Spectroscopic Evidence for the α-FeOOH-to-ε-FeOOH Phase Transition: Insights from High-Pressure and High-Temperature Raman Spectroscopy
Abstract
1. Introduction
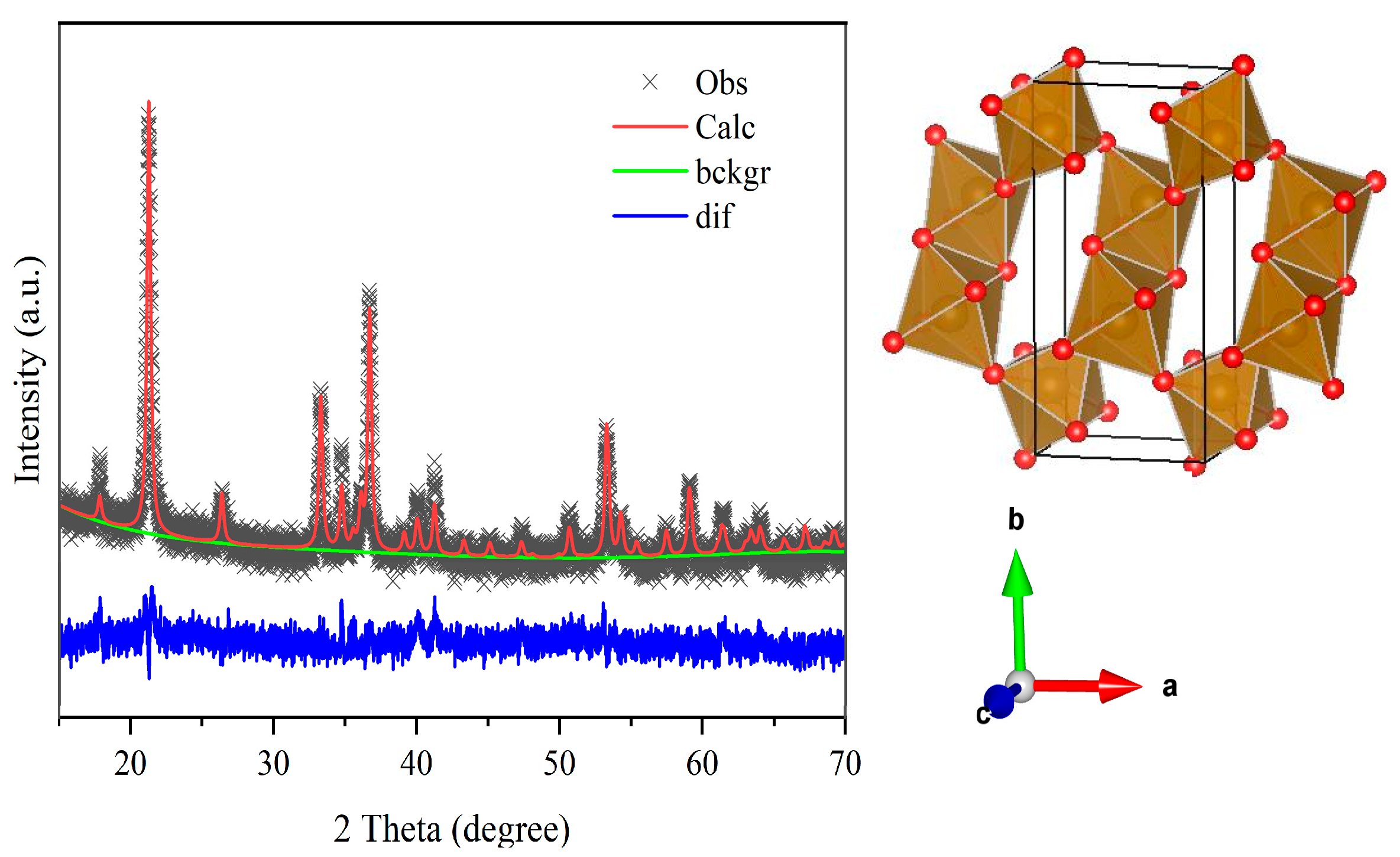
2. Materials and Methods
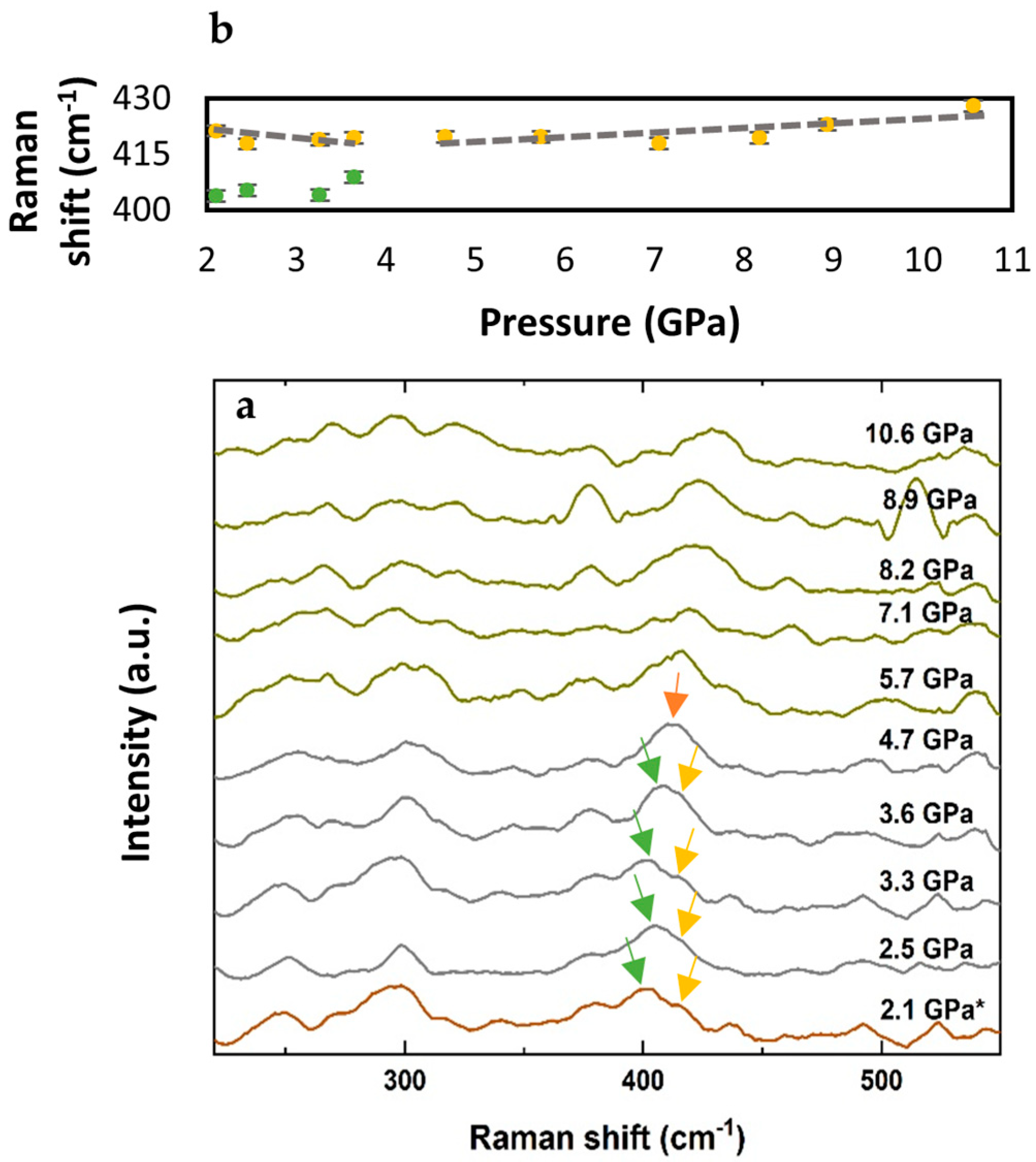
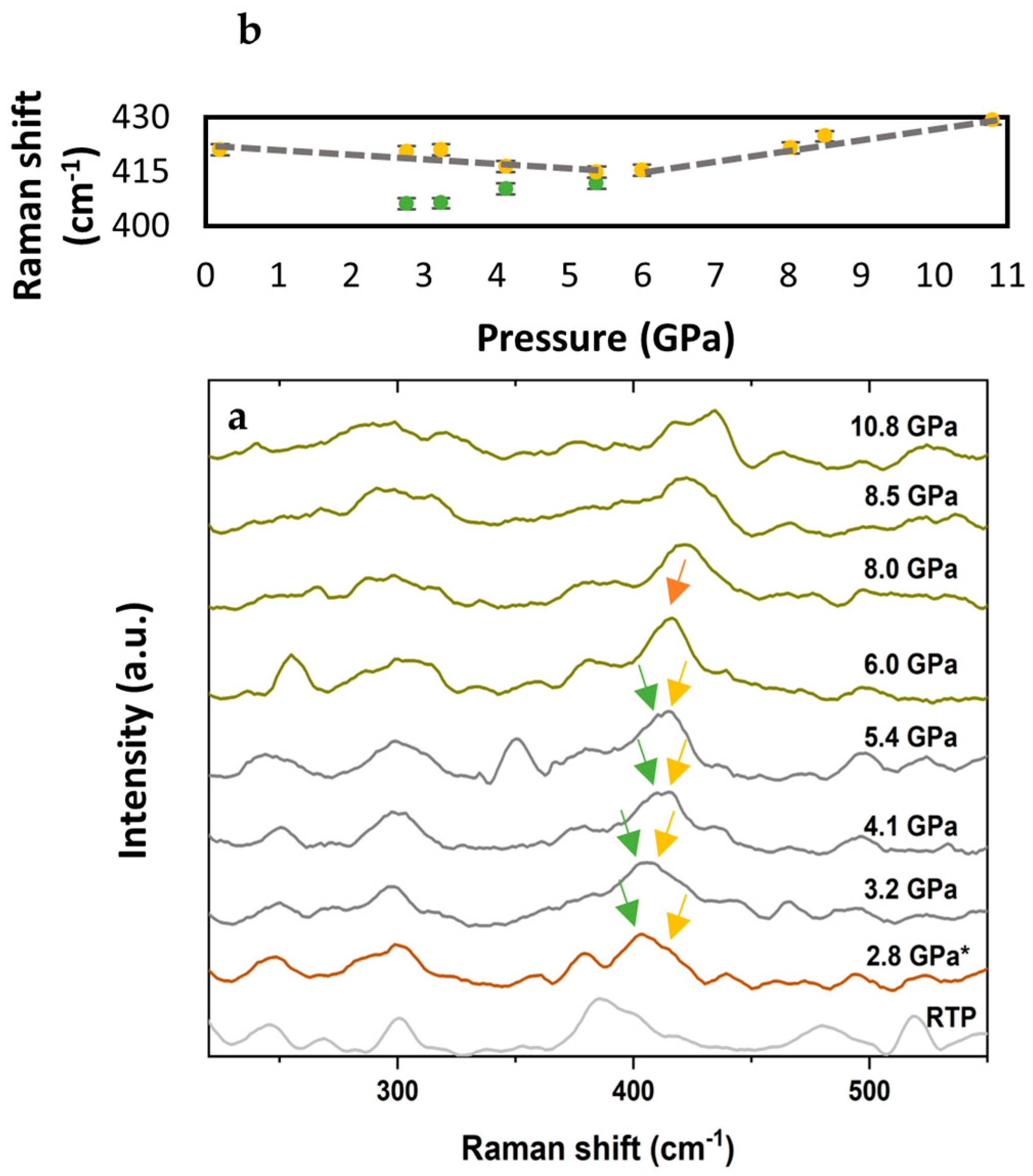
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| XRD | X-Ray Diffraction |
| DAC | Diamond Anvil Cell |
| GPa | Gigapascals |
References
- Majzlan, J.; Lang, B.E.; Stevens, R.; Navrotsky, A.; Woodfield, B.F.; Boerio-Goates, J. Thermodynamics of Fe oxides: Part I. Entropy at standard temperature and pressure and heat capacity of goethite (α-FeOOH), lepidocrocite (γ-FeOOH), and maghemite (γ-Fe2O3). Am. Mineral. 2003, 88, 846–854. [Google Scholar] [CrossRef]
- Gleason, A.E.; Jeanloz, R.; Kunz, M. Pressure-temperature stability studies of FeOOH using X-ray diffraction. Am. Mineral. 2008, 93, 1882. [Google Scholar] [CrossRef]
- Hu, Q.; Kim, D.Y.; Yang, W.; Yang, L.; Meng, Y.; Zhang, L.; Mao, H.-K. FeO2 and FeOOH under deep lower-mantle conditions and Earth’s oxygen–hydrogen cycles. Nature 2016, 534, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Nishi, M.; Kuwayama, Y.; Tsuchiya, J.; Tsuchiya, T. The pyrite-type high-pressure form of FeOOH. Nature 2017, 547, 205–208. [Google Scholar] [CrossRef]
- Pearson, D.G.; Brenker, F.E.; Nestola, F.; McNeill, J.; Nasdala, L.; Hutchison, M.T.; Matveev, S.; Mather, K.; Silversmit, G.; Schmitz, S.; et al. Hydrous mantle transition zone indicated by ringwoodite included within diamond. Nature 2014, 507, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Palot, M.; Jacobsen, S.D.; Townsend, J.P.; Nestola, F.; Marquardt, K.; Miyajima, N.; Harris, J.W.; Stachel, T.; McCammon, C.A.; Pearson, D.G. Evidence for H2O-bearing fluids in the lower mantle from diamond inclusion. Lithos 2016, 265, 237–243. [Google Scholar] [CrossRef]
- Dixon, J.E.; Dixon, T.H.; Bell, D.R.; Malservisi, R. Lateral variation in upper mantle viscosity: Role of water. Earth Planet. Sci. Lett. 2004, 222, 451–467. [Google Scholar] [CrossRef]
- Van der Meijde, M.; Marone, F.; Giardini, D.; van der Lee, S. Seismic Evidence for Water Deep in Earth’s Upper Mantle. Science 2003, 300, 1556–1558. [Google Scholar] [CrossRef]
- Tschauner, O.; Huang, S.; Greenberg, E.; Prakapenka, V.B.; Ma, C.; Rossman, G.R.; Shen, A.H.; Zhang, D.; Newville, M.; Lanzirotti, A.; et al. Ice-VII inclusions in diamonds: Evidence for aqueous fluid in Earth’s deep mantle. Science 2018, 359, 1136–1139. [Google Scholar] [CrossRef]
- Majzlan, J.; Grevel, K.-D.; Navrotsky, A. Thermodynamics of Fe oxides: Part II. Enthalpies of formation and relative stability of goethite (α-FeOOH), lepidocrocite (γ-FeOOH), and maghemite (γ-Fe2O3). Am. Mineral. 2003, 88, 855–859. [Google Scholar] [CrossRef]
- Tang, R.; Liu, J.; Kim, D.Y.; Hu, Q.; Yang, B.; Li, Y.; Pickard, C.; Needs, R.; He, Y.; Liu, H.; et al. Chemistry and P-V-T equation of state of FeO2H at the base of Earth’s lower mantle and their geophysical implications. Sci. Bull. 2021, 66, 1954–1958. [Google Scholar] [CrossRef]
- Tang, R.; Chen, J.; Zeng, Q.; Li, Y.; Liang, X.; Yang, B.; Wang, Y. Study on the High-Pressure Behavior of Goethite up to 32 GPa Using X-Ray Diffraction, Raman, and Electrical Impedance Spectroscopy. Minerals 2020, 10, 99. [Google Scholar] [CrossRef]
- Thompson, E.C.; Davis, A.H.; Brauser, N.M.; Liu, Z.; Prakapenka, V.B.; Campbell, A.J. Phase transitions in ε-FeOOH at high pressure and ambient temperature. Am. Mineral. 2020, 105, 1769–1777. [Google Scholar] [CrossRef]
- Thompson, E.C.; Campbell, A.J.; Tsuchiya, J. Elasticity of ε-FeOOH: Seismic implications for Earth’s lower mantle. J. Geophys. Res. Solid Earth 2017, 122, 5038–5047. [Google Scholar] [CrossRef]
- Suzuki, A. High-pressure X-ray diffraction study of ε-FeOOH. Phys. Chem. Miner. 2010, 37, 153–157. [Google Scholar] [CrossRef]
- Chen, J.; Esdaille, S. Is Earth’s core rusting? Eos 2022, 103, 34–39. [Google Scholar] [CrossRef]
- Esdaille, S.S.; Chen, J. Mineral physics constraints on ultra-low velocity zones in the lowermost mantle. Earth-Sci. Rev. 2024, 250, 104685. [Google Scholar] [CrossRef]
- Voigt, R.; Will, G. The system Fe2O3-H2O under high-pressures. Neues Jahrb. MineraIogie-Monatshefte 1981, 2, 89–96. [Google Scholar]
- Xu, C.; Nishi, M.; Inoue, T. Solubility behavior of δ-AlOOH and ε-FeOOH at high pressures. Am. Mineral. 2019, 104, 1416–1420. [Google Scholar] [CrossRef]
- Nishi, M.; Irifune, T.; Gréaux, S.; Tange, Y.; Higo, Y. Phase transitions of serpentine in the lower mantle. Phys. Earth Planet. Inter. 2015, 245, 52–58. [Google Scholar] [CrossRef]
- Nishi, M.; Tsuchiya, J.; Kuwayama, Y.; Arimoto, T.; Tange, Y.; Higo, Y.; Hatakeyama, T.; Irifune, T. Solid Solution and Compression Behavior of Hydroxides in the Lower Mantle. J. Geophys. Res. Solid Earth 2019, 124, 10231–10239. [Google Scholar] [CrossRef]
- Tsuchiya, J. First principles prediction of a new high-pressure phase of dense hydrous magnesium silicates in the lower mantle. Geophys. Res. Lett. 2013, 40, 4570–4573. [Google Scholar] [CrossRef]
- Ohtani, E.; Amaike, Y.; Kamada, S.; Sakamaki, T.; Hirao, N. Stability of hydrous phase H MgSiO4H2 under lower mantle conditions. Geophys. Res. Lett. 2014, 41, 8283–8287. [Google Scholar] [CrossRef]
- Wiethoff, F.; Grevel, K.-D.; Marler, B.; Herrmann, J.; Majzlan, J.; Kirste, J.; Lathe, C. P-V-T behavior of FeO(OH) and MnO(OH). Phys. Chem. Miner. 2017, 44, 567–576. [Google Scholar] [CrossRef]
- Yoshino, T.; Baker, E.; Duffey, K. Fate of water in subducted hydrous sediments deduced from stability fields of FeOOH and AlOOH up to 20 GPa. Phys. Earth Planet. Inter. 2019, 294, 106295. [Google Scholar] [CrossRef]
- Nishihara, Y.; Matsukage, K.N. Iron-titanium oxyhydroxides as water carriers in the Earth’s deep mantle. Am. Mineral. 2016, 101, 919–927. [Google Scholar] [CrossRef]
- Liu, X.; Matsukage, K.N.; Nishihara, Y.; Suzuki, T.; Takahashi, E. Stability of the hydrous phases of Al-rich phase D and Al-rich phase H in deep subducted oceanic crust. Am. Mineral. 2019, 104, 64–72. [Google Scholar] [CrossRef]
- Otte, K.; Pentcheva, R.; Schmahl, W.W.; Rustad, J.R. Pressure-induced structural and electronic transitions in FeOOH from first principles. Phys. Rev. B 2009, 80, 205116. [Google Scholar] [CrossRef]
- Suzuki, A. Thermal equation of state of goethite (α-FeOOH). High Press. Res. 2017, 37, 193–199. [Google Scholar] [CrossRef]
- Liu, K.; Dai, L.; Li, H.; Hu, H.; Zhuang, Y.; Linfei, Y.; Pu, C.; Hong, M. Pressure-induced phase transitions for goethite investigated by Raman spectroscopy and electrical conductivity. High Press. Res. 2019, 39, 106–116. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Dünnwald, J.; Otto, A. An investigation of phase transitions in rust layers using raman spectroscopy. Corros. Sci. 1989, 29, 1167–1176. [Google Scholar] [CrossRef]
- de Faria, D.L.A.; Venâncio Silva, S.; de Oliveira, M.T. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Schwertmann, U.; Cornerll, R.M. Goethite. In Iron Oxides in the Laboratory; Wiley-VCH: Weinheim, Germany, 2000; pp. 67–92. [Google Scholar]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Mao, H.K.; Xu, J.; Bell, P.M. Calibration of the ruby pressure gauge to 800 kbar under quasi-hydrostatic conditions. J. Geophys. Res. Solid Earth 1986, 91, 4673–4676. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Baneyeva, M.I. The Fe2O3-H2O system at high pressures and extemperatures. Geochem. Int. 1973, 10, 840–842. [Google Scholar]
- Suzuki, A. Pressure–volume–temperature equation of state of ε–FeOOH to 11 GPa and 700 K. J. Mineral. Petrol. Sci. 2016, 111, 420–424. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esdaille, S.S.; Drozd, V.; Durygin, A.; Li, W.; Chen, J. Spectroscopic Evidence for the α-FeOOH-to-ε-FeOOH Phase Transition: Insights from High-Pressure and High-Temperature Raman Spectroscopy. Crystals 2025, 15, 782. https://doi.org/10.3390/cryst15090782
Esdaille SS, Drozd V, Durygin A, Li W, Chen J. Spectroscopic Evidence for the α-FeOOH-to-ε-FeOOH Phase Transition: Insights from High-Pressure and High-Temperature Raman Spectroscopy. Crystals. 2025; 15(9):782. https://doi.org/10.3390/cryst15090782
Chicago/Turabian StyleEsdaille, Shanece S., Vadym Drozd, Andriy Durygin, Wenhao Li, and Jiuhua Chen. 2025. "Spectroscopic Evidence for the α-FeOOH-to-ε-FeOOH Phase Transition: Insights from High-Pressure and High-Temperature Raman Spectroscopy" Crystals 15, no. 9: 782. https://doi.org/10.3390/cryst15090782
APA StyleEsdaille, S. S., Drozd, V., Durygin, A., Li, W., & Chen, J. (2025). Spectroscopic Evidence for the α-FeOOH-to-ε-FeOOH Phase Transition: Insights from High-Pressure and High-Temperature Raman Spectroscopy. Crystals, 15(9), 782. https://doi.org/10.3390/cryst15090782





