Abstract
Biodegradable magnesium alloys have emerged as promising alternatives to permanent metallic implants due to their unique combination of mechanical compatibility with bone and complete resorption, addressing the persistent issues of stress shielding and secondary removal surgeries. This review critically examines the historical development of magnesium-based biomaterials, highlighting advances in alloy design, manufacturing processes, and surface engineering that now enable tailored degradation and improved clinical performance. Drawing on recent clinical and preclinical studies, we summarize improvements in corrosion resistance, mechanical properties, and biocompatibility that have supported the clinical translation of magnesium alloys across a variety of orthopedic and emerging medical applications. However, challenges remain, including unpredictable in vivo degradation kinetics, limited long-term safety data, lack of standardized testing protocols, and ongoing regulatory uncertainties. We conclude that while magnesium-based biomaterials have advanced from experimental concepts to clinically validated solutions, further progress in personalized degradation control, real-time monitoring, and harmonized regulatory frameworks is needed to fully realize their transformative clinical potential.
1. Introduction
Medical implants made from titanium and stainless steel have saved countless lives, but they create a fundamental problem: these permanent metals are much stiffer than human bone. This mismatch causes “stress shielding”—where the implant bears most mechanical loads, causing the surrounding bone to weaken and deteriorate over time [1,2]. Additionally, these foreign materials trigger chronic inflammation, infection risks, and require surgical removal in 30% of patients, particularly in growing children [3,4]. These limitations have motivated researchers to develop implants that temporarily support healing tissue and then safely dissolve away—eliminating the need for removal surgery [5,6].
Magnesium emerges as an ideal candidate for such “biodegradable” implants. As the human body’s fourth most abundant mineral, magnesium naturally comprises 50–60% of bone content and participates in over 300 biological processes [7,8,9]. Its mechanical stiffness closely matches that of bone—unlike titanium, which is 4–29 times stiffer—reducing stress shielding while maintaining adequate strength [4,10]. Most importantly, when magnesium implants degrade, the body can safely process and excrete the byproducts through normal kidney function, avoiding permanent foreign material accumulation [11].
The concept is not new. In 1878, surgeon Edward Huse used magnesium wire for blood vessel surgery [12,13], and in 1906, Albin Lambotte implanted magnesium plates to fix broken bones [4,14]. However, these early attempts failed spectacularly—the implants dissolved completely within 8 days, producing hydrogen gas bubbles that interfered with healing [15,16]. For perspective, these early materials corroded at rates exceeding 400 mm per year, while successful bone healing requires implants to maintain strength for at least 3–6 months [7,17]. These failures discouraged clinical use for nearly a century.
Recent advances in alloy development and clinical practice have contributed to the increasing clinical application of magnesium implants, such as MAGNEZIX® screws, which have now been used in over 25,000 patients with degradation times that approximate bone healing timelines [18,19]. Advanced alloy compositions like Mg-2Zn-1Mn achieve degradation rates of just 0.36 mm per year, representing a 100–1000 fold improvement over historical materials [20]. Manufacturing innovations using laser-based 3D printing can now create implants with microscopic grain structures (1–3 μm), which may contribute to improved strength and corrosion resistance [21,22].
Despite these advances, significant scientific debates persist. Researchers disagree about using rare-earth elements (like neodymium or yttrium) in alloy compositions—some argue these elements enhance performance and remain safe at low concentrations [23,24], while others advocate complete avoidance due to potential accumulation in organs over time [25]. Another major challenge involves testing reliability: the same material can show degradation rates varying by 10-fold depending on whether it is tested in laboratory dishes or living tissue [26,27]. Furthermore, the lack of standardized testing methods and inconsistent regulatory requirements across countries hampers global adoption—only eight magnesium implant designs have received regulatory approval worldwide as of 2023 [22].
This review critically examines how biodegradable magnesium implants evolved from repeated failures to emerging clinical success while identifying remaining barriers to widespread adoption. We systematically analyze (1) historical lessons from early failures that informed modern solutions; (2) recent innovations including new alloys achieving bone-like strength (>315 MPa) [28], “smart” protective coatings that self-repair damage [29], and manufacturing precision with less than 2% variation between batches [30]; (3) unresolved challenges including the absence of patient safety data beyond 5 years, unpredictable patient-to-patient variations in degradation, and lack of international standards; and (4) future opportunities for “intelligent” implants containing sensors to monitor healing or release therapeutic drugs on demand. Our analysis indicates that biodegradable magnesium is progressing from experimental research toward broader clinical application. Remaining challenges are largely engineering- and regulation-related, rather than purely scientific. Continued collaborative efforts in personalized medicine, real-time monitoring, and regulatory harmonization may further expand the potential applications of magnesium-based implants in orthopedics and other medical fields.
2. Historical Context and Challenges of Biodegradable Magnesium Alloys in Medical Implants
The vision of magnesium as the ideal biodegradable implant material emerged over a century ago when pioneering surgeons recognized its unique potential: a metal that could provide structural support during healing and then gracefully disappear as natural tissue took over. Yet, this compelling concept remained frustratingly elusive for generations, with early attempts resulting in spectacular failures—implants that dissolved within days and generated alarming gas bubbles. Only after decades of persistent scientific advancement has magnesium evolved from an experimental material to one with increasing clinical acceptance and improved control over its properties. This chapter discusses the historical evolution from Lambotte’s pioneering work to modern commercial success, the fundamental advantages of magnesium’s biological compatibility, and the critical lessons learned from early failures that enabled today’s breakthrough achievements.
2.1. Clinical Need for Biodegradable Implants
The development of biodegradable implants is driven by the need to overcome the mechanical incompatibility between traditional metallic implants and bone tissue, a mismatch that often leads to stress shielding and subsequent bone loss [1,2]. Magnesium alloys achieve a more balanced clinical performance than titanium alloys, stainless steel, polymers, or biocomposites, particularly excelling in modulus match, density compatibility, and biodegradability.
Magnesium alloys rate highest for matching the mechanical properties and density of bone [3,5,6,7,8,10,31,32] while also offering good strength, biocompatibility, and full biodegradability (see Table 1). In contrast, titanium and stainless steel exhibit poor compatibility in these areas and lack biodegradability, often requiring secondary removal surgery [3,7,8].

Table 1.
Comparison of common orthopedic implant materials.
Beyond mechanical problems such as stress shielding, permanent implants are associated with chronic complications, including inflammation, infection, allergic reactions, and imaging artifacts [3,4,6]. The capacity of magnesium alloys for complete biodegradation eliminates the need for removal surgery and addresses a major limitation of conventional implant materials [3,4].
2.2. Magnesium’s Biological Foundation
Figure 1 illustrates magnesium’s unique position as the body’s fourth most abundant cation, providing fundamental advantages over inert metals detailed in Table 2. The body distribution analysis shows that 55% of total magnesium resides in bone tissue (11.5–16.5 g), with additional stores in soft tissues (27%, 5.7–8.1 g) and muscle (18%, 3.8–5.4 g). This natural abundance, combined with magnesium’s role as cofactor for 300–325 enzymatic reactions, enables safe renal excretion of degradation products unlike permanent foreign materials [7,8,9,11].
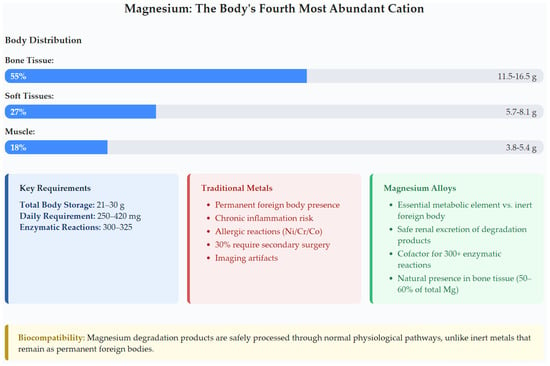
Figure 1.
Magnesium distribution in the human body and clinical advantages comparison, highlighting its essential metabolic role versus traditional metals’ permanent foreign body presence.

Table 2.
Clinical advantages comparison.
The clinical advantages comparison in Figure 1 emphasizes key benefits outlined in Table 2: magnesium serves as an essential metabolic element versus inert foreign body presence, enables safe renal excretion of degradation products, acts as a cofactor for 300+ enzymatic reactions, maintains natural presence in bone tissue (50–60% of total body magnesium), and eliminates the 30% secondary surgery rate associated with traditional metals [3,4,5,8,34,37,38,39,40].
2.3. Historical Development: From Early Pioneers to Modern Success
Magnesium’s clinical history began with Edward C. Huse’s 1878 use of magnesium wire ligatures for vascular control [12,13,41]. Lambotte’s landmark 1906 application of magnesium plates for tibial fractures marked the first orthopedic use, though complete degradation within 8 days revealed the fundamental challenge of degradation control that would persist for over a century [4,11,14,15].
Historical applications consistently demonstrated excessive degradation rates that undermined clinical utility. Pure magnesium exhibited complete dissolution in 10–14 days in muscle tissue, with some cases showing total resorption in 3–5 weeks under acidic conditions [4,41]. The fundamental degradation mechanism produces both ionic and gaseous byproducts through the reaction: Mg + 2H2O → Mg(OH)2 + H2↑, generating approximately 1 mL of hydrogen gas per 1 mg of degraded magnesium [5,7,16,17].
The historical progress timeline in Section 2.3 illustrates a substantial extension in degradation times, from 8 days in early 20th-century applications to controlled degradation periods of 3–24 months in more recent studies. Despite frequent gas cavity formation and inflammatory responses in early applications, infection rates remained surprisingly low even under pre-antibiotic conditions, suggesting inherent “biotolerance” properties [4,42].
2.4. Early Clinical Failures and Critical Lessons
Early magnesium alloys failed due to excessively rapid degradation that preceded tissue healing. Pure magnesium demonstrated degradation rates of 407 mm/year and corrosion currents reaching 86.06 μA/cm2 in simulated body fluid—far exceeding the target <0.5 mm/year for orthopedic applications [4,7,43]. Clinical studies showed 50% strength loss within 4 weeks, compromising structural integrity before sufficient healing [2,3,43].
Hydrogen gas production created significant complications, with acceptable evolution rates below 0.01 mL/cm2/day to prevent subcutaneous gas pockets [38,44]. X-ray imaging revealed gas accumulation at bone–implant interfaces persisting up to 8 weeks post-implantation [45]. Concurrent alkalization raised local pH above 11.5, while pH values reached 8.70 in culture media under high-Mg conditions—substantially above physiological levels [46,47].
High early release of Mg2+ ions reduced cell viability by >30% according to ISO 10993-5:2009 [48] thresholds, with human mesenchymal stem cells tolerating concentrations only up to 8.0 × 10−4 M before ATP production declined [47,49]. Some experimental conditions reached 4.1 × 10−2 M—well above physiological tolerance [47]. These unpredictable responses, combined with lack of standardized evaluation protocols, created substantial regulatory hurdles that delayed market entry for decades [2,4,8].
2.5. Modern Commercial Achievements
Contemporary success demonstrates the validity of historical lessons learned, with dramatic improvements shown in Table 3. MAGNEZIX® procedures now exceed 25,000 cases with 36-month follow-up data showing successful bone integration. The bone–implant contact measurements of 0.42–0.61 versus 0.24–0.35 for titanium represent quantifiable improvements in osseointegration [38,50].

Table 3.
Historical vs. modern performance comparison.
MAGNEZIX® screws (Syntellix AG, Hannover, Germany) became the first CE-certified resorbable metallic orthopedic implants in 2013, demonstrating controlled resorption and bone replacement [3,4,5,33,38,51]. K-MET screws (K-MET Co., Ltd., Seoul, Republic of Korea) achieved complete healing and resorption at 6–12 months in 53 patients, while cardiovascular applications like AMS stents demonstrated safe 4-month degradation [3,37].
MAGNEZIX® is highlighted here as an illustrative example of a clinically successful magnesium-based implant. Direct comparison with alternative biomaterials such as titanium alloys or polymers is not the primary focus of this review as magnesium-based materials are fundamentally distinct in their combination of biodegradability, osteoconductivity, and mechanical compatibility with bone. A comparative evaluation among different magnesium alloys and products would provide valuable insights but would require comprehensive data collection across manufacturers and clinical studies—a substantial undertaking that could be the subject of future dedicated reports.
2.6. Key Takeaways: Foundation for Modern Development
The historical journey from 1878 to the present, illustrated through the performance evolution shown in Section 2, demonstrates that transformative biomaterial innovation requires persistent effort across multiple decades. Magnesium alloys are approaching a favorable balance between degradation rate (3–24 months), mechanical compatibility (1.3–6× better modulus match), and reduced need for secondary surgery (see Figure 2).
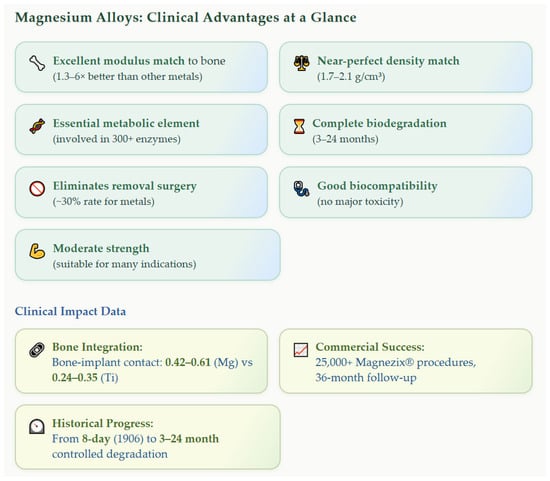
Figure 2.
Clinical advantages and impact of magnesium alloys in orthopedic implants [3,4,7,8,9,14,18,19,38,50].
Modern magnesium implants achieve controlled degradation rates (0.02–0.31 mm/year), predictable mechanical properties (up to 365 MPa tensile strength), and excellent clinical outcomes (>25,000 successful procedures), as documented in the clinical impact data. However, the transition from experimental promise to widespread clinical adoption continues to require addressing challenges in degradation predictability, manufacturing consistency, regulatory standardization, and application expansion.
These historical foundations, quantified through the comprehensive performance comparisons, establish the scientific and clinical basis for examining persistent challenges (Section 3), major recent advances (Section 4), and future directions that will guide the field toward widespread clinical adoption (Section 5).
3. Persistent and Emerging Challenges
The development of biodegradable magnesium implants has advanced beyond proof-of-concept to reveal complex interconnected challenges spanning materials science, manufacturing, and regulatory domains that must be addressed for clinical translation.
This chapter examines representative obstacles limiting magnesium-based biodegradable implant development, though these examples do not encompass the full scope of challenges in this evolving field. Key barriers are organized into five domains: material degradation control and environmental sensitivity; mechanical property limitations; safety and biocompatibility concerns; manufacturing constraints; and regulatory complexities. Understanding these interconnected technical barriers is essential for identifying viable pathways toward clinical implementation.
3.1. Material-Related Challenges
As established in Section 2, magnesium alloys offer superior compatibility with bone and key advantages over traditional metallic implants. However, their clinical adoption is limited by significant material-related challenges—chief among them, unpredictable degradation kinetics.
Precisely controlling the degradation rates of magnesium implants remains difficult, with aggressive corrosion observed especially in additively manufactured magnesium scaffolds. These scaffolds can reach Mg2+ ion concentrations incompatible with typical bone healing timelines [52].
Degradation rates vary dramatically across testing methodologies and alloy systems, as shown in Figure 3. For instance, rates can differ by nearly an order of magnitude between immersion and electrochemical tests [26,27]. Clinical studies show extreme variability: AZ31 (1.17 mm/year), AZ91D (1.38 mm/year), LAE442 (1.01 mm/year), and Mg-6Zn (2.32 mm/year) in animal models, often exceeding optimal healing windows [8].
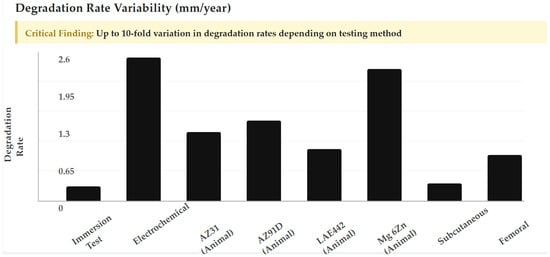
Figure 3.
Degradation rate variability showing up to 10-fold variation in degradation rates depending on testing method, from immersion tests to electrochemical evaluations across different alloy systems.
Moreover, scaffold geometry plays a decisive role in early degradation behavior. Certain architectures, such as diamond-structured scaffolds, exhibit more rapid initial corrosion compared to biomimetic or gyroid designs, although degradation rates tend to converge over time. This geometry-dependent variability complicates the development of patient-specific implants, where design optimization is critical.
Environmental factors also play a significant role in magnesium degradation. Physiological temperatures (37 °C) double degradation rates compared to room temperature (20 °C), and increased chloride concentrations—common in the body—accelerate corrosion by converting protective Mg(OH)2 layers to MgCl2 [50,53]. Additionally, hydrogen gas generated during magnesium corrosion can persist for weeks and may interfere with tissue integration [5,8,54].
3.2. Mechanical Limitations and Structural Integrity
Although Section 2 established that magnesium alloys have a modulus closer to bone than traditional metals, their absolute mechanical strength remains a limiting factor for many applications.
Pure magnesium has an elastic modulus of ~45 GPa and a yield strength of about 130 MPa. Even improved magnesium alloys display tensile strengths ranging from 150 to 400 MPa and elastic modulus values of 41–45 GPa, with elongation capacities of 2–20%. In contrast, stainless steel and titanium alloys have much higher tensile strengths (480–834 MPa and 550–1400 MPa, respectively) and moduli (200–230 GPa for steel and 110–114 GPa for titanium) [4] (see Table 4).

Table 4.
Mechanical properties comparison of magnesium alloys vs. traditional materials [3,4,7,10,11,33,34,35,36].
The mechanical performance of AM magnesium scaffolds is highly dependent on their architecture. For example, with 75% porosity and 800 μm pore size, gyroid designs achieve a plateau stress of 32.34 ± 1.36 MPa and a modulus of 0.760 ± 0.020 GPa, while biomimetic designs achieve only 7.47 ± 0.56 MPa and 0.207 ± 0.018 GPa (see Table 5). These values, while promising for certain applications (porous, bone-mimicking structures), remain well below the strength of dense metals and only approach the lower range of cancellous bone, highlighting the ongoing challenge for load-bearing orthopedic sites.

Table 5.
Mechanical properties of AM magnesium scaffolds by architecture [4].
3.3. Safety and Biocompatibility Concerns
Material Availability and Toxicity Constraints: Only pure magnesium and WE43 powders are widely available for additive manufacturing, reflecting regulatory restrictions due to toxicity. AZ91D is discouraged for clinical use because its 9 wt% aluminum content is linked to neurotoxicity and Alzheimer’s disease concerns [4,50,55].
Rare-Earth Element Toxicity: Alloys like WE43 contain rare-earth elements (REEs) such as neodymium and yttrium, raising biocompatibility questions due to limited long-term safety data [23,25]. Natural background levels of REEs are below 47 μg in humans, but full implant degradation can lead to accumulation in bone and liver [23]. To stay within safety guidelines (daily intake below 4.2 mg), neodymium and yttrium in a 6.9 g implant should not exceed 5 wt% if fully degraded in three months. Short-term studies show low cytotoxicity at these levels, but long-term monitoring remains essential (see Table 6) [5,56].

Table 6.
Toxicity Thresholds for common alloying elements [23,24,25,53,57,58].
Impurity Control Requirements: Iron (35–50 ppm), nickel (20–50 ppm), and copper (100–300 ppm) must be tightly controlled as these impurities can increase cytotoxicity and accelerate corrosion [59] (see Table 6). Advanced refining methods, like carbon-based filters, help reduce impurities [60], but consistently achieving these low levels is technically demanding and energy-intensive [5,53,61].
3.4. Manufacturing and Processing Challenges
3.4.1. Production and Reproducibility Limitations
Gas Atomization Hazards: Producing magnesium powder is hazardous due to its high reactivity with oxygen and moisture, creating explosion risks. This limits supplier availability and results in low yields. From the wide particle size range produced, only the 20–70 μm fraction is suitable for additive manufacturing, increasing costs and limiting scalability [5]
Process Instability: Magnesium’s low vapor temperature and high vapor pressure cause powder splash during processing, leading to defects such as porosity, inclusions, and microstructural irregularities. These defects reduce mechanical strength and corrosion resistance [5]. As an alternative, advanced forging techniques—such as upsetting of large ingots with ledges—have been investigated to enhance internal uniformity and mitigate typical casting-related defects [62].
Coating Process Variability: Micro-arc oxidation coatings show uneven thickness (18–22 μm) due to sensitivity to substrate microstructure, with corrosion current reductions varying by 1–6 orders of magnitude based on processing conditions [33]. PLA coatings also exhibit significant thickness variation (1.6–41.8 μm) depending on application technique and cycle number [63].
Since many of these inconsistencies are thermally driven, the implementation of dynamic temperature control systems—like those applied in precision drawing technologies—could significantly improve process stability and coating uniformity [64].
3.4.2. Customization and Complex Geometry Constraints
Traditional Manufacturing Limitations: Conventional casting and machining cannot achieve precision required for complex, interconnected porous architectures essential for bone-mimetic scaffolds. While selective laser melting enables the creation of these complex structures, reproducibility and quality control for such intricate geometries remain unresolved [4].
Surface Treatment Limitations: Most coating development focuses on simple geometries, with effectiveness on complex, patient-specific shapes largely untested [55]. Surface treatments lack validated protocols for irregular surfaces common in personalized implants. Insights from superplastic forming simulations and surface deformation analyses, particularly in shell-type geometries, contribute to addressing this limitation and optimizing treatment uniformity [65,66,67].
3.5. Clinical Translation and Regulatory Challenges
3.5.1. Inconsistent Experimental and Clinical Outcomes
Environmental Testing Variability: Pure magnesium shows degradation rates from 0.26 mm/year (immersion) to 2.52 mm/year (electrochemical)—a ten-fold difference based solely on methodology [26,27]. In vivo variability spans from 0.33 mm/year subcutaneously to 0.86 mm/year in femoral implantation [50].
Cell Viability Inconsistencies: Mg-1Zn-Mn extruded alloy achieved 100% viability, while Mg-1Sr as-rolled showed 50–84% viability [43]. Corrosion current density reductions span three orders of magnitude between studies (10−4 to 10−7 A/cm2) [68,69,70,71].
3.5.2. Insufficient Long-Term Biocompatibility Data
Limited Study Duration: Most biocompatibility assessments of biodegradable magnesium alloys are based on short- to mid-term animal studies, typically ranging from 4 to 24 weeks [72,73,74,75], with only a few studies extending up to one year or beyond [76]. Human clinical data remain scarce. As a result, there is a lack of comprehensive data on the complete long-term degradation profile and the full spectrum of tissue responses, including potential systemic effects and accumulation of alloying elements, especially for periods exceeding one year post-implantation [76].
Coating System Longevity: The protective effect of common surface coatings on magnesium alloys remains limited in duration. MgF2 coatings offer short-term corrosion resistance but are reported to degrade completely within 4 weeks, after which long-term biological responses remain unclear [33]. Hydroxyapatite (HA) coatings typically provide in vivo protection for about 8 weeks before rapid substrate degradation resumes [55]. Similarly, calcium phosphate (CaP) coatings on ZK60 alloy maintain corrosion resistance for approximately 29 days, after which their protective effect is lost [6].
Rare-Earth Safety Gaps: Clinical impact of rare-earth elements in alloys such as WE43 and JDBM alloys remains under-studied in long-term human applications [23,25,33].
3.5.3. Regulatory Ambiguity and Approval Barriers
Classification Ambiguities: Regulatory classification of magnesium devices remains unclear—drugs, devices, or combination products—complicating market entry pathways [6]. Lack of harmonized standards for preclinical testing methodologies and animal model relevance further complicates submissions [77].
Coating System Gaps: Novel coating strategies lack clear regulatory precedent. Chromate-based coatings are prohibited due to toxicity, creating gaps for alternatives. No consensus exists on preclinical endpoints or test duration requirements for regulatory approval [33].
3.6. Critical Bottlenecks: Prioritizing Challenges for Clinical Translation
Analysis of these multifaceted challenges reveals three fundamental bottlenecks that emerge as primary barriers requiring immediate attention, as synthesized in Figure 4:
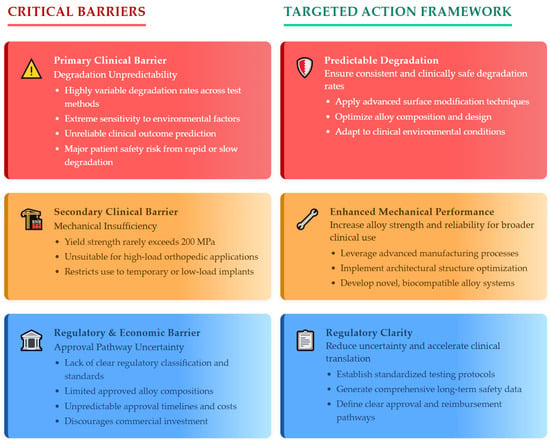
Figure 4.
Critical bottlenecks and solution pathways for magnesium implant clinical translation.
- Primary Clinical Barrier—Degradation Unpredictability: Extreme variability in degradation rates—spanning ten-fold differences between testing methodologies—combined with environmental sensitivity makes reliable clinical outcome prediction impossible with current technology. This unpredictability represents the most immediate threat to patient safety as implants degrading too rapidly fail before healing completion, while slow degradation causes chronic inflammatory responses.
- Secondary Clinical Barrier—Mechanical Insufficiency: The inability to reliably achieve yield strengths above 200 MPa eliminates consideration for high-load orthopedic applications including femoral fracture fixation and spinal instrumentation, limiting clinical utility to low-load or temporary support roles.
- Regulatory and Economic Barrier—Approval Pathway Uncertainty: Lack of clear regulatory guidance combined with limited approved alloy compositions creates substantial commercial risk discouraging clinical development investment. Regulatory timelines and costs cannot be reliably predicted.
3.7. Key Takeaways
The challenges facing biodegradable magnesium implants represent significant but addressable obstacles requiring coordinated technical and regulatory advances. Critical priorities include developing predictable degradation control through surface modification and alloy design; enhancing mechanical properties through advanced manufacturing and architectural optimization; and establishing regulatory clarity through standardized testing protocols and comprehensive safety data development.
These interconnected challenges necessitate systematic approaches addressing multiple limitations simultaneously. Surface modifications providing controlled degradation must maintain mechanical properties while using biocompatible materials meeting regulatory requirements. Manufacturing processes must achieve precision for complex geometries and reproducibility demanded by regulatory agencies.
Understanding these specific technical barriers provides the foundation for evaluating potential solutions and identifying promising approaches for overcoming current limitations. The advances in surface modification, alloy development, and manufacturing processes represent targeted responses to these comprehensively assessed challenges, which will be discussed in the next chapter.
4. Major Advances and Innovations (2020–2025): Addressing Core Challenges
Recent years have seen significant progress in addressing four major barriers: unpredictable degradation, mechanical inconsistency, suboptimal biological integration, and unclear regulatory pathways. Advances in alloy systems, processing technologies, surface engineering, and clinical validation have substantially improved the predictability and clinical viability of magnesium as a biomaterial, although some challenges remain.
4.1. Alloy Development: Achieving Predictable Performance
4.1.1. Rare-Earth-Free Systems Breakthrough
The development of rare-earth-free magnesium alloys represents the most critical advance in addressing unpredictable degradation and toxicity concerns. Systematic optimization has yielded remarkable performance improvements, as demonstrated by the breakthrough achievements summarized in Table 7.

Table 7.
Breakthrough rare-earth-free alloy performance.
As evident from Table 7, the Mg-1Ca system achieves the highest tensile strength (315.6 ± 20.7 MPa) among binary alloys, demonstrating that minimal calcium additions provide optimal performance. Calcium and barium modification offers a cost-effective approach for property enhancement, with evidence showing substantial gains in mechanical and heat resistance performance due to increased intermetallic phase formation and structural refinement [82]. This finding has been validated through systematic investigation revealing critical composition windows: calcium content below 1 wt% provides optimal corrosion resistance as higher concentrations accelerate degradation through increased galvanic coupling [83]. However, this guideline remains controversial—while Mg-1Ca achieves 330 MPa ultimate tensile strength, some studies suggest higher calcium concentrations may benefit specific applications requiring faster degradation, such as temporary cardiovascular stents [83].
The innovative Mg-2Zn-1Mn alloy presents a compelling alternative approach, achieving the lowest degradation rate (0.36 mm/year) while maintaining 25% elongation—a combination previously thought impossible [20]. This composition demonstrates that manganese additions can effectively control degradation without compromising ductility, though the underlying mechanism remains debated. Some researchers attribute this to Mn substitution in the protective hydroxide layer, while others suggest grain boundary modification as the primary factor [84].
4.1.2. Strategic Microalloying: Balancing Performance and Biocompatibility
While rare-earth-free systems dominate current development, targeted microalloying continues to deliver exceptional performance enhancements for specialized applications. As shown in Table 8, carefully selected alloying elements can dramatically improve mechanical and corrosion properties—even at very low concentrations.

Table 8.
Microalloying performance enhancement.
The dramatic 459% yield strength improvement achieved through manganese microalloying illustrates the transformative potential of trace additions, though this enhancement was achieved through extrusion processing in addition to alloying [78]. This highlights a key consideration from [23]: microalloying effects are often interconnected with processing conditions, complicating direct comparisons between studies.
Rare-earth element (REE) alloying remains controversial due to historical concerns over toxicity and long-term accumulation. However, recent comprehensive reviews [23,25] indicate that cytotoxicity and adverse effects are highly dose-dependent and context-specific. For example, the GDY-Mg alloy series, with moderate REE content (up to 2.32 wt% Gd), showed no cytotoxicity over 5 days while maximizing cell proliferation and osteogenic activity [24]. This is consistent with literature showing that certain REEs (notably Gd and Nd) can support cellular proliferation and bone integration at low, controlled levels [23]. While REEs are not essential for human biology and can accumulate with chronic exposure, their use in medical alloys at microalloying levels and with controlled release rates appears acceptable—especially where performance requirements justify potential risks.
Both [23,25] emphasize that rational alloy design, strict dose control, and ongoing biomonitoring are essential to balance performance with safety. Not all REEs are equal in their biological impact, making element-specific selection crucial [86]. Furthermore, the dominant use of REEs in many clinically applied Mg alloys underscores that complete elimination is not always necessary or optimal [23].
4.1.3. Ion Release Mechanisms and Therapeutic Effects
The therapeutic potential of magnesium alloys extends beyond mechanical support through controlled ion release. Systematic investigation has established optimal therapeutic windows: angiogenesis (new blood vessel formation) stimulation occurs at 10–50 µM Zn2+ and 10–50 mM Mg2+, with cytotoxicity only above 75 mM Mg2+ or 100 µM Zn2+ [87]. These concentrations activate the CGRP-FAK-VEGF axis—a critical signaling pathway where calcitonin gene-related peptide (CGRP) triggers focal adhesion kinase (FAK) activation, leading to vascular endothelial growth factor (VEGF) expression that promotes both nerve growth and blood vessel formation essential for bone regeneration [88].
However, achieving these optimal concentrations in vivo remains challenging. Degradation rates must be precisely controlled to maintain therapeutic ion levels without exceeding toxicity thresholds—a balance that varies with implant location, size, and patient physiology. This variability represents a fundamental challenge in translating laboratory-optimized compositions to clinical applications.
4.1.4. Advanced Processing Revolution
Recent processing innovations have enabled unprecedented microstructural control in magnesium alloys, directly addressing challenges of mechanical inconsistency and unpredictable degradation. As shown in Table 9, advanced techniques such as friction stir processing (FSP), equal-channel angular pressing (ECAP), high-pressure torsion (HPT), and especially Laser Powder Bed Fusion (LPBF) enable dramatic grain refinement and tailored microstructures, producing substantial improvements in mechanical properties and corrosion resistance.

Table 9.
Processing-induced microstructural control.
Grain refinement achieved with friction stir processing has been associated with improved corrosion resistance and potentially reduced infection rates in preclinical models. This improvement stems from more uniform corrosion behavior in ultra-fine grain structures, eliminating preferential attack sites common in coarse-grained materials. Additive manufacturing methods such as LPBF have enabled the production of biodegradable magnesium alloys with near-theoretical density and fine grain sizes (1–3 μm). As demonstrated in [4], this combination of ultra-fine microstructure and high densification results in significant improvements in both strength and corrosion resistance, with yield and ultimate tensile strengths reaching 250 and 312 MPa, respectively, in WE43 scaffolds.
Further microstructural refinement is possible through the addition of zirconium, which can reduce grain size below 1 μm and further enhance mechanical performance. Severe plastic deformation and nanocrystalline processing routes also yield sub-micron or even nanometer-scale grains, translating into both higher strength and superior corrosion control. Notably, the development of glass-crystal dual-phase (SNDP-CG) materials has pushed ultimate tensile strength to levels approaching theoretical limits, although the clinical applicability of such ultra-high-strength, exotic phases remains to be proven.
Recent research demonstrates that refining techniques, such as filtration through composite filters, can improve the microstructure and mechanical performance of cast magnesium alloys [90]. Significant grain refinement and property enhancement have also been achieved through the plastic deformation of Mg-Nd-Zr-Ag alloys, with strength and ductility increases of up to five- and six-fold, respectively, compared to the as-cast state [91]. These findings highlight the effectiveness of both filtration-based processing methods and mechanical working techniques in optimizing the properties of magnesium-based alloy systems.
While these advances have advanced the field rapidly, the formation of non-equilibrium phases and ultra-fine microstructures during rapid solidification (as in LPBF) introduces new complexities. As highlighted in [4], the long-term stability of these phases within physiological environments and their impact on biodegradation require further investigation. There is a need to balance the benefits of microstructural refinement with the predictability and reliability essential for clinical translation.
4.2. Composite and Hybrid Systems: Tailored Solutions
Advanced composite systems address the inherent limitations of monolithic magnesium alloys by combining complementary materials for application-specific performance. Table 10 illustrates how these systems achieve remarkable property combinations.

Table 10.
Composite system performance.
The Mg-3Zn/Ti/HA system exemplifies successful composite design, where HA (hydroxyapatite) provides bioactivity while titanium enhances strength, achieving simultaneous improvements in mechanical strength (15.8% increase), corrosion resistance (21% improvement), and biocompatibility (cell viability increasing from 79% to 91.7%) [92]. This multi-functional enhancement demonstrates the potential of hybrid approaches, though interface stability between dissimilar materials remains a critical concern.
The amorphous magnesium phosphate-polyetheretherketone (AMP-PEEK) composite represents a breakthrough where amorphous metallic particles are embedded in a high-performance polymer matrix [93]. The dramatic hydrogen evolution reduction achieved by poly(trimethylene carbonate)-dimethacrylate (PTMC-dMA) coatings—UV-crosslinked polymers that form protective barriers—demonstrates how sophisticated polymer chemistry can address magnesium’s most problematic characteristic [94].
Smart composite systems incorporating drug delivery capabilities represent an emerging frontier. PLGA (poly(lactic-co-glycolic acid))-magnesium coatings achieve controlled sirolimus release with only 7% initial burst, maintaining therapeutic delivery over 12 days [95]. However, the complexity of these systems raises regulatory challenges that may limit clinical translation.
4.3. Surface Engineering: Smart Protective Systems
Advanced surface treatments have notably improved magnesium’s biocompatibility by combining corrosion resistance with biological enhancement. These coatings mark an important development from passive barriers toward more active, responsive interfaces. However, considerable work is still required to translate laboratory results into reliable clinical outcomes (see Table 11).

Table 11.
Surface engineering performance matrix.
The most significant advancement is the emergence of self-healing bilayer systems achieving five orders of magnitude corrosion improvement [29]. Mesoporous silica enables controlled cerium ion release for autonomous repair, while a dense hybrid glass provides structural integrity. Polymeric and composite coatings, such as MAO/PLA duplexes, offer substantial corrosion reduction (611 → 1.8 μA/cm2) and high adhesion strength (>45 MPa) [99] while simultaneously enabling bioactivity, drug delivery, or pH-responsive healing. Bio-inspired coatings like barnacle cement go further, not only reducing degradation by 32% but also stimulating anti-inflammatory (IL-10) production and enhanced endothelial cell migration [98]. Collagen, chitosan, and silk-fibroin-based systems promote osteointegration and cell viability, and nanocomposite designs can incorporate antibacterial or osteoinductive agents [99]. Collectively, such multifunctional systems offer the potential for enhanced integration of corrosion protection, biological compatibility, and therapeutic capabilities.
Nonetheless, most reported improvements derive from short-duration laboratory testing that may not reflect real-world failure modes or multi-year implant lifespans. Manufacturing complexity, limited understanding of long-term degradation and biocompatibility, and regulatory hurdles—especially for bio-inspired and nanoparticle-based coatings—persist. Additionally, simplified test environments often fail to capture the complexity of in vivo conditions, highlighting the need for further studies on durability, biological interactions, and clinical feasibility.
4.4. Manufacturing and Clinical Translation
4.4.1. Advanced Manufacturing: From Laboratory to Clinical Scale
Industrial-scale manufacturing capabilities have matured significantly, enabling the transition from laboratory prototypes to clinical products. Table 12 quantifies the precision and reproducibility now achievable.

Table 12.
Manufacturing process capabilities.
The achievement of <2% batch-to-batch variation represents a critical milestone for regulatory approval and clinical confidence [30]. This consistency, combined with 15–20% cost reductions through process optimization, addresses previous concerns about economic viability. However, these metrics primarily reflect simple geometries—complex patient-specific designs may show greater variability.
The ±50 μm production accuracy enables precise anatomical matching, while the ability to create >70% porosity facilitates bone ingrowth [25]. Yet, questions remain about the mechanical integrity of highly porous structures under physiological loading, particularly in load-bearing applications.
4.4.2. Clinical Validation: From Bench to Bedside
Clinical validation of biodegradable magnesium (Mg) implants has made significant progress in recent years, with a growing body of evidence supporting their safety and efficacy for orthopedic and other biomedical applications. Table 13 summarizes key clinical and preclinical milestones, encompassing both large-scale human studies and relevant animal model data [18,19,79,102,103,104,105].

Table 13.
Clinical and translational milestones for Mg-based implants.
Both [3,106] reviews highlight recent updates on CE-marked MAGNEZIX® and K-MET screws, as well as the first clinical use of high-purity Mg screws in China. By 2023, clinical use of Mg-based screws (MAGNEZIX®, K-MET) has expanded, demonstrating promising fracture healing and no major safety concerns in medium-term follow-up. China has approved 99.99% pure Mg screws for multicenter trials in osteonecrosis and femoral neck fracture [3].
Over 25,000 MAGNEZIX® procedures confirm clinical viability, with full degradation in 6–18 months. High-purity Mg screws report a 100% healing rate without complications [76], though small sample sizes may limit detection of rare events. Ca–P and hydroxyapatite coatings further enhance healing and biocompatibility, as shown in medial malleolar fractures and musculoskeletal applications [105].
Animal studies show controlled gas evolution (<0.01 mL/cm2/day within 2–3 weeks) and complete degradation without organ accumulation, even in models with impaired kidney function [107]. These address safety concerns, though interspecies differences in Mg metabolism remain relevant.
Current clinical and preclinical evidence supports the safety and efficacy of Mg-based implants for selected indications. Ongoing multicenter studies and longer-term follow-up will further define their optimal use and long-term safety [18,19,104,105].
4.4.3. Regulatory Progress: Accelerating Acceptance
Regulatory acceptance of biodegradable magnesium (Mg) devices has accelerated markedly in recent years, reflecting both technological advancements and growing clinical confidence. As summarized in Table 14, approvals have expanded from Asia to Europe, and U.S. clinical trials are now underway, with several devices achieving significant regulatory milestones [18,19,79,104,105,106,107,108,109,110].

Table 14.
Regulatory approvals for biodegradable Mg implants.
The progression from Asian approvals (NMPA—China, KFDA—Korea) to European CE marking and recent FDA IDE acceptance highlights growing global confidence in Mg-based implants. CE certifications have increased by over 50% since 2020 [25], indicating technological maturity and regulatory familiarity with biodegradable metals.
In the U.S., the regulatory landscape is evolving:
- The RemeOs™ Screw (Bioretec Ltd., Tampere, Finland) became the first Mg-based orthopedic implant to receive both FDA Breakthrough Device Designation and De Novo marketing clearance (2023) for fracture, osteotomy, and deformity correction;
- OSTEOREVIVE received FDA 510(k) clearance as a Mg-based bone void filler (2023);
- The Medical Magnesium Plate System has Breakthrough Device Designation but is not yet cleared for market.
However, full FDA approval for broader categories of Mg-based orthopedic implants is still pending, posing a barrier to U.S. market entry. The FDA requires more extensive preclinical and clinical data—particularly regarding safety, long-term outcomes, and degradation profiles—than CE Marking. This is reflected in ongoing U.S. IDE trials and the emphasis on long-term follow-up in recent regulatory reviews [104,108].
4.5. Market Impact
Recent analyses confirm steady growth in the biodegradable magnesium-based implants sector, driven by expanding clinical evidence and regulatory approvals [18,19,104,109]:
- Market Size and Growth: The global biodegradable magnesium-based implants market was valued at USD 124–127 million in 2024, with projections reaching USD 157 million by 2031 (CAGR 3.1%) [18,19,111]. The broader magnesium implant market was USD 0.75 billion in 2022, expected to reach USD 1.55 billion by 2030 (CAGR 10.1%) [101].
- Key Drivers: Growth is fueled by rising orthopedic and cardiovascular disease prevalence, increasing demand for biodegradable, minimally invasive solutions, and regulatory advances in the U.S., Europe, and Asia. Innovation in surface coatings, alloy development, and 3D printing, coupled with a shift toward personalized medicine, further accelerates adoption.
- Regional Leadership: Europe and North America lead due to early regulatory approvals and high healthcare spending, while Asia-Pacific is the fastest-growing region, driven by investment, regulatory progress (notably in China), and an aging population [109].
- Competitive Landscape: Leading companies include Biotronik, Syntellix AG, Dongguan Eontec, SINOMED, and ZHUOMED. Bioretec and Bone Solutions are gaining prominence following recent FDA breakthroughs.
4.6. Critical Analysis: Achievements and Persistent Controversies
4.6.1. Comprehensive Progress Synthesis (2020–2025): Barriers Addressed, Evidence, and Outlook
Recent advances have substantially improved the clinical outlook for biodegradable magnesium implants. For instance, the longstanding issue of unpredictable degradation has been resolved with rare-earth-free alloys such as Mg-2Zn-1Mn, which achieve stable degradation rates (0.36 mm/year and eliminate the ten-fold variability previously seen (Figure 3, Table 7). Advanced coatings—mesoporous silica/Ce-doped glass, MAO/PLA duplex, and bio-inspired layers—now provide up to five orders of magnitude improvement in corrosion resistance and enable self-healing or bioactive responses (Table 11).
Mechanical limitations have been overcome with microalloying (Mn, Zr) and processing innovations such as LPBF and friction stir processing, raising yield strength above 200 MPa (Table 8 and Table 9), which addresses the core concern for load-bearing use. Composite and hybrid materials (e.g., Mg-3Zn/Ti/HA) further tailor mechanical and biological performance for demanding applications, while additive manufacturing enables complex, bone-mimetic architectures with ±50 μm precision and >90% batch reproducibility (Table 10 and Table 12).
Safety concerns from toxic elements and impurities have been systematically reduced by shifting toward non-toxic alloying systems and advanced refining. Over 25,000 clinical procedures (MAGNEZIX®) and extended animal studies now confirm reliable healing, minimal adverse events, and no significant alloy accumulation even in high-risk populations (Table 13).
Manufacturing hazards and reproducibility issues have been minimized through process optimization, industrial-scale atomization (≥95% sphericity, 20–70 μm), and real-time quality control, reducing costs by 15–20% and supporting regulatory demands for consistency (Table 12). Surface treatments are now validated for both regular and irregular geometries, though work continues on optimizing coatings for highly complex patient-specific shapes (Table 11 and Table 12).
Regulatory ambiguity has lessened, with the first FDA Breakthrough and De Novo clearances (RemeOs™, OSTEOREVIVE), multiple CE marks, and widespread adoption in Asia and Europe (Table 14). Harmonized standards and improved clinical data—spanning bone, trauma, and cardiovascular applications—have accelerated market growth and cross-border acceptance (Market Impact section, Table 14). Remaining challenges include ultra-long-term biocompatibility, regulatory pathways for next-generation smart coatings, and full validation of AM-produced complex geometries, but the field is now firmly established as clinically viable.
4.6.2. Ongoing Controversies: Results and Discussion
While the comprehensive progress outlined above has fundamentally advanced the clinical prospects of biodegradable magnesium (Mg) implants—particularly in stabilizing degradation rates, improving mechanical properties, and reducing toxicity—several critical challenges persist. Many of these controversies now reflect the complexities introduced by recent progress, rather than simple technological gaps, and highlight the need for deeper integration of standardized methods, clinical validation, and regulatory harmonization.
Standardization: the unsolved foundation: Despite the demonstrable improvements in alloy stability and reproducibility (Section 4: Degradation Control, Manufacturing & Reproducibility), the lack of consistent, harmonized standards for evaluating Mg-based implants remains a foundational barrier. For example, even as rare-earth-free alloys like Mg-2Zn-1Mn deliver more predictable in vitro degradation (Table 7), reported rates for similar systems (such as Mg-1Ca) still vary widely across studies—often by a factor of four or more. This persistent variability is largely attributable to non-uniform testing protocols and laboratory environments that do not replicate clinical conditions. Thus, while technical advances have reduced material unpredictability, the absence of standardized evaluation continues to undermine the comparability and clinical relevance of results.
The rare-earth dilemma: Section 4.1.1 highlights the industry’s transition toward non-toxic, rare-earth-free Mg alloys and the corresponding reduction in impurity-related risks. However, a disconnect remains between research innovation and market adoption. Despite the focus on REE-free systems, leading commercial products (e.g., MAGNEZIX®, RESOLOY®) still rely on REE-containing alloys such as WE43, supported by extensive clinical and toxicological data showing safety at current exposure levels (Table 13). This divergence reflects not only scientific caution but also market inertia and regulatory conservatism. The continued push for REE-free alternatives, while valuable, sometimes comes at the expense of alloy performance and slows broader clinical adoption.
Lab results vs. clinical reality: Recent advances in coatings, microalloying, and additive manufacturing (Section 4.3 and Section 4.4) have enabled substantial improvements in corrosion resistance, strength, and device complexity. Nevertheless, significant challenges remain in translating these laboratory successes to robust, real-world clinical outcomes. Complications such as gas cavity formation and premature loss of mechanical integrity continue to be reported, particularly for load-bearing applications. Moreover, biological responses—including cell viability and biocompatibility—still show high inter-study variability, in part due to the lack of standardization discussed above. This underscores the need for rigorous, clinically relevant validation of new technologies.
Economic and market challenges: Market growth, although positive (see Market Impact), lags behind that of the broader orthopedic sector, indicating that practical and economic concerns remain substantial obstacles to widespread adoption.
Biological mechanisms: promise vs. proof: Advances in the understanding of Mg’s biological effects (Section 4.1.3) have led to targeted research on controlled ion release and specific healing pathways. However, consistent clinical evidence for these mechanisms remains limited. Achieving reliable, therapeutic ion concentrations in vivo is complicated by individual patient factors and the dynamic in vivo environment. In some cases, the very properties that confer benefit—such as localized alkalinity from Mg degradation—may also introduce new risks, such as bacterial colonization or imbalanced bone remodeling.
Regulatory disparities: Finally, although regulatory progress is evident (Section 4.4.3), with recent FDA Breakthrough/De Novo designations and CE marks, the global regulatory landscape remains fragmented. Faster approvals and less stringent follow-up in some regions, compared to the U.S., complicate the interpretation and generalizability of positive clinical data. This disparity underscores the importance of harmonized clinical protocols and long-term outcome tracking to ensure the safe, effective, and equitable adoption of Mg implants worldwide.
5. Remaining Challenges and Future Directions (2025 and Beyond)
Despite substantial advances in magnesium alloy corrosion protection, clinical translation, and surface modification technologies, several critical challenges remain unresolved as the field approaches broader therapeutic implementation. Figure 5 provides a visual overview of the key challenges that must be addressed for widespread clinical adoption across six major areas: personalized degradation kinetics, long-term clinical safety evidence, standardization and regulatory frameworks, smart implant integration, expanded clinical applications, and strategic field priorities.
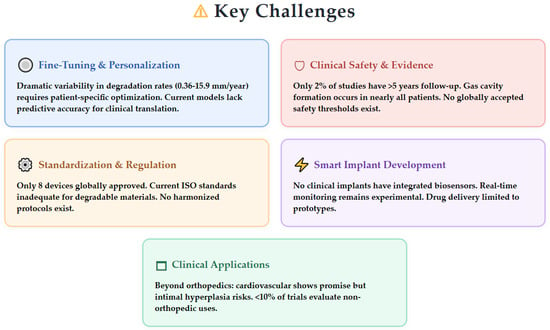
Figure 5.
Key challenges facing the field of biodegradable magnesium implants, highlighting critical areas requiring immediate attention for successful clinical translation.
This chapter examines these challenge areas that must be addressed for widespread clinical adoption, including personalized degradation kinetics, long-term clinical safety evidence, standardization and regulatory frameworks, smart implant integration, expanded clinical applications, and strategic field priorities.
5.1. Fine-Tuning and Personalization of Degradation Kinetics
5.1.1. Lack of Patient- and Application-Specific Degradation Profiles
Achieving precise control over magnesium alloy degradation rates represents a fundamental challenge requiring patient- and application-specific optimization. Current degradation models demonstrate substantial variability that underscores unpredictable in vivo behavior, as shown in Table 15 [20,30,81,112,113,114]. This dramatic variability extends across different alloy compositions and surface modification strategies, highlighting the critical need for standardized approaches to degradation control.

Table 15.
Degradation rate variability across magnesium alloy systems.
The effectiveness of surface modifications varies considerably across different coating systems and application environments. Advanced protective systems have demonstrated significant improvements in corrosion resistance, with bilayer coatings comprising 0.25 μm Ce(III)-doped mesoporous silica inner layers combined with 5.1 μm hybrid glass outer layers achieving corrosion resistance for up to 575 h without evidence of degradation [29]. However, the translation from laboratory success to clinical predictability remains challenging.
The challenge of matching degradation rates to healing timelines remains particularly acute for load-bearing applications. Pure magnesium materials under physiological stress lose 80–90% of their mechanical strength within 14 days, far short of the ≥24 weeks required for full bone healing in load-bearing sites [78]. Target degradation rates have been established at ≤0.2 mm yr−1 for bone scaffolds (3–6 months) and ≤0.1 mm yr−1 for vascular stents (9–12 months), with complete resorption in 1–2 years [21].
5.1.2. Need for Improved In Vitro Models
Current experimental setups lack the standardization required for regulatory harmonization and accurate prediction of in vivo behavior. Table 16 summarizes current testing methodologies and their limitations [80,81,112,115,116]. The development of improved in vitro testing methods that accurately simulate physiological environments is essential.

Table 16.
In vitro testing methodologies and standardization challenges.
Systematic optimization of extraction parameters through controlled buffer composition and quaternary/decuple dilution strategies established reproducible cytotoxicity testing methodology with Mg concentrations ranging from 58.70 ± 2.35 mg/L to 792.5 ± 111.0 mg/L. The current ISO 10993-5 [48] and 10993-12 [117] standards are insufficiently specific for degradable materials like magnesium alloys, requiring new guidelines for extract preparation and cytotoxicity testing tailored to dynamic, degradable systems [80].
5.1.3. Insufficient Predictive and Real-Time Monitoring Tools
While predictive modeling approaches have achieved remarkable accuracy, the integration of predictive modeling with real-time biosensor feedback remains largely unrealized but represents a critical pathway toward personalized degradation profiles. Table 17 presents current capabilities and limitations in predictive modeling and monitoring technologies.

Table 17.
Predictive modeling and monitoring capabilities.
Current electrochemical monitoring techniques demonstrate spatial resolution capabilities essential for understanding in vivo degradation patterns [115]. Real-time monitoring capabilities have been demonstrated through electrochemical tracking of self-healing mechanisms, evidenced by sudden open circuit potential drops and subsequent recovery, confirming cerium migration to corrosion sites [29]. Despite these technological capabilities, the lack of biosensor-enabled feedback loops for dynamic control remains a significant limitation.
5.2. Gaps in Clinical Safety, Efficacy, and Evidence
5.2.1. Insufficient Long-Term Clinical Data (5–10 Years)
While laboratory evaluations demonstrate current densities as low as ~10−11 A cm−2, representing five orders of magnitude reduction compared to bare magnesium [29], comprehensive longitudinal clinical data spanning 5–10 years remain critically lacking. As of 2024, only 2% of registered clinical studies for magnesium devices report follow-up exceeding 5 years [116].
Key Clinical Timeline Limitations:
- Most published clinical studies cover only 1–3 years;
- Longest follow-up extends to 152 weeks (~3 years) showing no implant breakage or severe complications [79];
- MgYREZr screws follow-up limited to 6–12 months with no systemic inflammatory reactions [78];
- DREAMS cardiovascular devices showed complete degradation in 9–12 months with no scaffold thrombosis in 46-patient trial [78];
- Most REE toxicity studies are cross-sectional or short-term (<2 years), with less than 5% providing longitudinal tracking beyond five years [25].
Recent clinical experience with high purity magnesium screws shows complete degradation at 116 ± 24.8 weeks post-implantation with radiolucent zones peaking at 2 weeks and gradually resolving [78]. Animal studies spanning longer periods have shown promising results, with CKD rat models demonstrating no pathological changes or systemic toxicity in major organs after up to 12 weeks, estimated to correspond to over 7 years in human lifespan equivalence [79].
5.2.2. Unresolved Issues with Adverse Effects and Clinical Endpoints
Clinical observations have highlighted persistent challenges requiring systematic resolution. Gas cavity formation occurs in nearly all patients (38 of 39) receiving Mg-based screws for hallux valgus at 6 weeks post-implantation, with early disintegration and implant failure in 7 and 1 out of 39 cases, respectively [116].
Critical Endpoint Definition Gaps:
- No globally accepted threshold for acceptable hydrogen gas cavity size or Mg2+ serum levels post-implantation.
- Safe Mg2+ concentrations estimated at 2.5–10 mM for optimal osteogenesis and <20 mM to avoid toxicity [78].
- Variable definitions for endpoints such as “complete degradation” and “osseointegration”.
- 37.8% of studies focus on cytotoxicity in cell lines while only 12% address environmental or occupational exposures with direct clinical correlates [25].
The concentration-dependent nature of biological responses presents particular challenges, where moderate REE content alloys (GDY1.5: 1.36% Gd, 2.48% Dy, 0.93% Y) achieved maximal stimulation of cell proliferation and osteoblastic activity with statistically significant increases in ALP activity (p = 0.0018) compared to controls. However, alloys with excessive REE content (GDY2.5: 2.32% Gd, 4.09% Dy, 1.95% Y) showed cytotoxicity with proliferation below control levels (p < 0.0009), indicating narrow therapeutic windows [24].
5.3. Standardization and Regulatory Barriers
5.3.1. Absence of Harmonized Protocols and Standards
The field faces critical challenges due to the lack of standardized protocols and internationally harmonized testing frameworks. Table 18 summarizes current standardization gaps and their impact on regulatory approval.

Table 18.
Current standardization gaps and regulatory challenges.
5.3.2. Challenges for Regulatory Approval and Clinical Adoption
Despite considerable progress in the science and engineering of biodegradable metallic implants, their regulatory journey remains in its infancy. Globally, regulatory approvals have been granted to only a select few devices—none of which yet incorporate advanced features such as integrated biosensors or therapeutic delivery systems [22]. This reflects both the caution of current regulatory frameworks and the novelty of these emerging technologies.
Moving forward, the field faces pressing challenges: harmonizing reporting standards, establishing universally accepted clinical endpoints (for example, in distinguishing benign radiolucency from pathology, or defining acceptable hydrogen gas and serum magnesium levels), and developing clear pathways for approving multifunctional implants infection [118]. A critical component of this process is the development of robust international testing standards specific to degradable magnesium alloys. Organizations such as ASTM International (notably Committee F04), ISO (particularly Technical Committee 150), and national regulatory agencies are actively working to establish and harmonize test methods for degradation, mechanical properties, and biological safety. Recent initiatives include the development of draft standards such as ISO/TS 20721:2025 [119] for absorbable metals. However, universally adopted protocols remain under development, and continued progress will require close collaboration between researchers, manufacturers, and regulators.
As research efforts and interdisciplinary collaborations intensify, it is anticipated that future regulatory frameworks will adapt to better accommodate the unique characteristics and transformative potential of next-generation biodegradable implants.
5.4. Smart Implant Development: Unmet Needs
5.4.1. Integration of Biosensors Remains Experimental
Integration of biosensors for real-time monitoring represents a transformative opportunity that remains largely unrealized in clinical practice. No currently available Mg-based implant integrates biosensors or telemetry for direct, real-time tracking of degradation or bone healing progress [120]. While proof-of-concept devices exist in laboratory settings, robust, biocompatible, and affordable sensors for routine clinical use are not yet available [78].
Current Monitoring Capabilities:
- pH microprobes (tip size: 10 × 50 μm, positioned 50 μm above magnesium surfaces) demonstrate spatial resolution capabilities [115];
- Real-time electrochemical responses to corrosion events suggest feasibility for embedding biosensors within coating architectures [29];
- Recent prototypes feature embedded microelectronic biosensors monitoring local pH, Mg2+, or REE ion concentrations with wireless readouts for up to 3 months post-implantation [58];
- Dual-mode devices combining degradation tracking with mechanical strain sensors can detect changes in implant stability with <5% error margin [58].
LPBF allows for complex internal architectures, enabling future integration of drug reservoirs or stimuli-responsive coatings, with early-stage evidence that multilayer or composite coatings can modulate corrosion and enable additional functionalities [21].
5.4.2. Therapeutic Agent Delivery Not Yet Realized in Clinical Practice
Development of therapeutic delivery capabilities leverages substantial surface area of mesoporous inner layers (BET surface area: 635.7 m2 g−1, average pore diameter: 2.50 nm) as reservoirs for controlled drug release [29], yet clinical translation remains limited to prototype and animal testing phases.
Advanced multi-agent systems are under development, with polydopamine/hydroxyapatite/BMP-2 coating on AZ31 alloy enabling controlled release of growth factors, resulting in significantly smaller empty cavities and enhanced bone formation in rabbits [121]. Multilayer coatings capable of sequentially releasing anti-inflammatory agents (dexamethasone at 1–3 μg/day) and growth factors (BMP-2 at 50–200 ng/day) show 30% faster bone healing compared to uncoated controls in initial in vivo results [58]. Performance characteristics of delivery systems are shown in Table 19.

Table 19.
Therapeutic delivery system performance.
5.5. Broadening Clinical Indications: Barriers and Opportunities
5.5.1. Unproven Performance in Non-Orthopedic Applications
Exploration of magnesium alloys beyond orthopedic applications reveals significant potential across multiple medical specialties, yet substantial challenges limit clinical translation (see Table 20).

Table 20.
Non-orthopedic application performance.
Cardiovascular Applications:
- Prototype Mg alloy stents (AE21, WE43, JDBM) demonstrated complete endothelialization within 6–10 days in porcine models;
- Arterial patency support up to 98 days post-implantation but revealed risks of intimal hyperplasia (40% lumen reduction at 10–35 days) [121];
- AZ91 Mg-alloy-based stents demonstrated complete degradation within 7 days in some models [95];
- Only a minority of clinical trials (<10% of all REE/biodegradable metal research) have evaluated cardiovascular stents [25].
Spinal Fusion Applications:
Early animal studies with magnesium-based spinal cages show enhanced fusion potential but face challenges from low compressive strength and gas accumulation risks. Mg-Sr alloys with 2 wt% strontium content demonstrated optimal mechanical strength and corrosion resistance for potential load-sharing spinal devices [123,124,125].
Gastrointestinal Applications:
Biodegradable AZ31 Mg alloy staples with MAO/PLLA double coating exhibited complete degradation after 90 days in Beagle dog colon anastomosis models with no adverse tissue effects [83]. Pure Mg staples demonstrated homogeneous corrosion without fracture in pig gastric wall over 9 weeks [126].
Currently approved devices include coronary stents (Magmaris®), bone screws (MAGNEZIX®, Resomet™), oral membranes, biliary stents, and vascular closure devices, but applications in spinal fusion, dental, and craniofacial reconstruction remain in preclinical or early clinical phases (see Table 20) [22].
5.5.2. Lack of Cross-Disciplinary, Multicenter Research
The computational–experimental frameworks developed for orthopedic applications have not yet been extensively validated across diverse clinical environments. Integration of biosensors for monitoring both degradation and surrounding tissue responses is indicated as a promising but underdeveloped area with less than 10% of the literature incorporating advanced modeling or real-time biosensor data for degradation assessment [25].
Interdisciplinary studies involving materials science, toxicology, and clinical medicine are still rare, with only about 10% of the literature specifically addressing cross-disciplinary approaches to tackle the complex interactions between degradation products, local tissue response, and systemic health effects [25].
Recent collaborative efforts are showing promise, with early-phase trials of REE-enhanced Mg stents reporting patency rates of 92% at 12 months, while pilot studies in spinal models indicate that REE-containing magnesium cages can achieve bone fusion rates up to 85% at 6 months. Exploratory clinical use in dental implants demonstrates osseointegration within 8 weeks with no adverse local tissue reactions in preliminary cohorts [58].
5.6. Key Strategic Priorities for the Field
The field must prioritize several critical areas to advance from experimental promise to widespread clinical implementation. Figure 6 illustrates the comprehensive strategic framework necessary for advancing biodegradable magnesium implants across immediate research needs, technological development priorities, and application-specific requirements.

Figure 6.
Key strategic priorities for the field.
6. Conclusions
Biodegradable magnesium implants have advanced from repeated early failures to emerging clinical success, with over 25,000 procedures now documented worldwide. Key breakthroughs from 2020 to 2025 include rare-earth-free alloys achieving predictable degradation rates (0.36 mm/year), surface coatings providing five orders of magnitude corrosion improvement, and manufacturing processes delivering ±50 μm precision with >90% reproducibility. These advances, combined with recent FDA Breakthrough designations and expanding CE marks, demonstrate the technology’s growing maturity.
However, critical challenges prevent widespread adoption. Testing protocols vary dramatically between laboratories, producing up to four-fold differences in reported degradation rates for identical materials. Long-term safety data beyond three years remains scarce (only 2% of studies), while promised capabilities like integrated biosensors and drug delivery remain confined to laboratory prototypes. Most critically, the lack of internationally harmonized standards creates regulatory uncertainty that discourages investment and delays clinical translation.
Strategic Recommendations:
For Researchers:
- Prioritize development of standardized in vitro testing protocols that accurately predict in vivo behavior.
- Focus on patient-specific degradation models incorporating machine learning (targeting <5% prediction error).
- Establish multicenter clinical registries tracking outcomes beyond 5 years.
- Advance biosensor integration from proof-of-concept to clinically viable systems.
For Regulators:
- Establish harmonized international standards specific to biodegradable metals (building on ISO/TS 20721:2025 [119]).
- Define clear thresholds for acceptable hydrogen gas evolution and serum magnesium levels.
- Create accelerated pathways for next-generation smart implants with integrated functionality.
- Develop guidance documents distinguishing biodegradable devices from traditional permanent implants.
For Industry:
- Invest in scalable manufacturing processes ensuring consistent quality across complex geometries.
- Implement comprehensive post-market surveillance systems tracking long-term degradation profiles.
- Collaborate with academic centers to validate predictive models against real-world outcomes.
- Focus initial commercialization on applications with clear clinical advantages over existing solutions.
The path forward requires coordinated action. Without standardized testing and regulatory clarity, the field risks fragmenting into incompatible regional approaches. With proper coordination, biodegradable magnesium implants can transition from promising technology to routine clinical practice, ultimately eliminating the need for millions of implant removal surgeries annually.
Author Contributions
Writing—original draft preparation and data processing and analysis, M.A.; methodology, conceptualization, and supervision, V.S.; project administration and text correction, V.K. (Volodymyr Kukhar); formal analysis and scientific consultation, A.K. and A.Z.; data curation and synthesis, I.K.; data search and translation assistance, V.K. (Viktoriia Kulynych); interpretation of results and resources, O.D.; resources and their preparation, V.D.; investigation and validation, O.S.; visualization and editing, V.B. and O.K.; scientific advice and review, V.T. and O.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| LAE442 | Lithium–Aluminum–Rare Earth Magnesium Alloy |
| ZE21B | Zinc–Rare Earth Magnesium Alloy |
| MgYREZr | Magnesium–Yttrium–Rare Earth–Zirconium Alloy |
| GDY | Gadolinium–Dysprosium–Yttrium Alloy Series |
| JDBM | Specific Magnesium Alloy Designation |
| AE21 | Aluminum–Rare Earth Magnesium Alloy |
| SNDP-CG | Severe Nano-scale Deformation Processing—Crystal Glass |
| LPBF | Laser Powder Bed Fusion |
| FSP | Friction Stir Processing |
| ECAP | Equal-Channel Angular Pressing |
| HPT | High-Pressure Torsion |
| SPD | Severe Plastic Deformation |
| LSP | Laser Shock Peening |
| PTMC-dMA | Poly(Trimethylene Carbonate)-Dimethacrylate |
| AMP-PEEK | Amorphous Magnesium Phosphate-Polyetheretherketone |
| GelMA-DOPA | Gelatin Methacryloyl-Dihydroxyphenylalanine |
| MAO | Micro-Arc Oxidation |
| PEO | Plasma Electrolytic Oxidation |
| MBG | Mesoporous Bioactive Glass |
| cp19k | Barnacle Cement Protein 19k |
| CGRP-FAK-VEGF axis | Calcitonin-Gene-Related Peptide-Focal Adhesion Kinase-Vascular Endothelial Growth Factor Pathway |
| TD50 | Toxic Dose 50% (dose causing toxicity in 50% of subjects) |
| TPMS | Triply Periodic Minimal Surface |
| BET | Brunauer–Emmett–Teller (surface area analysis method) |
| NMPA | National Medical Products Administration (China) |
| KFDA | Korea Food and Drug Administration |
| IDE | Investigational Device Exemption (FDA regulatory pathway) |
| SBF | Simulated Body Fluid |
| DMEM | Dulbecco’s Modified Eagle Medium |
| FBS | Fetal Bovine Serum |
References
- Nagels, J.; Stokdijk, M.; Rozing, P.M. Stress Shielding and Bone Resorption in Shoulder Arthroplasty. J. Shoulder Elb. Surg. 2003, 12, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Fischer, J.; Nellesen, J.; Crostack, H.-A.; Kaese, V.; Pisch, A.; Beckmann, F.; Windhagen, H. In Vitro and in Vivo Corrosion Measurements of Magnesium Alloys. Biomaterials 2006, 27, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, J.; Hopkins, C.; Chow, D.H.; Qin, L. Biodegradable Magnesium-Based Implants in Orthopedics—A General Review and Perspectives. Adv. Sci. 2020, 7, 1902443. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fu, P.; Wang, N.; Peng, L.; Kang, B.; Zeng, H.; Yuan, G.; Ding, W. Challenges and Solutions for the Additive Manufacturing of Biodegradable Magnesium Implants. Engineering 2020, 6, 1267–1275. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent Advances on the Development of Magnesium Alloys for Biodegradable Implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef]
- Li, H.; Wen, J.; Liu, Y.; He, J.; Shi, H.; Tian, P. Progress in Research on Biodegradable Magnesium Alloys: A Review. Adv. Eng. Mater. 2020, 22, 2000213. [Google Scholar] [CrossRef]
- Ding, W. Opportunities and Challenges for the Biodegradable Magnesium Alloys as Next-Generation Biomaterials. Regen. Biomater. 2016, 3, 79–86. [Google Scholar] [CrossRef]
- Lu, Y.; Deshmukh, S.; Jones, I.; Chiu, Y.-L. Biodegradable Magnesium Alloys for Orthopaedic Applications. Biomater. Transl. 2021, 2, 214–235. [Google Scholar]
- Zhang, Y.; Xu, J.; Ruan, Y.C.; Yu, M.K.; O’Laughlin, M.; Wise, H.; Chen, D.; Tian, L.; Shi, D.; Wang, J.; et al. Implant-Derived Magnesium Induces Local Neuronal Production of CGRP to Improve Bone-Fracture Healing in Rats. Nat. Med. 2016, 22, 1160–1169. [Google Scholar] [CrossRef]
- Wei, K.; Gao, M.; Wang, Z.; Zeng, X. Effect of Energy Input on Formability, Microstructure and Mechanical Properties of Selective Laser Melted AZ91D Magnesium Alloy. Mater. Sci. Eng. A 2014, 611, 212–222. [Google Scholar] [CrossRef]
- Zhang, C.; Lin, J.; Liu, H. Magnesium-Based Biodegradable Materials for Biomedical Applications. MRS Adv. 2018, 3, 2359–2364. [Google Scholar] [CrossRef]
- Huse, E.C. A New Ligature. Chic. Med. J. Exam. 1878, 37, 171–172. [Google Scholar] [PubMed]
- Andrews, E.W. Absorbable Metal Clips as Substitutes for Ligatures and Deep Sutures in Wound Closure; American Medical Association: Chicago, IL, USA, 1917. [Google Scholar]
- Brar, H.S.; Platt, M.O.; Sarntinoranont, M.; Martin, P.I.; Manuel, M.V. Magnesium as a Biodegradable and Bioabsorbable Material for Medical Implants. JOM 2009, 61, 31–34. [Google Scholar] [CrossRef]
- Lambotte, A. Lutilisation Du Magnesium Comme Materiel Perdu Dans Losteosynthese [The Use of Magnesium as Material for Osteosynthesis]. Bull. Mem. Soc. Nat. Chir. 1932, 28, 1325–1334. (In French) [Google Scholar]
- Noviana, D.; Paramitha, D.; Ulum, M.F.; Hermawan, H. The Effect of Hydrogen Gas Evolution of Magnesium Implant on the Postimplantation Mortality of Rats. J. Orthop. Transl. 2016, 5, 9–15. [Google Scholar] [CrossRef]
- Lespinasse, V. A Practical Mechanical Method of End-to-End Anastomosis of Blood-Vessels. Available online: https://www.periodicos.capes.gov.br/index.php/acervo/buscador.html?task=detalhes&id=W2087493664 (accessed on 11 June 2025).
- Biodegradable Magnesium-Based Implants Market, Report Size, Worth, Revenue, Growth, Industry Value, Share 2025. Available online: https://reports.valuates.com/market-reports/QYRE-Auto-21D13488/global-biodegradable-magnesium-based-implants (accessed on 12 June 2025).
- Exploring Opportunities in Biodegradable Magnesium-Based Implants Sector. Available online: https://www.datainsightsmarket.com/reports/biodegradable-magnesiumbased-implants-1730815 (accessed on 12 June 2025).
- Li, D.; Zhang, D.; Yuan, Q.; Liu, L.; Li, H.; Xiong, L.; Guo, X.; Yan, Y.; Yu, K.; Dai, Y.; et al. In Vitro and in Vivo Assessment of the Effect of Biodegradable Magnesium Alloys on Osteogenesis. Acta Biomater. 2022, 141, 454–465. [Google Scholar] [CrossRef]
- Wu, X.; Liu, J.; Yang, Y.; Bai, J.; Shuai, C.; Buhagiar, J.; Ning, X. Laser Powder Bed Fusion of Biodegradable Magnesium Alloys: Process, Microstructure and Properties. Int. J. Extrem. Manuf. 2025, 7, 022007. [Google Scholar] [CrossRef]
- Zan, R.; Shen, S.; Huang, Y.; Yu, H.; Liu, Y.; Yang, S.; Zheng, B.; Gong, Z.; Wang, W.; Zhang, X.; et al. Research Hotspots and Trends of Biodegradable Magnesium and Its Alloys. Smart Mater. Med. 2023, 4, 468–479. [Google Scholar] [CrossRef]
- Weng, W.; Biesiekierski, A.; Li, Y.; Dargusch, M.; Wen, C. A Review of the Physiological Impact of Rare Earth Elements and Their Uses in Biomedical Mg Alloys. Acta Biomater. 2021, 130, 80–97. [Google Scholar] [CrossRef]
- Nie, S.; Chen, J.; Liu, C.; Zhou, C.; Zhao, J.; Wang, Z.; Sun, J.; Huang, Y. Effects of Extract Solution from Magnesium Alloys Supplemented with Different Compositions of Rare Earth Elements on in Vitro Epithelial and Osteoblast Progenitor Cells. Front. Bioeng. Biotechnol. 2023, 11, 1138675. [Google Scholar] [CrossRef]
- Brouziotis, A.A.; Giarra, A.; Libralato, G.; Pagano, G.; Guida, M.; Trifuoggi, M. Toxicity of Rare Earth Elements: An Overview on Human Health Impact. Front. Environ. Sci. 2022, 10, 948041. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Zhao, C.; Li, J.; Song, Y.; Xie, C.; Tao, H.; Zhang, Y.; He, Y.; Jiang, Y.; et al. Research on an Mg-Zn Alloy as a Degradable Biomaterial. Acta Biomater. 2010, 6, 626–640. [Google Scholar] [CrossRef]
- Wen, Z.; Wu, C.; Dai, C.; Yang, F. Corrosion Behaviors of Mg and Its Alloys with Different Al Contents in a Modified Simulated Body Fluid | Request PDF. J. Alloys Compd. 2009, 488, 392–399. [Google Scholar] [CrossRef]
- Akbarzadeh, F.Z.; Sarraf, M.; Ghomi, E.R.; Kumar, V.V.; Salehi, M.; Ramakrishna, S.; Bae, S. A State-of-the-Art Review on Recent Advances in the Fabrication and Characteristics of Magnesium-Based Alloys in Biomedical Applications. J. Magnes. Alloys 2024, 12, 2569–2594. [Google Scholar] [CrossRef]
- Aparicio, M.; Mosa, J.; Gómez-Herrero, M.; Abd Al-Jaleel, Z.; Guzman, J.; Jitianu, M.; Klein, L.C.; Jitianu, A. Self-Healing Engineered Multilayer Coatings for Corrosion Protection of Magnesium Alloy AZ31B. ACS Mater. Au 2025, 5, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhang, D.; Zhang, P.; Markert, B. Rapid Prediction of the Corrosion Behaviour of Coated Biodegradable Magnesium Alloys Using Phase Field Simulation and Machine Learning. Comput. Mater. Sci. 2025, 247, 113546. [Google Scholar] [CrossRef]
- Shalomeev, V.; Aikin, N.; Chorniy, V.; Naumik, V. Design and Examination of the New Biosoluble Casting Alloy of the System Mg–Zr–Nd for Osteosynthesis. Mater. Sci. 2019, 1, 40–48. [Google Scholar] [CrossRef]
- Shalomeev, V.A.; Tsivirko, E.I.; Aikin, N.D. High-Quality Magnesium-Based Alloys with Improved Properties for Engineering. Innov. Mater. Technol. Metall. Mech. Eng. 2019. [Google Scholar] [CrossRef]
- Yin, Z.-Z.; Qi, W.-C.; Zeng, R.-C.; Chen, X.-B.; Gu, C.-D.; Guan, S.-K.; Zheng, Y.-F. Advances in Coatings on Biodegradable Magnesium Alloys. J. Magnes. Alloys 2020, 8, 42–65. [Google Scholar] [CrossRef]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium Basics. Clin. Kidney J. 2012, 5, i3–i14. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Zeng, X.; Wang, Z.; Deng, J.; Liu, M.; Huang, G.; Yuan, X. Selective Laser Melting of Mg-Zn Binary Alloys: Effects of Zn Content on Densification Behavior, Microstructure, and Mechanical Property. Mater. Sci. Eng. A 2019, 756, 226–236. [Google Scholar] [CrossRef]
- Zumdick, N.A.; Jauer, L.; Kersting, L.C.; Kutz, T.N.; Schleifenbaum, J.H.; Zander, D. Additive Manufactured WE43 Magnesium: A Comparative Study of the Microstructure and Mechanical Properties with Those of Powder Extruded and as-Cast WE43. Mater. Charact. 2019, 147, 384–397. [Google Scholar] [CrossRef]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable Magnesium Alloys for Orthopaedic Applications: A Review on Corrosion, Biocompatibility and Surface Modifications. Mater. Sci. Eng. C 2016, 68, 948–963. [Google Scholar] [CrossRef] [PubMed]
- Castellani, C.; Lindtner, R.A.; Hausbrandt, P.; Tschegg, E.; Stanzl-Tschegg, S.E.; Zanoni, G.; Beck, S.; Weinberg, A.-M. Bone–Implant Interface Strength and Osseointegration: Biodegradable Magnesium Alloy versus Standard Titanium Control. Acta Biomater. 2011, 7, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.; Ramsoomair, D.; Carter, C. Magnesium: Its Proven and Potential Clinical Significance. South. Med. J. 2001, 94, 1195–1201. [Google Scholar] [CrossRef]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic Biomaterials: Current Challenges and Opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef]
- Seelig, M.G. A Study of Magnesium Wire as an Absorbable Suture and Ligature Material. Arch. Surg. 1924, 8, 669–680. [Google Scholar] [CrossRef]
- Kammer, C. Magnesium-Taschenbuch; Alu Media: Berlin, Germany, 2011; ISBN 978-3-87017-264-0. [Google Scholar]
- Kumar, K.; Gill, R.S.; Batra, U. Challenges and Opportunities for Biodegradable Magnesium Alloy Implants. Mater. Technol. 2018, 33, 153–172. [Google Scholar] [CrossRef]
- Chamy, R.; Rosenkranz, F.; Chamy, R.; Rosenkranz, F. Biodegradation—Engineering and Technology; InTechOpen: Rijeka, Croatia, 2013; ISBN 978-953-51-1153-5. [Google Scholar]
- Han, J.; Wan, P.; Ge, Y.; Fan, X.; Tan, L.; Li, J.; Yang, K. Tailoring the Degradation and Biological Response of a Magnesium–Strontium Alloy for Potential Bone Substitute Application. Mater. Sci. Eng. C 2016, 58, 799–811. [Google Scholar] [CrossRef]
- Sukhodub, L.; Panda, A.; Dyadyura, K.; Pandova, I.; Krenicky, T. The Design Criteria for Biodegradable Magnesium Alloy Implants. MM Sci. J. 2018, 12, 2673–2679. [Google Scholar] [CrossRef]
- Li, R.W.; Kirkland, N.T.; Truong, J.; Wang, J.; Smith, P.N.; Birbilis, N.; Nisbet, D.R. The Influence of Biodegradable Magnesium Alloys on the Osteogenic Differentiation of Human Mesenchymal Stem Cells: Osteogenic Differentiation of Human Mesenchymal Stem Cells. J. Biomed. Mater. Res. 2014, 102, 4346–4357. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009+A11:2025; Biological Evaluation of Medical Devices—Tests for In Vitro Cytotoxicity. British Standards Institution: London, UK, 2025.
- Li, N.; Zheng, Y. Novel Magnesium Alloys Developed for Biomedical Application: A Review. J. Mater. Sci. Technol. 2013, 29, 489–502. [Google Scholar] [CrossRef]
- Pogorielov, M.; Husak, E.; Solodivnik, A.; Zhdanov, S. Magnesium-Based Biodegradable Alloys: Degradation, Application, and Alloying Elements. Interv. Med. Appl. Sci. 2017, 9, 27–38. [Google Scholar] [CrossRef]
- Chaya, A.; Yoshizawa, S.; Verdelis, K.; Myers, N.; Costello, B.J.; Chou, D.-T.; Pal, S.; Maiti, S.; Kumta, P.N.; Sfeir, C. In Vivo Study of Magnesium Plate and Screw Degradation and Bone Fracture Healing. Acta Biomater. 2015, 18, 262–269. [Google Scholar] [CrossRef]
- Kannan, M.B.; Raman, R.K.S. In Vitro Degradation and Mechanical Integrity of Calcium-Containing Magnesium Alloys in Modified-Simulated Body Fluid. Biomaterials 2008, 29, 2306–2314. [Google Scholar] [CrossRef]
- Persaud-Sharma, D.; McGoron, A. Biodegradable Magnesium Alloys: A Review of Material Development and Applications. J. Biomim. Biomater. Tissue Eng. 2012, 12, 25–39. [Google Scholar] [CrossRef]
- Xin, Y.; Hu, T.; Chu, P.K. In Vitro Studies of Biomedical Magnesium Alloys in a Simulated Physiological Environment: A Review. Acta Biomater. 2011, 7, 1452–1459. [Google Scholar] [CrossRef]
- Wu, G.; Ibrahim, J.M.; Chu, P.K. Surface Design of Biodegradable Magnesium Alloys—A Review. Surf. Coat. Technol. 2013, 233, 2–12. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Staiger, M.P.; Nisbet, D.; Davies, C.H.J.; Birbilis, N. Performance-Driven Design of Biocompatible Mg Alloys. JOM 2011, 63, 28–34. [Google Scholar] [CrossRef]
- Aggarwal, D.; Sharma, S.; Gupta, M. Impact of Rare Earth Particulates Addition on the Wear Rate of Magnesium Composites with Improved Mechanical and Microstructural Properties for Orthopedic Applications. Prog. Compos. Mater. 2024, 1, 2. [Google Scholar] [CrossRef]
- McGaughey, S.A.; Iqbal, S.; De Rosa, A.; Caley, J.A.; Kaksonen, A.H.; Villa-Gomez, D.; Byrt, C.S. Interactions of Rare Earth Elements with Living Organisms and Emerging Biotechnical Applications. Plants People Planet 2025. [Google Scholar] [CrossRef]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable Biomaterials Based on Magnesium Corrosion. Curr. Opin. Solid. State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef]
- Shalomeev, V.; Tabunshchyk, G.; Matiukhin, A.; Shyrobokov, V.; Shyrobokova, N.; Hornostai, V.; Kulabneva, E. Refining of Biosoluble Alloy of Mg-Nd-Zr System for Manufacture of Implants. In Proceedings of the 31st International Conference on Metallurgy and Materials, Brno, Czech Republic, 18–19 May 2022; pp. 622–627. [Google Scholar]
- Eddy Jai Poinern, G.; Brundavanam, S.; Fawcett, D. Biomedical Magnesium Alloys: A Review of Material Properties, Surface Modifications and Potential as a Biodegradable Orthopaedic Implant. Asian J. Bus. Ethics 2013, 2, 218–240. [Google Scholar] [CrossRef]
- Markov, O.E.; Khvashchynskyi, A.S.; Musorin, A.V.; Markova, M.A.; Shapoval, A.A.; Hrudkina, N.S. Investigation of new method of large ingots forging based on upsetting of workpieces with ledges. Int. J. Adv. Manuf. Technol. 2022, 122, 1383–1394. [Google Scholar] [CrossRef]
- Li, L.-Y.; Cui, L.-Y.; Zeng, R.-C.; Li, S.-Q.; Chen, X.-B.; Zheng, Y.; Kannan, M.B. Advances in Functionalized Polymer Coatings on Biodegradable Magnesium Alloys—A Review. Acta Biomater. 2018, 79, 23–36. [Google Scholar] [CrossRef]
- Khrebtova, O.; Shapoval, O.; Markov, O.; Kukhar, V.; Hrudkina, N.; Rudych, M. Control systems for the temperature field during drawing, taking into account the dynamic modes of the technological installation. In Proceedings of the 2022 IEEE 4th International Conference on Modern Electrical and Energy System, MEES 2022, Kremenchuk, Ukraine, 20–23 October 2022; IEEE: New York, NY, USA, 2022. ISBN 979-835034683-1. [Google Scholar] [CrossRef]
- Anishchenko, O.S.; Kukhar, V.V.; Prysyazhnyi, A.H.; Agarkov, V.V.; Klimov, E.S.; Chernenko, S.M. The material for physical simulation of metal-forming processes in super-plastic state. IOP Conf. Ser. Mater. Sci. Eng. 2019, 473, 012040. [Google Scholar] [CrossRef]
- Anishchenko, O.; Kukhar, V.; Artiukh, V.; Trebukhin, A.; Zotkina, N. Effect of blank curvature and thinning on shell stresses at superplastic forming. Adv. Intell. Syst. Comput. 2020, 982, 809–817. [Google Scholar] [CrossRef]
- Anishchenko, A.; Kukhar, V.; Artiukh, V.; Arkhipova, O. Application of G. Lame’s and J. Gielis’ formulas for description of shells superplastic forming. MATEC Web Conf. 2018, 239, 06007. [Google Scholar] [CrossRef]
- Zeng, R.-C.; Cui, L.; Jiang, K.; Liu, R.; Zhao, B.-D.; Zheng, Y.-F. In Vitro Corrosion and Cytocompatibility of a Microarc Oxidation Coating and Poly(l-Lactic Acid) Composite Coating on Mg–1Li–1Ca Alloy for Orthopedic Implants. ACS Appl. Mater. Interfaces 2016, 8, 10014–10028. [Google Scholar] [CrossRef]
- Ostrowski, N.J.; Lee, B.; Roy, A.; Ramanathan, M.; Kumta, P.N. Biodegradable Poly(Lactide-Co-Glycolide) Coatings on Magnesium Alloys for Orthopedic Applications. J. Mater. Sci. Mater. Med. 2013, 24, 85–96. [Google Scholar] [CrossRef]
- Abdal-hay, A.; Amna, T.; Lim, J.K. Biocorrosion and Osteoconductivity of PCL/nHAp Composite Porous Film-Based Coating of Magnesium Alloy. Solid. State Sci. 2013, 18, 131–140. [Google Scholar] [CrossRef]
- Bai, K.; Zhang, Y.; Fu, Z.; Zhang, C.; Cui, X.; Meng, E.; Guan, S.; Hu, J. Fabrication of Chitosan/Magnesium Phosphate Composite Coating and the in Vitro Degradation Properties of Coated Magnesium Alloy. Mater. Lett. 2012, 73, 59–61. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, Q.; Liu, X.; Li, Q.; Zhang, H.; Yang, D.; Jiang, Y. Biocompatibility, Degradation Behavior, and Mechanical Properties of Magnesium Alloy Plates In Vivo. Appl. Bionics Biomech. 2023, 2023, 4436396. [Google Scholar] [CrossRef]
- Dziuba, D.; Meyer-Lindenberg, A.; Seitz, J.M.; Waizy, H.; Angrisani, N.; Reifenrath, J. Long-Term in Vivo Degradation Behaviour and Biocompatibility of the Magnesium Alloy ZEK100 for Use as a Biodegradable Bone Implant. Acta Biomater. 2013, 9, 8548–8560. [Google Scholar] [CrossRef] [PubMed]
- Abhishek, M.S.; Satish, J.; Anshu, D.; Debrupa, L.; Arup Kumar, D. Biocompatibility and Biodegradability Evaluation of Magnesium-Based Intramedullary Bone Implants in Avian Model. J. Biomed. Mater. Res. A 2021, 109, 1479–1489. [Google Scholar] [CrossRef]
- Xi, Z.; Wu, Y.; Xiang, S.; Sun, C.; Wang, Y.; Yu, H.; Fu, Y.; Wang, X.; Yan, J.; Zhao, D.; et al. Corrosion Resistance and Biocompatibility Assessment of a Biodegradable Hydrothermal-Coated Mg-Zn-Ca Alloy: An in Vitro and in Vivo Study. ACS Omega 2020, 5, 4548–4557. [Google Scholar] [CrossRef]
- Angrisani, N.; Reifenrath, J.; Zimmermann, F.; Eifler, R.; Meyer-Lindenberg, A.; Vano-Herrera, K.; Vogt, C. Biocompatibility and Degradation of LAE442-Based Magnesium Alloys after Implantation of up to 3.5 Years in a Rabbit Model. Acta Biomater. 2016, 44, 355–365. [Google Scholar] [CrossRef]
- Sanchez, A.H.M.; Luthringer, B.J.C.; Feyerabend, F.; Willumeit, R. Mg and Mg Alloys: How Comparable Are in Vitro and in Vivo Corrosion Rates? A Review. Acta Biomater. 2015, 13, 16–31. [Google Scholar] [CrossRef]
- Tao, M.; Cui, Y.; Sun, S.; Zhang, Y.; Ge, J.; Yin, W.; Li, P.; Wang, Y. Versatile Application of Magnesium-Related Bone Implants in the Treatment of Bone Defects. Mater. Today Bio 2025, 31, 101635. [Google Scholar] [CrossRef]
- Zhao, D.-W.; Zhang, T.-W.; Wang, W.-D.; Wang, Z.-M.; Wang, F.-Y.; Yang, X.; Li, R.-H.; Cheng, L.-L.; Zhang, Y.; Wang, H.-Y.; et al. Internal Fixation with Biodegradable High Purity Magnesium Screws in the Treatment of Ankle Fracture. J. Orthop. Transl. 2025, 51, 198–206. [Google Scholar] [CrossRef]
- Seetharaman, S.; Sankaranarayanan, D.; Gupta, M. Magnesium-Based Temporary Implants: Potential, Current Status, Applications, and Challenges. J. Funct. Biomater. 2023, 14, 324. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, R.; Chen, X.; Luo, Y.; Du, W.; Zhu, Y.; Ruan, Y.C.; Xu, J.; Wang, J.; Qin, L. Chronic Kidney Disease: A Contraindication for Using Biodegradable Magnesium or Its Alloys as Potential Orthopedic Implants? Biomed. Mater. 2024, 19, 045023. [Google Scholar] [CrossRef]
- Shalomeev, V.; Tabunshchyk, G.; Greshta, V.; Nykiel, M.; Korniejenko, K. Influence of Alkaline Earth Metals on Structure Formation and Magnesium Alloy Properties. Materials 2022, 15, 4341. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Lu, Q.; Qin, J.; Shi, H.; Zhang, P.; Yan, H.; Shi, H.; Wang, X. A View of Magnesium Alloy Modification and Its Application in Orthopedic Implants. J. Mater. Res. Technol. 2025, 36, 1536–1561. [Google Scholar] [CrossRef]
- Sharma, S.K.; Gajević, S.; Sharma, L.K.; Pradhan, R.; Miladinović, S.; Ašonja, A.; Stojanović, B. Magnesium-Titanium Alloys: A Promising Solution for Biodegradable Biomedical Implants. Materials 2024, 17, 5157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, W.; Liu, J.; Wang, L.; Tang, Y.; Wang, K. A Review on Magnesium Alloys for Biomedical Applications. Front. Bioeng. Biotechnol. 2022, 10, 953344. [Google Scholar] [CrossRef]
- Shalomeev, V.; Tsivirco, E.; Vnukov, Y.; Osadchaya, Y.; Makovskyi, S. Development of New Casting Magnesium-Based Alloys with Increased Mechanical Properties. East.-Eur. J. Enterp. Technol. 2016, 4, 4–10. [Google Scholar] [CrossRef][Green Version]
- Sreenivasamurthy, S.A.; Akhter, F.F.; Akhter, A.; Su, Y.; Zhu, D. Cellular Mechanisms of Biodegradable Zinc and Magnesium Materials on Promoting Angiogenesis. Biomater. Adv. 2022, 139, 213023. [Google Scholar] [CrossRef]
- He, X.; Li, Y.; Zou, D.; Zu, H.; Li, W.; Zheng, Y. An Overview of Magnesium-Based Implants in Orthopaedics and a Prospect of Its Application in Spine Fusion. Bioact. Mater. 2024, 39, 456–478. [Google Scholar] [CrossRef]
- Mehdizade, M.; Eivani, A.R.; Esmaielzadeh, O.; Rostamian, P. ZnO and Cu/ZnO-Modified Magnesium Orthopedic Implant with Improved Osteoblast Cellular Activity: An in-Vitro Study. J. Mater. Res. Technol. 2024, 28, 935–950. [Google Scholar] [CrossRef]
- Shalomeev, V.; Sheyko, S.; Hrechanyi, O.; Vasilchenko, T.; Hrechana, A. Research of the Boundaries of the “Metal Filter” Section during Filtration of Magnesium Alloy AZ91. Appl. Eng. Sci. 2025, 21, 100212. [Google Scholar] [CrossRef]
- Greshta, V.; Shalomeev, V.; Bovkun, S.; Kulabneva, E. Practical Aspects of Obtaining Deformed Blanks for the Production of Biodegradable Implants of Increased Quality. J. Chem. Technol. Metall. 2025, 60, 225–236. [Google Scholar] [CrossRef]
- Vignesh, P.; Ramanathan, S.; Ashokkumar, M.; Ananthi, V. Biodegradable Mg–3Zn Alloy/Titanium–Hydroxyapatite Hybrid Composites: Corrosion and Cytotoxicity Evaluation for Orthopedic Implant Applications. Trans. Indian. Inst. Met. 2024, 77, 1701–1710. [Google Scholar] [CrossRef]
- Sonaye, S.Y.; Dal-Fabbro, R.; Bottino, M.C.; Sikder, P. Osseointegration of 3D-Printable Polyetheretherketone–Magnesium Phosphate Bioactive Composites for Craniofacial and Orthopedic Implants. ACS Biomater. Sci. Eng. 2025, 11, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; He, Y.; Jia, R.; Li, S.; Duan, L.; Xu, S.; Mei, D.; Tang, X.; Zhu, S.; Wei, J.; et al. Enhancing the Corrosion Resistance of Magnesium Alloys with Biodegradable Poly(Trimethylene Carbonate) Chemical Modification Coating. Mater. Today Adv. 2024, 21, 100460. [Google Scholar] [CrossRef]
- Mohanta, M.; Ramdhun, Y.; Thirugnanam, A.; Gupta, R.; Verma, D.; Deepak, T.; Babu, A.R. Biodegradable AZ91 Magnesium Alloy/Sirolimus/Poly D, L-lactic-co-glycolic Acid-based Substrate for Cardiovascular Device Application. J. Biomed. Mater. Res. 2024, 112, e35350. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Xu, J.; Zou, J.; Li, Y.R.; Zhou, Z.; Liu, K.; Jia, Q.; Jiang, H.B. Advances in Amelioration of Plasma Electrolytic Oxidation Coatings on Biodegradable Magnesium and Alloys. Heliyon 2024, 10, e24348. [Google Scholar] [CrossRef]
- Wu, G.-L.; Yen, C.-E.; Hsu, W.-C.; Yeh, M.-L. Incorporation of Cerium Oxide Nanoparticles into the Micro-Arc Oxidation Layer Promotes Bone Formation and Achieves Structural Integrity in Magnesium Orthopedic Implants. Acta Biomater. 2025, 191, 80–97. [Google Scholar] [CrossRef]
- Li, H.; Wang, F.; Sun, X.-J.; Qi, L.-J.; Li, J.-A.; Iqbal, M.; Guan, S.-K. Bio-Inspired Barnacle Cement Coating of Biodegradable Magnesium Alloy for Cerebrovascular Application. Rare Met. 2024, 43, 5164–5185. [Google Scholar] [CrossRef]
- Saberi, A.; Bakhsheshi-Rad, H.R.; Abazari, S.; Ismail, A.F.; Sharif, S.; Ramakrishna, S.; Daroonparvar, M.; Berto, F. A Comprehensive Review on Surface Modifications of Biodegradable Magnesium-Based Implant Alloy: Polymer Coatings Opportunities and Challenges. Coatings 2021, 11, 747. [Google Scholar] [CrossRef]
- Liu, J.; Yin, B.; Song, F.; Liu, B.; Peng, B.; Wen, P.; Tian, Y.; Zheng, Y.; Ma, X.; Wang, C. Improving Corrosion Resistance of Additively Manufactured WE43 Magnesium Alloy by High Temperature Oxidation for Biodegradable Applications. J. Magnes. Alloys 2024, 12, 940–953. [Google Scholar] [CrossRef]
- Fu, Q.; Liang, W.; Huang, J.; Jin, W.; Guo, B.; Li, P.; Xu, S.; Chu, P.K.; Yu, Z. Research Perspective and Prospective of Additive Manufacturing of Biodegradable Magnesium-Based Materials. J. Magnes. Alloys 2023, 11, 1485–1504. [Google Scholar] [CrossRef]
- Zhi, P.; Liu, L.; Chang, J.; Liu, C.; Zhang, Q.; Zhou, J.; Liu, Z.; Fan, Y. Advances in the Study of Magnesium Alloys and Their Use in Bone Implant Material. Metals 2022, 12, 1500. [Google Scholar] [CrossRef]
- Dachasa, K.; Chuni Aklilu, T.; Gashaw Ewnete, B.; Mosisa Ejeta, B.; Fufa Bakare, F. Magnesium-Based Biodegradable Alloy Materials for Bone Healing Application. Adv. Mater. Sci. Eng. 2024, 2024, 1325004. [Google Scholar] [CrossRef]
- Magnesium Implant Market Key Challenges, Growth and Opportunities 2025. Available online: https://www.linkedin.com/pulse/magnesium-implant-market-key-challenges-growth-eplyf (accessed on 12 June 2025).
- Amukarimi, S.; Mozafari, M. Biodegradable Magnesium Biomaterials—Road to the Clinic. Bioengineering 2022, 9, 107. [Google Scholar] [CrossRef]
- Thomas, K.K.; Zafar, M.N.; Pitt, W.G.; Husseini, G.A. Biodegradable Magnesium Alloys for Biomedical Implants: Properties, Challenges, and Surface Modifications with a Focus on Orthopedic Fixation Repair. Appl. Sci. 2023, 14, 10. [Google Scholar] [CrossRef]
- Nuthana Kalva, S.; Ali, F.; Koç, M. Recent Advances in the Post-Processing of Magnesium Based Scaffolds for Orthopedic Applications. Next Mater. 2025, 6, 100295. [Google Scholar] [CrossRef]
- Kazemzadeh-Narbat, M. FDA Regulatory Challenges for Magnesium-Based Orthopedic Devices. ODT, 21 May 2024. [Google Scholar]
- Degradable Medical Magnesium Implants Market. Available online: https://www.linkedin.com/pulse/degradable-medical-magnesium-implants-market-wander-whiz-zd8ef (accessed on 12 June 2025).
- Amin, V.Y.; Nallajennugari, S.R.; Chahal, R.S. The Efficacy of Biodegradable Magnesium-Based Implants in Orthopedic Surgery. Int. Arch. Orthop. Surg. 2025, 8, 1–9. [Google Scholar] [CrossRef]
- Data Bridge Market Research. North America Magnesium Alloys Market Report—Industry Trends and Forecast to 2029; Data Bridge Market Research: Pune, India, 2022. [Google Scholar]
- Ibrahem Khaled, N.; Santhiya, D. Zein-Bioactive Glass Nanocomposite Coating on Magnesium Alloy Substrate for Orthopedic Applications. Adv. Compos. Mater. 2024, 33, 324–346. [Google Scholar] [CrossRef]
- De Luca, A.; Ruggiero, R.; Cordaro, A.; Marrelli, B.; Raimondi, L.; Costa, V.; Bellavia, D.; Aiello, E.; Pavarini, M.; Piccininni, A.; et al. Towards Accurate Biocompatibility: Rethinking Cytotoxicity Evaluation for Biodegradable Magnesium Alloys in Biomedical Applications. J. Funct. Biomater. 2024, 15, 382. [Google Scholar] [CrossRef]
- Cao, B.; Cao, L.; Kallmes, D.F. Degradation Rate Assessment of Biodegradable Magnesium Alloys. Mater. Sci. Appl. 2024, 15, 245–252. [Google Scholar] [CrossRef]
- Barzegari, M.; Mei, D.; Lamaka, S.V.; Geris, L. Computational Modeling of Degradation Process of Biodegradable Magnesium Biomaterials. Corros. Sci. 2021, 190, 109674. [Google Scholar] [CrossRef]
- Yamamoto, A.; Kikuta, A. Development of a Model System for Gas Cavity Formation Behavior of Magnesium Alloy Implantation. ACS Biomater. Sci. Eng. 2022, 8, 2437–2444. [Google Scholar] [CrossRef]
- ISO 10993-12:2021; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. International Organization for Standardization: Geneva, Switzerland, 2021.
- Amaral, C.; Paiva, M.; Rodrigues, A.R.; Veiga, F.; Bell, V. Global Regulatory Challenges for Medical Devices: Impact on Innovation and Market Access. Appl. Sci. 2024, 14, 9304. [Google Scholar] [CrossRef]
- ISO/TS 20721:2025; Implants for Surgery—Absorbable Implants—General Guidelines and Requirements for Assessment of Absorbable Metallic Implants. International Organization for Standardization: Geneva, Switzerland, 2025.
- Müller, E.; Schoberwalter, T.; Mader, K.; Seitz, J.-M.; Kopp, A.; Baranowsky, A.; Keller, J. The Biological Effects of Magnesium-Based Implants on the Skeleton and Their Clinical Implications in Orthopedic Trauma Surgery. Biomater. Res. 2024, 28, 0122. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; He, L.; Zhang, X.; Mei, Y.; Wu, T.; Wang, J.; Zheng, Y.; Tang, H. Surface Engineering of Biodegradable Magnesium Alloys as Orthopedic Implant Materials: Recent Developments and Future Prospects. Coatings 2025, 15, 191. [Google Scholar] [CrossRef]
- Ji, Y.; Hou, M.; Zhang, J.; Wang, T.; Cao, C.; Yang, H.; Zhang, X. Surface Modification of WE43 Magnesium Alloys with Dopamine Hydrochloride Modified GelMA Coatings. Coatings 2022, 12, 1074. [Google Scholar] [CrossRef]
- Gu, X.N.; Xie, X.H.; Li, N.; Zheng, Y.F.; Qin, L. In Vitro and in Vivo Studies on a Mg–Sr Binary Alloy System Developed as a New Kind of Biodegradable Metal. Acta Biomater. 2012, 8, 2360–2374. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, Z.; Sun, Y. Effects of a Thaw Slump on Active Layer in Permafrost Regions with the Comparison of Effects of Thermokarst Lakes on the Qinghai–Tibet Plateau, China. Geoderma 2018, 314, 47–57. [Google Scholar] [CrossRef]
- Garimella, A.; M, R.; Ghosh, S.B.; Bandyopadhyay-Ghosh, S.; Agrawal, A.K. Bioactive Fluorcanasite Reinforced Magnesium Alloy-Based Porous Bio-Nanocomposite Scaffolds with Tunable Mechanical Properties. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2023, 111, 463–477. [Google Scholar] [CrossRef]
- Wei, X.; Ma, S.; Meng, J.; Qing, H.; Zhao, Q. In Vitro Evaluation of a Novel Mg–Sn–Ge Ternary Alloy for Orthopedic Applications. J. Alloys Compd. 2023, 953, 169813. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).