Micro-Arc Coatings with Different Types of Microparticles on Titanium Alloy: Formation, Structure, and Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Structural, Morphological, and Mechanical Properties of Coatings
3. Results and Discussions
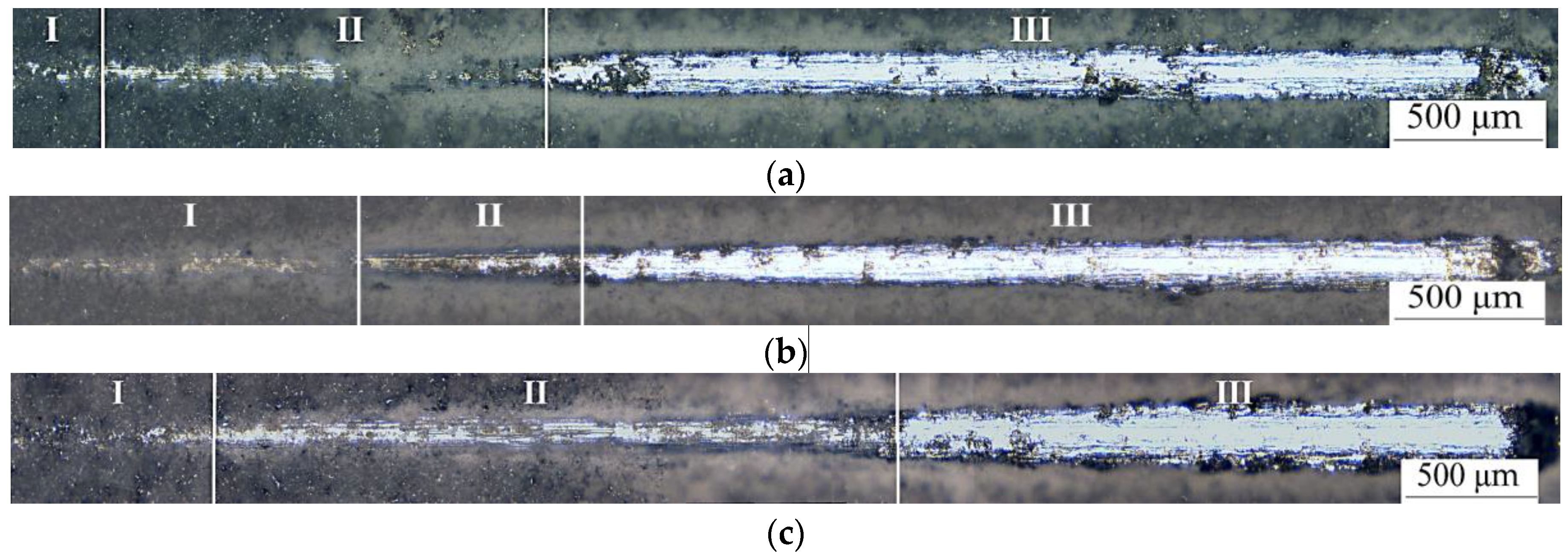
3.1. Coating Formation
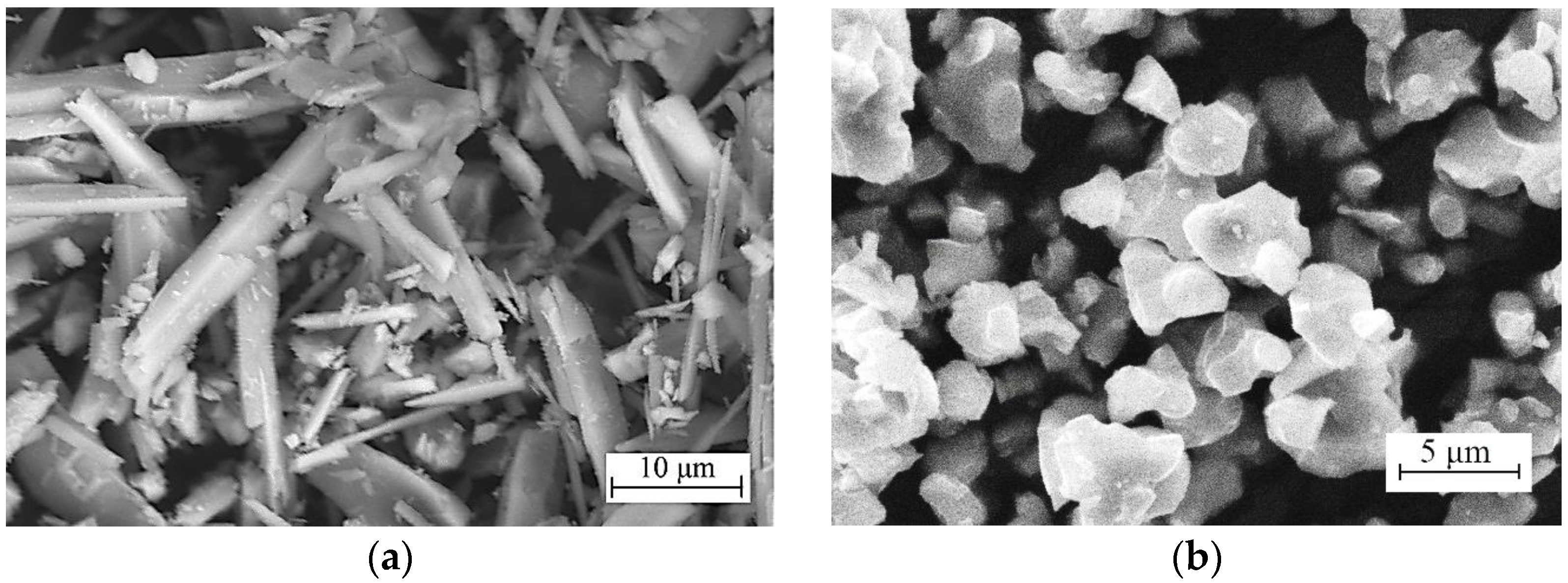
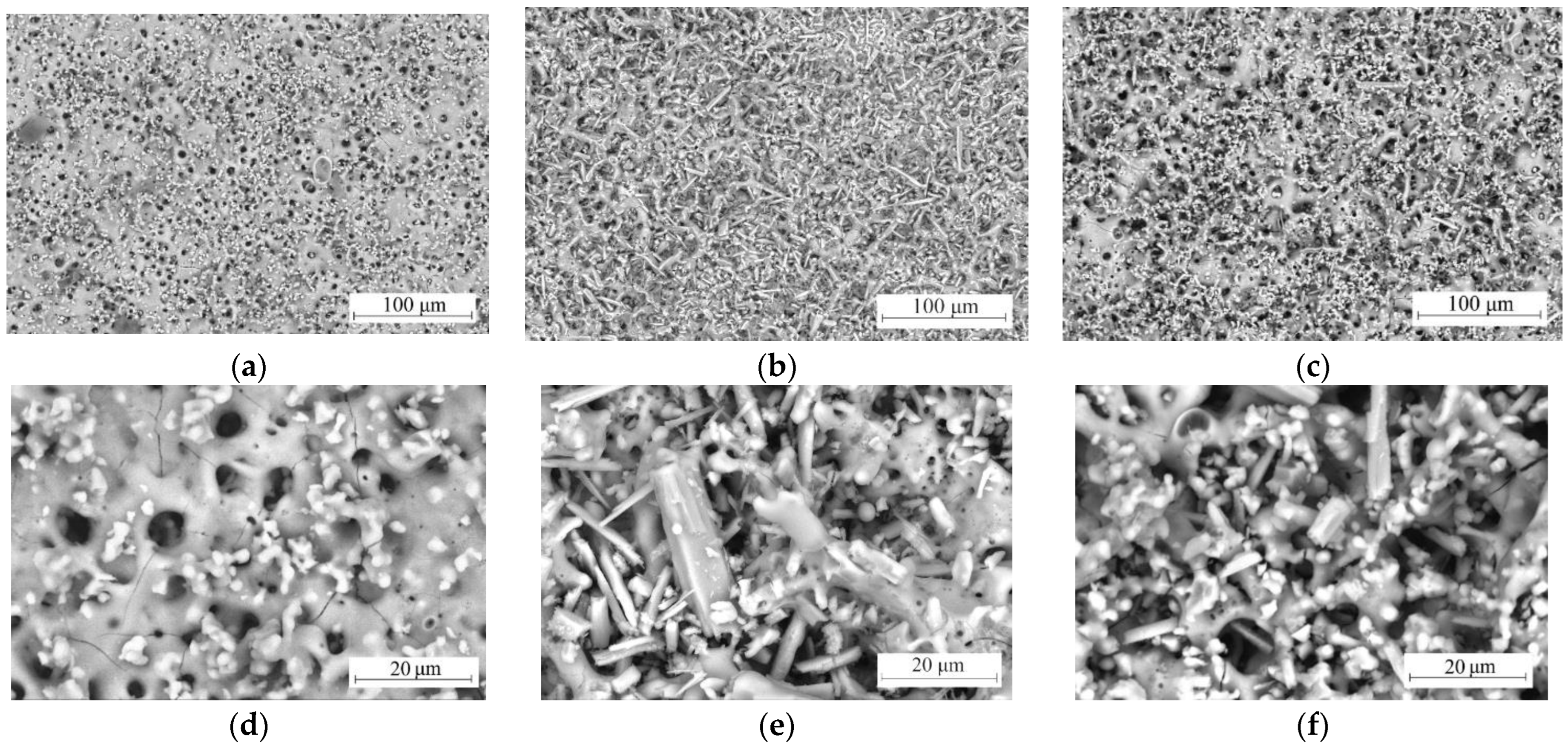
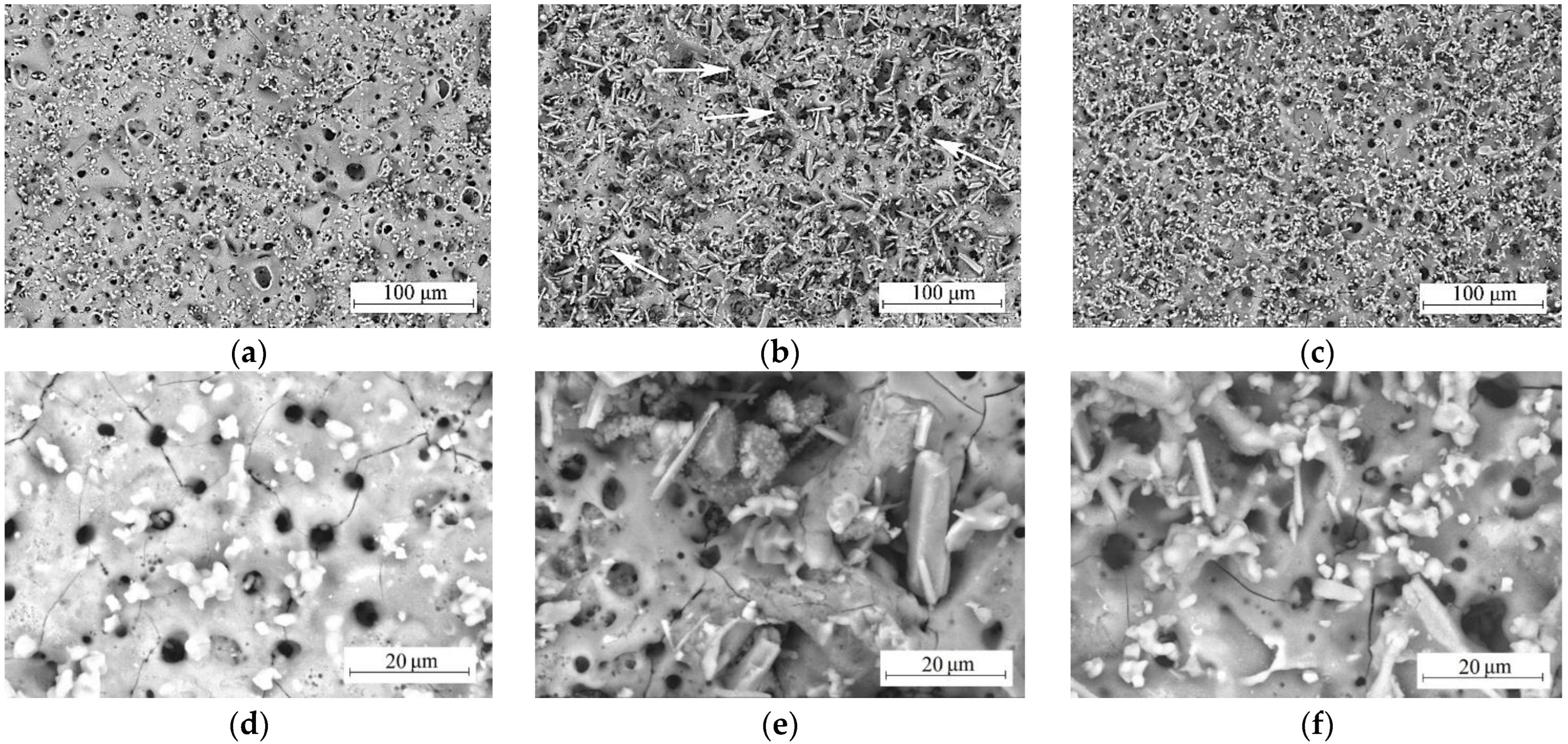
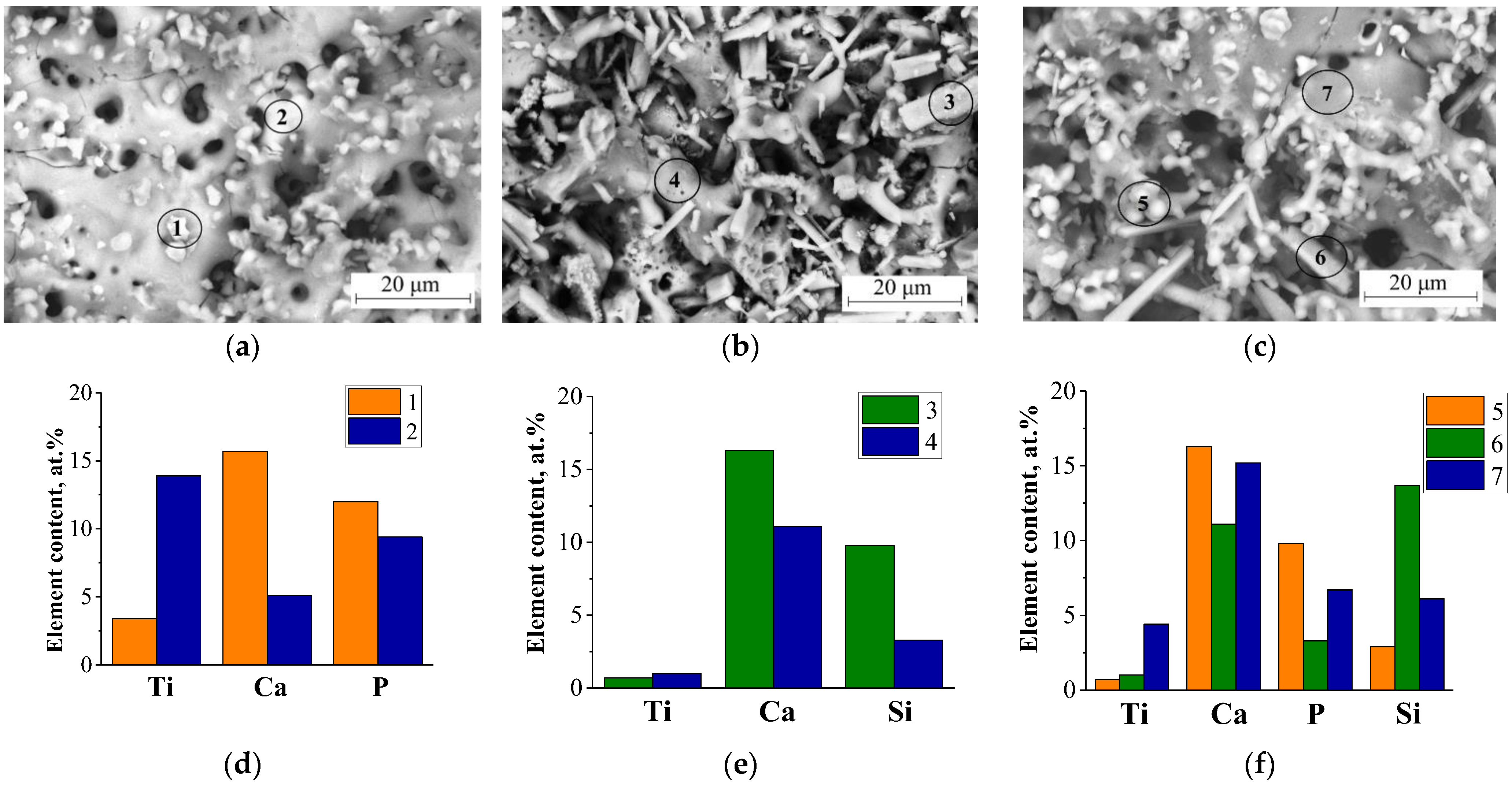
3.2. Morphology, Structure, and Elemental Composition of Coatings
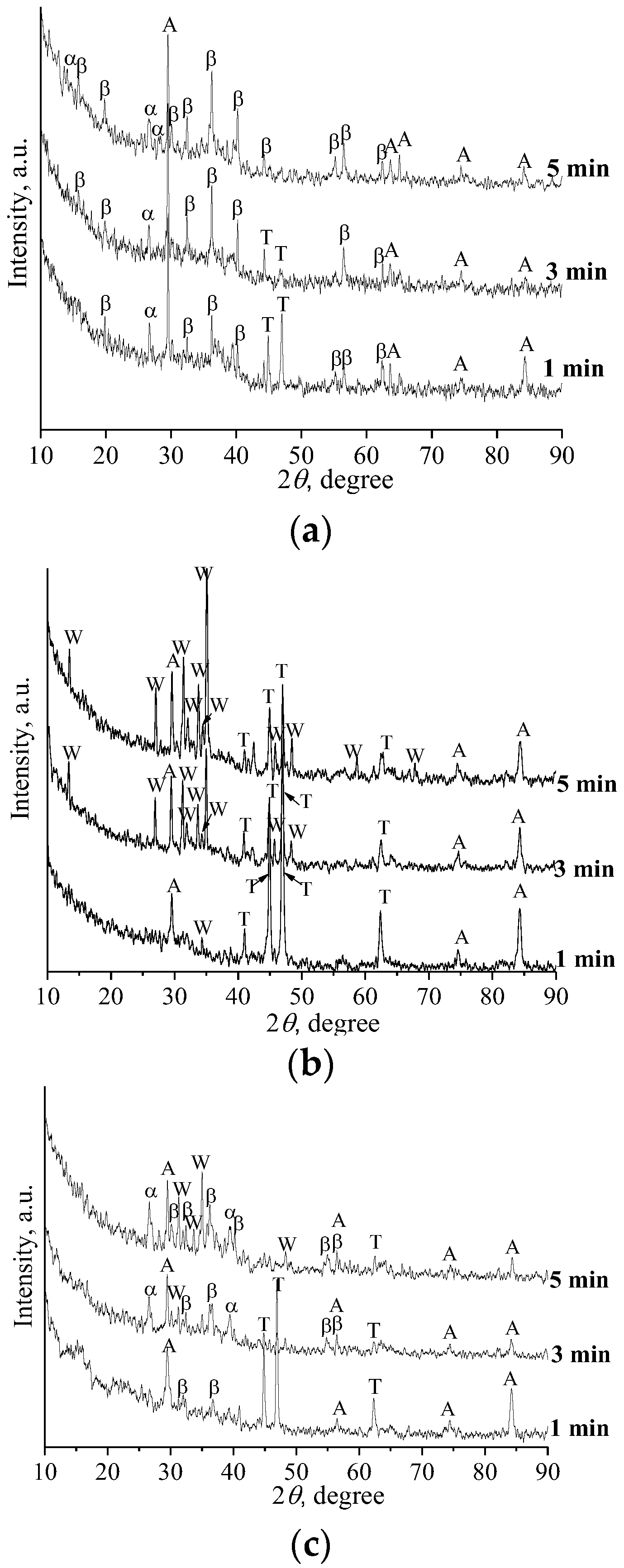
3.3. Phase Composition and Fine Structure of Coatings
3.4. Physico-Mechanical Properties of Coatings
4. Conclusions
- (1)
- The specific conductivity of electrolytes I and III was found to be 2.87 and 2.52 Sm/m, respectively, while the specific conductivity of electrolyte II (with wollastonite particles) took an intermediate value of 2.6 Sm/m. This phenomenon can be explained by the fact that β-TCP particles included in the electrolytes I and III are characterized by a more negative zeta potential value, which enhances their electrophoretic properties.
- (2)
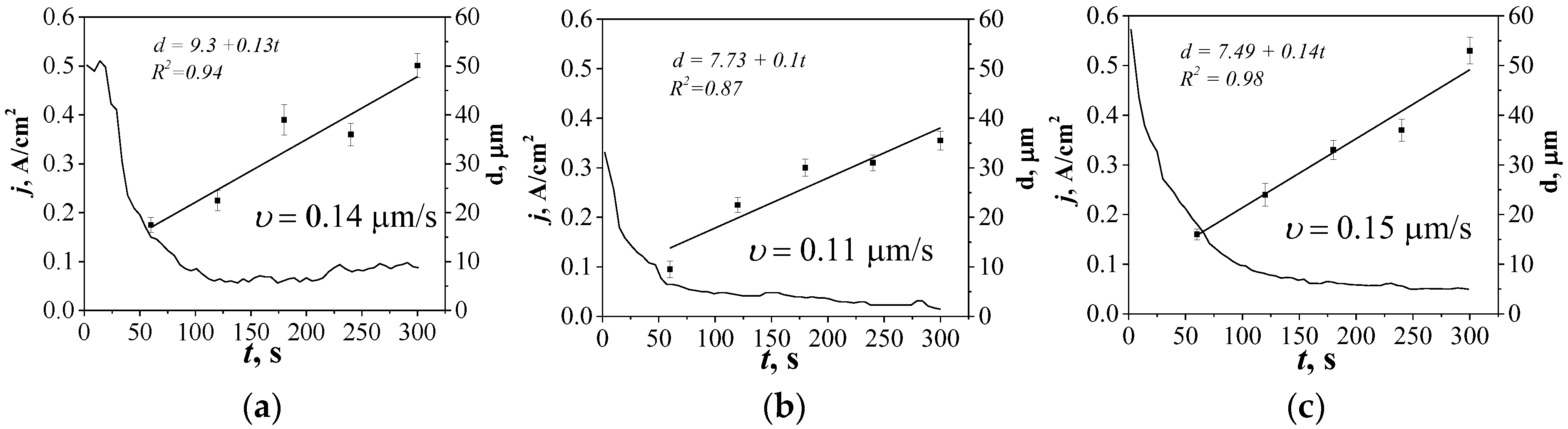
- It was found that in the medium of electrolytes with higher specific conductivity, the MAO process occurred at higher values of initial current density (0.50 and 0.58 A/cm2, for CP and SP coatings, respectively), and a higher growth rate of coatings was observed (0.14 and 0.15 μm/s for CP and SP coatings, respectively). It is evident that with a higher specific conductivity of the electrolyte, more intense micro-arc discharges take place, and therefore, the rate of coating growth increases.
- (3)
- The coatings were found to have an amorphous crystalline structure. The following were identified as the main crystalline phases: β-TCP, α-TCP, wollastonite, and TiO2 (anatase).
- (4)
- Crystallization of calcium phosphate compounds in CP and SP coatings started during the first minute of the MAO process. In CS coatings, only oxide coating was formed during the first minutes of the MAO process, which is explained by the lower mobility of wollastonite particles due to their needle shape and their lower zeta potential value.
- (5)
- When the voltage of the MAO process was increased to 500 V, the thickness of CP, CS, and SP coatings increased to 80, 50, and 50 μm, respectively, and the roughness of these coatings by Ra parameter increased to 4.8, 5.5 and 5.0 μm, respectively.
- (6)
- The values of critical load during scratch testing for CP, CS, and SP coatings synthesized at 500 V were 10.0, 10.3, and 22.0 N, respectively. Thus, the combined coatings formed in the electrolyte with the addition of β-TCP and wollastonite particles were characterized by the highest adhesion strength. Therefore, these coatings are the most promising for use in load-bearing medical implants where it is critical to prevent delamination under mechanical loads.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MAO | Micro-arc oxidation |
| CP | Calcium phosphate coating |
| CS | Calcium silicate coating |
| SP | Silicate phosphate coating |
References
- Zhang, L.C.; Chen, L.Y.; Wang, L.Q. Surface modification of titanium and titanium alloys: Technologies, developments, and future interests. Adv. Eng. Mater. 2020, 22, 1901258. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Shi, Y.; Tang, J.; Huang, D.; Yan, M.; Dargusch, M.S. Surface modification of biomedical Ti and Ti alloys: A Review on current advances. Materials 2022, 15, 1749. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Chen, Y.M.; Biao, M.N.; Zhang, X.D.; Yang, B.C. Bio-functionalization of biomedical metals. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 1057–1070. [Google Scholar] [CrossRef]
- Li, Y.Y.; Zou, L.M.; Yang, C.; Li, Y.H.; Li, L.J. Ultrafine-grained Ti-based composites with high strength and low modulus fabricated by spark plasma sintering. Mater. Sci. Eng. A 2013, 560, 857–861. [Google Scholar] [CrossRef]
- Liu, C.F.; Lee, T.H.; Liu, J.F.; Hou, W.T.; Li, S.J.; Hao, Y.L.; Pan, H.B.; Huang, H.H. A unique hybrid-structured surface produced by rapid electrochemical anodization enhances bio-corrosion resistance and bone cell responses of beta-type Ti-24Nb-4Zr-8Sn alloy. Sci. Rep. 2018, 8, 6623. [Google Scholar] [CrossRef]
- Liu, G.C.; Li, R.Y.; Liang, H.J.; Qin, Y.G. Research advance in the osteointegration of surface bioactive coating on titanium alloys. Chin. J. Tissue Eng. Res. 2017, 21, 969–974. [Google Scholar] [CrossRef]
- Mosas, K.K.A.; Chandrasekar, A.R.; Dasan, A.; Pakseresht, A.; Galusek, D. Recent advancements in materials and coatings for biomedical implants. Gels 2022, 8, 323. [Google Scholar] [CrossRef]
- Jugowiec, D.; Kot, M.; Moskalewicz, T. Electrophoretic deposition and characterisation of chitosan coatings on near-β titanium alloy. Arch. Metall. Mater. 2016, 61, 657–664. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Z.; Fu, Y.; Dunne, N.; Liang, C.; Luo, X.; Liu, K.; Li, X.; Pang, X.; Lu, K. Improved osteogenic differentiation of human amniotic mesenchymal stem cells on gradient nanostructured Ti surface. J. Biomed. Mater. Res. Part A 2020, 108, 1824–1833. [Google Scholar] [CrossRef]
- Lin, Q.; Zhou, Y.; Yin, M.; Cheng, Y.; Wei, Y.; Hu, Y.; Lian, X.; Chen, W.; Huang, D. Hydroxyapatite/tannic acid composite coating formation based on Ti modified by TiO2 nanotubes. Colloids Surf. B Biointerfaces 2020, 196, 111304. [Google Scholar] [CrossRef]
- Bajda, S.; Liu, Y.; Tosi, R.; Cholewa-Kowalska, K.; Krzyzanowski, M.; Dziadek, M.; Kopyscianski, M.; Dymek, S.; Polyakov, A.V.; Semenova, I.P.; et al. Laser cladding of bioactive glass coating on pure titanium substrate with highly refined grain structure. J. Mech. Behav. Biomed. Mater. 2021, 119, 104519. [Google Scholar] [CrossRef] [PubMed]
- Filipović, U.; Dahmane, R.G.; Ghannouchi, S.; Zore, A.; Bohinc, K. Bacterial adhesion on orthopedic implants. Adv. Colloid Interface Sci. 2020, 283, 102228. [Google Scholar] [CrossRef]
- Fosca, M.; Streza, A.; Antoniac, I.V.; Vadalà, G.; Rau, J.V. Ion-doped calcium phosphate-based coatings with antibacterial properties. J. Funct. Biomater. 2023, 14, 250. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Kung, H.-P.; Lin, H.-C. Effects of electrical parameters on micro-arc oxidation coatings on pure titanium. Micromachines 2023, 14, 1950. [Google Scholar] [CrossRef]
- Yasui, T.; Hayashi, K.; Fukumoto, M. Behaviors of micro-arcs, bubbles, and coating growth during plasma electrolytic oxidation of titanium alloy. Materials 2023, 16, 360. [Google Scholar] [CrossRef]
- Nahum, E.Z.; Lugovskoy, S.; Lugovskoy, A.; Kazanski, B.; Sobolev, A. The study of hydroxyapatite growth kinetics on CP–Ti and Ti65Zr treated by plasma electrolytic oxidation process. J. Mater. Res. Technol. 2023, 24, 2169–2186. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O.; Yerokhin, A.; Matthews, A. Spectroscopic study of electrolytic plasma and discharging behaviour during the plasma electrolytic oxidation (PEO) process. J. Phys. D Appl. Phys. 2010, 43, 105203. [Google Scholar] [CrossRef]
- Matykina, E.; Berkani, A.; Skeldon, P.; Thompson, G.E. Real-time imaging of coating growth during plasma electrolytic oxidation of titanium. J. Electrochim. Acta 2007, 53, 1987–1994. [Google Scholar] [CrossRef]
- Sedelnikova, M.; Bakina, O.; Ugodchikova, A.; Tolkacheva, T.; Khimich, M.; Uvarkin, P.; Kashin, A.; Miller, A.; Egorkin, V.; Schmidt, J.; et al. The role of microparticles of β-TCP and wollastonite in the creation of biocoatings on Mg0.8Ca Alloy. Metals 2022, 12, 1647. [Google Scholar] [CrossRef]
- Galvis, O.A.; Quintero, D.; Castaño, J.G.; Liu, H.; Thompson, G.E.; Skeldon, P.; Echeverría, F. Formation of grooved and porous coatings on titanium by plasma electrolytic oxidation in H2SO4/H3PO4 electrolytes and effects of coating morphology on adhesive bonding. Surf. Coat. Technol. 2015, 269, 238–249. [Google Scholar] [CrossRef]
- Li, Q.; Yang, W.; Liu, C.; Wang, D.; Liang, J. Correlations between the growth mechanism and properties of micro-arc oxidation coatings on titanium alloy: Effects of electrolytes. Surf. Coat. Technol. 2017, 316, 162–170. [Google Scholar] [CrossRef]
- Abbas, A.; Wang, T.-Y.; Lin, H.-C. Effects of electrolyte compositions and electrical parameters on micro-arc oxidation coatings on 7075 aluminum alloy. J. Compos. Sci. 2023, 7, 472. [Google Scholar] [CrossRef]
- Bykova, A.D.; Markov, M.A.; Kuznetsov, Y.A.; Kravchenko, I.N.; Belyakov, A.N.; Makarov, A.M. Effect of electrolyte composition on the structure and tribological properties of ceramic coatings obtained by micro-arc oxidation. Refract. Ind. Ceram. 2024, 64, 541–549. [Google Scholar] [CrossRef]
- Simchen, F.; Sieber, M.; Kopp, A.; Lampke, T. Introduction to plasma electrolytic oxidation—An overview of the process and applications. Coatings 2020, 10, 628. [Google Scholar] [CrossRef]
- Fattah-Alhosseini, A.; Keshavarz, M.K.; Molaei, M.; Gashti, S.O. Plasma electrolytic oxidation (PEO) process on cmmercially pure Ti surface: Effects of electrolyte on the microstructure and corrosion behavior of coatings. J. Metall. Mater. Trans. A 2018, 49, 4966–4979. [Google Scholar] [CrossRef]
- Sikdar, S.; Menezes, P.V.; Maccione, R.; Jacob, T.; Menezes, P.L. Plasma electrolytic oxidation (PEO) process—Processing, properties, and applications. Nanomaterials 2021, 11, 1375. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, J.; He, J.; Jiang, J.; Zhang, Q. Effect of electrolyte concentration on the tribological performance of MAO coatings on aluminum alloys. J. Front. Chem. Eng. 2020, 14, 1065–1071. [Google Scholar] [CrossRef]
- Fattah-Alhosseini, A.; Molaei, M.; Babaei, K. Influence of electrolyte composition and voltage on the microstructure and growth mechanism of plasma electrolytic oxidation (PEO) coatings on tantalum: A Review. J. Anal. Bioanal. Electrochem. 2020, 12, 517–535. [Google Scholar]
- Sandhyarani, M.; Prasadrao, T.; Rameshbabu, N. Role of electrolyte composition on structural, morphological and in-vitro biological properties of plasma electrolytic oxidation films formed on zirconium. J. Appl. Surf. Sci. 2014, 317, 198–209. [Google Scholar] [CrossRef]
- Sedelnikova, M.B.; Ugodchikova, A.V.; Uvarkin, P.V.; Chebodaeva, V.V.; Tolkacheva, T.V.; Schmidt, J.; Sharkeev, Y.P. Structural-morphological and adhesive properties of calcium-phosphate coatings formed on a magnesium alloy by micro-arc oxidation in an electrolyte containing disperse particles. Russ. Phys. J. 2021, 64, 830–837. [Google Scholar] [CrossRef]
- Kashin, A.D.; Sedelnikova, M.B.; Chebodaeva, V.V.; Uvarkin, P.V.; Luginin, N.A.; Dvilis, E.S.; Kazmina, O.V.; Sharkeev, Y.P.; Khlusov, I.A.; Miller, A.A.; et al. Diatomite-based ceramic biocoating for magnesium implants. Ceram. Int. 2022, 48, 28059–28071. [Google Scholar] [CrossRef]
- Awasthi, S.; Pandey, S.K.; Arunan, E.; Srivastava, C. A Review on hydroxyapatite coatings for biomedical application: Experimental and theoretical perspectives. J. Mater. Chem. B 2021, 9, 228–249. [Google Scholar] [CrossRef]
- Mamaeva, A.; Kenzhegulov, A.; Panichkin, A.; Abdulvaliyev, R.; Fischer, D.; Bakhytuly, N.; Toiynbaeva, N. Influence of current duty cycle and voltage of micro-arc oxidation on the microstructure and composition of calcium phosphate coating. Coatings 2024, 14, 766. [Google Scholar] [CrossRef]
- Molaei, M.; Fattah-alhosseini, A.; Nouri, M.; Nourian, A. Systematic optimization of corrosion, bioactivity, and biocompat-ibility behaviors of calcium-phosphate plasma electrolytic oxidation (PEO) coatings on titanium substrates. Ceram. Int. 2022, 48, 6322–6337. [Google Scholar] [CrossRef]
- Sayed, M.; Mahmoud, E.M.; Bondioli, F.; Naga, S.M. Developing porous diopside/hydroxyapatite bio-composite scaffolds via a combination of freeze-drying and coating process. Ceram. Int. 2019, 45, 9025–9031. [Google Scholar] [CrossRef]
- Bragg, W.L.; Claringbull, G.F. Crystal Structure of Minerals; Cornell University Press: Ithaca, NY, USA, 1965; Volume 4. [Google Scholar]
- Palakurthy, S.; Reddy, K.V.G.; Samudrala, R.K.; Azeem, P.A. In vitro bioactivity and degradation behaviour of β-wollastonite derived from natural waste. Mater. Sci. Eng. C 2019, 98, 109–117. [Google Scholar] [CrossRef]
- Sedelnikova, M.B.; Ugodchikova, A.V.; Tolkacheva, T.V.; Chebodaeva, V.V.; Cluklhov, I.A.; Khimich, M.A.; Bakina, O.V.; Lerner, M.I.; Egorkin, V.S.; Schmidt, J.; et al. Surface modification of Mg0.8Ca alloy via wollastonite micro-arc coatings: Significant improvement in corrosion resistance. Metals 2021, 11, 754. [Google Scholar] [CrossRef]
- Sedelnikova, M.B.; Mayer, V.V.; Bakina, O.V.; Kashin, A.D.; Uvarkin, P.V.; Khimich, M.A.; Luginin, N.A.; Glukhov, I.A.; Tolkacheva, T.V.; Ugodchikova, A.V.; et al. Antibacterial amorphous-crystalline coatings based on wollastonite and ZnO particles. Crystals 2024, 14, 886. [Google Scholar] [CrossRef]
- Osborn, E.F.; Muan, A.; Levin, E.M.; Robbins, C.R.; McMurdie, H.F. Phase Diagrams for Ceramists; American Ceramic Society: Columbus, OH, USA, 1964; p. 598. [Google Scholar]
- Brazete, D.; Torres, P.M.C.; Abrantes, J.C.C.; Ferreira, J.M.F. Influence of the Ca/P ratio and cooling rate on the allotropic α↔β-tricalcium phosphate phase transformations. J. Ceram. Int. 2018, 44, 8249–8256. [Google Scholar] [CrossRef]
- Kim, S.A.; Hussain, S.K.; Abbas, M.A.; Bang, J.H. High-temperature solid-state rutile-to-anatase phase transformation in TiO2. J. Solid State Chem. 2022, 315, 123510. [Google Scholar] [CrossRef]
- Karunadasa, K.S.P.; Manoratne, C.H. Microstructural view of anatase to rutile phase transformation examined by in-situ high-temperature X-ray powder diffraction. J. Solid State Chem. 2022, 314, 123377. [Google Scholar] [CrossRef]





| Compound | Content in the Electrolyte Composition, g/L | ||
|---|---|---|---|
| I | II | III | |
| CaSiO3 (wollastonite) | – | 90 | 45 |
| β-Ca3(PO4)2 (β-TCP) | 90 | – | 45 |
| Na2HPO4·12H2O | 20 | – | 10 |
| NaOH | 10 | 10 | 10 |
| Na2SiO3 | – | 5 | 2.5 |
| NaF | 5 | 5 | 5 |
| pH | 11 | 12 | 11 |
| Coating designator | CP | CS | SP |
| Elements | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| O | 67.8 ± 1.2 | 68.4 ± 1.1 | 68.1 ± 0.9 | 63.7 ± 1.3 | 69.2 ± 0.8 | 69.0 ± 1.2 | 65.4 ± 1.1 |
| Na | 1.2 ± 0.1 | 3.3 ± 0.9 | 0.7 ± 0.03 | 4.6 ± 0.7 | 4.1 ± 0.4 | 1.9 ± 0.6 | 5.4 ± 0.9 |
| P | 12 ± 0.9 | 9.4 ± 0.5 | – | – | 9.3 ± 0.7 | 3.3 ± 0.4 | 6.0 ± 0.5 |
| Si | – | – | 15.7 ± 0.5 | 14.6 ± 0.8 | 2.0 ± 0.2 | 13.7 ± 0.9 | 5.9 ± 0.3 |
| Ca | 15.7 ± 0.9 | 5.1 ± 0.2 | 14.7 ± 0.7 | 15.7 ± 0.6 | 13.5 ± 0.4 | 11.1 ± 0.3 | 10.1 ± 0.7 |
| Ti | 3.4 ± 0.2 | 13.9 ± 0.8 | 0.7 ± 0.09 | 2.3 ± 0.1 | 1.9 ± 0.08 | 1.0 ± 0.05 | 7.3 ± 0.6 |
| Coating designator | CP | CS | SP | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ugodchikova, A.V.; Tolkacheva, T.V.; Uvarkin, P.V.; Khimich, M.A.; Sharkeev, Y.P.; Kashin, A.D.; Glukhov, I.A.; Sedelnikova, M.B. Micro-Arc Coatings with Different Types of Microparticles on Titanium Alloy: Formation, Structure, and Properties. Crystals 2025, 15, 811. https://doi.org/10.3390/cryst15090811
Ugodchikova AV, Tolkacheva TV, Uvarkin PV, Khimich MA, Sharkeev YP, Kashin AD, Glukhov IA, Sedelnikova MB. Micro-Arc Coatings with Different Types of Microparticles on Titanium Alloy: Formation, Structure, and Properties. Crystals. 2025; 15(9):811. https://doi.org/10.3390/cryst15090811
Chicago/Turabian StyleUgodchikova, Anna V., Tatiana V. Tolkacheva, Pavel V. Uvarkin, Margarita A. Khimich, Yurii P. Sharkeev, Alexander D. Kashin, Ivan A. Glukhov, and Mariya B. Sedelnikova. 2025. "Micro-Arc Coatings with Different Types of Microparticles on Titanium Alloy: Formation, Structure, and Properties" Crystals 15, no. 9: 811. https://doi.org/10.3390/cryst15090811
APA StyleUgodchikova, A. V., Tolkacheva, T. V., Uvarkin, P. V., Khimich, M. A., Sharkeev, Y. P., Kashin, A. D., Glukhov, I. A., & Sedelnikova, M. B. (2025). Micro-Arc Coatings with Different Types of Microparticles on Titanium Alloy: Formation, Structure, and Properties. Crystals, 15(9), 811. https://doi.org/10.3390/cryst15090811









