Mechanical and Corrosion Properties of Ultrafine-Grained TC4-0.55Fe Alloy Processed by Equal-Channel Angular Pressing
Abstract
1. Introduction
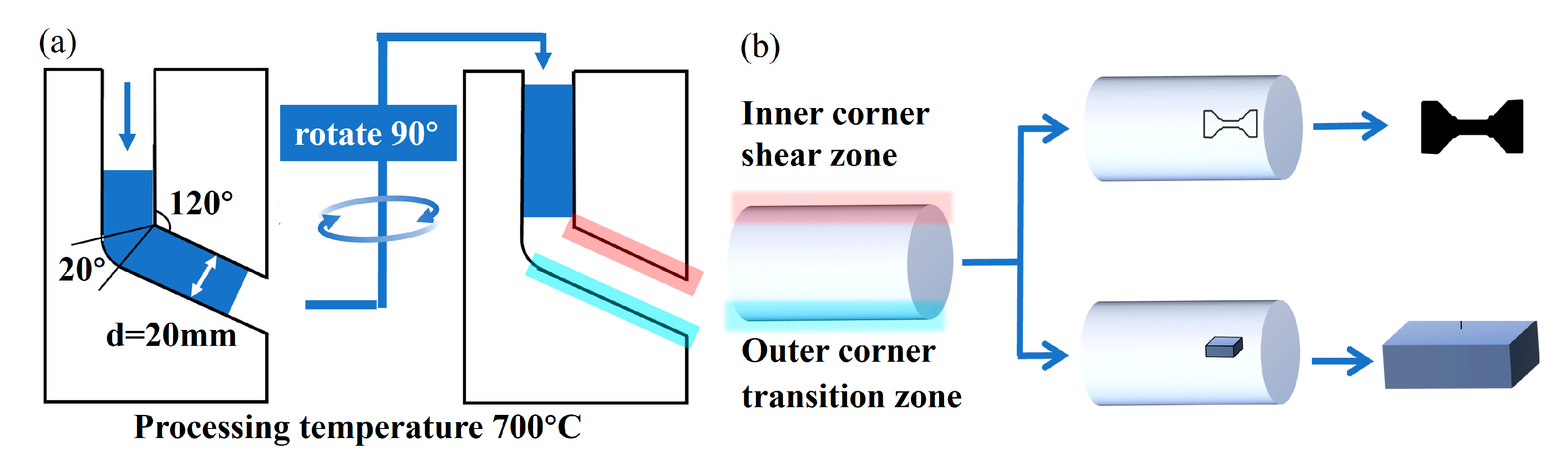
2. Materials and Methods
3. Results
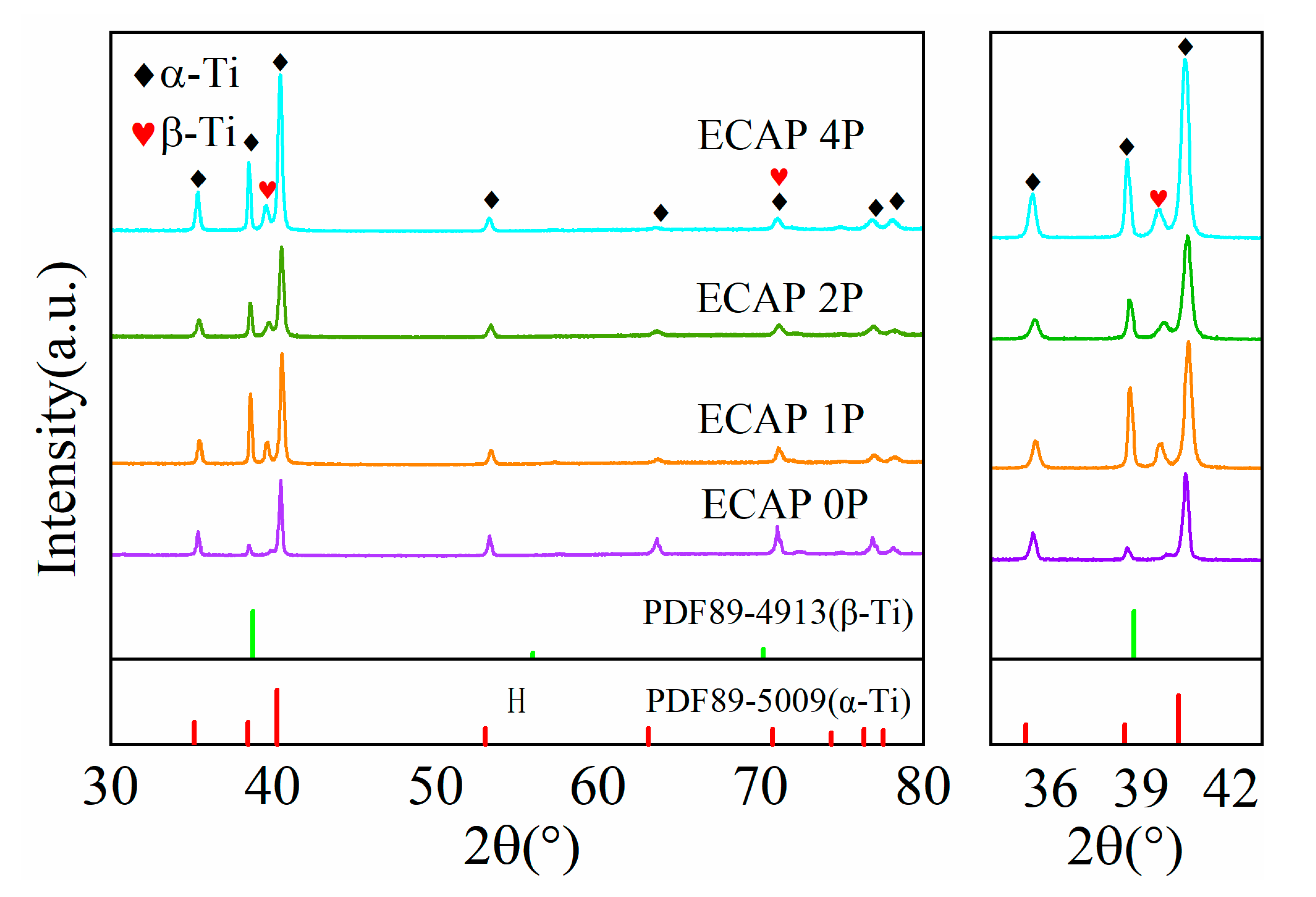
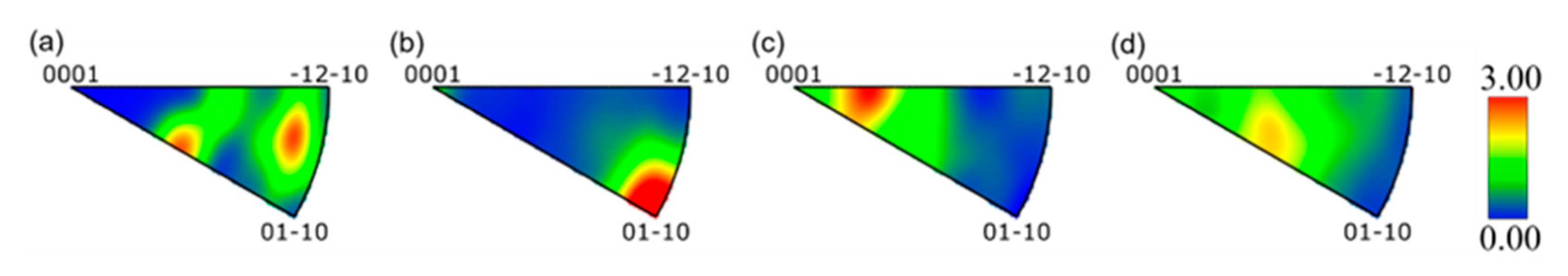
3.1. Microstructure
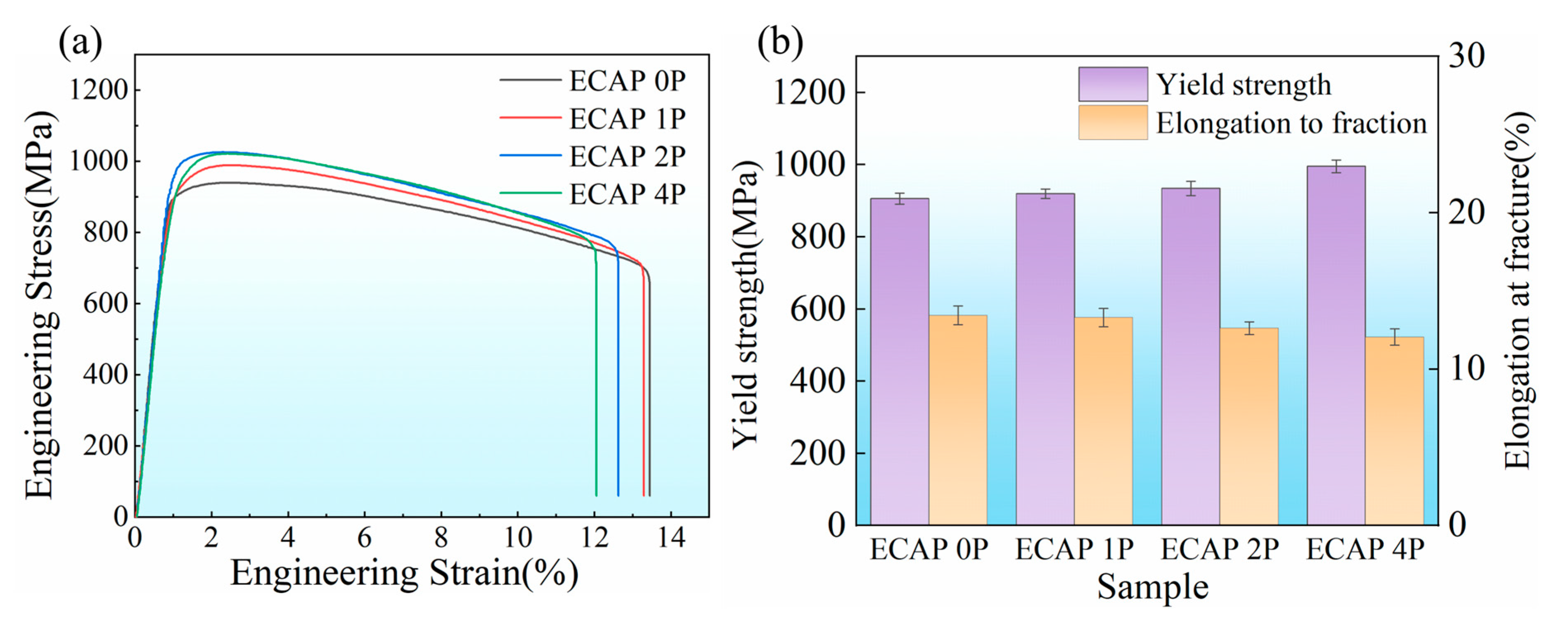
3.2. Mechanical Properties
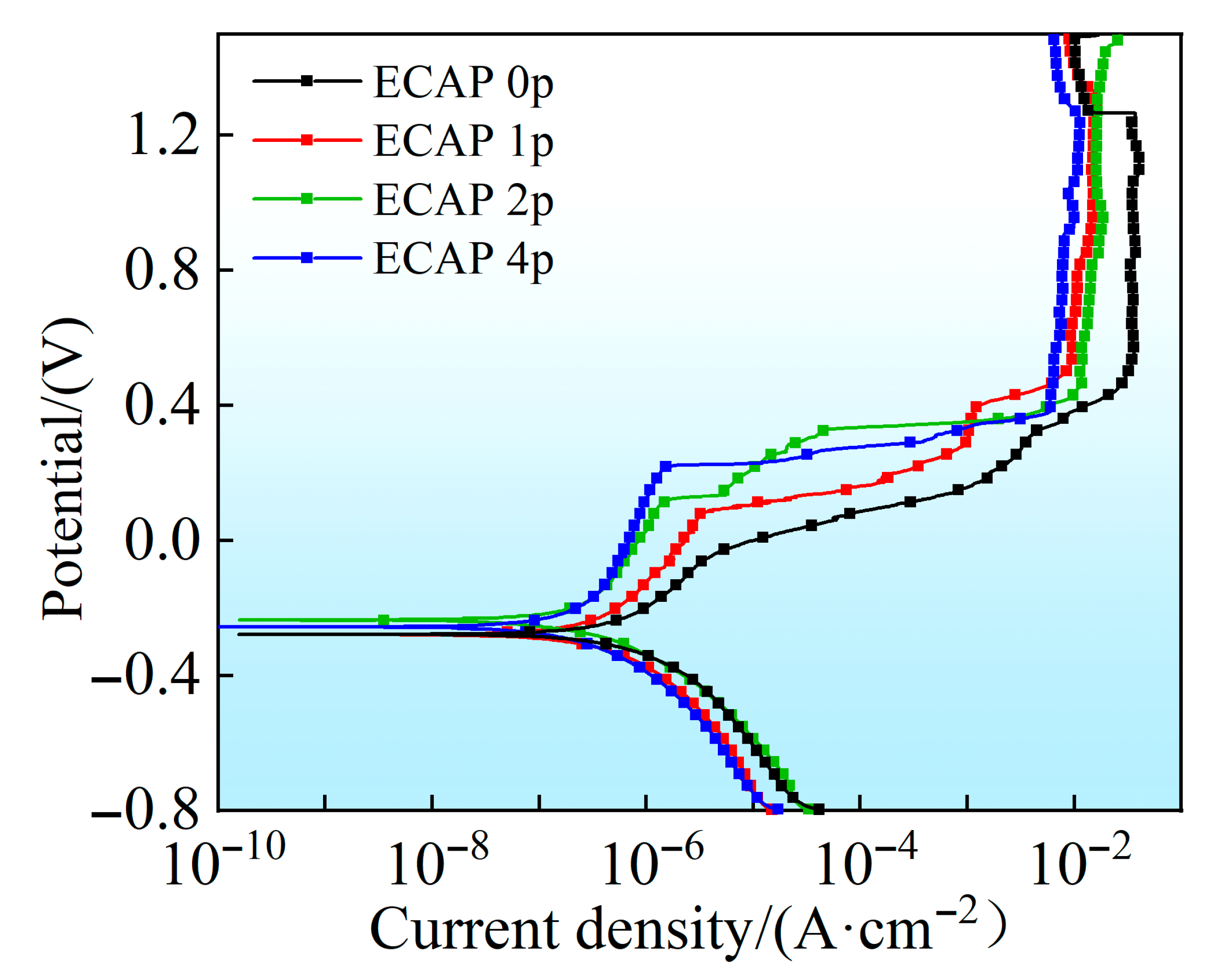
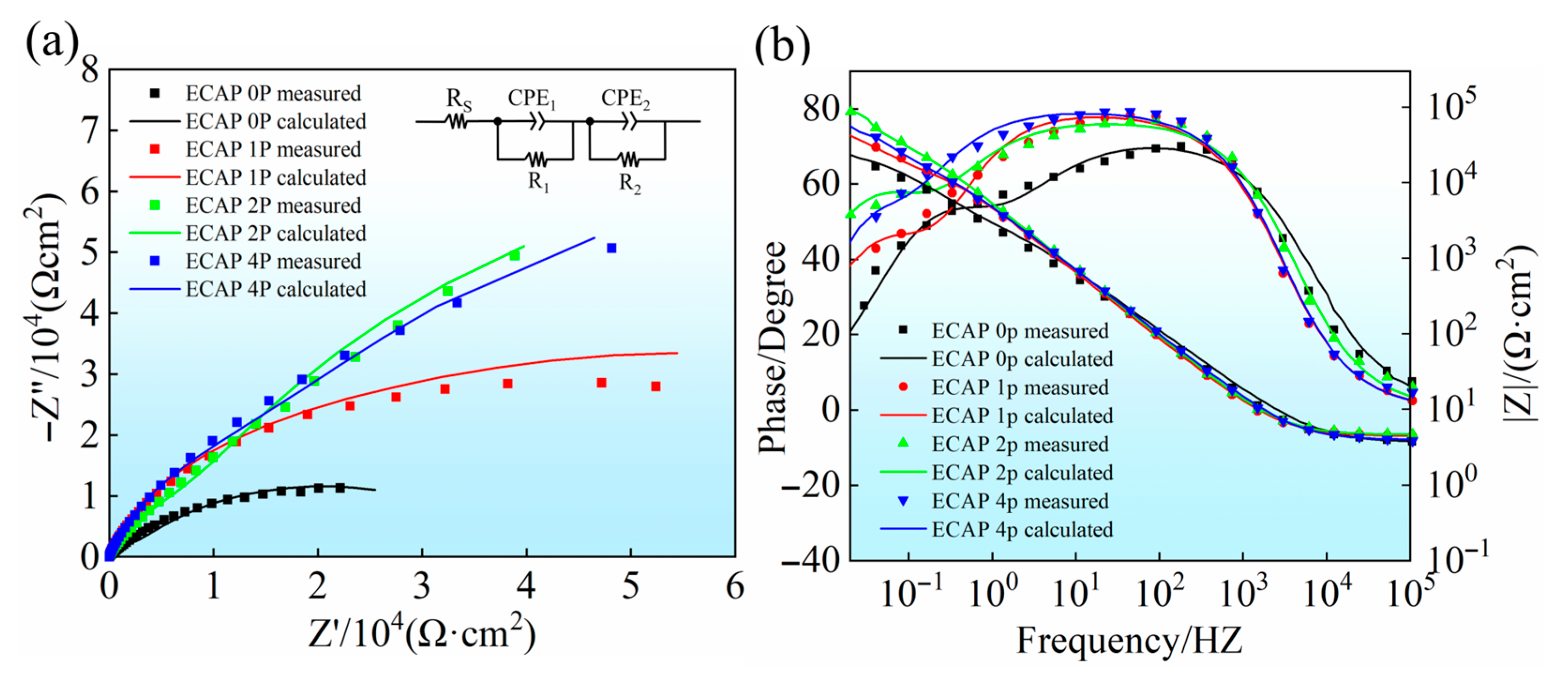
3.3. Corrosion Properties
4. Discussion
5. Conclusions
- The TC4-0.55Fe alloy was processed by ECAP for 0, 1, 2, and 4 passes, resulting in grain sizes of 3.8 μm, 2.5 μm, 2.2 μm, and 1.8 μm, respectively. During ECAP, the fraction of dynamic recrystallization increased from 3.5% to 14.0%, while the dislocation density exhibited an overall increasing trend. Concurrently, the proportion of LAGBs increased from 54.5% to 69.6%, followed by a decrease to 62.0%.
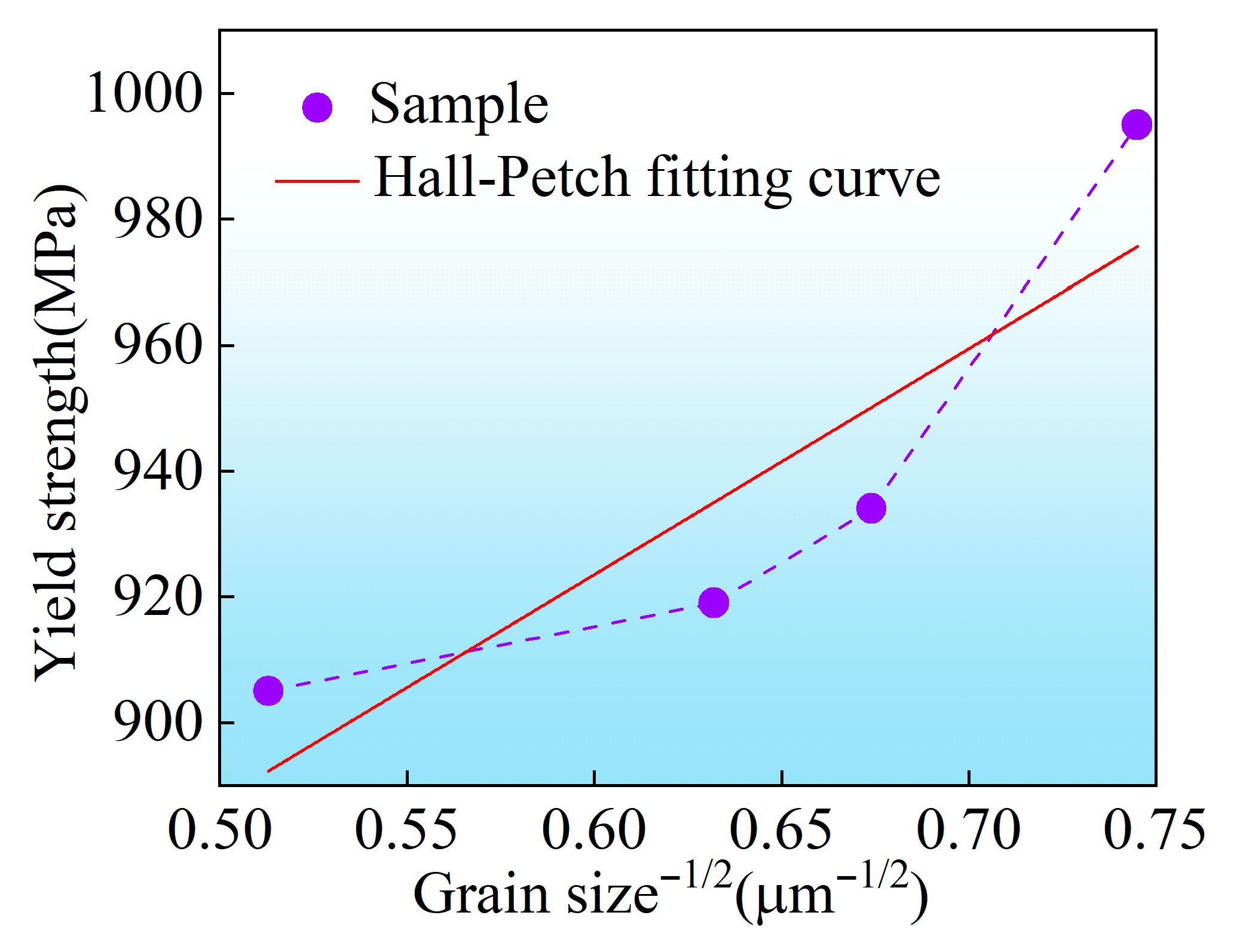
- Room-temperature tensile tests were conducted on TC4-0.55Fe titanium alloys with different grain sizes. With an increasing number of ECAP passes, both the yield strength and ultimate tensile strength of TC4-0.55Fe improved, rising from 906 MPa and 939 MPa (ECAP 0P) to 995 MPa and 1022 MPa (ECAP 4P), respectively. In contrast, the ductility exhibited an opposite trend, decreasing from 13.5% (ECAP 0P) to 12.0% (ECAP 4P).
- ECAP treatment significantly improved the alloy’s corrosion resistance in a simulated seawater environment, with two-pass-processed specimens exhibiting optimal performance. The corrosion current density (icorr) decreases to 0.0961 μA·cm−2, representing a 9.3-fold reduction in corrosion rate compared to untreated samples, along with significantly enhanced passive film stability and pitting resistance.
- The enhanced corrosion resistance was attributed to microstructural changes from grain refinement, including increased dislocation density, stronger basal texture, and improved grain boundary characteristics. The ECAP 2P specimen had the best corrosion resistance because it has a relatively high dislocation density, the strongest basal texture, and the most low-angle grain boundaries (69.6%). Conversely, the corrosion resistance of the ECAP 4P specimen was slightly inferior to that of the 2P specimen due to its somewhat weaker texture and a lower proportion of low-angle grain boundaries.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, Q.Y.; Sun, Q.Y.; Xin, S.W.; Chen, Y.N.; Wu, C.; Wang, H.; Xu, J.W.; Wan, M.P.; Zeng, W.D.; Zhao, Y.Q. High-strength titanium alloys for aerospace engineering applications: A review on melting-forging process. Mater. Sci. Eng. A 2022, 845, 143260. [Google Scholar] [CrossRef]
- Jia, Z.Y.; Zhao, Q.Y.; Zhang, Y.; Xu, Y.K.; Chen, Y.N.; Deng, X.T.; Zhang, F.Y.; Wang, L.; Guo, D.Z. Hot and cold rolling of a novel near-α titanium alloy: Mechanical properties and underlying deformation mechanism. Mater. Sci. Eng. A 2023, 863, 144543. [Google Scholar] [CrossRef]
- Wang, R.; Shi, M.; Xu, F.; Qiu, Y.; Zhang, P.; Shen, K.; Zhao, Q.; Yu, J.; Zhang, Y. Graphdiyne-modified TiO2 nanofibers with osteoinductive and enhanced photocatalytic antibacterial activities to prevent implant infection. Nat. Commun. 2020, 11, 4465. [Google Scholar] [CrossRef]
- Yang, X.; Lin, B.; Zhang, H.; Tang, J.; Zhou, T.; Wang, Y.; Zheng, H.; Kuang, Y. Influence of stress on the corrosion behavior of Ti alloys: A review. J. Alloys Compd. 2024, 985, 173346. [Google Scholar] [CrossRef]
- Seo, D.-I.; Lee, J.-B. Effects of competitive anion adsorption (Br− or Cl−) and semiconducting properties of the passive films on the corrosion behavior of the additively manufactured Ti-6Al-4V alloys. Corros. Sci. 2020, 173, 108789. [Google Scholar] [CrossRef]
- Chen, L.; Liang, M.; Wan, W.; Tang, J.; Lin, B.; Yang, X.; Chen, Y.; Chen, Y. Corrosion of commercial pure titanium and two titanium alloys in extremely high-chloride and high-alkali seawater electrolysis environment. J. Alloys Compd. 2025, 1020, 179431. [Google Scholar] [CrossRef]
- Chen, F.; Gu, Y.; Xu, G.; Cui, Y.; Chang, H.; Zhou, L. Improved fracture toughness by microalloying of Fe in Ti-6Al-4V. Mater. Des. 2020, 185, 108251. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Q.; Liu, S.; Li, F.; Chen, F.; Wang, H.; Chang, H. Phase transformation and microstructure evolution of Ti6Al4V-0.55 Fe alloy with different initial microstructure during continuous heating. J. Mater. Res. Technol. 2022, 18, 1704–1716. [Google Scholar] [CrossRef]
- Liao, Y.; Bai, J.; Chen, F.; Xu, G.; Cui, Y. Microstructural strengthening and toughening mechanisms in Fe-containing Ti-6Al-4V: A comparison between homogenization and aging treated states. J. Mater. Sci. Technol. 2022, 99, 114–126. [Google Scholar] [CrossRef]
- Sinong, L.; Jingqi, Z.; Bowei, L.; Xin, L.; Feng, L.; Hui, C. Effect of heat treatment and pre-stretching on microstructure and mechanical properties of TC4-0.55 Fe alloy. Rare Met. Mater. Eng. 2023, 52, 3485–3494. [Google Scholar]
- Dai, G.; Niu, J.; Guo, Y.; Sun, Z.; Dan, Z.; Chang, H.; Zhou, L. Microstructure evolution and grain refinement behavior during hot deformation of Fe micro-alloyed Ti-6Al-4V. J. Mater. Res. Technol. 2021, 15, 1881–1895. [Google Scholar] [CrossRef]
- Niu, J.; Guo, Y.; Li, K.; Liu, W.; Dan, Z.; Sun, Z.; Chang, H.; Zhou, L. Improved mechanical, bio-corrosion properties and in vitro cell responses of Ti-Fe alloys as candidate dental implants. Mater. Sci. Eng. C 2021, 122, 111917. [Google Scholar] [CrossRef]
- Sun, Y.; Alexandrov, I.V.; Dong, Y.; Valiev, R.Z.; Chang, H.; Zhou, L. Optimized low-cycle fatigue behavior and fracture characteristics of Ti–6Al–4V alloy by Fe microalloying. J. Mater. Res. Technol. 2021, 15, 5277–5287. [Google Scholar] [CrossRef]
- Estrin, Y.; Vinogradov, A. Extreme grain refinement by severe plastic deformation: A wealth of challenging science. Acta Mater. 2013, 61, 782–817. [Google Scholar] [CrossRef]
- Segal, V. Review: Modes and processes of severe plastic deformation (SPD). Materials 2018, 11, 1175. [Google Scholar] [CrossRef]
- Edalati, K.; Horita, Z. A review on high-pressure torsion (HPT) from 1935 to 1988. Mater. Sci. Eng. A 2016, 652, 325–352. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Wang, Q. Accumulative roll-bonding of aluminum alloys and composites: An overview of properties and performance. J. Mater. Res. Technol. 2022, 19, 4381–4403. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, W.; Fu, D.; Teng, J.; Zhang, H. Improvement of the mechanical properties of Al-Mg-Si alloys with nano-scale precipitates after repetitive continuous extrusion forming and T8 tempering. J. Mater. Res. Technol. 2019, 8, 5950–5960. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, G.; Zhang, Y.; Gao, J.; Hou, H. Microstructure evolution and mechanical properties of Ti-6Al-4V alloy prepared by multipass equal channel angular pressing. J. Mater. Eng. Perform. 2020, 29, 905–913. [Google Scholar] [CrossRef]
- Radnia, A.; Ketabchi, M.; He, A.; Li, D. Effects of ECAP and subsequent recovery on microstructure, mechanical, tribological and corrosion properties of Ti-6Al-4V alloy. J. Mater. Res. Technol. 2025, 35, 4534–4542. [Google Scholar] [CrossRef]
- Liu, G.H.; Li, T.R.; Xu, M.; Fu, T.L.; Li, Y.; Wang, G.D.; Wang, Z.D. Microstructural evolution and mechanical properties of TC4 titanium alloy during acculative roll bonding process. Acta Metall. Sin. 2017, 53, 1038–1046. [Google Scholar]
- Hoseini, M.; Shahryari, A.; Omanovic, S.; Szpunar, J.A. Comparative effect of grain size and texture on the corrosion behaviour of commercially pure titanium processed by equal channel angular pressing. Corros. Sci. 2009, 51, 3064–3067. [Google Scholar] [CrossRef]
- Li, L.; Hao, L.; Zhang, S.; Shen, S.; Liu, X.; Fu, E. Biocompatible TA4 and tc4eli with excellent mechanical properties and corrosion resistance via multiple ECAP. Biomed. Mater. 2024, 20, 015026. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Hu, T.; Gao, A.; Gao, N.; Starink, M.J.; Chen, Y.; Sun, W.; Liao, Q.; Tong, L.; Xu, X. Homogeneous anodic TiO2 nanotube layers on Ti–6Al–4V alloy with improved adhesion strength and corrosion resistance. Adv. Mater. Interfaces 2019, 6, 1801964. [Google Scholar] [CrossRef]
- Sakai, T.; Belyakov, A.; Kaibyshev, R.; Miura, H.; Jonas, J.J. Dynamic and post-dynamic recrystallization under hot, cold and severe plastic deformation conditions. Prog. Mater. Sci. 2014, 60, 130–207. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, W.; Wei, K.X.; Zhong, Y.; Cheng, G.; Hu, J. Finite element analysis of deformation behavior in continuous ecap process. Mater. Sci. Eng. A 2009, 516, 111–118. [Google Scholar] [CrossRef]
- Sahoo, P.S.; Meher, A.; Mahapatra, M.M.; Vundavilli, P.R.; Pandey, C. Analysis of mechanical and microstructural characteristics of plunger-assisted ECAP strengthened Ti-6Al-4V alloy sheets. Arch. Civ. Mech. Eng. 2023, 23, 194. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, B.; Xue, J.; Li, F.; Su, S.; Chang, H. Microstructural characteristics and recrystallization mechanism of Ti-6.5Al-2Zr-1Mo-1V alloy during two-stage hot deformation. J. Mater. Res. Technol. 2024, 30, 769–781. [Google Scholar] [CrossRef]
- Arabi, H.; Ketabchi, M.; Alhosseini, S.H.N. Mechanical and microstructural variations in ECAP of Ti–6Al–4V alloy with equiaxed microstructure. Rare Met. 2022, 41, 2732–2738. [Google Scholar]
- Su, B.; Luo, L.; Wang, B.; Su, Y.; Wang, L.; Ritchie, R.O.; Guo, E.; Li, T.; Yang, H.; Huang, H. Annealed microstructure dependent corrosion behavior of Ti-6Al-3Nb-2Zr-1Mo alloy. J. Mater. Sci. Technol. 2021, 62, 234–248. [Google Scholar]
- Çomaklı, O.; Yazıcı, M.; Yetim, T.; Yetim, A.; Çelik, A. The effect of calcination temperatures on structural and electrochemical properties of TiO2 film deposited on commercial pure titanium. Surf. Coat. Technol. 2016, 285, 298–303. [Google Scholar]
- Lei, J.; Xiu, S.; Gang, L.; Lian, Z. Effects of surface nanocrystallization on corrosion resistance of β-type titanium alloy. Trans. Nonferrous Met. Soc. China 2014, 24, 2529–2535. [Google Scholar] [CrossRef]
- Yang, X.; Du, C.; Wan, H.; Liu, Z.; Li, X. Influence of sulfides on the passivation behavior of titanium alloy TA2 in simulated seawater environments. Appl. Surf. Sci. 2018, 458, 198–209. [Google Scholar] [CrossRef]
- Zhao, Z.; Ji, H.; Zhong, Y.; Han, C.; Tang, X. Mechanical properties and fracture behavior of a TC4 titanium alloy sheet. Materials 2022, 15, 8589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Z.; Lv, Z.; Cao, J.; Tong, Y.; Sun, M.; Cui, C.; Wang, X. Effect of SiC and TiC content on microstructure and wear behavior of Ni-based composite coating manufactured by laser cladding on Ti-6Al-4V. Wear 2024, 552, 205431. [Google Scholar] [CrossRef]
- Liu, D.; Li, C.; Huang, J.; Wei, P.; Xu, J.; Zhang, L.; Qin, J. Influence of Mo additive on microstructure and corrosion behavior of TC4 metal-cord welding overlay. J. Mater. Res. Technol. 2025, 36, 2554–2567. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Y.; Fang, Y.; Lei, J.; Song, H.; Wang, T. Microstructure and electrochemical corrosion behavior of TA2 with nano/micro TiC particle reinforcement. Mater. Charact. 2024, 212, 113972. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.; Cheng, K.; Yang, K.; Zhang, W. Nano hierarchical hill-like structure with TA1 surface manufactured by lipss for anti-corrosion and anti-icing. J. Mater. Res. Technol. 2025, 35, 3655–3667. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, J.; Wu, B.; Guo, X.; Wang, Y.; Chen, D.; Zhang, Y.; Du, K.; Oguzie, E.; Ma, X. Unmasking chloride attack on the passive film of metals. Nat. Commun. 2018, 9, 2559. [Google Scholar] [CrossRef]
- Cui, Y.-W.; Chen, L.-Y.; Chu, Y.-H.; Zhang, L.; Li, R.; Lu, S.; Wang, L.; Zhang, L.-C. Metastable pitting corrosion behavior and characteristics of passive film of laser powder bed fusion produced Ti-6Al-4V in NaCl solutions with different concentrations. Corros. Sci. 2023, 215, 111017. [Google Scholar] [CrossRef]
- Sotniczuk, A.; Kuczyńska, D.; Kubacka, D.; Królikowski, A.; Garbacz, H. Influence of nanostructure on titanium corrosion resistance in fluoridated medium. Mater. Sci. Technol. 2019, 35, 288–296. [Google Scholar] [CrossRef]
- Kim, H.S.; Yoo, S.J.; Ahn, J.W.; Kim, D.H.; Kim, W.J. Ultrafine grained titanium sheets with high strength and high corrosion resistance. Mater. Sci. Eng. A 2011, 528, 8479–8485. [Google Scholar] [CrossRef]
- Rodriguez-Calvillo, P.; Cabrera, J. Microstructure and mechanical properties of a commercially pure Ti processed by warm equal channel angular pressing. Mater. Sci. Eng. A 2015, 625, 311–320. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Q.; Liu, J.; Yang, R. Characterizations of microstructure and crystallographic orientation in a near-α titanium alloy billet. J. Alloys Compd. 2017, 712, 179–184. [Google Scholar] [CrossRef]
- Hu, C.L.; Xia, S.; Li, H.; Liu, T.G.; Zhou, B.X.; Chen, W.J.; Wang, N. Improving the intergranular corrosion resistance of 304 stainless steel by grain boundary network control. Corros. Sci. 2011, 53, 1880–1886. [Google Scholar] [CrossRef]
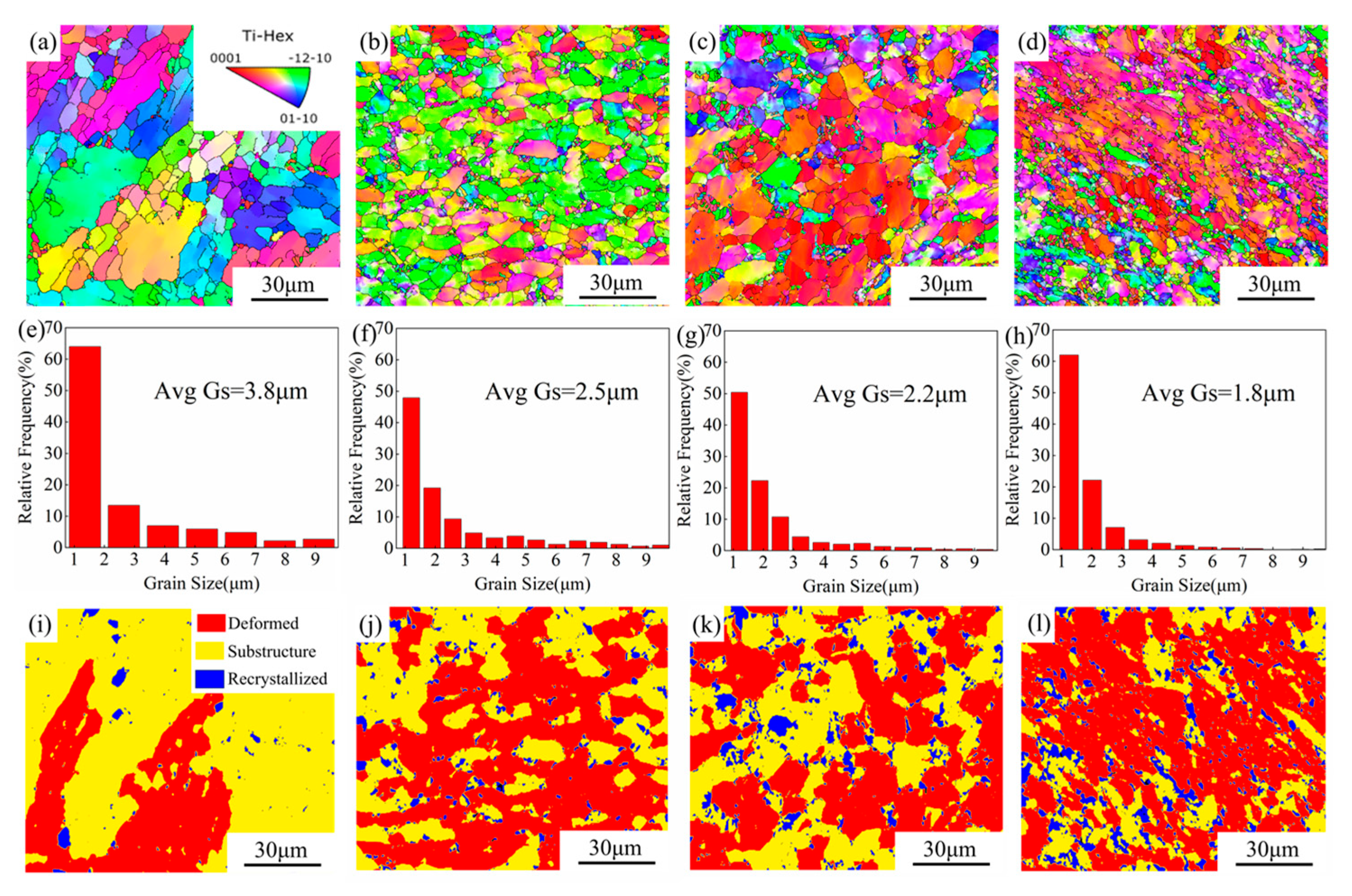

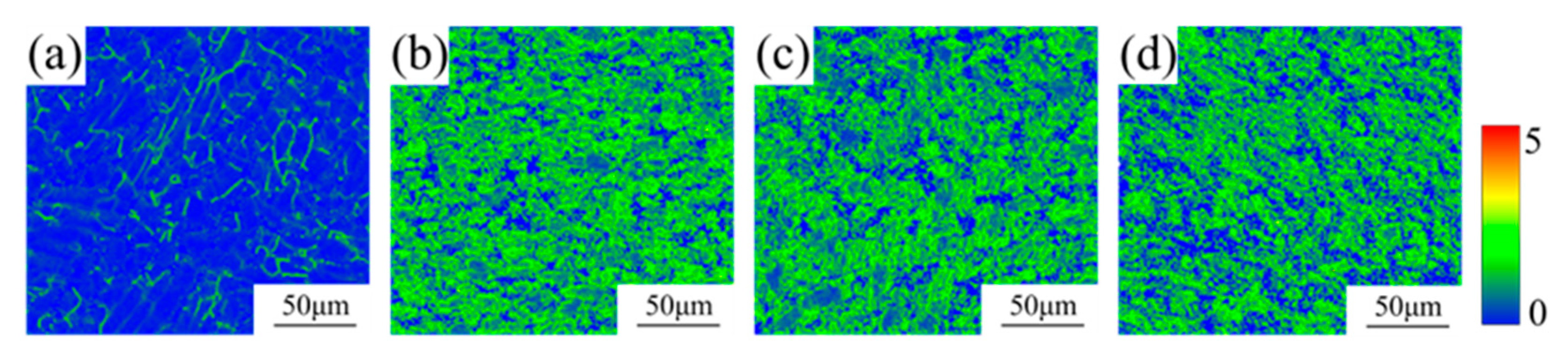

| Samples | Average Grain Size (μm) | Recrystallized Grains (%) | Substructure Grains (%) | Deformed Grains (%) | β-Phase (%) |
|---|---|---|---|---|---|
| ECAP 0P | 3.8 | 3.5 | 66.3 | 37.2 | 0.4 |
| ECAP 1P | 2.5 | 5.7 | 41.0 | 53.3 | 0.8 |
| ECAP 2P | 2.2 | 10.1 | 35.1 | 54.8 | 2.0 |
| ECAP 4P | 1.8 | 14.0 | 28.1 | 57.9 | 2.2 |
| Sample | Yield Strength (MPa) | Ultimate Tensile Strength (MPa) | Elongation at Fracture (%) | Hardness (HV) |
|---|---|---|---|---|
| ECAP 0P | 906 ± 15 | 939 ± 9 | 13.5 ± 0.6 | 325 ± 6.1 |
| ECAP 1P | 919 ± 13 | 988 ± 10 | 13.3 ± 0.6 | 326 ± 8.6 |
| ECAP 2P | 934 ± 20 | 1024 ± 9 | 12.6 ± 0.4 | 329 ± 5.9 |
| ECAP 4P | 995 ± 18 | 1022 ± 7 | 12.0 ± 0.5 | 330 ± 7.2 |
| Sample | Ecorr (V) | Epit (V) | icorr (µA·cm−2) | ipass (µA·cm−2) | R (10−3 mm·a−1) |
|---|---|---|---|---|---|
| ECAP 0P | −0.279 | −0.0783 | 0.890 | 1.23 | 7.73 |
| ECAP 1P | −0.279 | 0.0812 | 0.512 | 1.67 | 4.45 |
| ECAP 2P | −0.236 | 0.114 | 0.0961 | 0.468 | 0.832 |
| ECAP 4P | −0.256 | 0.215 | 0.108 | 0.402 | 0.946 |
| Sample | RS (Ω·cm2) | CPE1 (10−5 S·sn·cm2) | n1 | R1 (105 Ω·cm2) | CPE2 (10−5 S·sn·cm2) | n2 | R2 (105 Ω·cm2) | Rp (105 Ω·cm2) |
|---|---|---|---|---|---|---|---|---|
| ECAP 0P | 4.325 | 4.705 | 0.896 | 0.0181 | 10.75 | 0.804 | 0.375 | 0.3931 |
| ECAP 1P | 4.645 | 2.857 | 0.913 | 0.222 | 3.815 | 0.810 | 0.796 | 1.018 |
| ECAP 2P | 3.952 | 7.652 | 0.856 | 0.0783 | 8.498 | 0.859 | 1.71 | 1.784 |
| ECAP 4P | 4.642 | 5.115 | 0.858 | 0.244 | 10.31 | 0.998 | 1.15 | 1.396 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Lu, Y.; He, M.; Wang, Y.; Dong, Y.; Alexandrov, I.V. Mechanical and Corrosion Properties of Ultrafine-Grained TC4-0.55Fe Alloy Processed by Equal-Channel Angular Pressing. Crystals 2025, 15, 795. https://doi.org/10.3390/cryst15090795
Guo Y, Lu Y, He M, Wang Y, Dong Y, Alexandrov IV. Mechanical and Corrosion Properties of Ultrafine-Grained TC4-0.55Fe Alloy Processed by Equal-Channel Angular Pressing. Crystals. 2025; 15(9):795. https://doi.org/10.3390/cryst15090795
Chicago/Turabian StyleGuo, Yumeng, Yu Lu, Miaoxia He, Yu Wang, Yuecheng Dong, and Igor V. Alexandrov. 2025. "Mechanical and Corrosion Properties of Ultrafine-Grained TC4-0.55Fe Alloy Processed by Equal-Channel Angular Pressing" Crystals 15, no. 9: 795. https://doi.org/10.3390/cryst15090795
APA StyleGuo, Y., Lu, Y., He, M., Wang, Y., Dong, Y., & Alexandrov, I. V. (2025). Mechanical and Corrosion Properties of Ultrafine-Grained TC4-0.55Fe Alloy Processed by Equal-Channel Angular Pressing. Crystals, 15(9), 795. https://doi.org/10.3390/cryst15090795






