Mineralogical and Thermochemical Characteristics of Dolomite Induced by Two Marine Microorganisms: Further Insights into Biomineralization
Abstract
1. Introduction
2. Materials and Methods
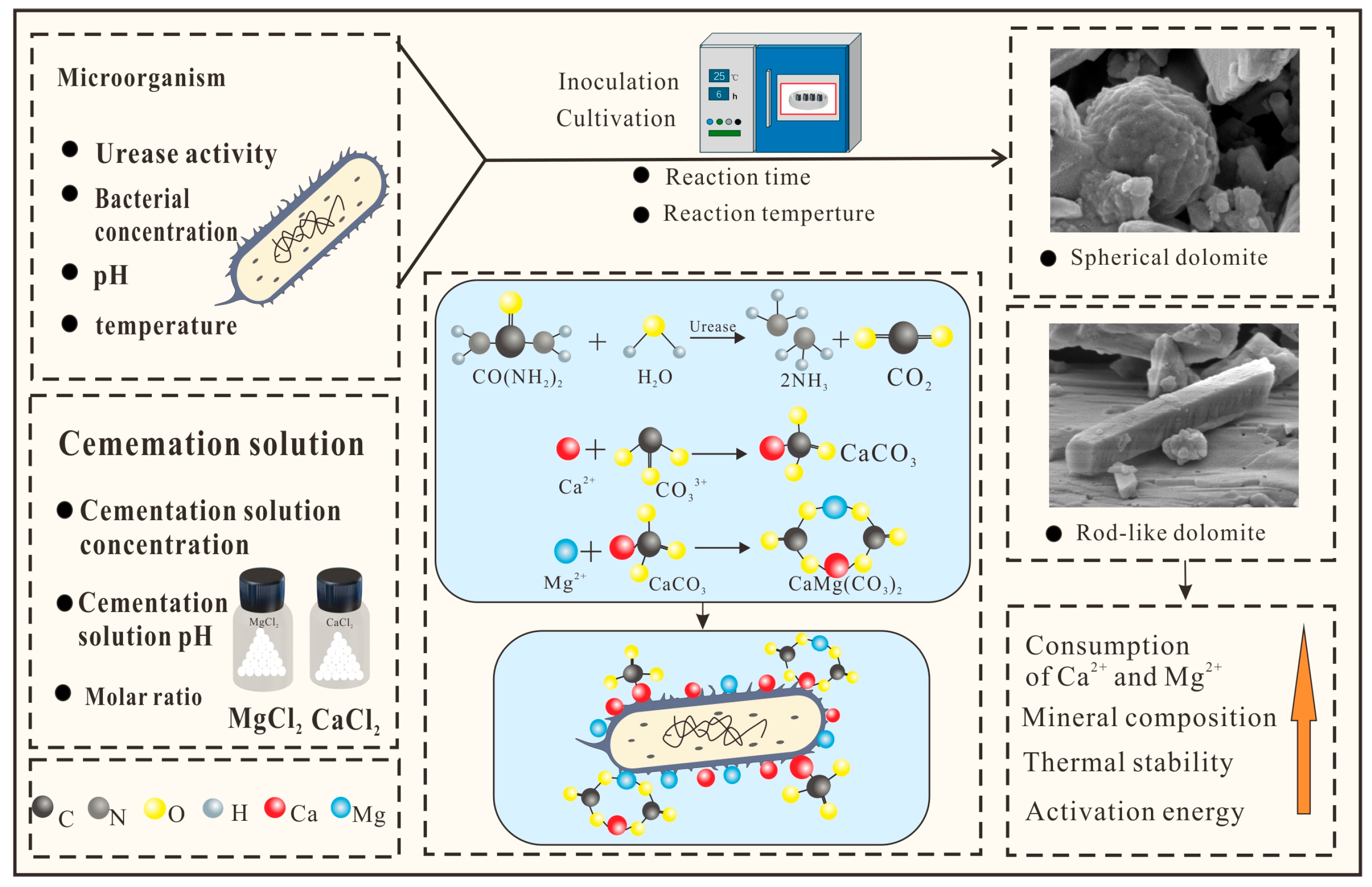
2.1. Source of Microorganisms
2.2. The Design Process of Experimental Scheme
2.3. Activate and Inoculate Strains
2.4. Sampling of Supernatant and Precipitation
2.5. The Ca2+ and Mg2+ Concentration Changes
2.6. Phase Compositions of Minerals
2.7. Morphology and Elemental Composition of Minerals
2.8. Thermochemical Analysis
2.9. Kinetic Analysis
3. Results
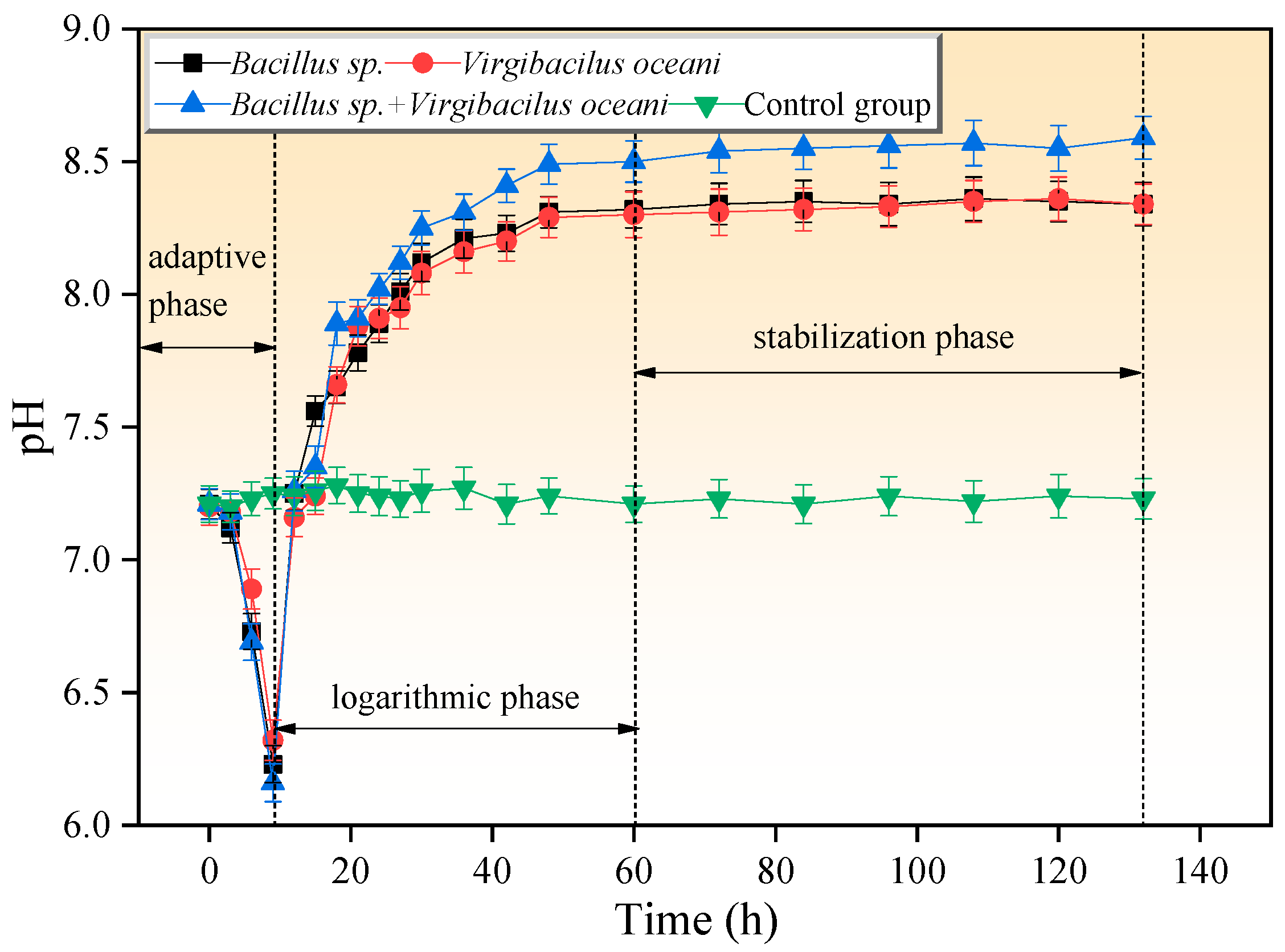
3.1. Dynamic Changes in pH Value
3.2. Consumption of Ca2+ and Mg2+
3.3. Morphological Changes and Elemental Composition in Different Systems
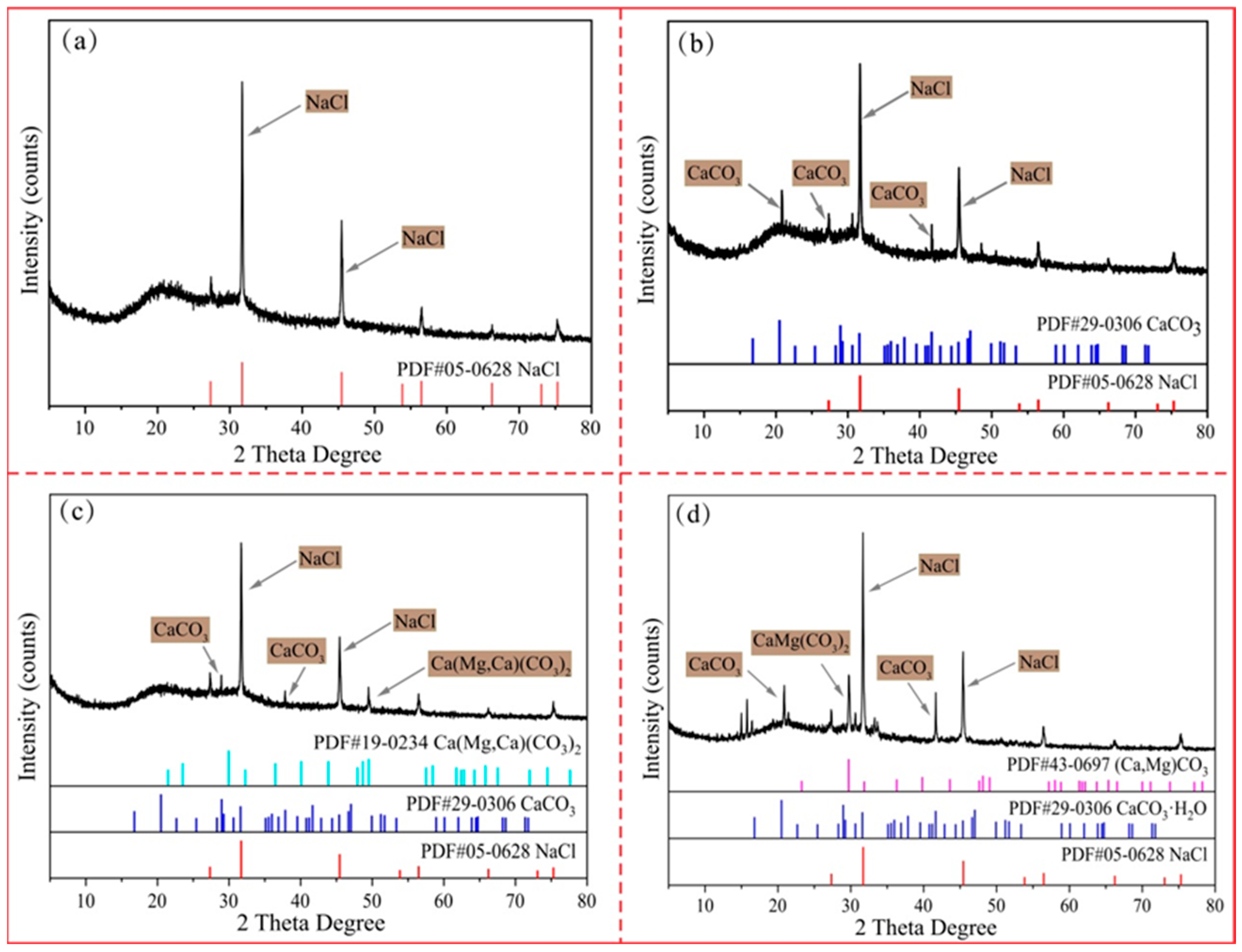
3.4. XRD Analysis
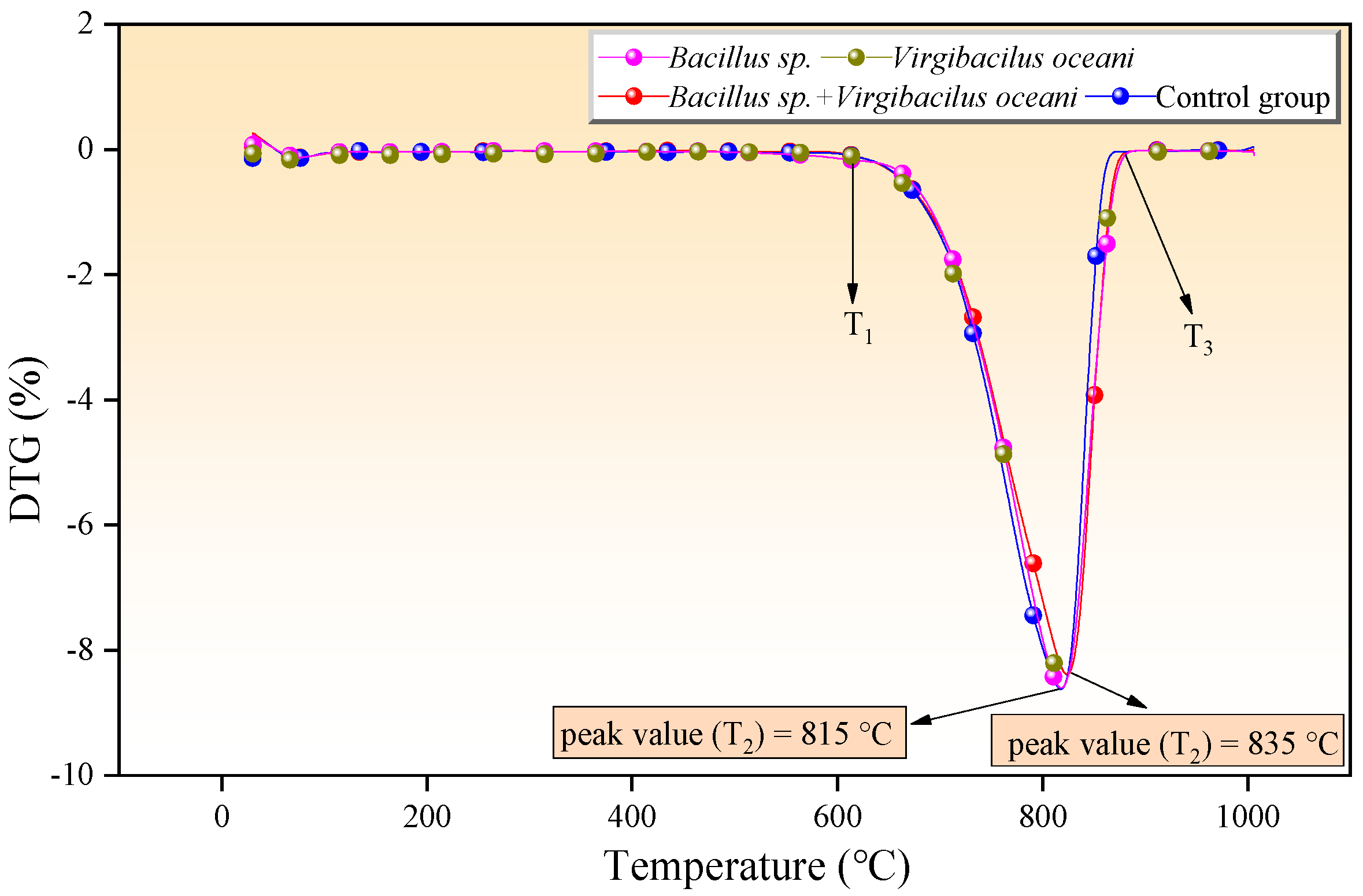
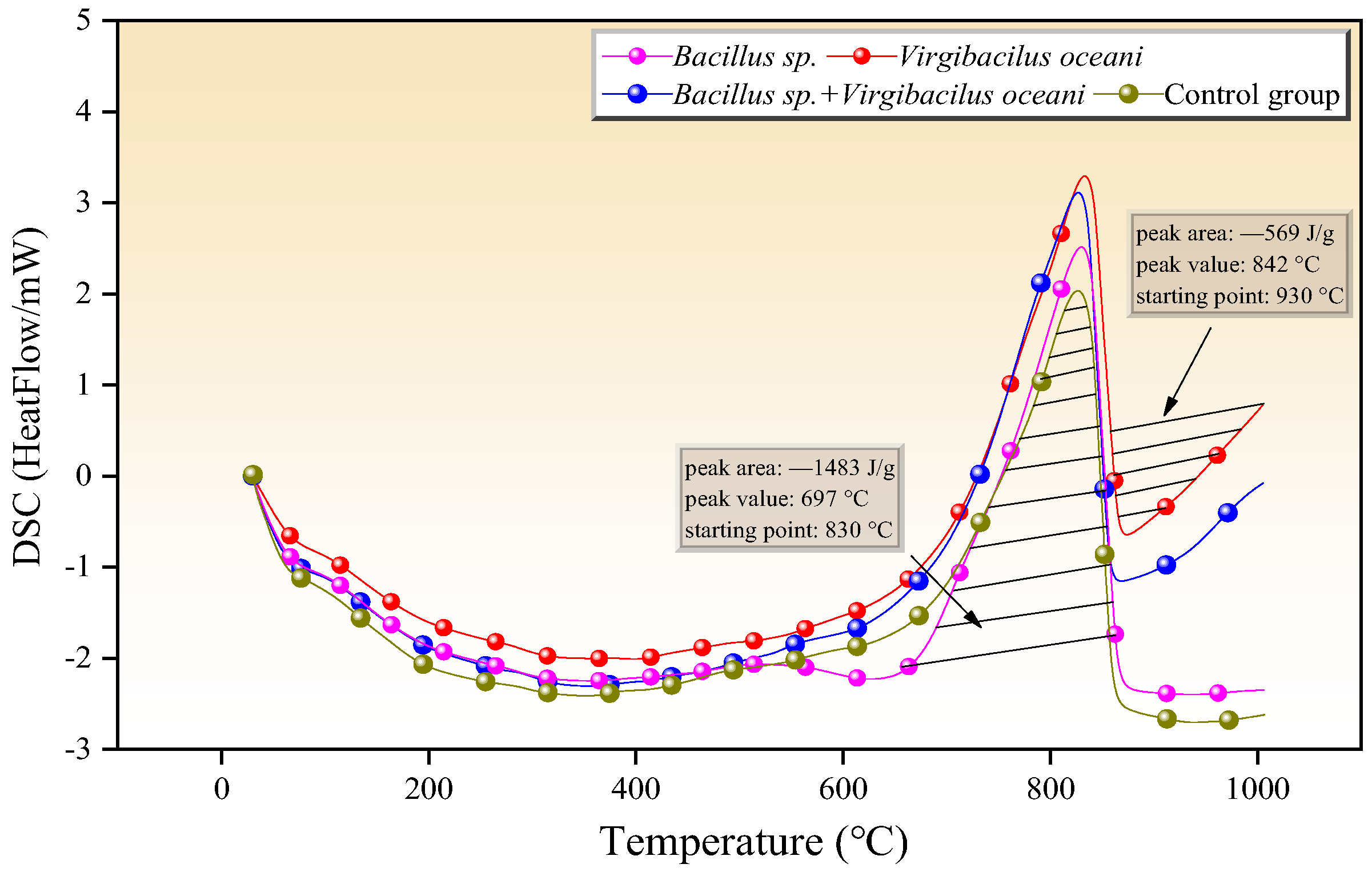
3.5. Thermal Decomposition Characteristics and Kinetic Study in Different Systems
4. Discussion
4.1. Precipitation Mechanism of Dolomite
4.2. Bioremediation of Adsorbed Ca2+ and Mg2+
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cai, W.K.; Liu, J.H.; Zhou, C.H.; Keeling, J.; Glasmacher, U.A. Structure, genesis and resources efficiency of dolomite: New insights and remaining enigmas. Chem. Geol. 2021, 573, 120191. [Google Scholar] [CrossRef]
- Chen, C.; Zhong, H.; Wang, X.; Ning, M.; Wang, X.; Ge, Y.; Wang, H.; Tang, R.; Hou, M. Thermodynamic and kinetic studies of dolomite formation: A review. Minerals 2023, 13, 1479. [Google Scholar] [CrossRef]
- Agrawal, Y.; Gupta, T.; Siddique, S.; Sharma, R.K. Potential of dolomite industrial waste as construction material: A review. Innov. Infrastruct. Solut. 2021, 6, 205. [Google Scholar] [CrossRef]
- Wan, Y.; Lin, J.; Zhao, Z.; Wang, Z. Origin of the dolomite in the Buqu Formation (Mid-Jurassic) in the south depression of the Qiangtang Basin, Tibet: Evidence from petrographic and geochemical constraints. Front. Earth Sci. 2022, 10, 944701. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, B.; Zhang, S.; Wang, H. Autochthonous Dolomitization and Dissolution in the Microbial Carbonate Rocks of the Fengjiawan Formation in the Ordos Basin. Acta Geol. Sin.-Engl. Ed. 2022, 96, 1376–1387. [Google Scholar] [CrossRef]
- Jurado, I.V.; Paese, G.; Schneider, I.H.; Féris, L. Phosphate removal from aqueous solutions using natural and thermic treated dolomites: Equilibrium, kinetic, and thermodynamic. Int. J. Environ. Sci. Technol. 2021, 19, 1739–1752. [Google Scholar] [CrossRef]
- Resio, L.C. Dolomite thermal behaviour: A short review. Phys. Chem. Miner. 2024, 51, 19. [Google Scholar] [CrossRef]
- Meister, P.; Frisia, S.; Dódony, I.; Pekker, P.; Molnár, Z.; Neuhuber, S.; Gier, S.; Kovács, I.; Demény, A.; Pósfai, M. Nanoscale pathway of modern dolomite formation in a Shallow, Alkaline Lake. Cryst. Growth Des. 2023, 23, 3202–3212. [Google Scholar] [CrossRef] [PubMed]
- Rivers, J.M. Warm acidified seawater: A dolomite solution. J. Sediment. Res. 2023, 93, 187–201. [Google Scholar] [CrossRef]
- Fang, Y.; Xu, H. Dissolved silica-catalyzed disordered dolomite precipitation. Am. Mineral. 2022, 107, 443–452. [Google Scholar] [CrossRef]
- Santos, H.S.; Nguyen, H.; Venâncio, F.; Ramteke, D.; Zevenhoven, R.; Kinnunen, P. Mechanisms of Mg carbonates precipitation and implications for CO2 capture and utilization/storage. Inorg. Chem. Front. 2023, 10, 2507–2546. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, Y.; Teng, H. Relative contributions of mg hydration and molecular structural restraints to the barrier of dolomite crystallization: A comparison of aqueous and non-aqueous crystallization in (BaMg)CO3 and (CaMg)CO3 systems. Minerals 2021, 11, 1214. [Google Scholar] [CrossRef]
- Aufort, J.; Raiteri, P.; Gale, J.D. Computational insights into Mg2+ dehydration in the presence of carbonate. ACS Earth Space Chem. 2022, 6, 733–745. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, H.; Shelobolina, E.S.; Konishi, H.; Roden, E.E. Precipitation of low-temperature disordered dolomite induced by extracellular polymeric substances of methanogenic Archaea Methanosarcina barkeri: Implications for sedimentary dolomite formation. Am. Mineral. 2021, 106, 69–81. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, F.; Liu, J.; Chen, L.; Li, Y.; Ding, H.; Lu, A. Microbial community response to photoelectrons and regulation on dolomite precipitation in marine sediments of Yellow Sea. Minerals 2023, 13, 753. [Google Scholar] [CrossRef]
- Sun, X.; Wai, O.W.H.; Xie, J.; Li, X. Biomineralization to prevent microbially induced corrosion on concrete for sustainable marine infrastructure. Environ. Sci. Technol. 2023, 58, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, M.; Feng, Z.; Zhang, Y.; Huang, X.; Xue, X. Efficient removal of Al (III) and P507 from high concentration MgCl2 solution based on in-situ reaction strategy. Trans. Nonferrous Met. Soc. China 2024, 34, 3042–3053. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, N.; Shi, J.; Xia, Y.; Zhu, B.; Shao, R.; Min, C.; Xu, Z.; Deng, H. Extra-thin composite nanofiltration membranes tuned by γ-cyclodextrins containing amphipathic cavities for efficient separation of magnesium/lithium ions. Sep. Purif. Technol. 2022, 286, 120419. [Google Scholar] [CrossRef]
- Fan, Q.; Liu, D.; Papineau, D.; Qiu, X.; Wang, H.; She, Z.; Zhao, L. Precipitation of high Mg-calcite and protodolomite using dead biomass of aerobic halophilic bacteria. J. Earth Sci. 2023, 34, 456–466. [Google Scholar] [CrossRef]
- Teng, J.; Qiu, L.; Zhang, S.; Ma, C. Origin and diagenetic evolution of dolomites in paleogene Shahejie Formation lacustrine organic shale of Jiyang depression, Bohai Bay Basin, east China. Pet. Explor. Dev. 2022, 49, 1251–1265. [Google Scholar] [CrossRef]
- Zhuang, D.; Yao, W.; Guo, Y.; Chen, Z.; Gui, H.; Zhao, Y. Bioremediation of Heavy Metal-Contaminated Solution and Aged Refuse by Microbially Induced Calcium Carbonate Precipitation: Further Insights into Sporosarcina pasteurii. Microorganisms 2025, 13, 64. [Google Scholar] [CrossRef]
- Zhuang, D.; Yao, W.; Guo, Y.; Wu, C.; Cao, J.; Sun, B. Comparative study on geochemical and thermodynamic characteristics among wormkalk, micrite, thrombolite, and stromatolite. Carbonates Evaporites 2025, 40, 22. [Google Scholar] [CrossRef]
- Jarwar, M.A.; Dumontet, S.; Pasquale, V.; Chen, C. Microbial induced carbonate precipitation: Environments, applications, and mechanisms. Geomicrobiol. J. 2022, 39, 833–851. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Li, D.; Qi, P.; Gao, X.; Guo, N.; Meng, R.; Tucker, M.E.; Yan, H.; Han, Z. Extreme halophilic bacteria promote the surface dolomitization of calcite crystals in solutions with various magnesium concentrations. Chem. Geol. 2022, 606, 120998. [Google Scholar] [CrossRef]
- Li, J.; Sun, Y.; Chen, F.; Hu, X.; Dong, L. Pressure and temperature combined with microbial supernatant effectively inactivate Bacillus subtilis spores. Front. Microbiol. 2021, 12, 642501. [Google Scholar] [CrossRef]
- Xue, Z.F.; Cheng, W.C.; Wang, L.; Hu, W. Effects of bacterial inoculation and calcium source on microbial-induced carbonate precipitation for lead remediation. J. Hazard. Mater. 2022, 426, 128090. [Google Scholar] [CrossRef] [PubMed]
- Fatimah, S.; Ragadhita, R.; Al Husaeni, D.F.; Nandiyanto, A.B.D. How to calculate crystallite size from x-ray diffraction (XRD) using Scherrer method. ASEAN J. Sci. Eng. 2022, 2, 65–76. [Google Scholar] [CrossRef]
- Ali, A.; Chiang, Y.W.; Santos, R.M. X-ray diffraction techniques for mineral characterization: A review for engineers of the fundamentals, applications, and research directions. Minerals 2022, 12, 205. [Google Scholar] [CrossRef]
- Liu, A.; Liu, S.; Liu, Y.; Liu, B.; Liu, T. Characterizing mechanical heterogeneity of coal at nano-to-micro scale using combined nanoindentation and FESEM-EDS. Int. J. Coal Geol. 2022, 261, 104081. [Google Scholar] [CrossRef]
- Qureshi, F.; Yusuf, M.; Tahir, M.; Haq, M.; Mohamed, M.M.I.; Kamyab, H.; Nguyen, H.-H.T.; Vo, D.-V.N.; Ibrahim, H. Renewable hydrogen production via biological and thermochemical routes: Nanomaterials, economic analysis and challenges. Process Saf. Environ. Prot. 2023, 179, 68–88. [Google Scholar] [CrossRef]
- Zhuang, D.; Yang, F.; Xiang, Q.; Wei, Q.; Jiang, W.; Zhao, J.; Guo, Y. Differences in thermal decomposition and crystallinity of dark organic laminae and light mineral laminae in same stromatolite. Thermochim. Acta 2023, 728, 179576. [Google Scholar] [CrossRef]
- Zhuang, D.; Cao, A.; Pan, L.; Jiang, W.; Zhao, J. Comparative study on thermal decomposition of stromatolite and micrite using the technique of TG and DSC. J. Therm. Anal. Calorim. 2023, 148, 5529–5541. [Google Scholar] [CrossRef]
- Arumsari, A.G.; Hernowo, P.; Wibowo, J.; Fadlulloh, M.Y.; Setiawan, R. Study of Reaction Kinetics by Flynn Wall Ozawa Method on Sawdust Pyrolysis Process. Indones. J. Appl. Chem. 2022, 24, 9–14. [Google Scholar]
- Ivanov, V.B.; Kalugina, E.V.; Pomerantsev, A.L.; Samoryadov, A.; Shebanov, M. Kinetic features of nonisothermal degradation of polyarylene ketones. Thermochim. Acta 2023, 730, 179608. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Kohout, J. Modified Arrhenius equation in materials science, chemistry and biology. Molecules 2021, 26, 7162. [Google Scholar] [CrossRef]
- Michael, P.S. Positive and negative effects of addition of organic carbon and nitrogen for management of sulfuric soil material acidity under general soil use conditions. Pol. J. Soil Sci. 2021, 54, 71–87. [Google Scholar] [CrossRef]
- Bayazitova, Z.E.; Kurmanbayeva, A.S.; Tleuova, Z.O.; Temirbekova, N.G. Application of the thermophilic fermentation method to obtain environmentally friendly organic fertilizer. J. Ecol. Eng. 2023, 24, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Fisher, P.R.; Argo, W.R.; Jeong, K.Y.; Altland, J. Macronutrient solubility in response to the pH of soilless container substrates. J. Soil Sci. Plant Nutr. 2025, 25, 3224–3240. [Google Scholar] [CrossRef]
- Xu, X.; Xu, L.; Yang, Z.; Chen, L.; Wang, Y.; Ren, H.; Zhang, Z.; El-Kassaby, Y.A.; Wu, S. Identification of key gene networks controlling organic acid and sugar metabolism during star fruit (Averrhoa carambola) development. BMC Plant Biol. 2024, 24, 943. [Google Scholar] [CrossRef] [PubMed]
- Gupta, T.; Cho, J.; Prakash, J. Hydrothermal synthesis of TiO2 nanorods: Formation chemistry, growth mechanism, and tailoring of surface properties for photocatalytic activities. Mater. Today Chem. 2021, 20, 100428. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Pan, Y.; Li, Y.; Chen, C.T.A.; Jiang, Z.-P.; Cai, W.-J.; Shen, Y.; Ding, Z.; Chen, Q.; Di, Y.; Fan, W.; et al. Unveiling the secrets of diatom-mediated calcification: Implications for the biological pump. Sci. China Earth Sci. 2024, 67, 2895–2909. [Google Scholar] [CrossRef]
- Klumpp, K.; Marcolli, C.; Peter, T. The impact of (bio-) organic substances on the ice nucleation activity of the K-feldspar microcline in aqueous solutions. Atmos. Chem. Phys. 2022, 22, 3655–3673. [Google Scholar] [CrossRef]
- Zhuang, D.; Wang, R.J.; Chen, S.; Li, X. The geochemical and thermodynamic characteristics of waste sand reinforced by microbially induced calcium carbonate precipitation. Environ. Technol. Innov. 2024, 36, 103828. [Google Scholar] [CrossRef]
- Zhuang, D.; Chen, S.; Li, J.; Han, S.; Guo, Y. Recycled coarse aggregate from waste concrete strengthened by microbially induced calcium carbonate precipitation. Environ. Technol. Innov. 2025, 37, 103981. [Google Scholar] [CrossRef]
- Han, Z.; Li, J.; Zhao, Y.; Chen, Q.; Gao, X.; Hu, K.; Guo, N.; Wei, X.; Meng, R.; Zhu, C.; et al. Dissolved Mn2+ promotes microbially-catalyzed protodolomite precipitation in brackish oxidized water. Chem. Geol. 2024, 650, 121986. [Google Scholar] [CrossRef]
- Liu, D.; Xu, Y.; Papineau, D.; Yu, N.; Fan, Q.; Qiu, X.; Wang, H. Experimental evidence for abiotic formation of low-temperature proto-dolomite facilitated by clay minerals. Geochim. Cosmochim. Acta 2019, 247, 83–95. [Google Scholar] [CrossRef]
- Zhuang, D.; Chen, Z.; Sun, B. Thermal decomposition of calcium carbonate at multiple heating rates in different atmospheres using the techniques of TG, DTG, and DSC. Crystals 2025, 15, 108. [Google Scholar] [CrossRef]





| System | MgCl2·6H2O (g) | CaCl2 (g) | NaCl (g) | NB Medium (g) |
|---|---|---|---|---|
| Bacillus sp. | 10.15 | 1.11 | 30 | 18 |
| Virgibacilus oceani | 10.15 | 1.11 | 30 | 18 |
| Bacillus sp. + Virgibacilus oceani | 10.15 | 1.11 | 30 | 18 |
| Control group | 10.15 | 1.11 | 30 | 18 |
| Material Composition | Composition Mass/Volume (g/L) |
|---|---|
| Ferric citrate | 0.1 |
| Potassium chloride | 0.55 |
| Sodium carbonate | 0.16 |
| Potassium bromide | 0.08 |
| Strontium chloride | 0.034 |
| Boric acid | 0.022 |
| Disodium hydrogen phosphate | 0.008 |
| Sodium sulfate | 0.016 |
| Tryptone | 10 |
| Beef Powder | 3.0 |
| Sodium chloride | 5.0 |
| Methods | FWO | KAS | Popescu | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Different Systems | Conversion Rate/α | R | E | lnA | R | E | lnA | R | E | lnA | Average |
| Bacillus sp. | 0.1 | 0.988 | 253.8 | 22.2 | 0.967 | 228.4 | 20.24 | 0.967 | 244.1 | 23.1 | |
| 0.2 | 0.967 | 248.9 | 21.0 | 0.958 | 232.3 | 18.54 | 0.978 | 249.8 | 23.9 | ||
| 0.3 | 0.945 | 245.2 | 21.4 | 0.981 | 224.8 | 26.24 | 0.927 | 250.8 | 21.9 | _ | |
| 0.4 | 0.964 | 250.1 | 18.9 | 0.945 | 258.0 | 10.94 | 0.951 | 255.4 | 26.4 | _ | |
| 0.5 | 0.938 | 245.0 | 18.5 | 0.972 | 223.2 | 26.44 | 0.990 | 223.7 | 13.0 | _ | |
| 0.6 | 0.974 | 243.7 | 21.3 | 0.938 | 243.6 | 10.34 | 0.944 | 262.9 | 17.9 | _ | |
| 0.7 | 0.991 | 226.8 | 17.9 | 0.989 | 264.1 | 15.24 | 0.972 | 233.4 | 13.4 | _ | |
| 0.8 | 0.923 | 222.9 | 21.9 | 0.908 | 242.8 | 9.14 | 0.926 | 258.8 | 17.7 | _ | |
| 0.9 | 0.919 | 244.7 | 26.9 | 0.923 | 226.4 | 17.14 | 0.937 | 246.9 | 19.6 | _ | |
| 242.3 ± 10.4 | 238.1 ± 14.9 | 247.3 ± 12.3 | 241.5 ± 4.6 | ||||||||
| Virgibacilus oceani | 239.5 ± 15.2 | 249.7 ± 12.5 | 239.7 ± 14.5 | 243.9 ± 5.9 | |||||||
| Bacillus sp. + Virgibacilus oceani | 261.2 ± 13.2 | 254.8 ± 10.2 | 257.2 ± 13.1 | 257.7 ± 3.2 | |||||||
| Control group | 221.6 ± 12.7 | 219.4 ± 14.2 | 226.5 ± 12.6 | 222.5 ± 3.6 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, D.; Yao, W.; Feng, S. Mineralogical and Thermochemical Characteristics of Dolomite Induced by Two Marine Microorganisms: Further Insights into Biomineralization. Crystals 2025, 15, 767. https://doi.org/10.3390/cryst15090767
Zhuang D, Yao W, Feng S. Mineralogical and Thermochemical Characteristics of Dolomite Induced by Two Marine Microorganisms: Further Insights into Biomineralization. Crystals. 2025; 15(9):767. https://doi.org/10.3390/cryst15090767
Chicago/Turabian StyleZhuang, Dingxiang, Weiheng Yao, and Songbao Feng. 2025. "Mineralogical and Thermochemical Characteristics of Dolomite Induced by Two Marine Microorganisms: Further Insights into Biomineralization" Crystals 15, no. 9: 767. https://doi.org/10.3390/cryst15090767
APA StyleZhuang, D., Yao, W., & Feng, S. (2025). Mineralogical and Thermochemical Characteristics of Dolomite Induced by Two Marine Microorganisms: Further Insights into Biomineralization. Crystals, 15(9), 767. https://doi.org/10.3390/cryst15090767




