Structural Characteristics of Homoleptic Zinc Complexes Incorporating Asymmetric Aminopyridinates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. NMR Spectroscopy
2.3. Single Crystal X-Ray Diffraction
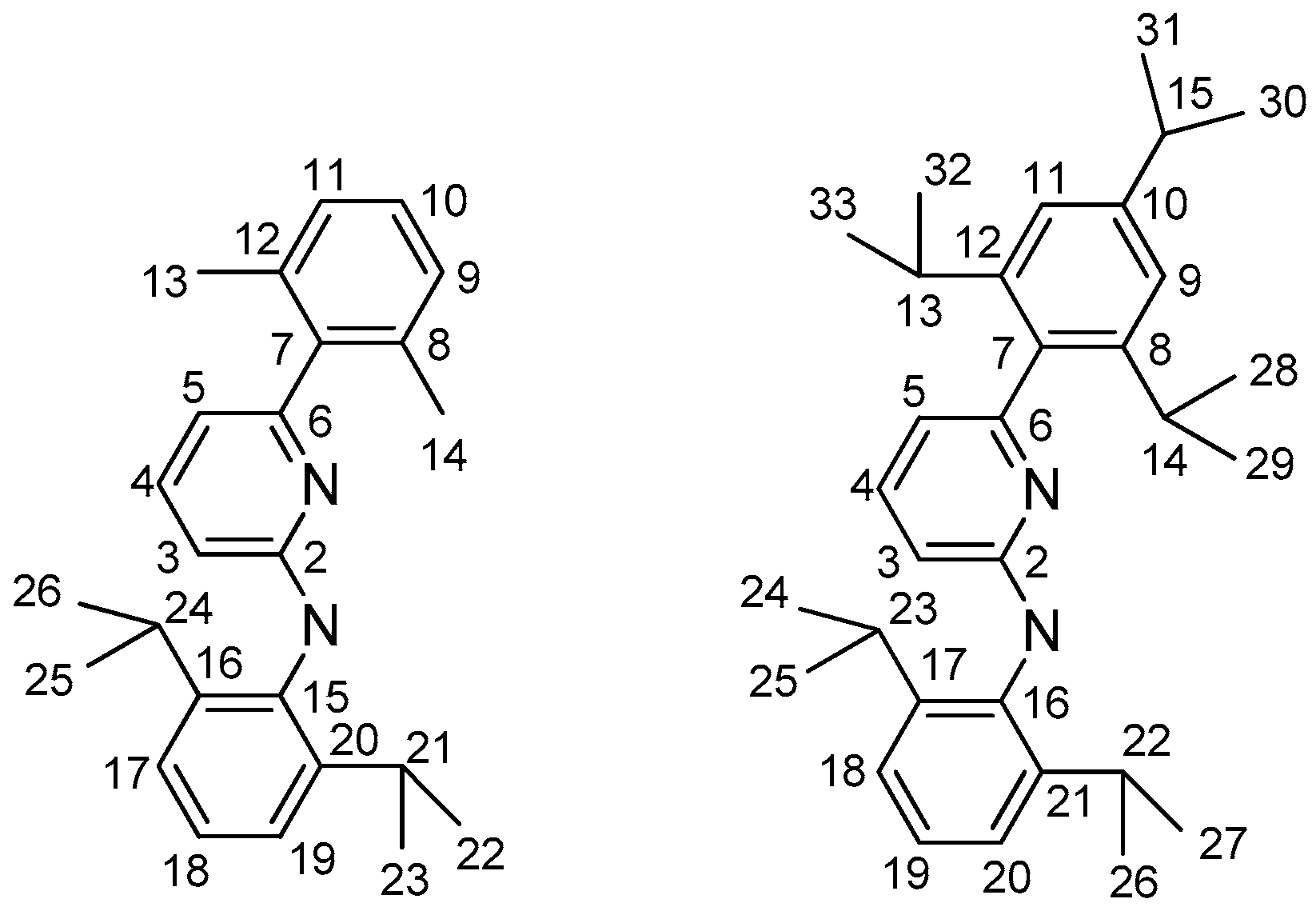
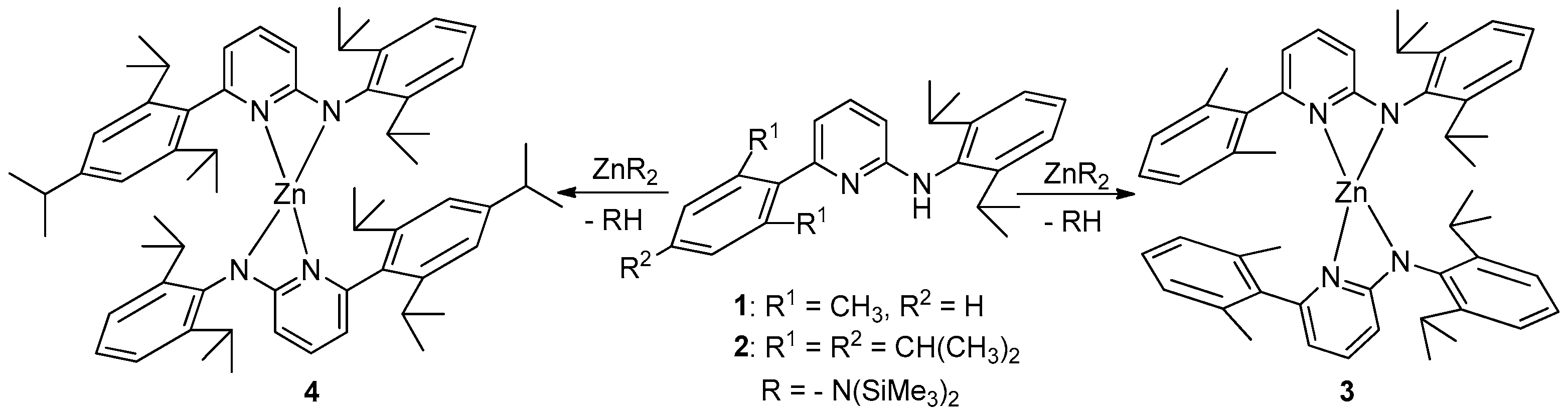
2.4. Syntheses
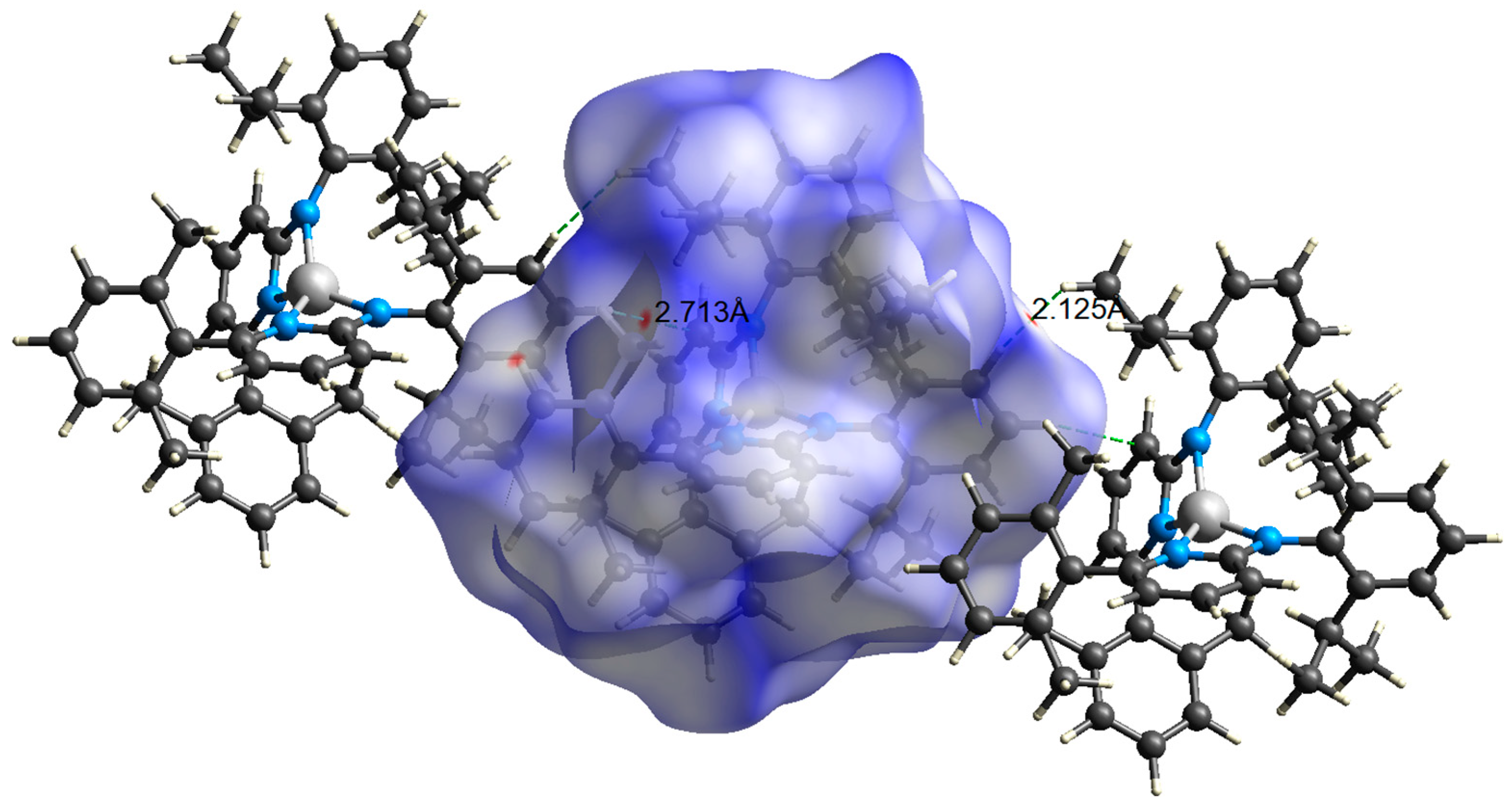
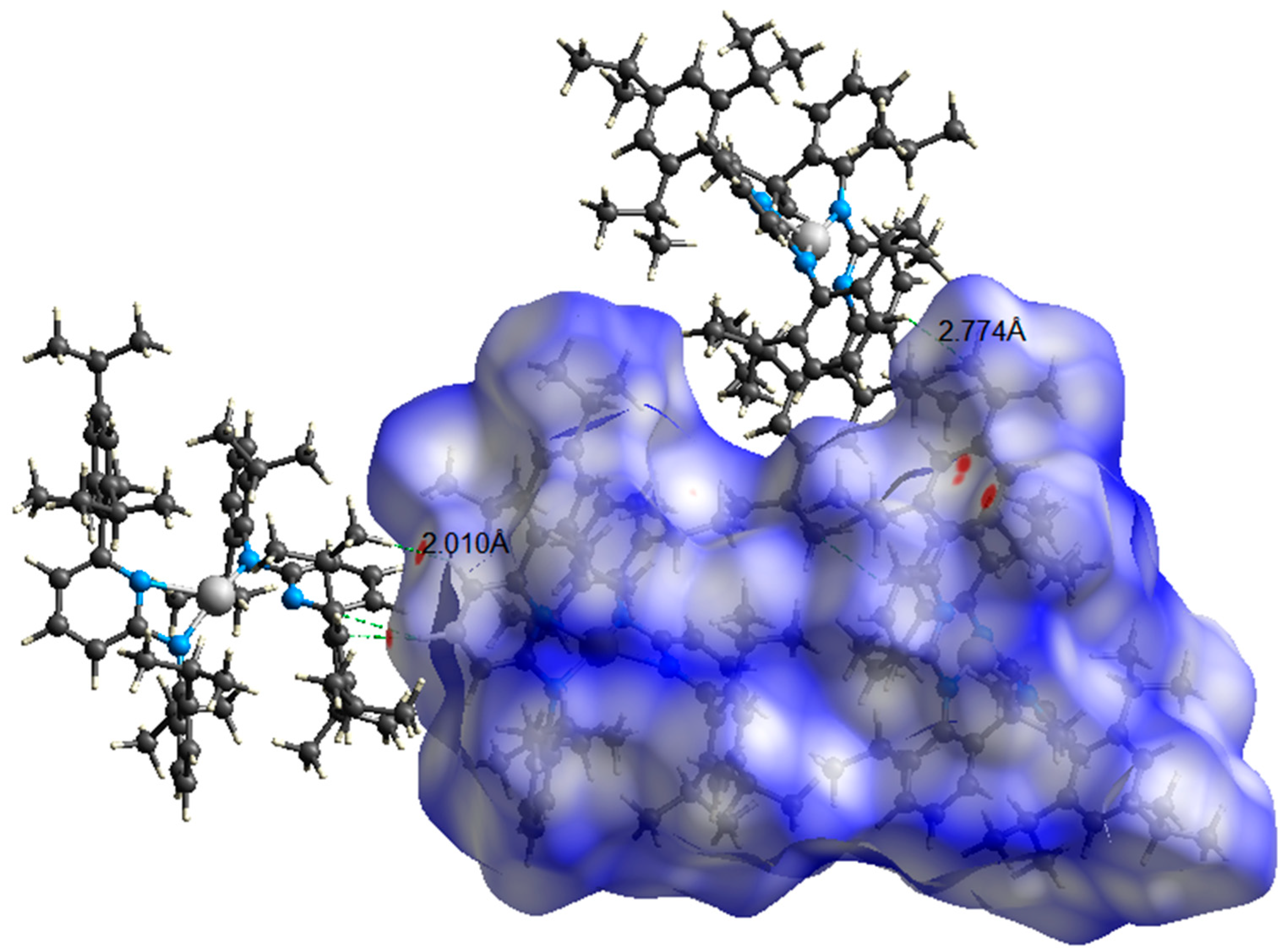
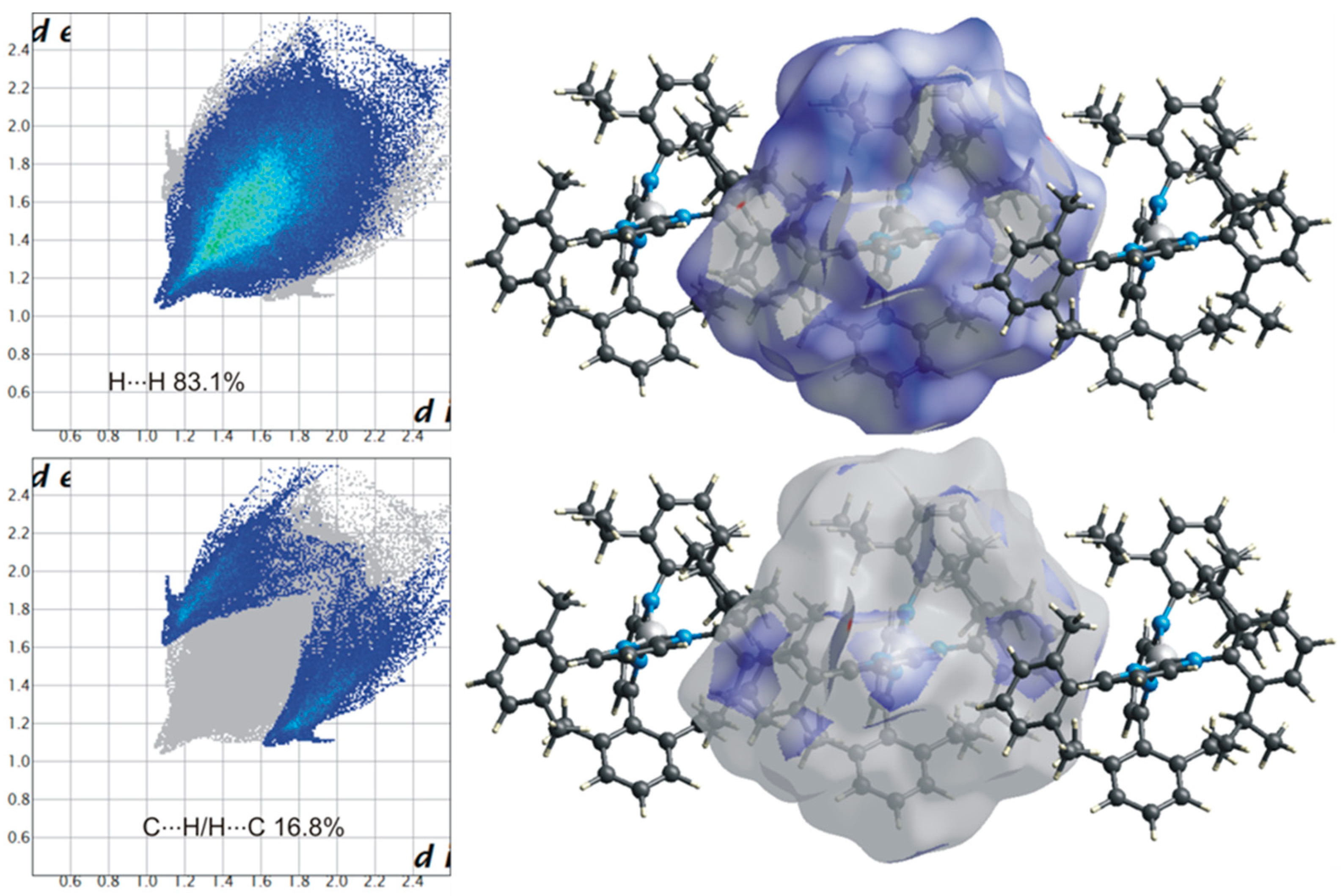
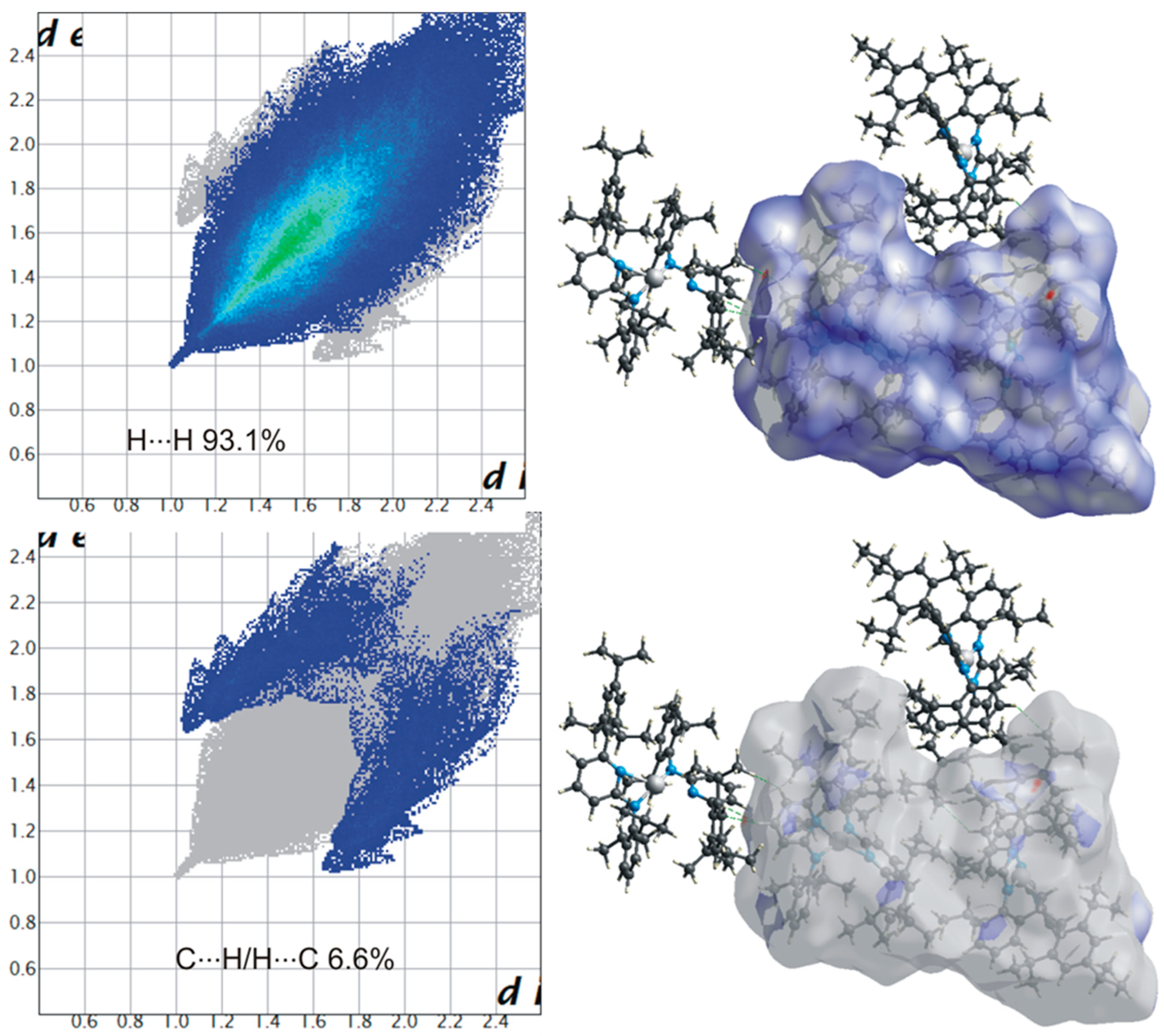
2.5. Hirshfeld Surface Analysis
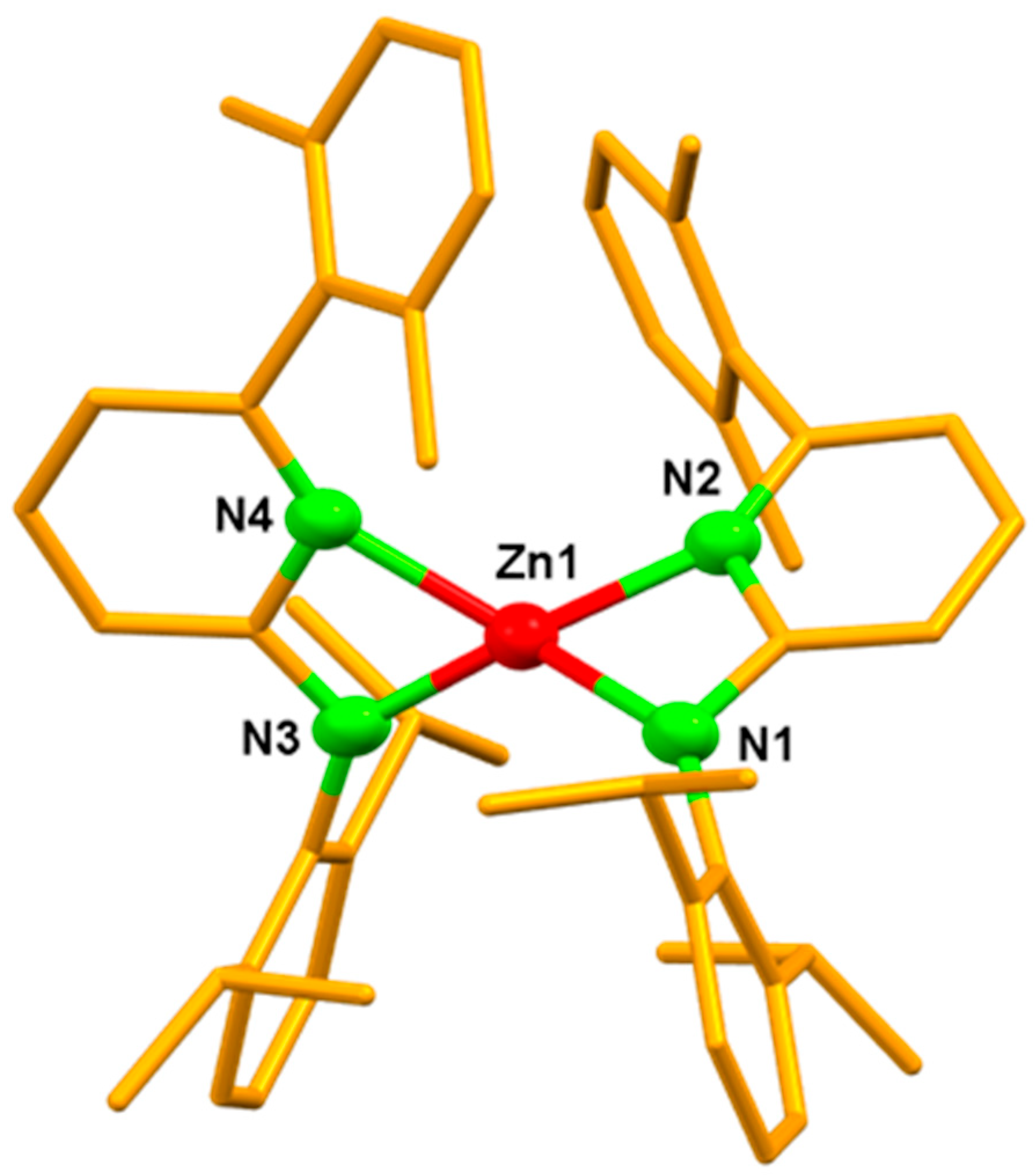
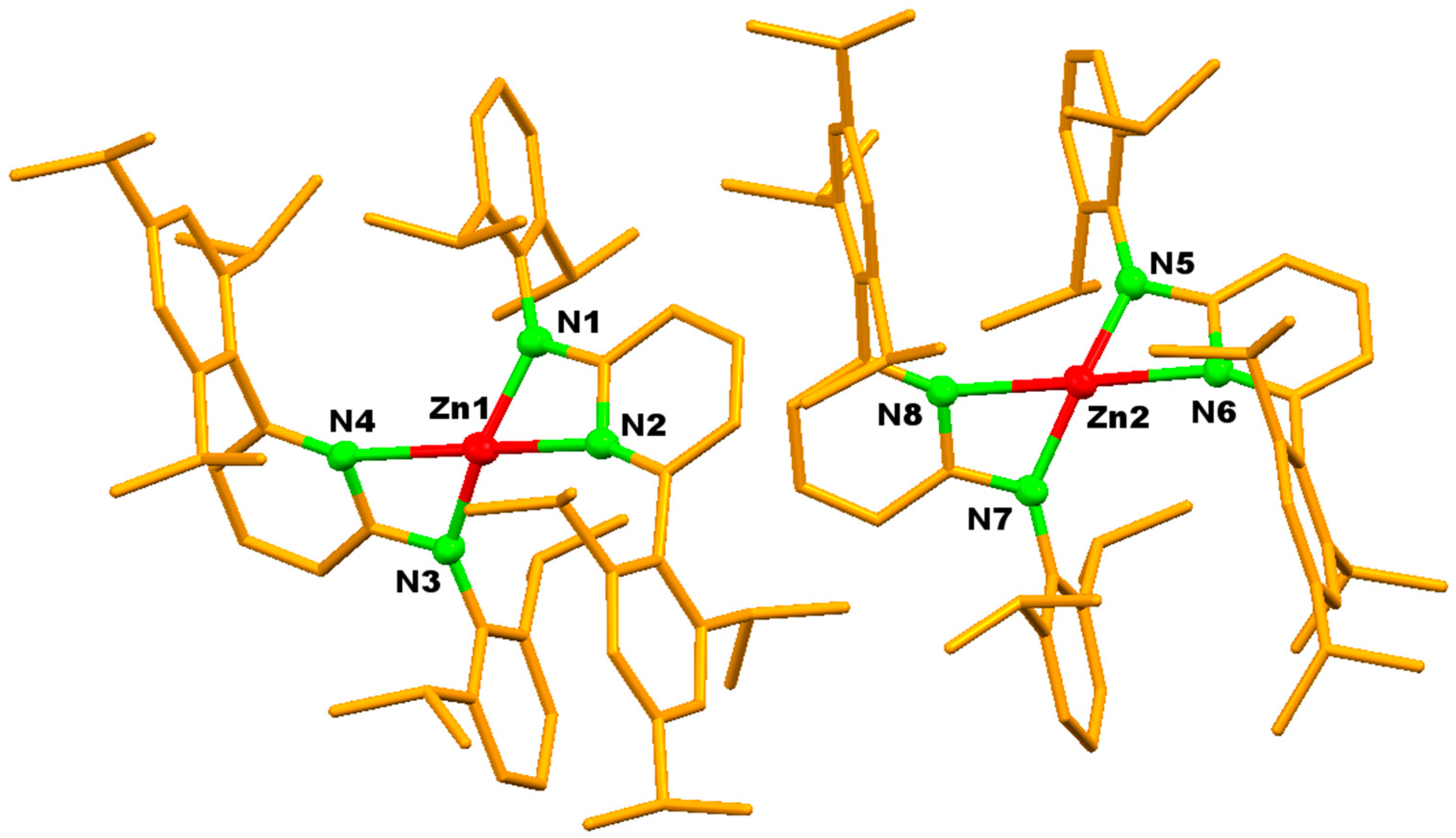
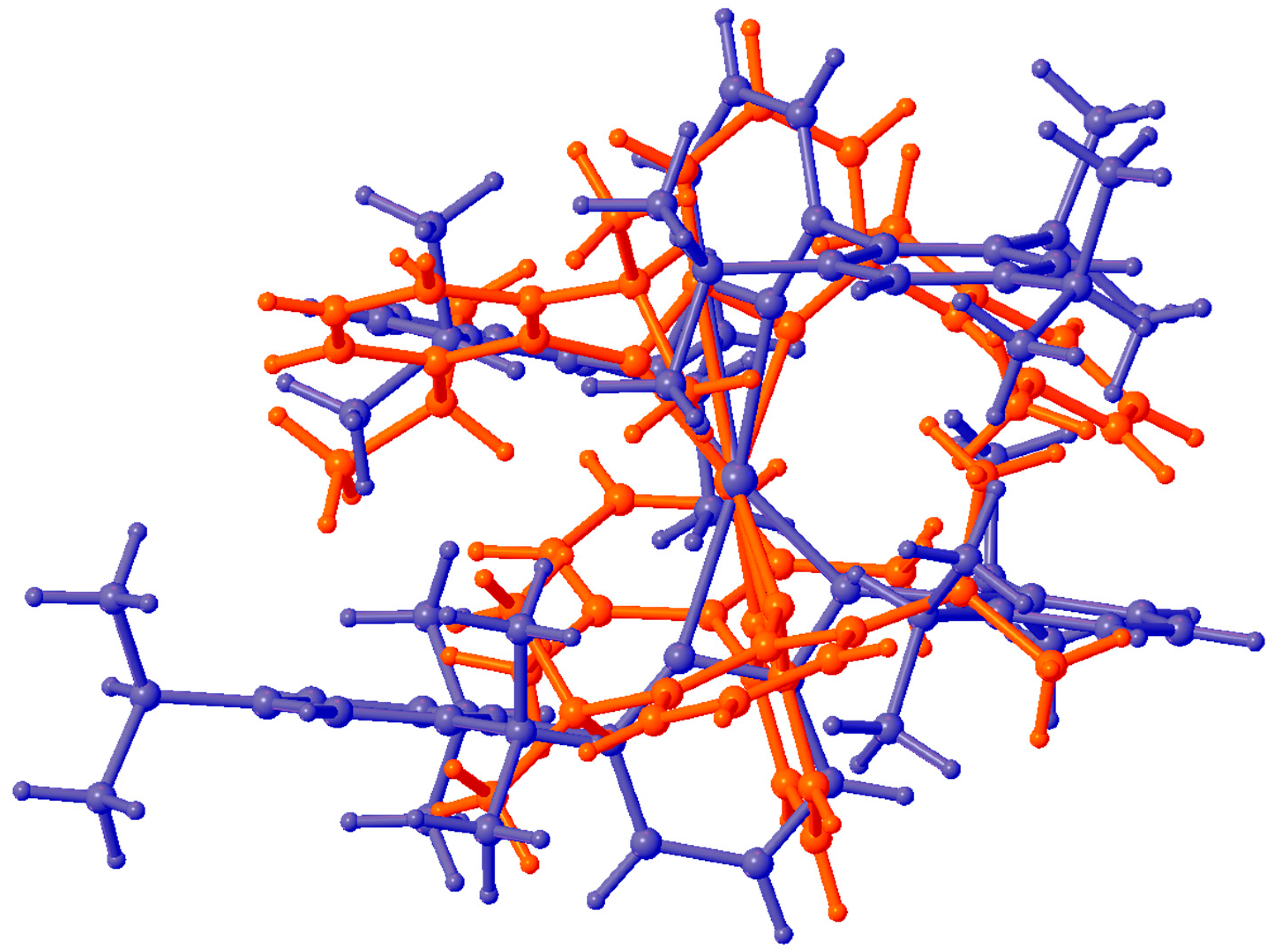
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kempe, R.; Brenner, S.; Arndt, P. Mononuclear Tris(aminopyridinato)zirconium Alkyl, Aryl, and Alkynyl Complexes. Organometallics 1996, 15, 1071–1074. [Google Scholar] [CrossRef]
- Kempe, R.; Arndt, P. Mononuclear Titanium Complexes That Contain Aminopyridinato Ligands. Inorg. Chem. 1996, 35, 2644–2649. [Google Scholar] [CrossRef]
- Noor, A. Coordination Chemistry of Bulky Aminopryridinates with Main Group and Transition Metals. Top. Curr. Chem. 2021, 379, 6. [Google Scholar] [CrossRef]
- Kempe, R. Aminopyridinato ligand complexes—Precursors for nanocomposite catalysts, quintuple bonding and on-purpose olefin synthesis. Adv. Inorg. Chem. 2023, 82, 41–67. [Google Scholar] [CrossRef]
- Wagner, F.R.; Noor, A.; Kempe, R. Ultrashort metal–metal distances and extreme bond orders. Nat. Chem. 2009, 1, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Dietel, T.; Lukas, F.; Kretschmer, W.P.; Kempe, R. Elongation and branching of α–olefins by two ethylene molecules. Science 2022, 375, 1021–1024. [Google Scholar] [CrossRef]
- Noor, A. A Rare Example of Manganese Aminopyridinate—Synthesis and Structure. J. Chem. Crystallogr. 2025, 55, 85–91. [Google Scholar] [CrossRef]
- Glatz, G.; Demeshko, S.; Motz, G.; Kempe, R. First Row Transition Metal Aminopyridinates–the Missing Complexes. Eur. J. Inorg. Chem. 2009, 2009, 1385–1392. [Google Scholar] [CrossRef]
- Lee, H.K.; Lam, C.H.; Li, S.L.; Zhang, Z.Y.; Mak, T.C.W. Low-Valent Chemistry of Cobalt Amide. Synthesis and Structural Characterization of Cobalt(II) Amido, Aryloxide, and Thiolate Compounds. Inorg. Chem. 2001, 40, 4691–4695. [Google Scholar] [CrossRef]
- Deeken, S.; Proch, S.; Casini, E.; Braun, H.F.; Mechtler, C.; Marschner, C.; Motz, G.; Kempe, R. Group 10 Metal Aminopyridinato Complexes: Synthesis, Structure, and Application as Aryl-Cl Activation and Hydrosilane Polymerization Catalysts. Inorg. Chem. 2006, 45, 1871–1879. [Google Scholar] [CrossRef]
- Engelhardt, L.M.; Jacobsen, G.E.; Patalinghug, W.Y.; Skelton, B.W.; Raston, C.L.; White, A.H. Synthesis and structure of copper(I), silver(I) and zinc(II) amides [Cu2(mpsa)2], [Cu6X2(mpsa)4](X = Cl or Br), [Ag4(mpsa)4] and [(ZnEt)2(mpsa)2][mpsa = 2-N(SiMe3)C5H3N-6-Me]. J. Chem. Soc. Dalton Trans. 1991, 2859–2868. [Google Scholar] [CrossRef]
- Aghabozorg, H.; Gambarotta, S.; Bensimon, C. Preparation and Molecular Structure of 2-Benzylaminopyridine Copper(I) Complex. J. Sci. Islam. Repub. Iran 1994, 5, 158–162. [Google Scholar]
- Birch, S.J.; Boss, S.R.; Cole, S.C.; Coles, M.P.; Haigh, R.; Hitchcock, P.B.; Wheatley, A.E.H. The structural characteristics of organozinc complexes incorporating N,N′-bidentate ligands. Dalton Trans. 2004, 3568–3574. [Google Scholar] [CrossRef]
- Alvarez, C.S.; Boss, S.R.; Burley, J.C.; Humphry, S.M.; Layfield, R.A.; Kowenicki, R.A.; McPartlin, M.; Rawson, J.M.; Wheatley, A.E.H.; Wood, P.T.; et al. Syntheses, structures and magnetic properties of Mn(II) dimers [CpMn(μ-X)]2 (Cp = C5H5; X = RNH, R1R2N, C≡CR). Dalton Trans. 2004, 3481–3487. [Google Scholar] [CrossRef]
- Zanders, D.; Boysen, N.; Land, M.A.; Obenlüneschloß, J.; Masuda, J.D.; Mallick, B.; Barry, S.T.; Devi, A. Co(II) Amide, Pyrrolate, and Aminopyridinate Complexes: Assessment of their Manifold Structural Chemistry and Thermal Properties. Eur. J. Inorg. Chem. 2021, 2021, 5119–5136. [Google Scholar] [CrossRef]
- Kempe, R. The strained η2−NAmido−NPyridine coordination of aminopyridinato ligands. Eur. Inorg. Chem. 2003, 2003, 791–803. [Google Scholar] [CrossRef]
- Davies, R.P.; Linton, D.J.; Schooler, P.; Snaith, R.; Wheatley, A.E.H. Oxygen Capture by Lithiated Organozinc Reagents Containing Aromatic 2-Pyridylamide Ligands. Chem. Eur. J. 2001, 7, 3696–3704. [Google Scholar] [CrossRef]
- Boss, S.R.; Haigh, R.; Linton, D.J.; Wheatley, A.E.H. Oxygen scavenging by lithium zincates: The synthesis, structural characterisation and derivatisation of [Ph(2-C5H4N)N]2ZnRLi·nthf (R = But, Bun; n = 1, 2). J. Chem. Soc. Dalton Trans. 2002, 3129–3134. [Google Scholar] [CrossRef]
- Barman, M.K.; Baishya, A.; Nembenna, S. Bulky guanidinate stabilized homoleptic magnesium, calcium and zinc complexes and their catalytic activity in the Tishchenko reaction. J. Organomet. Chem. 2015, 785, 52–60. [Google Scholar] [CrossRef]
- Li, J.; Shi, J.; Han, H.; Guo, Z.; Tong, H.; Wei, X.; Liu, D.; Lappert, M.F. Synthesis, Structures, and Reactivities of Guanidinatozinc Complexes and Their Catalytic Behavior in the Tishchenko Reaction. Organometallics 2013, 32, 3721–3727. [Google Scholar] [CrossRef]
- Coles, M.P.; Hitchcock, P.B. Zinc Guanidinate Complexes and Their Application in Ring-Opening Polymerisation Catalysis. Eur. J. Inorg. Chem. 2004, 2004, 2662–2672. [Google Scholar] [CrossRef]
- Barman, M.K.; Baishya, A.; Nembenna, S. Bulky guanidinate calcium and zinc complexes as catalysts for the intramolecular hydroamination. J. Organomet. Chem. 2019, 887, 40–47. [Google Scholar] [CrossRef]
- Scott, N.M.; Schareina, T.; Tok, O.; Kempe, R. Lithium and potassium amides of sterically demanding aminopyridines. Eur. J. Inorg. Chem. 2004, 2004, 3297–3304. [Google Scholar] [CrossRef]
- Burger, H.; Sawodny, W.; Wannagat, U. Darstellung und schwinkungsspektren von silylamiden der elemente zink, cadmium und quecksilber. J. Organomet. Chem. 1965, 3, 113–120. [Google Scholar] [CrossRef]
- Kottke, T.; Stalke, D. Crystal handling at low temperatures. J. Appl. Cryst. 1993, 26, 615–619. [Google Scholar] [CrossRef]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR 97: A new tool for crystal determination and refinement. J. Appl. Cryst. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX Suite for small–molecule single–crystal crystallography. J. Appl. Cryst. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer 17; University of Western Australia: Pert, WA, Australia, 2017. [Google Scholar]
- Kretschmer, W.P.; Hessen, B.; Noor, A.; Scott, N.M.; Kempe, R. Highly active/selective and adjustable zirconium polymerization catalysts stabilized by aminopyridinato ligands. J. Organom. Chem. 2007, 692, 4569–4579. [Google Scholar] [CrossRef]
- Noor, A. Homoleptic chromium(II) aminopyridinates: Transoid vs. cisoid coordination. Inorg. Chim. Acta 2025, 574, 122371. [Google Scholar] [CrossRef]
- Deeken, S.; Motz, G.; Kempe, R. How common are true aminopyridinato complexes? Z. Anorg. Allg. Chem. 2007, 633, 320–325. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(i) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4 (2007). Dalton Trans. 2007, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Noor, A. Crystallographic Evidence of κ1-coordination of bulky aminopyridine in halide-containing iron (II) complexes. Crystals 2022, 12, 697. [Google Scholar] [CrossRef]
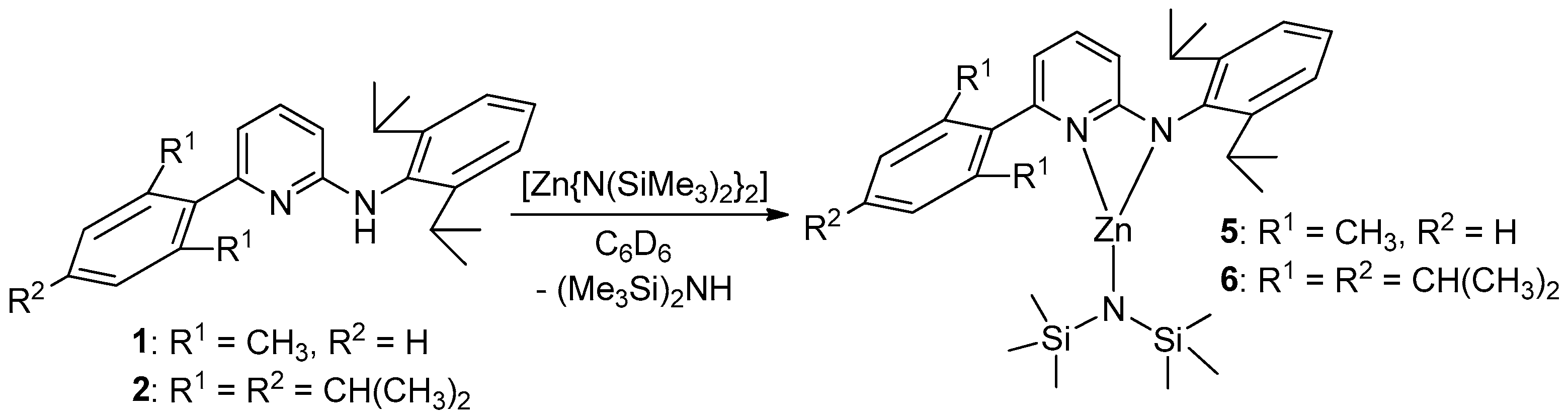



| 3 | 4 | |
|---|---|---|
| Empirical formula | C50H58N4Zn | C64H86N4Zn |
| Formula weight | 780.37 | 976.73 |
| Crystal system | triclinic | monoclinic |
| Space group | P-1 | P21/n |
| a [Å] | 10.244(2) | 20.7130(8) |
| b [Å] | 10.879(2) | 23.0200(7) |
| c [Å] | 20.759(4) | 24.7280(8) |
| α [°] | 82.30(3) | 90 |
| β [°] | 86.28(3) | 97.655(3) |
| γ [°] | 64.44(3) | 90 |
| V, [Å3] | 2064.2(9) | 11,685.6(7) |
| Crystal size, [mm3] | 0.23 × 0.13 × 0.12 | 0.51 × 0.36 × 0.31 |
| ρcalcd, [g cm−3] | 1.256 | 1.110 |
| µ, [mm−1] (Mo Kα) | 0.635 | 0.461 |
| T, [K] | 133(2) | 133(2) |
| 2θ range, [°] | 3.95–51.20 | 2.41–53.27 |
| No. of reflections unique | 8290 | 23,353 |
| No. of reflections obs. [I > 2σ (I)] | 4040 | 12,956 |
| No. of parameters | 509 | 1283 |
| wR2 (all data) | 0.1675 | 0.1498 |
| R value [I > 2σ (I)] | 0.0566 | 0.0571 |
| Goodness of fit | 0.908 | 0.998 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noor, A.; Qayyum, S. Structural Characteristics of Homoleptic Zinc Complexes Incorporating Asymmetric Aminopyridinates. Crystals 2025, 15, 821. https://doi.org/10.3390/cryst15090821
Noor A, Qayyum S. Structural Characteristics of Homoleptic Zinc Complexes Incorporating Asymmetric Aminopyridinates. Crystals. 2025; 15(9):821. https://doi.org/10.3390/cryst15090821
Chicago/Turabian StyleNoor, Awal, and Sadaf Qayyum. 2025. "Structural Characteristics of Homoleptic Zinc Complexes Incorporating Asymmetric Aminopyridinates" Crystals 15, no. 9: 821. https://doi.org/10.3390/cryst15090821
APA StyleNoor, A., & Qayyum, S. (2025). Structural Characteristics of Homoleptic Zinc Complexes Incorporating Asymmetric Aminopyridinates. Crystals, 15(9), 821. https://doi.org/10.3390/cryst15090821







