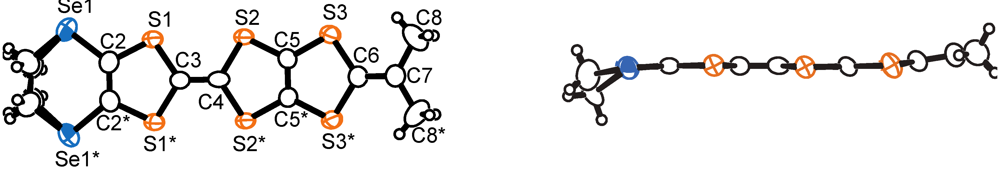
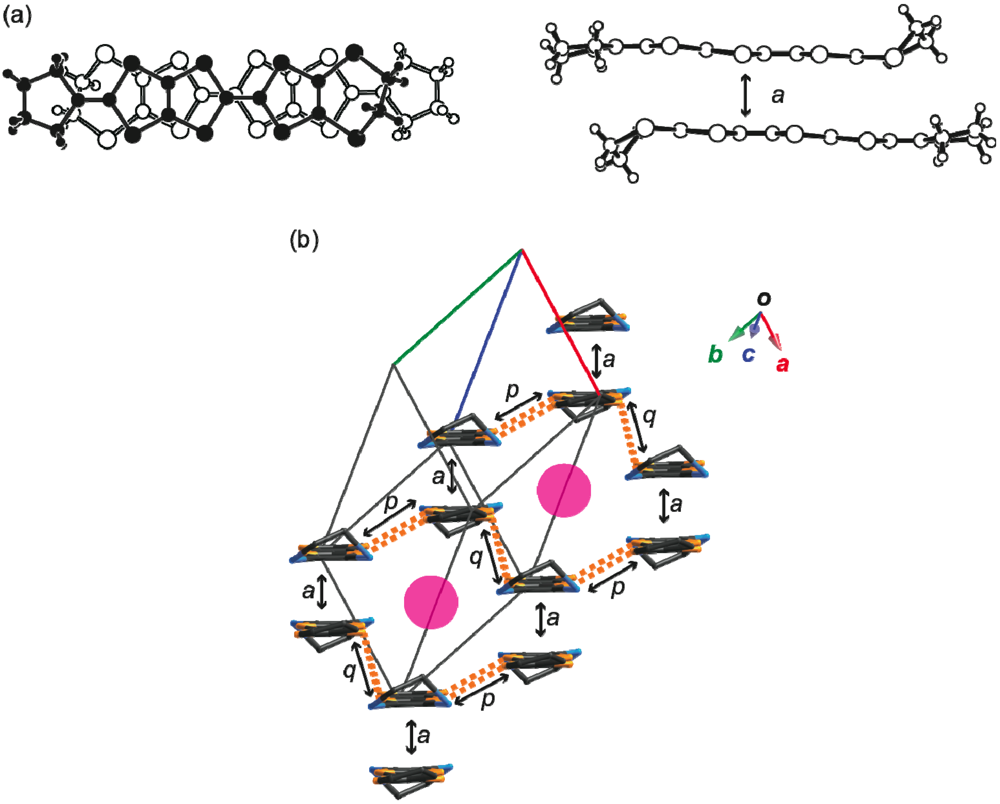
 . There are crystallographically independent two MeDTES molecules. The ORTEP drawings of Molecules A and B of MeDTES are shown in Figure 13. TTF moiety of Molecule A shows high planarity, and the 2-isopropylidene-1,3-dithiole unit slightly bends with dihedral angle of 6.3(3)°. Molecule B also has flat chair-like structure with dihedral angles 5.9(1) and 7.3(3)° as shown in Figure 13b.
. There are crystallographically independent two MeDTES molecules. The ORTEP drawings of Molecules A and B of MeDTES are shown in Figure 13. TTF moiety of Molecule A shows high planarity, and the 2-isopropylidene-1,3-dithiole unit slightly bends with dihedral angle of 6.3(3)°. Molecule B also has flat chair-like structure with dihedral angles 5.9(1) and 7.3(3)° as shown in Figure 13b. 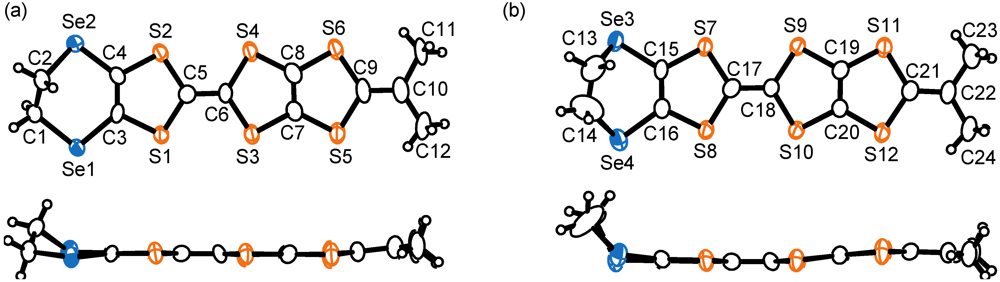

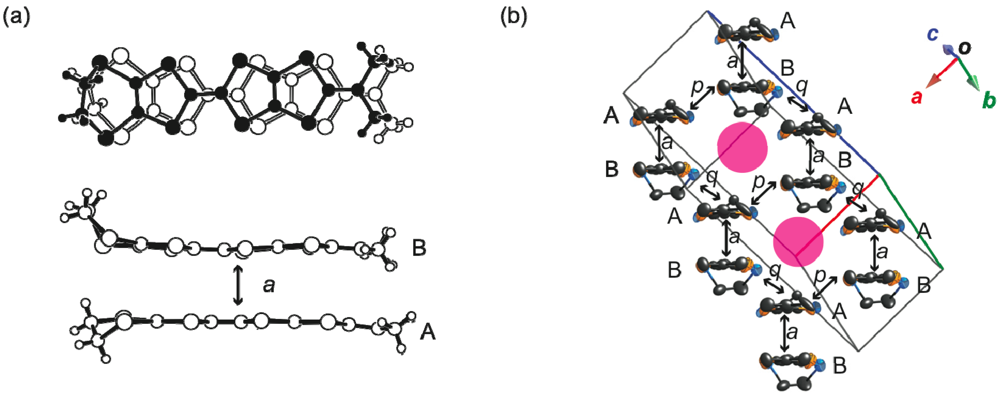
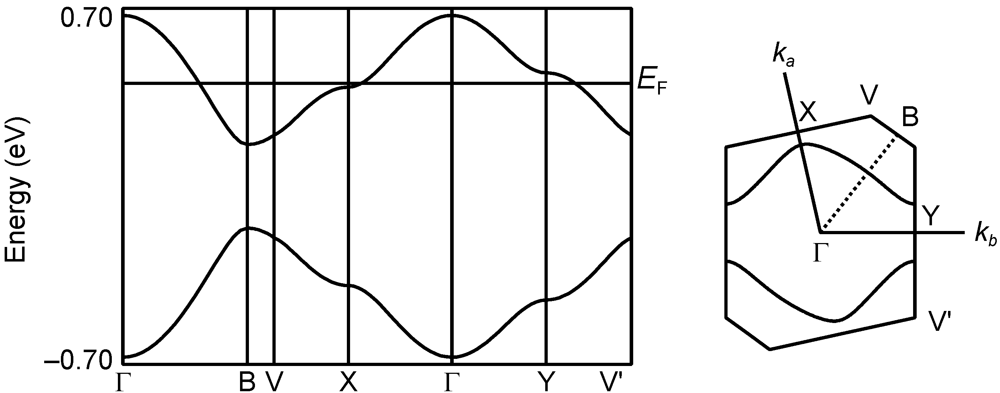
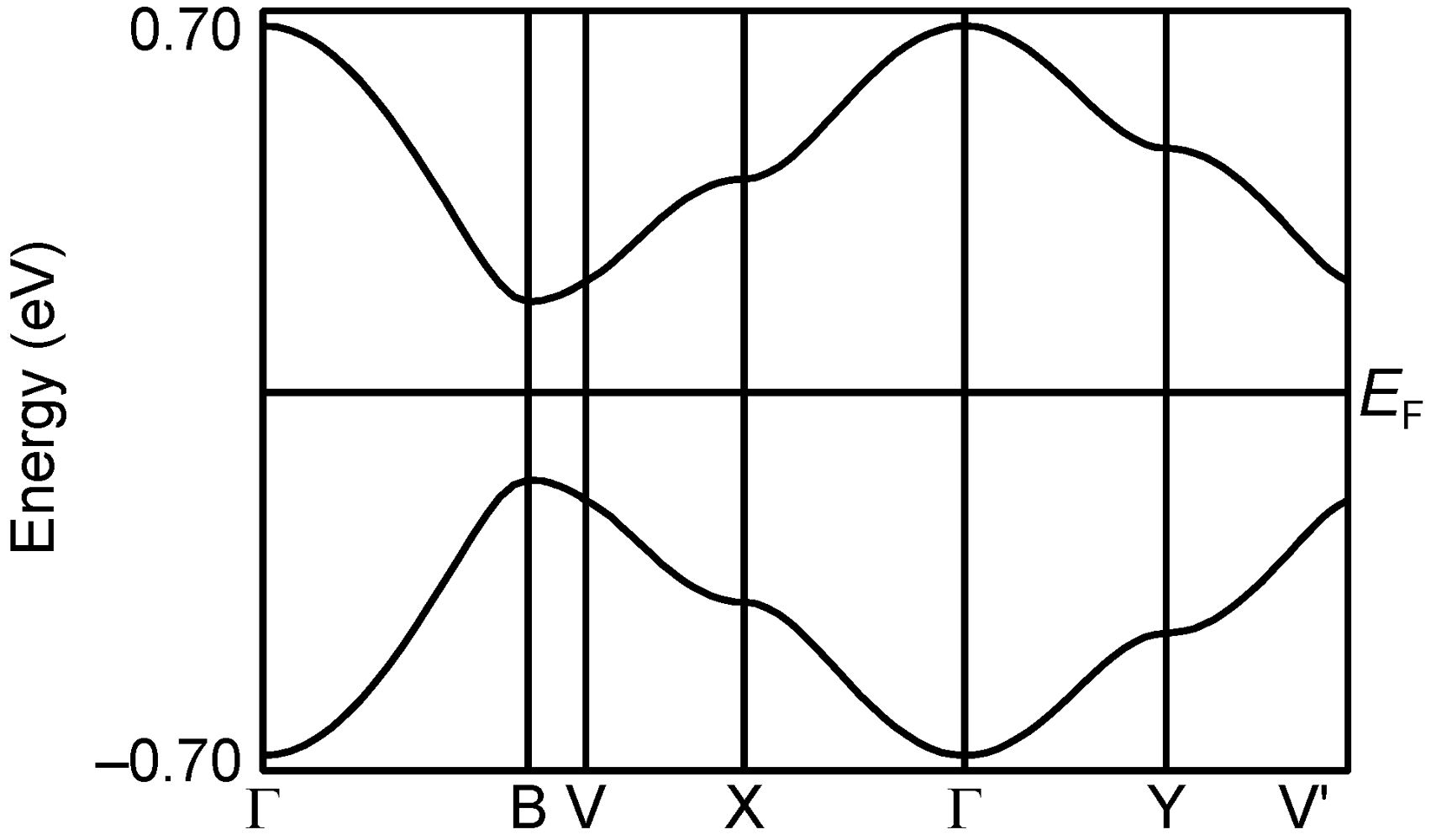
2.2.5. Structures and Electrical Properties of (CPDTES)(I3) (7)
 . The ORTEP drawings of the CPDTES molecule are shown in Figure 18. The molecule takes a flat chair-like structure and the dihedral angles of the TTP moiety composed of the S3−S6 and C6−C9 atoms are 5.7(1) and 6.9(2)°. One donor molecule is crystallographically independent. There are two types of I3− ion in the crystal and two halves of the ions are also crystallographically independent (Figure 19).
. The ORTEP drawings of the CPDTES molecule are shown in Figure 18. The molecule takes a flat chair-like structure and the dihedral angles of the TTP moiety composed of the S3−S6 and C6−C9 atoms are 5.7(1) and 6.9(2)°. One donor molecule is crystallographically independent. There are two types of I3− ion in the crystal and two halves of the ions are also crystallographically independent (Figure 19). 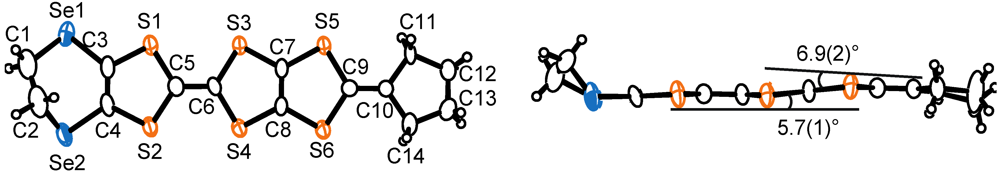
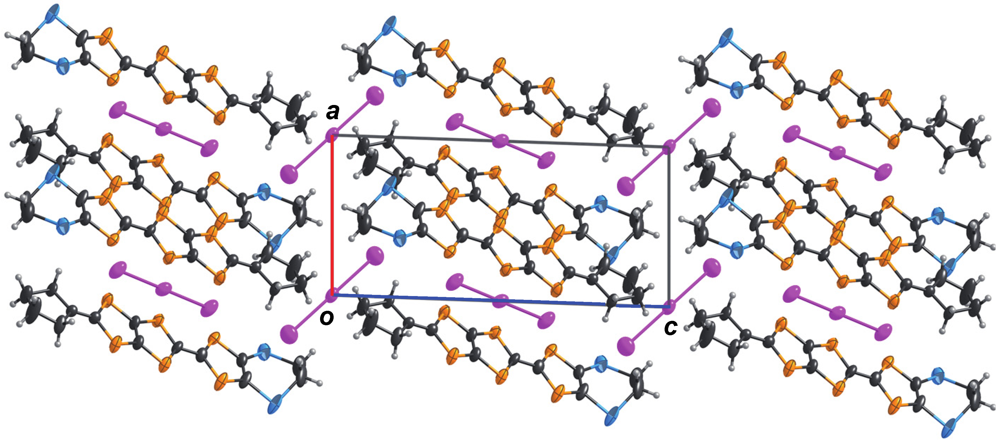
3. Experimental Section
3.1. General
3.2. Synthesis
3.2.1. Diethyl (5-(5,6-dihydro-[1,4]diselenino[2,3-d][1,3]dithiol-2-ylidene)-[1,3]dithiolo[4,5-d][1,3]dithiol-2-yl)phosphonate (3)
3.2.2. 2-Isopropylidene-1,3-Dithiolo[4,5-d]-4,5-ethylenediselenotetrathiafulvalene (MeDTES)
3.2.3. 2-(Pentan-3-ylidene)-1,3-dithiolo[4,5-d]-4,5-ethylenediselenotetrathiafulvalene (EtDTES)
3.2.4. 2-Cyclopentanylidene-1,3-dithiolo[4,5-d]-4,5-ethylenediselenotetrathiafulvalene (CPDTES)
3.3. Preparation of Cation Radical Salts
3.4. X-Ray Crystallographic Analysis
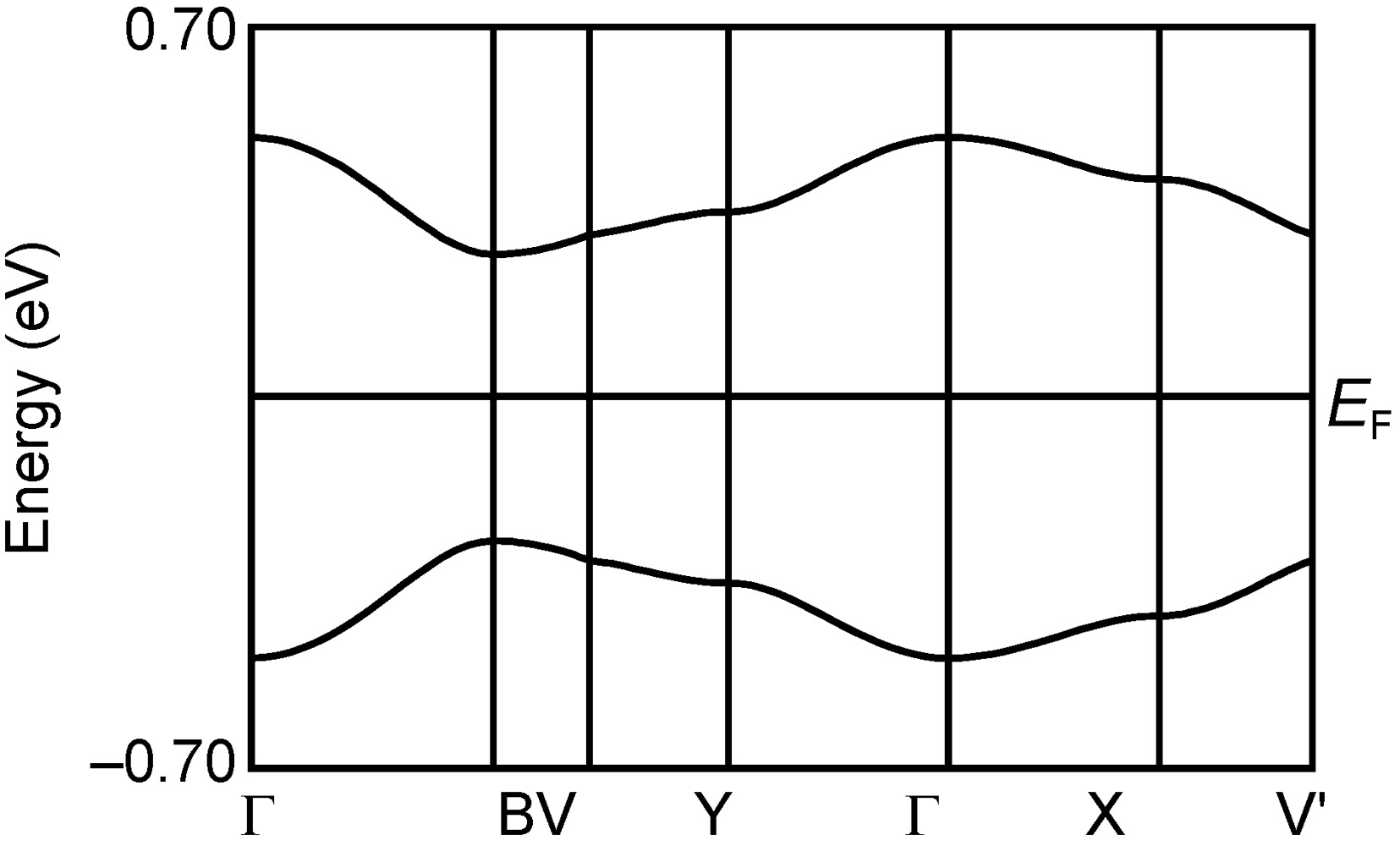
3.5. Band Calculations
3.6. Electrical Resistivity Measurement
4. Conclusions
Acknowledgments
Supplementary Files
References
- Ishiguro, T.; Yamaji, K.; Saito, G. Organic Superconductors, 2nd; Cardona, M., Fulde, P., von Klitzing, K., Queisser, H.-J., Eds.; Springer-Verlag: Berlin, Germany, 1998. [Google Scholar]
- Williams, J.M.; Thorn, R.J.; Ferraro, J.R.; Carlson, K.D.; Geiser, U.; Wang, H.H.; Kini, A.M.; Whangbo, M.-H. Organic Superconductors (Including Fullerenes) Synthesis, Structure, Properties, and Theory; Grimes, R.N., Ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 1992. [Google Scholar]
- Urayama, H.; Yamochi, H.; Saito, G.; Nozawa, K.; Sugano, T.; Kinoshita, M.; Sato, S.; Oshima, K.; Kawamoto, A.; Tanaka, J. A new ambient pressure organic superconductor based on BEDT-TTF with Tc higher than 10 K (Tc = 10.4 K). Chem. Lett. 1988, 17, 55–58. [Google Scholar]
- Williams, J.M.; Kini, A.M.; Wang, H.H.; Carlson, K.D.; Geiser, U.; Montgomery, L.K.; Pyrka, G.J.; Watkins, D.M.; Kommers, J.M. From semiconductor-semiconductor transition (42 K) to the highest-Tc organic superconductor, κ-(ET)2Cu[N(CN)2]Cl (Tc = 12.5 K). Inorg. Chem. 1990, 29, 3272–3274. [Google Scholar]
- Mori, T. Structural genealogy of BEDT-TTF-based organic conductors I. Parallel Molecules: β and β'' Phases. Bull. Chem. Soc. Jpn. 1998, 71, 2509–2526. [Google Scholar] [CrossRef]
- Mori, T. Structural genealogy of BEDT-TTF-based organic conductors II. Inclined molecules: θ, α, and κ phases. Bull. Chem. Soc. Jpn. 1999, 72, 179–197. [Google Scholar] [CrossRef]
- Aonuma, S.; Okano, Y.; Sawa, H.; Kato, R.; Kobayashi, H. Stable molecular metals based on a novel unsymmetrical diselenadithiafulvalene. J. Chem. Soc. Chem. Commun. 1992, 1992, 1193–1195. [Google Scholar]
- Misaki, Y.; Nishikawa, H.; Fujiwara, H.; Kawakami, K.; Yamabe, T.; Yamochi, H.; Saito, G. (2-Methylidene-1,3-dithiolo[4,5-d])tetrathiafulvalene (DT-TTF): New unsymmetrical TTFs condensed with 1,3-dithiol-2-ylidene moieties. J. Chem. Soc. Chem. Commun. 1992, 1992, 1408–1409. [Google Scholar]
- Misaki, Y.; Taniguchi, M.; Miura, T.; Fujiwara, H.; Yamabe, T.; Kawamoto, T.; Mori, T. Structures and properties of MeDTDM salts. Adv. Mater. 1997, 9, 663–635. [Google Scholar]
- Misaki, Y.; Nishikawa, H.; Yamabe, T.; Mori, T.; Inokuchi, H.; Mori, H.; Tanaka, S. Structure and Electrical Properties of MeDTET Salts. Chem. Lett. 1993, 22, 1341–1344. [Google Scholar]
- Fujiwara, H.; Misaki, Y.; Taniguchi, M.; Yamabe, T.; Kawamoto, T.; Mori, T.; Mori, H.; Tanaka, S. Preparation, structures and physical properties of κ-type two-dimensional conductors based on unsymmetrical extended tetrathiafulvalene: 2-cyclopentanylidene-1,3-dithiolo[4,5-d]-4,5-ethylenedithiotetrathiafulvalene (CPDTET). J. Mater. Chem. 1998, 8, 1711–1717. [Google Scholar] [CrossRef]
- Takimiya, K.; Otsubo, T. Selenium-containing π-conjugated compounds for electronic molecular materials. Phosphorus Sulfur Silicon Relat. Elem. 2005, 180, 873–881. [Google Scholar]
- Otsubo, T.; Takimiya, K. Selenium Analogues of TTFs. In TTF Chemistry—Fundamentals and Applications of Tetrathiafulvalene: Selenium Analogues of TTFs; Yamada, J., Sugimoto, T., Eds.; Kodansha & Springer: Tokyo, Japan, 2004; pp. 119–136, Chapter 5. [Google Scholar]
- Imakubo, T.; Shirahata, T.; Kibune, M.; Yoshino, H. Hybrid organic/inorganic supramolecular conductors D2[Au(CN)4] [D = diiodo(ethylenedichalcogeno)tetrachalcogenofulvalene], including a new ambient pressure superconductor. Eur. J. Inorg. Chem. 2007, 2007, 4727–4735. [Google Scholar]
- Wang, H.H.; Montgomery, L.K.; Geiser, U.; Porter, L.C.; Carlson, K.D.; Ferraro, J.R.; Williams, J.M.; Cariss, C.S.; Rubinstein, R.L.; Whitworth, J.R.; et al. Syntheses, structures, selected physical properties and band electronic structures of the bis(ethylenediseleno)tetrathiafulvalene salts, (BEDSe-TTF)2X, X− = I3−, AuI2−, and IBr2−. Chem. Mater. 1989, 1, 140–148. [Google Scholar] [CrossRef]
- Sakata, J.; Sato, H.; Miyazaki, A.; Enoki, T.; Okano, Y.; Kato, R. Superconductivity in new organic conductor κ-(BEDSe-TTF)2CuN(CN)2Br. Solid State Commun. 1999, 108, 377–381. [Google Scholar]
- Garín, J.; Orduna, J.; Savirón, M.; Bryce, M.R.; Moore, A.J.; Morisson, V. Synthesis and characterization of functionalized ethylenediselenotetrathiafulvalenes: A comparative study with their all-sulfur analogues. Tetrahedron 1996, 52, 11063–11074. [Google Scholar]
- Misaki, Y.; Ohta, T.; Higuchi, N.; Fujiwara, H.; Yamabe, T.; Mori, T.; Mori, H.; Tanaka, S. A vinylog of bis-fused tetrathiafulvalene: Novel π-electron framework for two-dimensional organic metals. J. Mater. Chem. 1995, 5, 1571–1579. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanioka, H.; Fueno, H.; Misaki, Y.; Tanaka, K. Preparation and characterization of novel aromatic-inserted tris-fused tetrathiafulvalenes. Chem. Lett. 2002, 31, 1002–1003. [Google Scholar]
- Imakubo, T.; Shirahata, T.; Kibune, M. Synthesis, crystal structure and electrochemical properties of bis(ethylenedioxy)tetraselenafulvalene (BEDO-TSeF). Chem. Commun. 2004, 2004, 1590–1591. [Google Scholar]
- Bondi, A. van der Waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar]
- Kistenmacher, T.J.; Phillips, T.E.; Cowan, D.O. The crystal structure of the 1:1 radical cation-radical anion salt of 2,2'-bis-l,3-dithiole (TTF) and 7,7,8,8-tetracyanoquinodimethane (TCNQ). Acta Cryst. 1974, B30, 763–768. [Google Scholar]
- Mori, T.; Kobayashi, A.; Sasaki, Y.; Kobayashi, H.; Saito, G.; Inokuchi, H. The intermolecular interaction of tetrathiafulvalene and bis(ethylenedithio)tetrathiafulvalene in organic metals. Calculation of orbital overlaps and models of energy-band structures. Bull. Chem. Soc. Jpn. 1984, 57, 627–633. [Google Scholar] [CrossRef]
- Svensson, P.H.; Kloo, L. Synthesis, structure, and bonding in polyiodide and metal iodide-iodine systems. Chem. Rev. 2003, 103, 1649–1684. [Google Scholar]
- Mizuno, M.; Tanaka, J.; Harada, I. Electronic spectra and structures of polyiodide chain complexes. J. Phys. Chem. 1981, 85, 1789–1794. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self-consistent molecular orbital methods. XII. Further extensions of Gaussian—Type basis sets for use in molecular orbital studies of organic molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1998, 37, 785–789. [Google Scholar]
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; Caro, L.D.; Giacovazzo, C.; Polidori, G.; Spagna, R. SIR2004: An improved tool for crystal structure determination and refinement. J. Appl. Cryst. 2005, 38, 381–388. [Google Scholar] [CrossRef]
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; de Caro, L.; Giacovazzo, C.; Polidori, G.; Siliqi, D.; Spagna, R. IL MILIONE: A suite of computer programs for crystal structure solution of proteins. J. Appl. Cryst. 2007, 40, 609–613. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar]
- Rigaku, M. CrystalStructure, Version 4.0; Rigaku Corporation: Tokyo, Japan, 2010. [Google Scholar]
- Misaki, Y. Tetrathiapentalene-based organic conductors. Sci. Technol. Adv. Mater. 2009, 10, 024301:1–024301:22. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Furuta, K.; Kohno, S.; Shirahata, T.; Yamasaki, K.; Hino, S.; Misaki, Y. Synthesis and Properties of 2-Alkylidene-1,3-dithiolo[4,5-d]-4,5-ethylenediselenotetrathiafulvalene Derivatives and Crystal Structures of Their Cation Radical Salts. Crystals 2012, 2, 393-412. https://doi.org/10.3390/cryst2020393
Furuta K, Kohno S, Shirahata T, Yamasaki K, Hino S, Misaki Y. Synthesis and Properties of 2-Alkylidene-1,3-dithiolo[4,5-d]-4,5-ethylenediselenotetrathiafulvalene Derivatives and Crystal Structures of Their Cation Radical Salts. Crystals. 2012; 2(2):393-412. https://doi.org/10.3390/cryst2020393
Chicago/Turabian StyleFuruta, Keisuke, Shuhei Kohno, Takashi Shirahata, Koya Yamasaki, Shojun Hino, and Yohji Misaki. 2012. "Synthesis and Properties of 2-Alkylidene-1,3-dithiolo[4,5-d]-4,5-ethylenediselenotetrathiafulvalene Derivatives and Crystal Structures of Their Cation Radical Salts" Crystals 2, no. 2: 393-412. https://doi.org/10.3390/cryst2020393
APA StyleFuruta, K., Kohno, S., Shirahata, T., Yamasaki, K., Hino, S., & Misaki, Y. (2012). Synthesis and Properties of 2-Alkylidene-1,3-dithiolo[4,5-d]-4,5-ethylenediselenotetrathiafulvalene Derivatives and Crystal Structures of Their Cation Radical Salts. Crystals, 2(2), 393-412. https://doi.org/10.3390/cryst2020393




