Polydianiline (PDANI) from a Safe Precursor: Dopant-Driven Control of Structure and Electroactivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
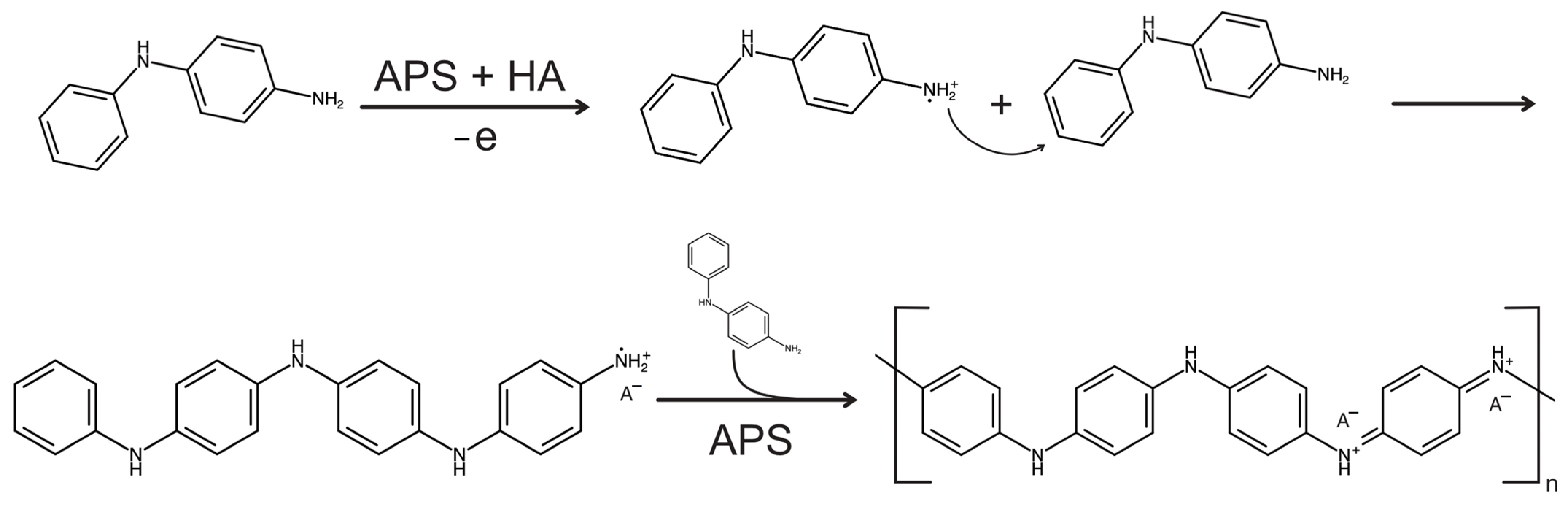
2.2. Synthesis of Pdani Polymers
2.3. Characterization Techniques
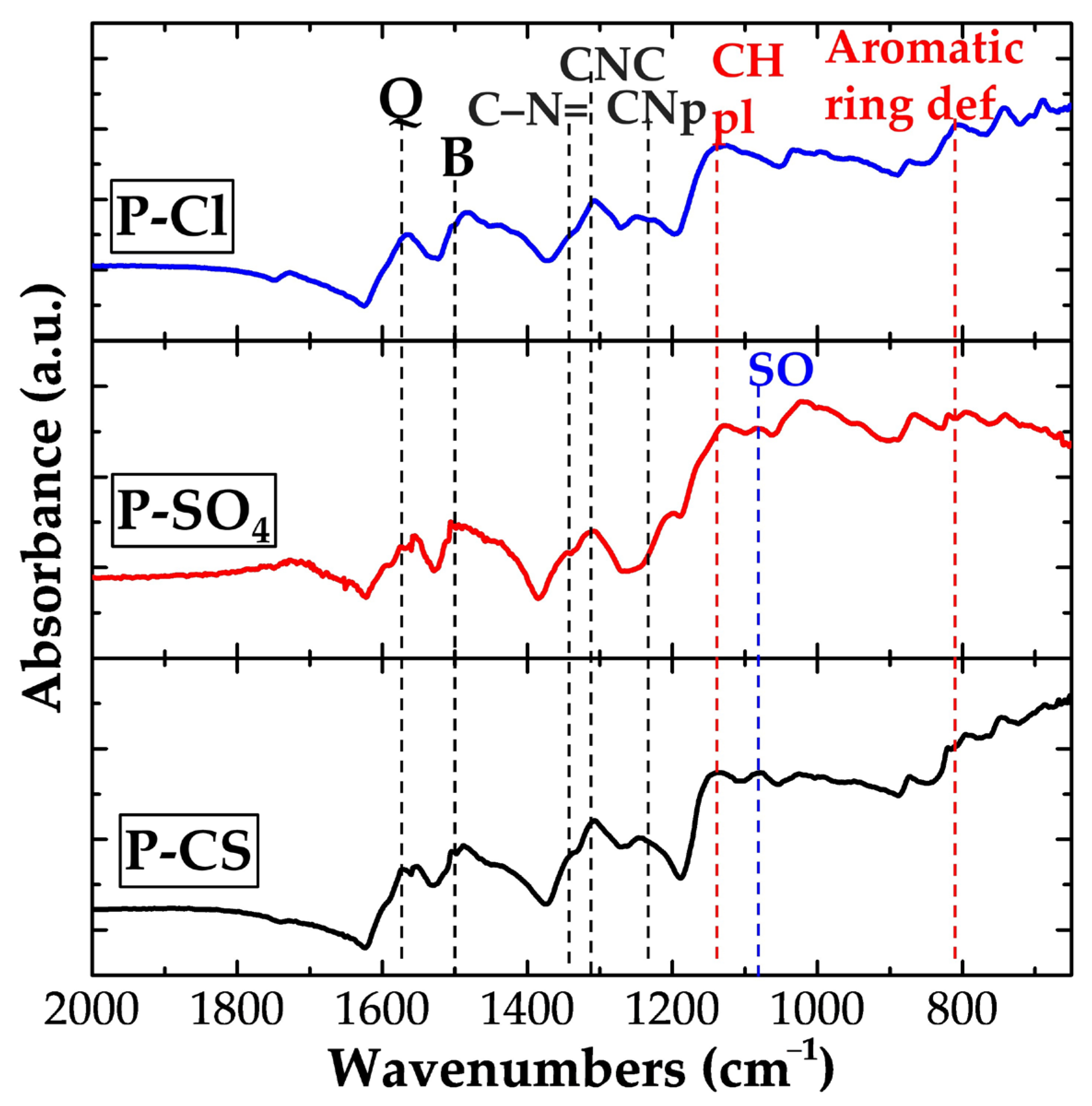
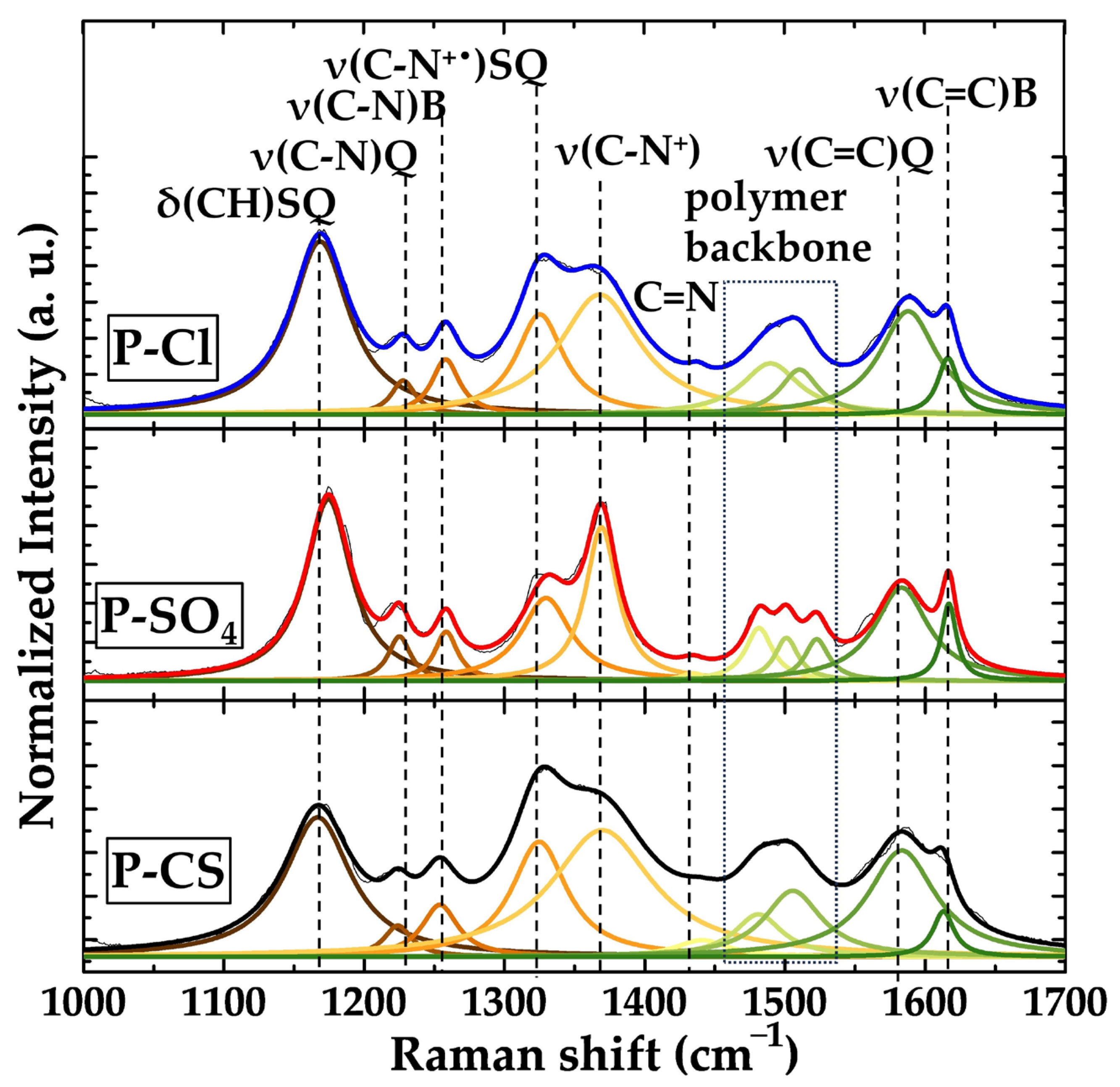
3. Results
- Anion exchange (typical for small dopants like Cl− and SO42−):
- Cation exchange (expected for bulky dopants like camphor sulfonate, CS−):
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of Electrically Conducting Organic Polymers: Halogen Derivatives of Polyacetylene, (CH) X. J. Chem. Soc. Chem. Commun. 1977, 16, 578–580. [Google Scholar] [CrossRef]
- Popov, A.; Brasiunas, B.; Mikoliunaite, L.; Bagdziunas, G.; Ramanavicius, A.; Ramanaviciene, A. Comparative Study of Polyaniline (PANI), Poly(3,4-Ethylenedioxythiophene) (PEDOT) and PANI-PEDOT Films Electrochemically Deposited on Transparent Indium Thin Oxide Based Electrodes. Polymer 2019, 172, 133–141. [Google Scholar] [CrossRef]
- Kumar, V.; Mirzaei, A.; Bonyani, M.; Kim, K.H.; Kim, H.W.; Kim, S.S. Advances in Electrospun Nanofiber Fabrication for Polyaniline (PANI)-Based Chemoresistive Sensors for Gaseous Ammonia. TrAC Trends Anal. Chem. 2020, 129, 115938. [Google Scholar] [CrossRef]
- Mazzara, F.; Patella, B.; D’agostino, C.; Bruno, M.G.; Carbone, S.; Lopresti, F.; Aiello, G.; Torino, C.; Vilasi, A.; O’riordan, A.; et al. Pani-Based Wearable Electrochemical Sensor for Ph Sweat Monitoring. Chemosensors 2021, 9, 169. [Google Scholar] [CrossRef]
- Politi, S.; Carcione, R.; Tamburri, E.; Matassa, R.; Lavecchia, T.; Angjellari, M.; Terranova, M.L. Graphene Platelets from Shungite Rock Modulate Electropolymerization and Charge Storage Mechanisms of Soft-Template Synthetized Polypyrrole-Based Nanocomposites. Sci. Rep. 2018, 8, 17045. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.H.; Niu, C.M.; Shi, J.Q.; Wang, Y.Y.; Yang, Y.M.; Wang, H.B. Novel Conductive Polypyrrole/Silk Fibroin Scaffold for Neural Tissue Repair. Neural Regen. Res. 2018, 13, 1455. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, Y.; Ng, E.L.L.; Zhang, Y.; Owh, C.; Wang, F.; Song, Q.; Feng, T.; Zhang, B.; Li, P.; et al. Progress and Opportunities in Additive Manufacturing of Electrically Conductive Polymer Composites. Mater. Today Adv. 2023, 17, 100333. [Google Scholar] [CrossRef]
- Liao, G.; Li, Q.; Xu, Z. The Chemical Modification of Polyaniline with Enhanced Properties: A Review. Prog. Org. Coat. 2019, 126, 35–43. [Google Scholar] [CrossRef]
- Carcione, R.; Marasso, S.L.; Guglielmotti, V.; Cocuzza, M.; Tamburri, E.; Battistoni, S. One-Pot and Mask-Less Realization Approach for Polypyrrole-Polydopamine-Based Organic Electrochemical Transistors. ACS Appl. Electron. Mater. 2025, 7, 2619–2628. [Google Scholar] [CrossRef]
- Scordo, G.; Bertana, V.; Ballesio, A.; Carcione, R.; Marasso, S.L.; Cocuzza, M.; Pirri, C.F.; Manachino, M.; Gomez, M.G.; Vitale, A.; et al. Effect of Volatile Organic Compounds Adsorption on 3D-Printed Pegda:Pedot for Long-Term Monitoring Devices. Nanomaterials 2021, 11, 94. [Google Scholar] [CrossRef]
- Cavallo, A.; Losi, P.; Buscemi, M.; Kayal, T.A.; Beccatelli, M.; Soldani, G.; Coppedè, N. Biocompatible Organic Electrochemical Transistor on Polymeric Scaffold for Wound Healing Monitoring. Flex. Print. Electron. 2022, 7, 035009. [Google Scholar] [CrossRef]
- Colucci, R.; Feitosa, B.A.; Faria, G.C. Impact of Ionic Species on the Performance of Pedot:PSS-Based Organic Electrochemical Transistors. Adv. Electron. Mater. 2024, 10, 2300235. [Google Scholar] [CrossRef]
- Battistoni, S.; Erokhin, V.; Iannotta, S. Frequency Driven Organic Memristive Devices for Neuromorphic Short Term and Long Term Plasticity. Org. Electron. 2019, 65, 434–438. [Google Scholar] [CrossRef]
- Doan, T.C.D.; Ramaneti, R.; Baggerman, J.; Van Der Bent, J.F.; Marcelis, A.T.M.; Tong, H.D.; Van Rijn, C.J.M. Carbon Dioxide Sensing with Sulfonated Polyaniline. Sens. Actuators B Chem. 2012, 168, 123–130. [Google Scholar] [CrossRef]
- Passeri, D.; Tamburri, E.; Terranova, M.L.; Rossi, M. Polyaniline–Nanodiamond Fibers Resulting from the Self-Assembly of Nano-Fibrils: A Nanomechanical Study. Nanoscale 2015, 7, 14358–14367. [Google Scholar] [CrossRef] [PubMed]
- Medi, B.; Bahramian, A.; Nazari, V. Synthesis and Characterization of Conducting Polyaniline Nanostructured Thin Films for Solar Cell Applications. JOM 2021, 73, 504–514. [Google Scholar] [CrossRef]
- Kamarudin, S.; Rani, M.S.A.; Mohammad, M.; Mohammed, N.H.; Su’ait, M.S.; Ibrahim, M.A.; Asim, N.; Razali, H. Investigation on Size and Conductivity of Polyaniline Nanofiber Synthesised by Surfactant-Free Polymerization. J. Mater. Res. Technol. 2021, 14, 255–261. [Google Scholar] [CrossRef]
- Wei, X.; Epstein, A.J. Synthesis of Highly Sulfonated Polyaniline. Synth. Met. 1995, 74, 123–125. [Google Scholar] [CrossRef]
- Chiolerio, A.; Bocchini, S.; Scaravaggi, F.; Porro, S.; Perrone, D.; Beretta, D.; Caironi, M.; Fabrizio Pirri, C. Synthesis of Polyaniline-Based Inks for Inkjet Printed Devices: Electrical Characterization Highlighting the Effect of Primary and Secondary Doping. Semicond. Sci. Technol. 2015, 30, 104001. [Google Scholar] [CrossRef]
- Pyarasani, R.D.; Jayaramudu, T.; John, A. Polyaniline-Based Conducting Hydrogels. J. Mater. Sci. 2018, 54, 974–996. [Google Scholar] [CrossRef]
- Yasuda, A.; Shimidzu, T. Chemical and Electrochemical Analyses of Polyaniline Prepared with FeCl3. Synth. Met. 1993, 61, 239–245. [Google Scholar] [CrossRef]
- Suendo, V.; Lau, Y.; Hidayat, F.; Reza, M.; Qadafi, A.; Rochliadi, A. Effect of Face-to-Face and Side-to-Side Interchain Interactions on the Electron Transport in Emeraldine Salt Polyaniline. Phys. Chem. Chem. Phys. 2021, 23, 7190–7199. [Google Scholar] [CrossRef]
- Banjar, M.F.; Joynal Abedin, F.N.; Fizal, A.N.S.; Muhamad Sarih, N.; Hossain, M.S.; Osman, H.; Khalil, N.A.; Ahmad Yahaya, A.N.; Zulkifli, M. Synthesis and Characterization of a Novel Nanosized Polyaniline. Polymers 2023, 15, 4565. [Google Scholar] [CrossRef]
- Xia, Y.; Wiesinger, J.M.; MacDiarmid, A.G.; Epstein, A.J. Camphorsulfonic acid fully doped polyaniline emeraldine salt: Conformations in different solvents studied by an ultraviolet/visible/near-infrared spectroscopic method. Chem. Mater. 1995, 7, 433–445. [Google Scholar] [CrossRef]
- Jamadade, V.S.; Dhawale, D.S.; Lokhande, C.D. Studies on Electrosynthesized Leucoemeraldine, Emeraldine and Pernigraniline Forms of Polyaniline Films and Their Supercapacitive Behavior. Synth. Met. 2010, 160, 955–960. [Google Scholar] [CrossRef]
- Sanches, E.A.; Soares, J.C.; Iost, R.M.; Marangoni, V.S.; Trovati, G.; Batista, T.; Mafud, A.C.; Zucolotto, V.; Mascarenhas, Y.P. Structural Characterization of Emeraldine-Salt Polyaniline/Gold Nanoparticles Complexes. J. Nanomater. 2011, 2011, 697071. [Google Scholar] [CrossRef]
- Junker, K.; Luginbühl, S.; Schüttel, M.; Bertschi, L.; Kissner, R.; Schuler, L.D.; Rakvin, B.; Walde, P. Efficient Polymerization of the Aniline Dimer p -Aminodiphenylamine (PADPA) with Trametes Versicolor Laccase/O2 as Catalyst and Oxidant and AOT Vesicles as Templates. ACS Catal. 2014, 4, 3421–3434. [Google Scholar] [CrossRef]
- Mir, A.; Kumar, A.; Riaz, U. A Short Review on the Synthesis and Advance Applications of Polyaniline Hydrogels. RSC Adv. 2022, 12, 19122–19132. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Nandy, S.; Fortunato, E.; Martins, R. Polyaniline and Its Composites Engineering: A Class of Multifunctional Smart Energy Materials. J. Solid State Chem. 2023, 317, 123679. [Google Scholar] [CrossRef]
- Montaina, L.; Carcione, R.; Pescosolido, F.; Montalto, M.; Battistoni, S.; Tamburri, E. Three-Dimensional-Printed Polyethylene Glycol Diacrylate-Polyaniline Composites by in Situ Aniline Photopolymerization: An Innovative Biomaterial for Electrocardiogram Monitoring Systems. ACS Appl. Electron. Mater. 2022, 5, 164–172. [Google Scholar] [CrossRef]
- Demin, V.A.; Erokhin, V.V.; Emelyanov, A.V.; Battistoni, S.; Baldi, G.; Iannotta, S.; Kashkarov, P.K.; Kovalchuk, M.V. Hardware Elementary Perceptron Based on Polyaniline Memristive Devices. Org. Electron. 2015, 25, 16–20. [Google Scholar] [CrossRef]
- Li, H.; Zhou, J.; Lu, X.; Wang, J.; Qu, S.; Weng, J.; Feng, B. Camphorsulfonic Acid-Doped Polyaniline/TiO2 Nanotube Hybrids: Synthesis Strategy and Enhanced Visible Photocatalytic Activity. J. Mater. Sci. Mater. Electron. 2015, 26, 7723–7730. [Google Scholar] [CrossRef]
- Poddar, A.K.; Patel, S.S.; Patel, H.D. Synthesis, Characterization and Applications of Conductive Polymers: A Brief Review. Polym. Adv. Technol. 2021, 32, 4616–4641. [Google Scholar] [CrossRef]
- Carcione, R.; Pescosolido, F.; Montaina, L.; Toschi, F.; Orlanducci, S.; Tamburri, E.; Battistoni, S. Self-Standing 3D-Printed PEGDA–PANIs Electroconductive Hydrogel Composites for PH Monitoring. Gels 2023, 9, 784. [Google Scholar] [CrossRef]
- Politi, S.; Battistoni, S.; Carcione, R.; Montaina, L.; Macis, S.; Lupi, S.; Tamburri, E. PANI-Modified Ti-Doped CVD Diamond As Promising Conductive Platform to Mimic Bioelectricity Functions. Adv. Mater. Interfaces 2021, 8, 2101401. [Google Scholar] [CrossRef]
- Korent, A.; Žagar Soderžnik, K.; Šturm, S.; Žužek Rožman, K. A Correlative Study of Polyaniline Electropolymerization and Its Electrochromic Behavior. J. Electrochem. Soc. 2020, 167, 106504. [Google Scholar] [CrossRef]
- Giacobbe, S.; Pezzella, C.; Della Ventura, B.; Giacobelli, V.G.; Rossi, M.; Fontanarosa, C.; Amoresano, A.; Sannia, G.; Velotta, R.; Piscitelli, A. Green Synthesis of Conductive Polyaniline by Trametes Versicolor Laccase Using a DNA Template. Eng. Life Sci. 2019, 19, 631–642. [Google Scholar] [CrossRef]
- Ranjith Kumar, D.; Dhakal, G.; Muhammed Shafi, P.; Saad Sayed, M.; Lee, J.; Lee, Y.R.; Shim, J.J. Sulfite Food Additive Electrochemical Determination by Nucleophilic Addition on Poly(4-Aminodiphenylamine)-4-Aminothiophenol-Au Composite Electrode. Microchem. J. 2022, 181, 107635. [Google Scholar] [CrossRef]
- Carcione, R.; Roppolo, I.; Chiappone, A.; Cocuzza, M.; Marasso, S.L.; Tamburri, E.; Battistoni, S. Role of Precursors and Doping Agents in Producing 3D-Printed PEGDA-PDANI Electroactive Composites by an In Situ Polymerization Approach. ACS Appl. Polym. Mater. 2024, 6, 1159–1168. [Google Scholar] [CrossRef]
- Li, X.G.; Huang, M.R.; Duan, W.; Yang, Y.L. Novel Multifunctional Polymers from Aromatic Diamines by Oxidative Polymerizations. Chem. Rev. 2002, 102, 2925–3030. [Google Scholar] [CrossRef]
- Zhao, R.; Peng, H.; Wang, F.; Zhen, J.; Li, L.; Ma, G.; Lei, Z. Regulating the Species and the Counter-Ion Size of Proton Acids to Prepare Novel Poly(4-Aminodiphenylamine) Nanomaterials for Supercapacitors. Mater. Chem. Front. 2021, 5, 6145–6151. [Google Scholar] [CrossRef]
- Lin, C.W.; Mak, W.H.; Chen, D.; Wang, H.; Aguilar, S.; Kaner, R.B. Catalytic Effects of Aniline Polymerization Assisted by Oligomers. ACS Catal. 2019, 9, 6596–6606. [Google Scholar] [CrossRef]
- Peng, H.; Zhao, R.; Liang, J.; Wang, S.; Wang, F.; Zhou, J.; Ma, G.; Lei, Z. Template-Confined Growth of Poly(4-Aminodiphenylamine) Nanosheets as Positive Electrode toward Superlong-Life Asymmetric Supercapacitor. ACS Appl. Mater. Interfaces 2018, 10, 37125–37134. [Google Scholar] [CrossRef]
- Pescosolido, F.; Montaina, L.; Carcione, R.; Politi, S.; Matassa, R.; Carotenuto, F.; Nottola, S.A.; Nardo, P.D.; Tamburri, E. A New Strong-Acid Free Route to Produce Xanthan Gum-PANI Composite Scaffold Supporting Bioelectricity. Macromol. Biosci. 2023, 23, 2300132. [Google Scholar] [CrossRef]
- Politi, S.; Tamburri, E.; Carcione, R.; Lavecchia, T.; Angjellari, M.; Terranova, M.L. Innovative Preparation Processes and Structural Characteristics of 3D Printable Polymer-Based Nanocomposites. In Proceedings of the AIP Conference Proceedings, Lakeland, FL, USA, 17–19 January 2019; Volume 2196. [Google Scholar]
- Galloni, M.G.; Della Pina, C.; Bortolotto, V.; Nikonova, V.; Falletta, E.; Bianchi, C.L. Highly Porous Polyaniline (PANI): A Novel Green Catalytic Method for Morphology Control. J. Mater. Sci. 2025, 60, 5300–5325. [Google Scholar] [CrossRef]
- Zhu, D.; Cheng, K.; Wang, Y.; Sun, D.; Gan, L.; Chen, T.; Jiang, J.; Liu, M. Nitrogen-Doped Porous Carbons with Nanofiber-like Structure Derived from Poly (Aniline-Co-p-Phenylenediamine) for Supercapacitors. Electrochim. Acta 2017, 224, 17–24. [Google Scholar] [CrossRef]
- Levi, M.D.; Lapkowski, M. Mechanism of Electron Transfer during the Course of Oxidation of N-Phenyl-p-Phenylenediamine Polythiophene and Poly-3-Methylthiophene Coated Electrodes: Redox Catalysis versus Outer-Sphere Heterogeneous Electron Transfer. Electrochim. Acta 1993, 38, 271–279. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Ko, T.H.; Huang, W.Y.; Hsieh, T.H.; Ho, K.S.; Chen, Y.Y.; Hsieh, S.J. Preparation of Pt-Catalyst by Poly(p-Phenylenediamine) Nanocomposites Assisted by Microwave Radiation for Proton Exchange Membrane Fuel Cell. Polymers 2018, 10, 1388. [Google Scholar] [CrossRef]
- Tsai, M.J.; Hsieh, T.H.; Wang, Y.Z.; Ho, K.S.; Chang, C.Y. Microwave Assisted Reduction of Pt-Catalyst by N-Phenyl-p-Phenylenediamine for Proton Exchange Membrane Fuel Cells. Polymers 2017, 9, 104. [Google Scholar] [CrossRef]
- Yao, C.J.; Xie, J.; Wu, Z.; Xu, Z.J.; Zhang, S.; Zhang, Q. A Conjugated Copolymer of N-Phenyl-p-Phenylenediamine and Pyrene as Promising Cathode for Rechargeable Lithium–Ion Batteries. Chem. Asian J. 2019, 14, 2210–2214. [Google Scholar] [CrossRef]
- Stejskal, J.; Trchová, M.; Bober, P.; Humpolíček, P.; Kašpárková, V.; Sapurina, I.; Shishov, M.A.; Varga, M. Conducting Polymers: Polyaniline. Encycl. Polym. Sci. Technol. 2015, 35, 1–44. [Google Scholar] [CrossRef]
- Yuan, J.; Hu, X.; Zhao, X.; Yin, J. Electrorheological Effect of Suspensions of Polyaniline Nanoparticles with Different Morphologies. Polymers 2023, 15, 4568. [Google Scholar] [CrossRef]
- Su, N. Polyaniline-Doped Spherical Polyelectrolyte Brush Nanocomposites with Enhanced Electrical Conductivity, Thermal Stability, and Solubility Property. Polymers 2015, 7, 1599–1616. [Google Scholar] [CrossRef]
- Shao, W.; Jamal, R.; Xu, F.; Ubul, A.; Abdiryim, T. The Effect of a Small Amount of Water on the Structure and Electrochemical Properties of Solid-State Synthesized Polyaniline. Materials 2012, 5, 1811–1825. [Google Scholar] [CrossRef]
- Prabhu, R.; Jeevananda, T.; Reddy, K.R.; Raghu, A.V. Polyaniline-Fly Ash Nanocomposites Synthesized via Emulsion Polymerization: Physicochemical, Thermal and Dielectric Properties. Mater. Sci. Energy Technol. 2021, 4, 107–112. [Google Scholar] [CrossRef]
- Aziz, D.M.; Hassan, S.A.; Aziz, S.B. Synthesis and Characterization of Enhanced Azo-Azomethine Doped PANI/HCl Conducting Polymers for Electrochemical Applications. Sci. Rep. 2024, 14, 18122. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Yang, Z.; Wang, Z.; Zhang, F.; Wang, S. A Novel Strategy for the Synthesis of Polyaniline Nanostructures with Controlled Morphology. React. Funct. Polym. 2008, 68, 1435–1440. [Google Scholar] [CrossRef]
- Tamburri, E.; Guglielmotti, V.; Orlanducci, S.; Terranova, M.L.; Sordi, D.; Passeri, D.; Matassa, R.; Rossi, M. Nanodiamond-Mediated Crystallization in Fibers of PANI Nanocomposites Produced by Template-Free Polymerization: Conductive and Thermal Properties of the Fibrillar Networks. Polymer 2012, 53, 4045–4053. [Google Scholar] [CrossRef][Green Version]
- Lindfors, T.; Ivaska, A. Raman Based PH Measurements with Polyaniline. J. Electroanal. Chem. 2005, 580, 320–329. [Google Scholar] [CrossRef]
- Shakoor, A.; Rizvi, T.Z.; Nawaz, A. Raman Spectroscopy and AC Conductivity of Polyaniline Montmorillonite (PANI-MMT) Nanocomposites. J. Mater. Sci. Mater. Electron. 2011, 22, 1076–1080. [Google Scholar] [CrossRef]
- Bednarczyk, K.; Matysiak, W.; Tański, T.; Janeczek, H.; Schab-Balcerzak, E.; Libera, M. Effect of Polyaniline Content and Protonating Dopants on Electroconductive Composites. Sci. Rep. 2021, 11, 7487. [Google Scholar] [CrossRef]
- Zhou, S.X.; Tao, X.Y.; Ma, J.; Qu, C.H.; Zhou, Y.; Guo, L.T.; Feng, P.Z.; Zhu, Y.B.; Wei, X.Y. Facile Synthesis of Self-Assembled Polyaniline Nanorods Doped with Sulphuric Acid for High-Performance Supercapacitors. Vacuum 2017, 143, 63–70. [Google Scholar] [CrossRef]
- Shen, Y.; Qin, Z.; Li, T.; Zeng, F.; Chen, Y.; Liu, N. Boosting the Supercapacitor Performance of Polyaniline Nanofibers through Sulfonic Acid Assisted Oligomer Assembly during Seeding Polymerization Process. Electrochim. Acta 2020, 356, 136841. [Google Scholar] [CrossRef]
- Krinichnyi, V.I.; Roth, H.K.; Schrödner, M.; Wessling, B. EPR Study of Polyaniline Highly Doped by P-Toluenesulfonic Acid. Polymer 2006, 47, 7460–7468. [Google Scholar] [CrossRef]
- Gupta, S.K.; Luthra, V.; Singh, R. Electrical Transport and EPR Investigations: A Comparative Study for d.c. Conduction Mechanism in Monovalent and Multivalent Ions Doped Polyaniline. Bull. Mater. Sci. 2012, 35, 787–794. [Google Scholar] [CrossRef]
- Krinichnyi, V.; Chemerisov, S.; Lebedev, Y.S. EPR and Charge-Transport Studies of Polyaniline. Phys. Rev. B 1997, 55, 16233. [Google Scholar] [CrossRef]
- Krinichnyi, V.I.; Roth, H.K.; Hinrichsen, G.; Lux, F.; Lüders, K. EPR and Charge Transfer in H2SO4-Doped Polyaniline. Phys. Rev. B 2002, 65, 155205. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, Z.; Wan, M. Nanostructures of Polyaniline Doped with Inorganic Acids. Macromolecules 2002, 35, 5937–5942. [Google Scholar] [CrossRef]
- Sinha, S.; Bhadra, S.; Khastgir, D. Effect of Dopant Type on the Properties of Polyaniline. J. Appl. Polym. Sci. 2009, 112, 3135–3140. [Google Scholar] [CrossRef]
- Arora, M.; Luthra, V.; Singh, R.; Gupta, S.K. Vibrational Spectra of Acid-Doped Polyaniline Study of Vibrational Spectra of Polyaniline Doped with Sulfuric Acid and Phosphoric Acid. Appl. Biochem. Biotechnol. 2001, 96, 173–181. [Google Scholar] [CrossRef]
- Nunes, W.G.; Pires, B.M.; Thaines, E.H.N.S.; Pereira, G.M.A.; da Silva, L.M.; Freitas, R.G.; Zanin, H. Operando Raman Spectroelectrochemical Study of Polyaniline Degradation: A Joint Experimental and Theoretical Analysis. J. Energy Storage 2022, 55, 105770. [Google Scholar] [CrossRef]
- Udayaraj, S.; Annappa, M.; Roopa, K.V.; Mathad, G.; Subramanya, K.; Pavithra, G.M. Optical, Structural and Conductivity Properties of Ferrous Sulfate Doped Polyaniline. Mater. Today Proc. 2024, 100, 132–138. [Google Scholar] [CrossRef]
- Ting, C.-Y.; Wu, P.-L.; Huang, C.-C.; Andreas, R.; Lesbani, A.; Yusuf, F.A. The Character Istics (Compositions, Morphological, and Structure) of Nanocomposites Polyaniline (PANI)/ZnO. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 012126. [Google Scholar] [CrossRef]
- Moulton, S.E.; Innis, P.C.; Kane-Maguire, L.A.P.; Ngamna, O.; Wallace, G.G. Polymerisation and Characterisation of Conducting Polyaniline Nanoparticle Dispersions. Curr. Appl. Phys. 2004, 4, 402–406. [Google Scholar] [CrossRef]
- Wong, P.Y.; Phang, S.W.; Baharum, A. Effects of Synthesised Polyaniline (PAni) Contents on the Anti-Static Properties of PAni-Based Polylactic Acid (PLA) Films. RSC Adv. 2020, 10, 39693–39699. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Wen, T.C.; Hu, C.C.; Gopalan, A. Identification of Inductive Behavior for Polyaniline via Electrochemical Impedance Spectroscopy. Electrochim. Acta 2002, 47, 1305–1315. [Google Scholar] [CrossRef]
- Kuzmany, H.; Sariciftci, N.S. In Situ Spectro-Electrochemical Studies of Polyaniline. Synth. Met. 1987, 18, 353–358. [Google Scholar] [CrossRef]
- Biabangard, F.; Nazari, H.; Arefinia, R. Effect of PH on the Electrochemical Properties of Polyaniline Nanoparticle Suspension in Strongly Acidic Solution: An Experimental and Theoretical Study. J. Solid State Electrochem. 2021, 25, 881–893. [Google Scholar] [CrossRef]
- Yan, B.; Yang, J.; Li, Y.; Cao, Y. Electrochemical Adsorption of Hydrogen and Various Ions on Polyaniline Film. Reactions Concerning the First Pair of Cyclic Voltammetric Peaks. Synth. Met. 1991, 44, 189–197. [Google Scholar] [CrossRef]
- Garrudo, F.F.F.; Ferreira, L.F.V.; Ferraria, A.M.; do Rego, A.M.B.; Charas, A.; André, V.; Duarte, M.T.; Linhardt, R.J.; Ferreira, F.C.; Morgado, J. Pseudo-Doping Effect on Structural and Electrical Properties of Polyaniline-Camphorsulfonic Acid. Synth. Met. 2024, 301, 117523. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, R.; Li, T.; Li, F.; Liu, Z.; Zheng, M.; Wang, B.; Yang, Z.; Luo, H.; Wan, Y. Application of Polyaniline for Li-Ion Batteries, Lithium–Sulfur Batteries, and Supercapacitors. ChemSusChem 2019, 12, 1591–1611. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.S.; Jeong, S.K.; Joo, J.; Kim, K.M. Polyaniline Doped with Dimethyl Sulfate as a Nucleophilic Dopant and Its Electrochemical Properties as an Electrode in a Lithium Secondary Battery and a Redox Supercapacitor. J. Phys. Chem. B 2007, 111, 731–739. [Google Scholar] [CrossRef] [PubMed]
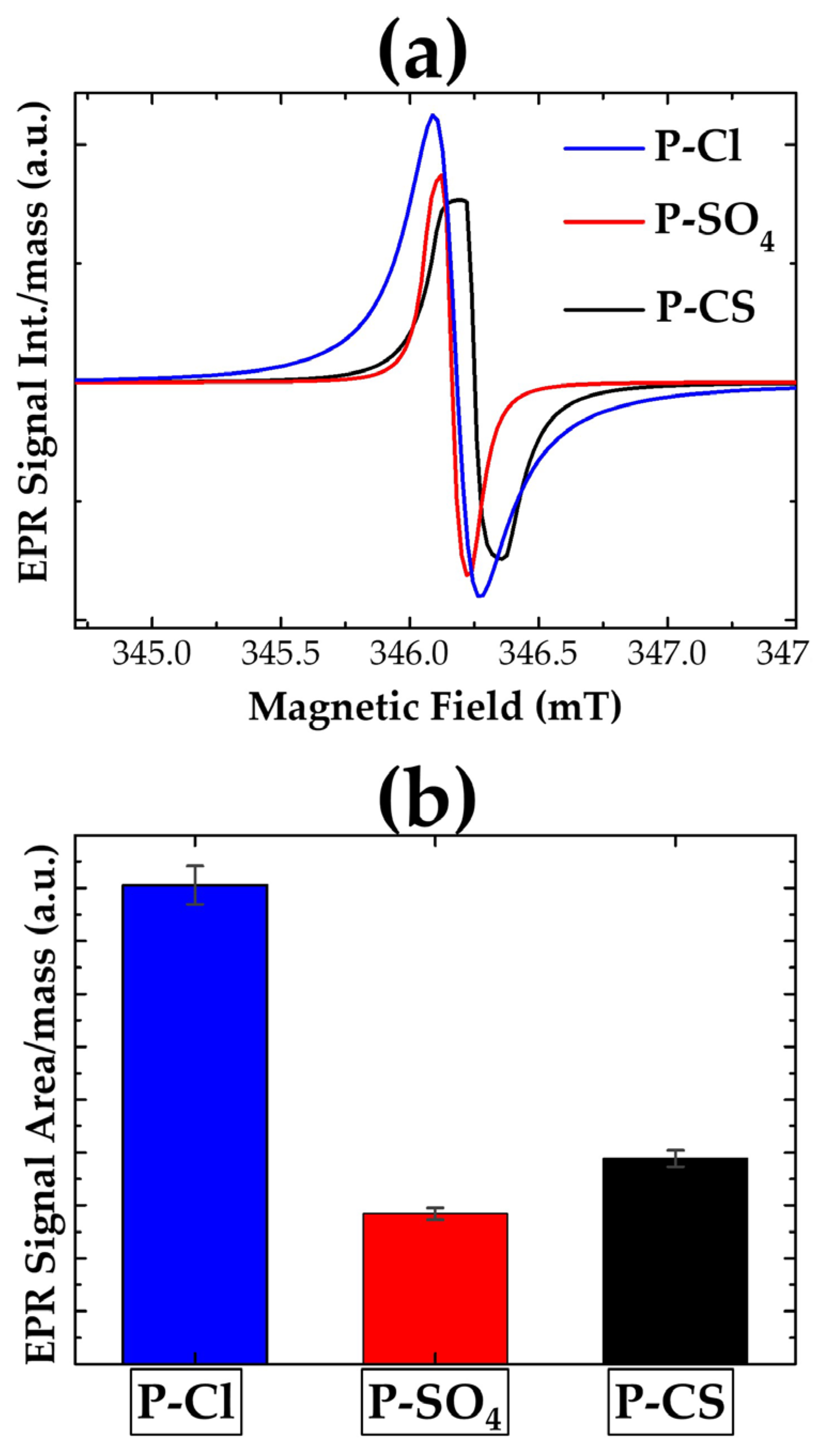
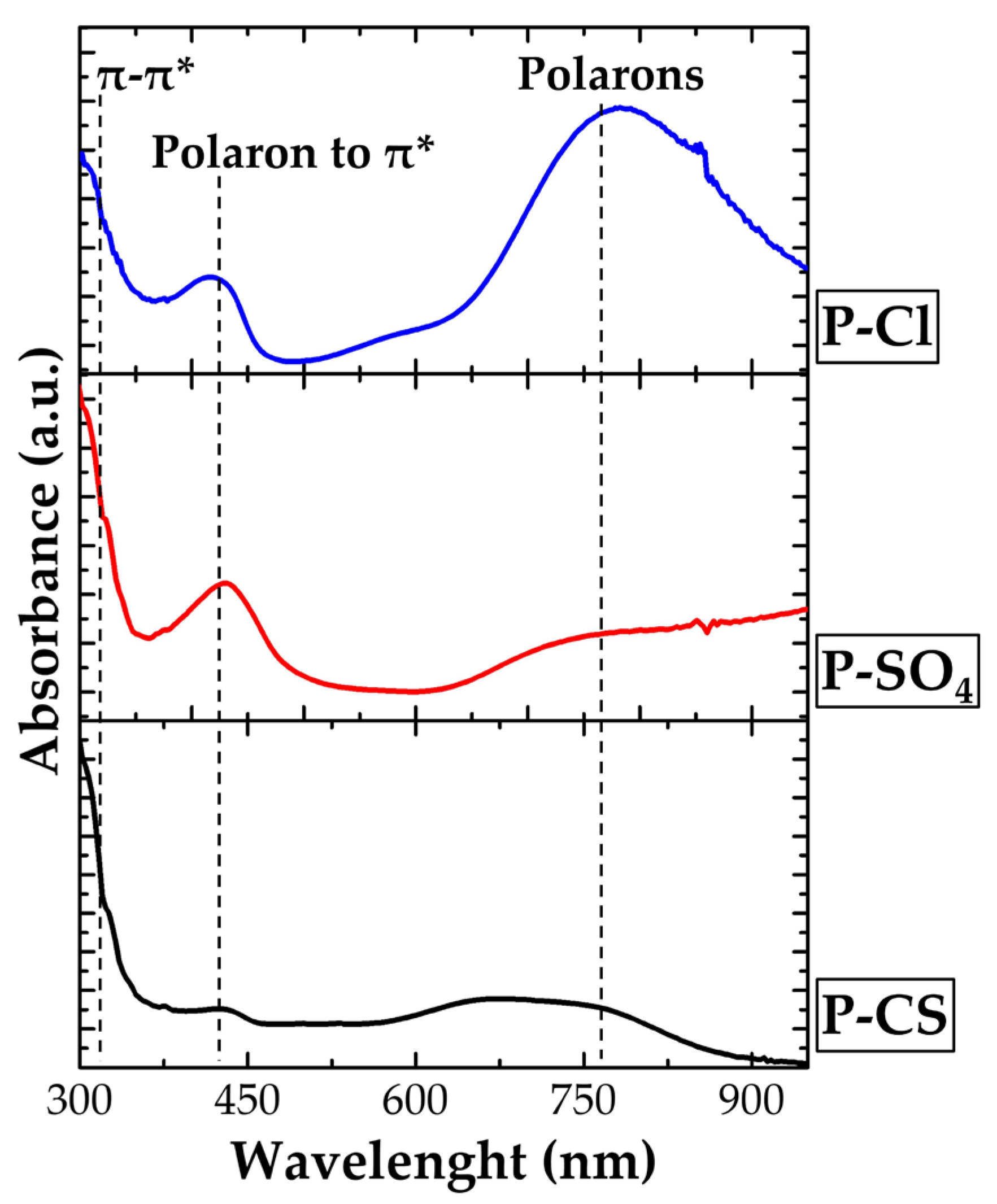
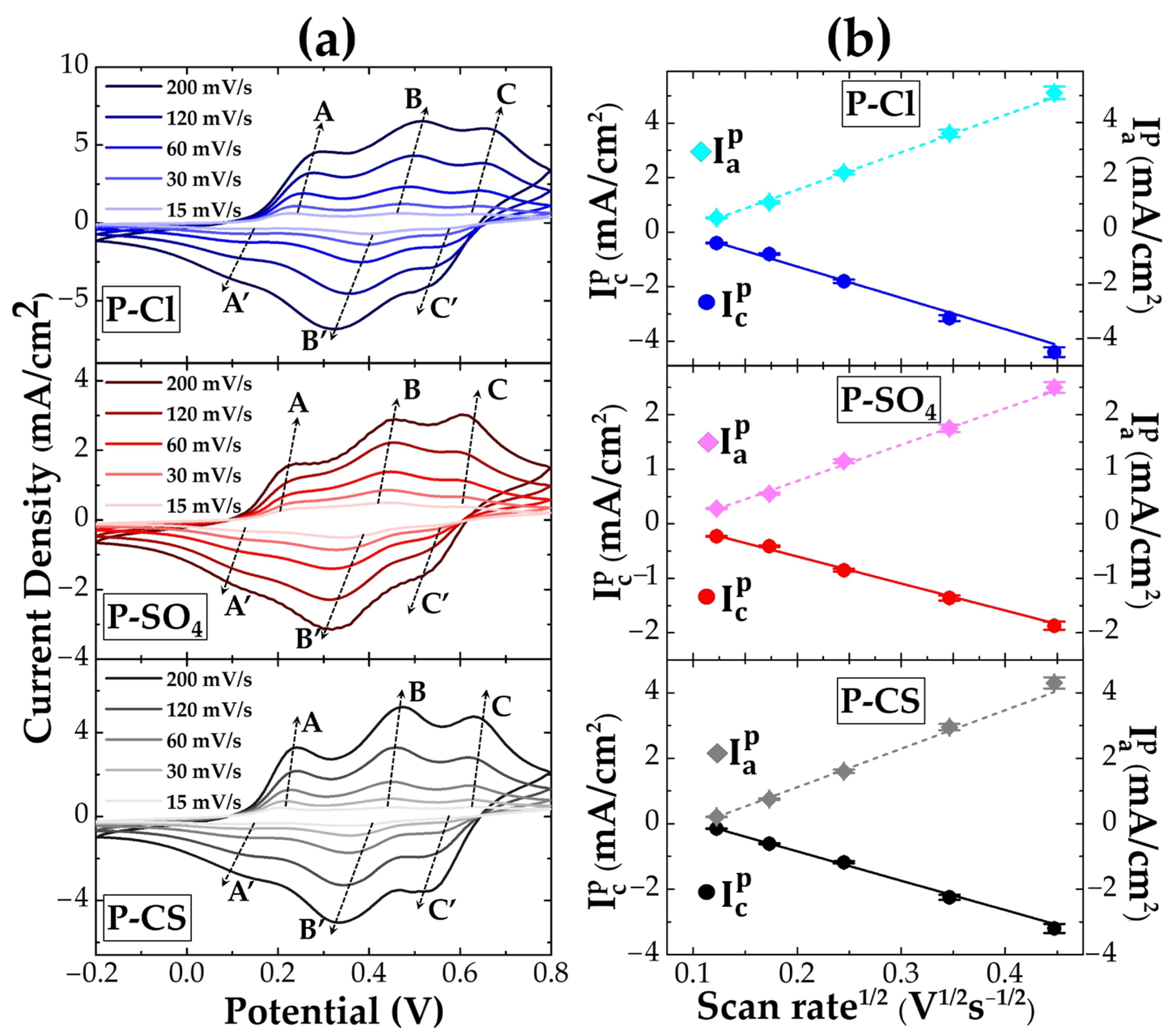
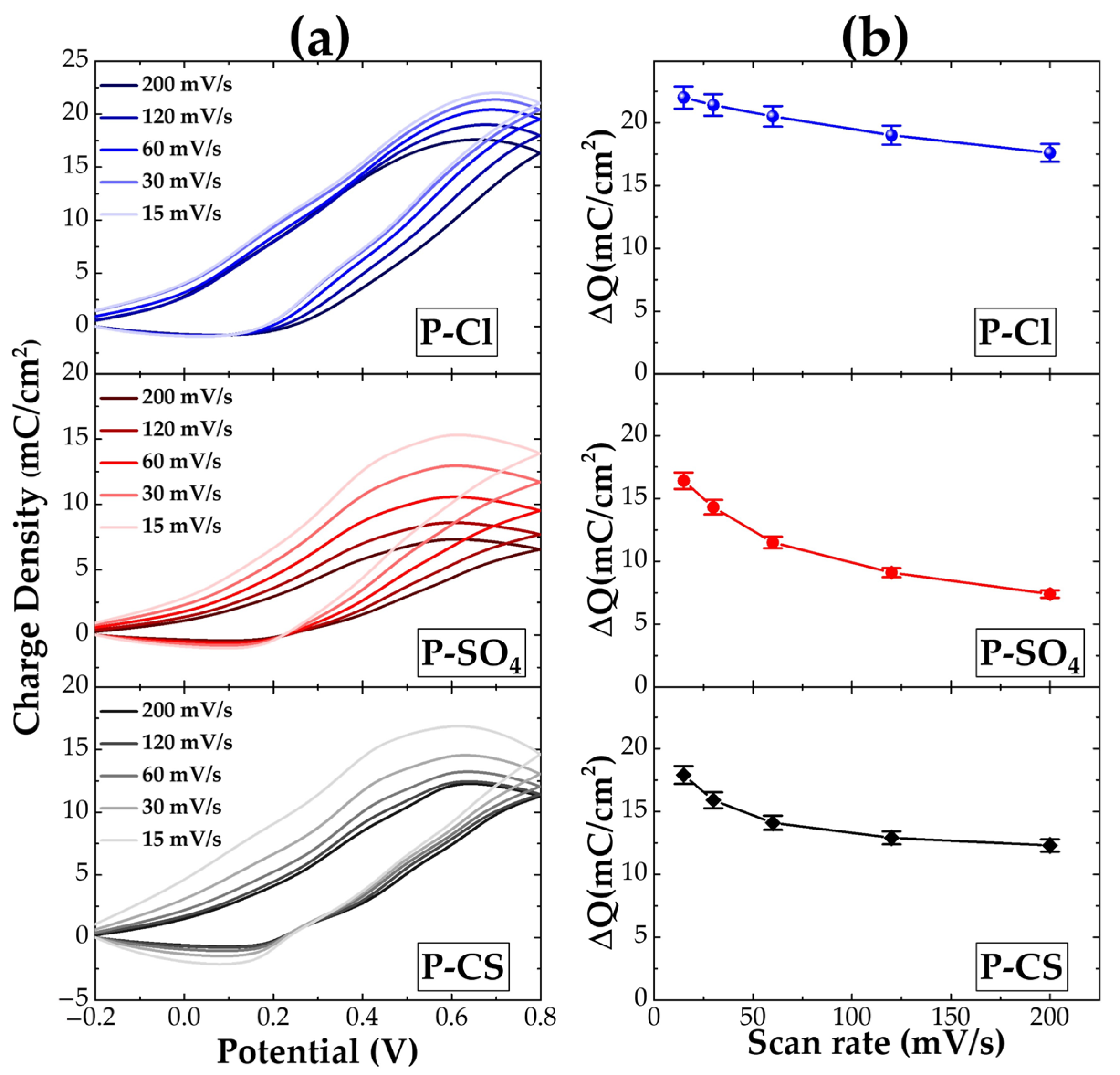
| Sample Name | Starting Solution | Oxidant Solution | Total Volume | Dopant Agent | Final Solution | ||
|---|---|---|---|---|---|---|---|
| DANI (mmol) | DMSO (mL) | APS (mmol) | Acidic Solution | mL | [DANI] (M) | ||
| P-Cl | 4 | 2 | 4 | HCl 1M | 80 | Cl− | 0.05 |
| P-SO4 | 4 | 2 | 4 | H2SO4 1M | 80 | SO42− | 0.05 |
| P-CS | 4 | 2 | 4 | CSA 1M | 80 | CS− | 0.05 |
| Sample | Equation | R2 |
|---|---|---|
| P-Cl | : y = −11.5x + 1.1 | 0.993 |
| : y = 13.8x − 1.2 | 0.989 | |
| P-SO4 | : y = −5x + 0.4 | 0.995 |
| : y = 6.7x − 0.5 | 0.994 | |
| P-CS | : y = −7.6x + 0.8 | 0.986 |
| : y = 10.3x − 1.1 | 0.991 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carcione, R.; Tamburri, E.; Scordo, G.; Pescosolido, F.; Montaina, L.; Palmieri, E.; Cemmi, A.; Battistoni, S. Polydianiline (PDANI) from a Safe Precursor: Dopant-Driven Control of Structure and Electroactivity. Crystals 2025, 15, 814. https://doi.org/10.3390/cryst15090814
Carcione R, Tamburri E, Scordo G, Pescosolido F, Montaina L, Palmieri E, Cemmi A, Battistoni S. Polydianiline (PDANI) from a Safe Precursor: Dopant-Driven Control of Structure and Electroactivity. Crystals. 2025; 15(9):814. https://doi.org/10.3390/cryst15090814
Chicago/Turabian StyleCarcione, Rocco, Emanuela Tamburri, Giorgio Scordo, Francesca Pescosolido, Luca Montaina, Elena Palmieri, Alessia Cemmi, and Silvia Battistoni. 2025. "Polydianiline (PDANI) from a Safe Precursor: Dopant-Driven Control of Structure and Electroactivity" Crystals 15, no. 9: 814. https://doi.org/10.3390/cryst15090814
APA StyleCarcione, R., Tamburri, E., Scordo, G., Pescosolido, F., Montaina, L., Palmieri, E., Cemmi, A., & Battistoni, S. (2025). Polydianiline (PDANI) from a Safe Precursor: Dopant-Driven Control of Structure and Electroactivity. Crystals, 15(9), 814. https://doi.org/10.3390/cryst15090814










