Luminescent Arylalkynyltitanocenes: Effect of Modifying the Electron Density at the Arylalkyne Ligand, or Adding Steric Bulk or Constraint to the Cyclopentadienyl Ligand
Abstract
1. Introduction
2. Materials and Methods
2.1. General Methods
2.2. Computational Methods
2.3. Single-Crystal X-Ray Diffraction
2.4. Syntheses
3. Results and Discussion
3.1. Syntheses and Spectroscopic Characterization
3.2. Structural Characterization
3.2.1. General Structural and Packing Discussion
3.2.2. Comparison of Key Structural Metrics
3.3. Photochemical and Photophysical Characterization
3.3.1. Comparison of the Cp* Complexes
3.3.2. Replacing Cp* with Indenyl or ansa-Cp
3.3.3. Impact of CuBr Coordination on Photostability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-Me-THF | 2-methyltetrahydrofuran |
| ansa-Cp | Me2Si(η5-C5H4)2 |
| Cp | Cyclopentadienyl |
| Cp* | Pentamethylcyclopentadienyl |
| DMA | Dimethylaniline |
| Φdecomp | Quantum yield for photodecomposition |
| ΦPL | Quantum yield for photoluminescence |
| Ind | Indenyl |
| LL’CT | Ligand-to-ligand charge transfer |
| LMCT | Ligand-to-metal charge transfer |
| MC | Metal-centered |
| MXMCT | Metal halide-to-metal charge transfer |
| RT | Room temperature |
References
- Yamazaki, Y.; Takeda, H.; Ishitani, O. Photocatalytic reduction of CO2 using metal complexes. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 106–137. [Google Scholar] [CrossRef]
- Brennaman, M.K.; Dillon, R.J.; Alibabaei, L.; Gish, M.K.; Dares, C.J.; Ashford, D.L.; House, R.L.; Meyer, G.J.; Papanikolas, J.M.; Meyer, T.J. Finding the Way to Solar Fuels with Dye-Sensitized Photoelectrosynthesis Cells. J. Am. Chem. Soc. 2016, 138, 13085–13102. [Google Scholar] [CrossRef]
- Zhang, X.; Yamauchi, K.; Sakai, K. Earth-Abundant Photocatalytic CO2 Reduction by Multielectron Chargeable Cobalt Porphyrin Catalysts: High CO/H2 Selectivity in Water Based on Phase Mismatch in Frontier MO Association. ACS Catal. 2021, 11, 10436–10449. [Google Scholar] [CrossRef]
- Hedstrand, D.M.; Kruizinga, W.H.; Kellogg, R.M. Light induced and dye accelerated reduction of phenacyl onium salts by 1,4-dihydropyridines. Tetrahedron Lett. 1978, 19, 1255–1258. [Google Scholar] [CrossRef]
- Stephenson, C.; Yoon, T. Enabling Chemical Synthesis with Visible Light. Acc. Chem. Res. 2016, 49, 2059–2060. [Google Scholar] [CrossRef]
- Marzo, L.; Pagire, S.K.; Reiser, O.; Konig, B. Visible-Light Photocatalysis: Does It make a Difference in Organic Synthesis? Angew. Chem. Int. Ed. 2018, 57, 10034–10072. [Google Scholar] [CrossRef] [PubMed]
- Glaser, F.; Wenger, O.S. Recent progress in the development of transition-metal based photoredox catalysts. Coord. Chem. Rev. 2020, 405, 213129. [Google Scholar] [CrossRef]
- Chan, A.Y.; Perry, I.B.; Bissonnette, N.B.; Buksh, B.F.; Edwards, G.A.; Frye, L.I.; Garry, O.L.; Lavagnino, M.N.; Li, B.X.; Liang, Y.; et al. Metallaphotoredox: The Merger of Photoredox and Transition Metal Catalysis. Chem. Rev. 2022, 122, 1485–1542. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, B.C.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Kalyanasundaram, K.; Grätzel, M. Applications of Functionalized Transition Metal Complexes in Photonic and Optoelectronic Devices. Coord. Chem. Rev. 1998, 177, 347–414. [Google Scholar] [CrossRef]
- Housecraft, C.E.; Constable, E.C. Solar energy conversion using first row d-block metal coordination compound sensitizers and redox mediators. Chem. Sci. 2022, 13, 1225–1262. [Google Scholar] [CrossRef] [PubMed]
- Singh-Rachford, T.N.; Castellano, F.N. Photon upconversion based on sensitized triplet-triplet annihilation. Coord. Chem. Rev. 2010, 254, 2560–2573. [Google Scholar] [CrossRef]
- Zeng, L.; Huang, L.; Han, J.; Han, G. Enhancing Triplet—Triplet Annihilation Upconversion: From Molecular Design to Present Applications. Acc. Chem. Res. 2022, 55, 2604–2615. [Google Scholar] [CrossRef]
- DeRosa, M.C.; Crutchley, R.J. Photosensitized singlet oxygen and its applications. Coord. Chem. Rev. 2002, 233–234, 351–371. [Google Scholar] [CrossRef]
- Ford, P.C.; van Eldik, R. (Eds.) Photochemistry and Photophysics of Earth-Abundant Transition-Metal Complexes; Advances in Inorganic Chemistry; Elsevier: Amsterdam, The Netherlands, 2024; Volume 83. [Google Scholar]
- Wegeberg, C.; Wenger, O. Luminescent First-Row Transition Metal Complexes. J. Am. Chem. Soc. Au 2021, 1, 1860–1876. [Google Scholar] [CrossRef]
- Morselli, G.; Rebeer, C.; Wenger, O.S. Molecular Design Principles for Photoactive Transition Metal Complexes: A Guide for “Photo-Motivated” Chemists. J. Am. Chem. Soc. 2025, 147, 11608–11624. [Google Scholar] [CrossRef] [PubMed]
- Förster, C.; Heinze, K. Photophysics and photochemistry with Earth-abundant metals—Fundamentals and concepts. Chem. Soc. Rev. 2020, 49, 1057–1070. [Google Scholar] [CrossRef]
- Giobbio, G.; Costa, R.D.; Gaillard, S. Earth-abundant transition metal complexes in light-emitting electrochemical cells: Successes, challenges and perspectives. Dalton. Trans. 2025, 54, 3573–3580. [Google Scholar] [CrossRef]
- Wagenknecht, P.S.; Ford, P.C. Metal Centered Ligand Field Excited States: Their Roles in the Design and Performance of Transition Metal Based Photochemical Molecular Devices. Coord. Chem. Rev. 2011, 255, 591–616. [Google Scholar] [CrossRef]
- Wagenknecht, P.S. Ligand-to-metal charge-transfer states in emissive d0 metallocenes: Design strategies for titanocene based photocatalysts. In Photochemistry and Photophysics of Earth-Abundant Transition-Metal Complexes; Ford, P.C., van Eldik, R., Eds.; Advances in Inorganic Chemistry; Elsevier: Amsterdam, The Netherlands, 2024; Volume 83, pp. 63–109. [Google Scholar] [CrossRef]
- Zhang, Y.; Petersen, J.L.; Milsmann, C. A Luminescent Zirconium(IV) Complex as a Molecular Photosensitizer for Visible Light Photoredox Catalysis. J. Am. Chem. Soc. 2016, 138, 13115–13118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lee, T.S.; Favale, J.M.; Leary, D.C.; Petersen, J.L.; Scholes, G.D.; Castellano, F.N.; Milsmann, C. Delayed fluorescence from a zirconium(IV) photosensitizer with ligand-to-metal charge-transfer excited states. Nat. Chem. 2020, 12, 345–352. [Google Scholar] [CrossRef]
- Yang, M.; Sheykhi, S.; Zhang, Y.; Milsmann, C.; Castellano, F.N. Low power threshold photochemical upconversion using a zirconium(IV) LMCT photosensitizer. Chem. Sci. 2021, 12, 9069–9077. [Google Scholar] [CrossRef]
- Urbán, B.; Dunlop, D.; Gyepes, R.; Kubát, P.; Lang, K.; Horáček, M.; Pinkas, J.; Šimková, L.; Lamač, M. Luminescent Zirconocene Complexes with Pendant Phosphine Chalcogenide Donor Groups. Organometallics 2023, 42, 1373–1385. [Google Scholar] [CrossRef]
- Dunlop, D.; Večeřa, M.; Gyepes, R.; Kubát, P.; Lang, K.; Horáček, M.; Pinkas, J.; Šimková, L.; Liška, A.; Lamač, M. Luminescent Cationic Group 4 Metallocene Complexes Stabilized by Pendant N-Donor Groups. Inorg. Chem. 2021, 60, 7315–7328. [Google Scholar] [CrossRef] [PubMed]
- Lamač, M.; Dunlop, D.; Lang, K.; Kubát, P. Group 4 metallocene derivatives as a new class of singlet oxygen photo- sensitizers. J. Photochem. Photobiol. A Chem. 2022, 424, 113619. [Google Scholar] [CrossRef]
- Romain, C.; Choua, S.; Collin, J.-P.; Heinrich, M.; Bailly, C.; Karmazin-Brelot, L.; Bellemin-Laponnaz, S.; Dagorne, S. Redox and Luminescent Properties of Robust and Air-Stable N-Heterocyclic Carbene Group 4 Metal Complexes. Inorg. Chem. 2014, 53, 7371–7376. [Google Scholar] [CrossRef]
- London, H.C.; Pritchett, D.Y.; Pienkos, J.A.; McMillen, C.D.; Whittemore, T.J.; Bready, C.J.; Myers, A.R.; Vieira, N.C.; Harold, S.; Shields, G.C.; et al. Photochemistry and Photophysics of Charge-Transfer Excited States in Emissive d10/d0Heterobimetallic Titanocene Tweezer Complexes. Inorg. Chem. 2022, 61, 10986–10998. [Google Scholar] [CrossRef] [PubMed]
- Barker, M.; Whittemore, T.J.; London, H.C.; Sledesky, J.M.; Harris, E.A.; Smith Pellizzeri, T.M.; McMillen, C.D.; Wagenknecht, P.S. Design Strategies for Luminescent Titanocenes: Improving the Photoluminescence and Photostability of Arylethynyltitanocenes. Inorg. Chem. 2023, 62, 17870–17882. [Google Scholar] [CrossRef]
- Sledesky, J.M.; Zimmerman, J.H.; London, H.C.; Lambert, E.C.; McMillen, C.D.; Barker, M.; Hanson, K.; Wagenknecht, P.S. Xylylethynyl titanocene with a microsecond emission lifetime photosensitizes singlet-oxygen formation and photon upconversion. Inorg. Chem. 2025, 64, 14977–14988. [Google Scholar] [CrossRef]
- Suzuki, K.; Kobayashi, A.; Kaneko, S.; Takehira, K.; Yoshihara, T.; Ishida, H.; Shiina, Y.; Oishic, S.; Tobita, S. Reevaluation of absolute luminescence quantum yields of standard solutions using a spectrometer with an integrating sphere and a back-thinned CCD detector. Phys. Chem. Chem. Phys. 2009, 11, 9850–9860. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision, C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, version 6; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- O’Boyle, N.M.; Tenderholt, A.L.; Langner, K.M. cclib: A library for package-independent computational chemistry algorithms. J. Comp. Chem. 2008, 29, 839–845. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A At. Mol. Opt. Phys. 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B Condens. Matter Mater. Phys. 1988, 37, 785–789. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian-basis sets for molecular calculations. 1. 2nd row atoms, Z = 11−18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Wachters, A.J.H. Gaussian basis set for molecular wavefunctions containing third-row atoms. J. Chem. Phys. 1970, 52, 1033–1036. [Google Scholar] [CrossRef]
- Hay, P.J. Gaussian basis sets for molecular calculations-representation of 3d orbitals in transition-metal atoms. J. Chem. Phys. 1977, 66, 4377–4384. [Google Scholar] [CrossRef]
- Clark, T.; Chandrasekhar, J.; Spitznagel, G.W.; Schleyer, P.V.R. Efficient diffuse function-augmented basis-sets for anion calculations. 3. The 3- 21+G basis set for 1st-row elements, Li-F. J. Comput. Chem. 1983, 4, 294–301. [Google Scholar] [CrossRef]
- Frisch, M.J.; Pople, J.A.; Binkley, J.S. Self-Consistent Molecular Orbital Methods. 25. Supplementary Functions for Gaussian Basis Sets. J. Chem. Phys. 1984, 80, 3265–3269. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef]
- APEX 4, version 2022.10-0; Bruker-AXS Inc.: Madison, WI, USA, 2022.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Pinkas, J.; Kubista, J.; Horacek, M.; Mach, K.; Varga, V.; Gyepes, R. Low-valent ansa-dimethylsilylene-, dimethylmethylene-bis(cyclopentadienyl) titanium compounds and ansa-titanium-magnesium complexes. J. Organomet. Chem. 2019, 889, 15–26. [Google Scholar] [CrossRef]
- Pienkos, J.A.; Agakidou, A.D.; Trindle, C.O.; Herwald, D.W.; Altun, Z.; Wagenknecht, P.S. Titanocene as a New Acceptor (A) for Arylamine Donors (D) in D-π-A Chromophores. Organometallics 2016, 35, 2575–2578. [Google Scholar] [CrossRef]
- London, H.C.; Whittemore, T.J.; Gale, A.G.; McMillen, C.D.; Pritchett, D.Y.; Myers, A.R.; Thomas, H.D.; Shields, G.C.; Wagenknecht, P.S. Ligand-to-Metal Charge-Transfer Photophysics and Photochemistry of Emissive d0 Titanocenes: A Spectroscopic and Computational Investigation. Inorg. Chem. 2021, 60, 14399–14409. [Google Scholar] [CrossRef] [PubMed]
- Lang, H.; Köhler, K.; Rheinwald, G.; Zsolnai, L.; Büchner, M.; Driess, A.; Huttner, G.; Strähle, J. Monomeric Alkyne-Stabilized Complexes of Organo−Copper(I) and −Silver(I). Organometallics 1999, 18, 598–605. [Google Scholar] [CrossRef]
- Dias, H.V.; Flores, J.A.; Wu, J.; Kroll, P. Monomeric Copper(I), Silver(I), and Gold(I) Alkyne Complexes and the Coinage Metal Family Group Trends. J. Am. Chem. Soc. 2009, 131, 11249–11255. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; London, H.C.; Pattadar, D.; Worster, C.; Salpage, S.R.; Jakubikova, E.; Saavedra, S.S.; Hanson, K. Increasing excited state lifetimes of Cu(I) coordination complexes via strategic surface binding. Inorg. Chem. Front. 2025, 12, 1295–1302. [Google Scholar] [CrossRef]
- Englman, R.; Jortner, J. The Energy Gap Law for Non-Radiative Decay in Large Molecules. J. Lumin. 1970, 1–2, 134–142. [Google Scholar] [CrossRef]
- Livshits, M.Y.; Turlington, M.D.; Trindle, C.O.; Wang, L.; Altun, Z.; Wagenknecht, P.S.; Rack, J.J. Picosecond to Nanosecond Manipulation of Excited State Lifetimes in Complexes with an FeII to TiIV Metal-to-Metal Charge-Transfer: The Role of Ferrocene-Centered Excited States. Inorg. Chem. 2019, 58, 15320–15329. [Google Scholar] [CrossRef]
- Shields, G.C. Twenty years of exceptional success: The molecular education and research consortium in undergraduate computational chemistry (MERCURY). Int. J. Quantum Chem. 2020, 120, 26274. [Google Scholar] [CrossRef]

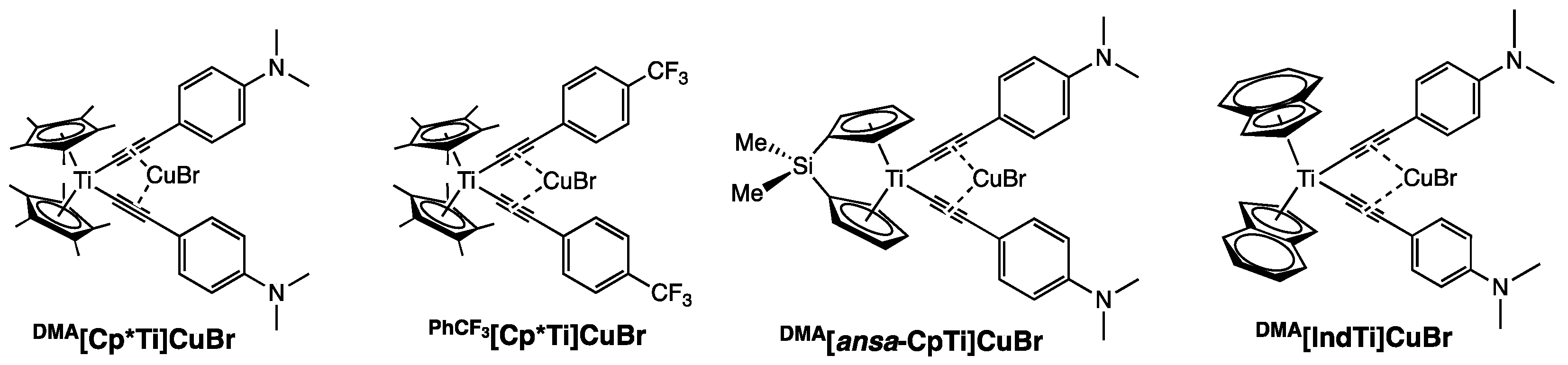


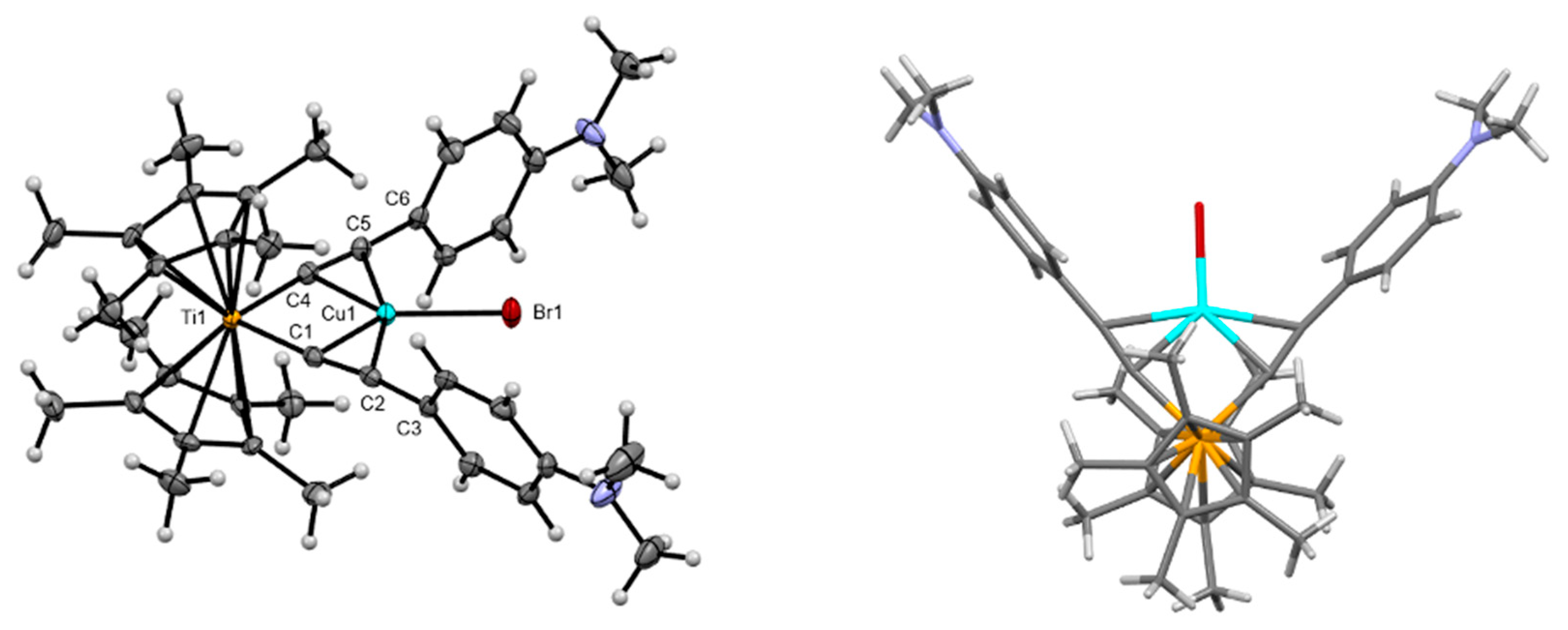
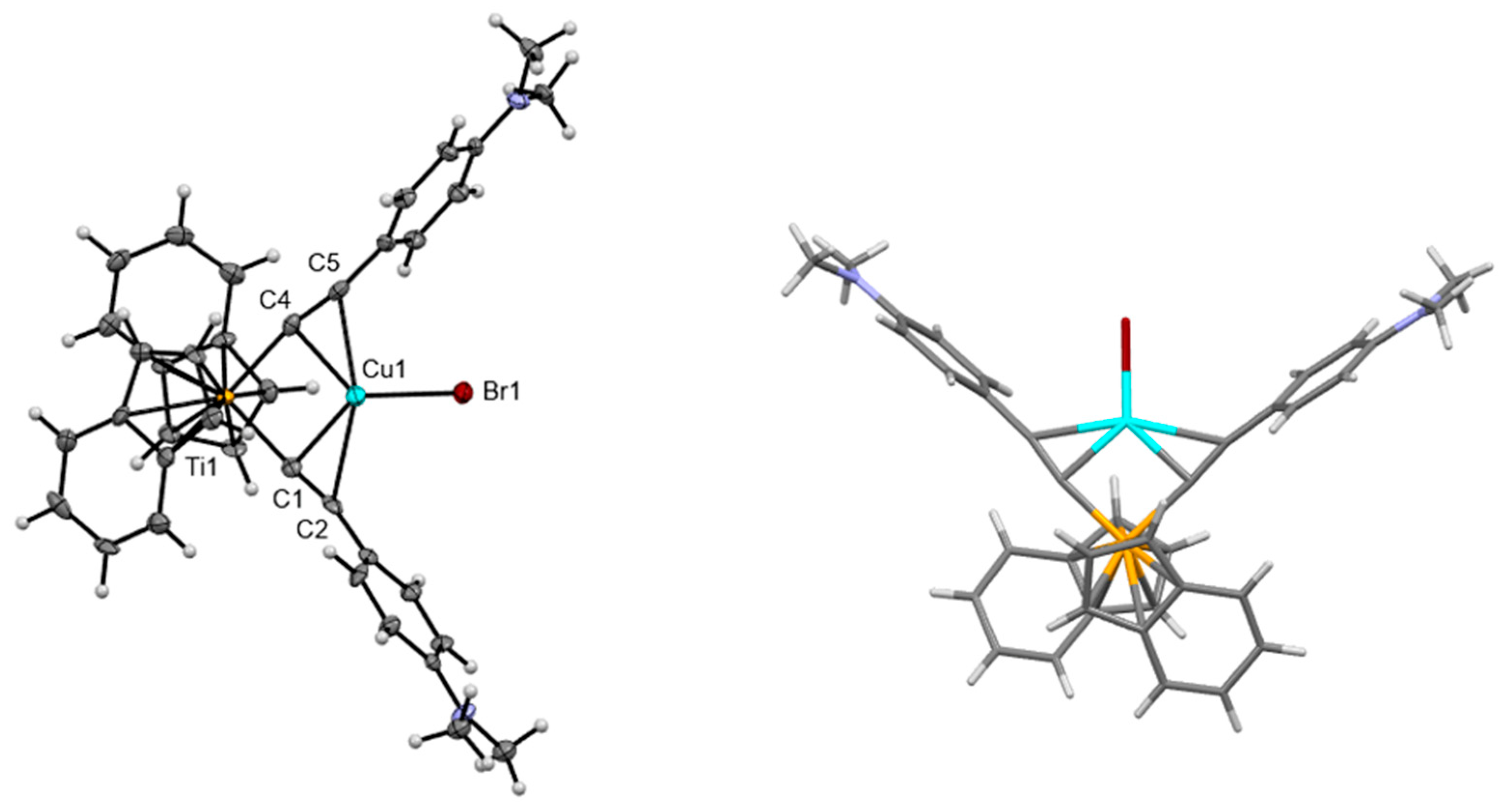
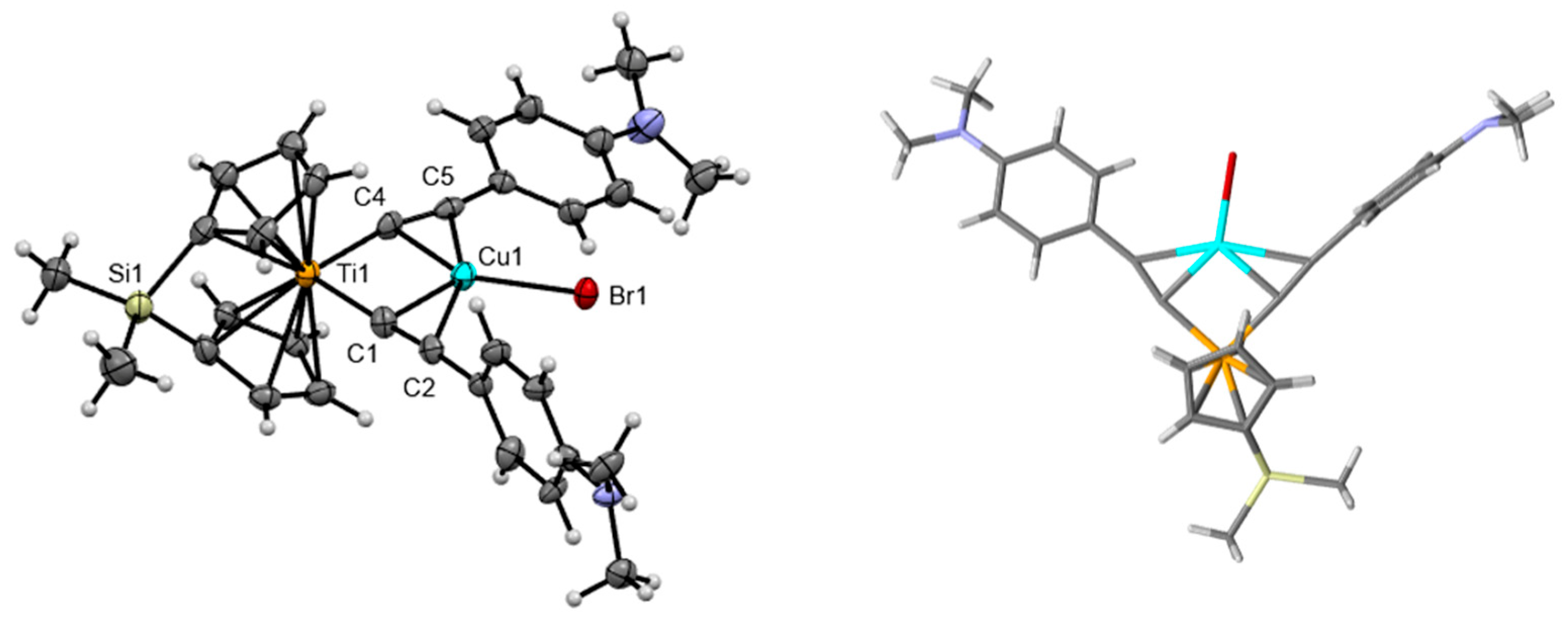


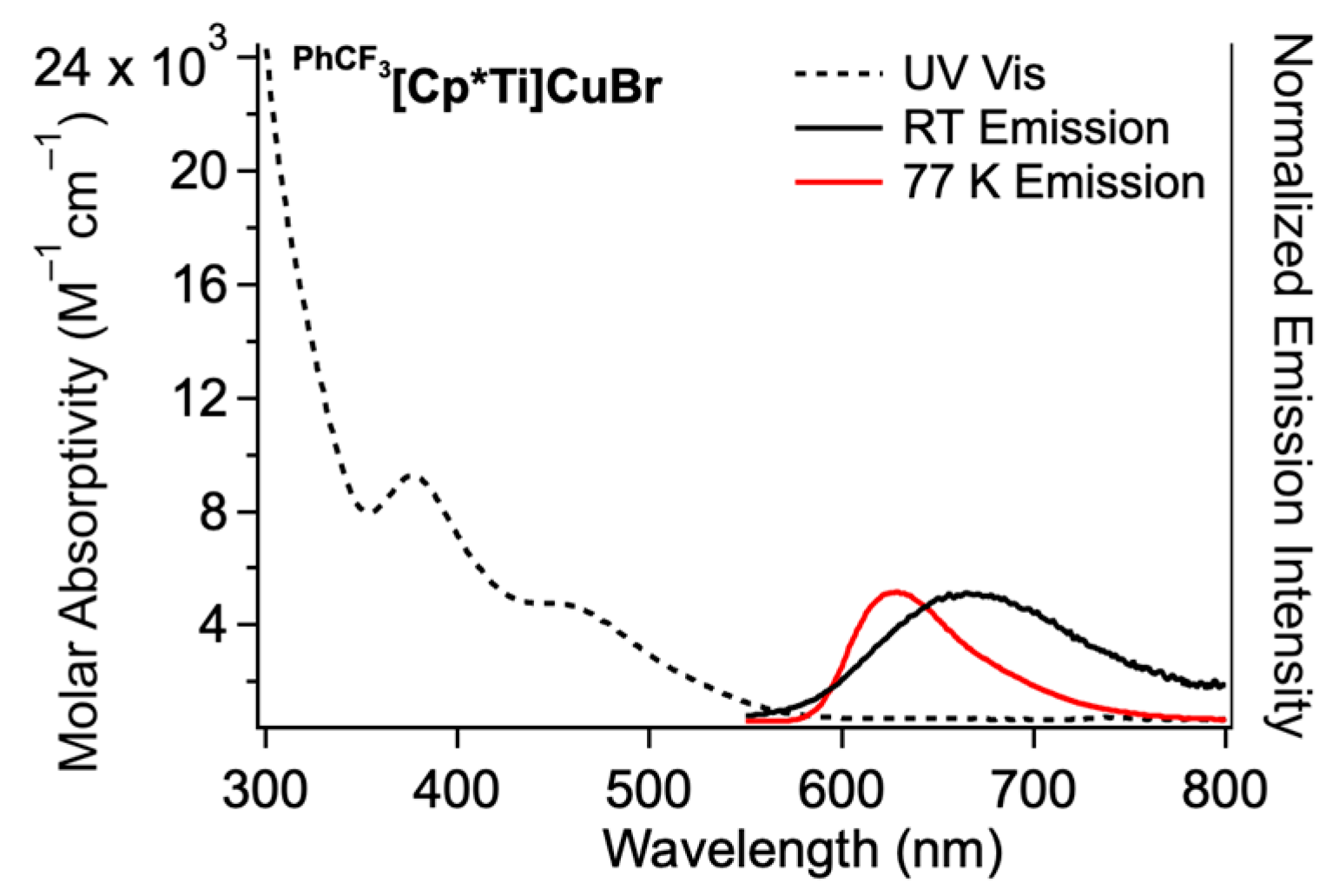


| PhCF3[Cp*Ti]CuBr THF | DMA[Cp*Ti]CuBr | DMA[IndTi]CuBr | DMA[ansa-CpTi]CuBr | |
|---|---|---|---|---|
| empirical formula | C42H46BrCuF6OTi | C40H50BrCuN2Ti | C38H34.19Br0.81CuN2O0.19Ti | C32H34.33Br0.67CuN2O0.33SiTi |
| formula wt. (g/mol) | 872.14 | 750.17 | 698.07 | 645.29 |
| crystal system | triclinic | monoclinic | monoclinic | orthorhombic |
| space group, Z | P-1 | P21/c, 8 | P21/c, 4 | Pna21, 4 |
| temperature (K) | 100(2) | 100(2) | 100(2) | 100(2) |
| a (Å) | 9.4166(4) | 15.5719(8) | 6.6123(5) | 22.7878(15) |
| b (Å) | 13.8760(6) | 16.9073(9) | 23.8625(17) | 9.3747(6) |
| c (Å) | 15.7364(6) | 27.5471(14) | 19.5013(14) | 13.6177(9) |
| α (°) | 75.7461(18) | 90 | 90 | 90 |
| β (°) | 74.2782(18) | 103.5177(17) | 97.482(2) | 90 |
| γ (°) | 74.9301(19) | 90 | 90 | 90 |
| volume (Å3) | 1876.72(14) | 7051.7(6) | 3050.8(4) | 2090.1(3) |
| Dcalc (g/cm3) | 1.543 | 1.413 | 1.520 | 1.473 |
| crystal size (mm) | 0.04 × 0.08 × 0.10 | 0.07 × 0.08 × 0.10 | 0.02 × 0.10 × 0.20 | 0.07 × 0.08 × 0.10 |
| abs. coeff. (mm−1) | 1.907 | 1.993 | 2.051 | 1.992 |
| F(000) | 892 | 3120 | 1428 | 1326 |
| Tmax, Tmin | 1.000, 0.923 | 1.000, 0.951 | 1.000, 0.858 | 1.000, 0.510 |
| Θ range for data (°) | 3.14 to 25.73 | 1.94 to 26.03 | 2.27 to 25.70 | 3.81 to 25.70 |
| reflections coll. | 79,466 | 207,807 | 53,511 | 41,647 |
| data/restr./param. | 7140/0/479 | 13,887/0/839 | 5768/2/399 | 5502/2/354 |
| R(int) | 0.1487 | 0.1311 | 0.1861 | 0.0947 |
| final R [I > 2σ(I)] R1, wR2 | 0.0544, 0.1021 | 0.0465, 0.1024 | 0.0603, 0.1219 | 0.0471, 0.0985 |
| final R (all data) R1, wR2 | 0.0797, 0.1220 | 0.0954, 0.1253 | 0.1223, 0.1510 | 0.0632, 0.1075 |
| goodness-of-fit on F2 | 1.067 | 1.015 | 1.015 | 1.033 |
| larg. diff. peak, hole (eÅ−3) | 0.780, −0.641 | 1.243, −1.103 | 0.862, −0.780 | 0.738, −0.320 |
| CCDC Deposition No. | 2475385 | 2475386 | 2475387 | 2475388 |
| Ph[Cp*Ti]CuBr [30] | PhCF3[Cp*Ti]CuBr | DMA[Cp*Ti]CuBr a | DMA[IndTi]CuBr | DMA[ansa-CpTi]CuBr | ||
|---|---|---|---|---|---|---|
| (1) | (2) | |||||
| ∠ Cp–Ti–Cp b | 141.6° | 141.23(19)° | 141.24(15)° | 141.46(15)° | 135.7(2)° | 131.5(3)° |
| ∠ C1–Ti–C4 | 88.0(4)° | 88.93(16)° | 92.89(15)° | 91.48(15)° | 92.6(2)° | 92.5(3)° |
| ∠ Ti–CαC | 172.0(7)° | 167.8(4)°, 169.6(4)° | 168.8(3)°, 167.5(3)° | 167.9(3)°, 169.3(3)° | 171.9(5)°, 165.0(5)° | 176.6(7)°, 165.3(6)° |
| ∠ C≡C–C | 160.1(9)° | 158.1(4)°, 165.5 (4)° | 173.9(4)°, 170.1(4)° | 172.3(4)°, 168.8(4)° | 167.3(6)°, 168.1(6)° | 172.8(8)°, 162.6(7)° |
| ∠ Ti–Cu–Br | 180° | 173.77(3)° | 176.17(3)° | 166.10(3)° | 177.07(5)° | 168.91(12)° |
| Ti–Cp c (Å) | 2.04(2) | 2.099(4), 2.095(4) | 2.099(4), 2.104(4) | 2.104(4), 2.100(4) | 2.083(6), 2.072(6) | 2.054(7), 2.065(7) |
| Ti–CC≡C (Å) | 2.080(8) | 2.084(4), 2.093(4) | 2.090(4), 2.090(4) | 2.085(4), 2.082(4) | 2.081(6), 2.081(6) | 2.069(8), 2.090(8) |
| Dihedral d | 31.1(7)° | 64.90(11)°, 19.6(2)° | 70.41(11)°, 83.18(12)° | 68.42(12)°, 88.12(12)° | 84.75(13)°, 78.55(15)° | 82.36(18)°, 22.01(12)° |
| Me/Cp dev e (Å) | 0.20 | 0.22 | 0.20 | 0.20 | - | - |
| λmax 77 K | τ 77 K (μs) | λmax RT | τ RT (μs) | ΦPL RT a | |
|---|---|---|---|---|---|
| DMA[Cp*Ti]CuBr | 679 nm | 124 b | 744 nm | 0.16 c | 3.7 × 10−3 |
| Ph[Cp*Ti]CuBr d | 619 nm | 655 | 693 nm | 0.18 | 1.3 × 10−3 |
| PhCF3[Cp*Ti]CuBr | 629 nm | 1130 b | 635 nm | – e | 4.5 × 10−4 |
| Complex (% HOMO-LUMO) | λ(nm) b | Ti | Cp* | C2Aryl | CuBr |
|---|---|---|---|---|---|
| DMA[Cp*Ti]CuBr (93%) | 687 | 1 → 49 (48) | 3 → 8 (5) | 83 → 38 (−45) | 14 → 5 (−9) |
| Ph[Cp*Ti]CuBr c (80%) | 577 | 3 → 50 (47) | 27 → 8 (−19) | 39 → 35 (−4) | 31 → 7 (−24) |
| PhCF3[Cp*Ti]CuBr (88%) | 591 | 10 → 49 (39) | 56 → 8 (−48) | 26 → 34 (8) | 8 → 8 (0) |
| Parent | CuBr Complex | |||
|---|---|---|---|---|
| λmax 77 K | Φdecomp RT a | λmax 77 K | Φdecomp RT a | |
| DMA[ansa-CpTi] | 675 nm | 0.15 | 770 nm | <1 × 10−4 |
| DMA[IndTi] | 705 nm | 0.24 | 793 nm | 3.6 × 10−4 |
| DMA[Cp*Ti] | 633 nm | 0.36 | 679 nm | 5.1 × 10−4 |
| PhCF3[Cp*Ti] | 644 nm | 0.48 | 626 nm | 6.1 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barker, M.; Walter, S.C.; McCallum, E.A.; Golden, R.S.; Zimmerman, J.H.; McCarthy, J.S.; McMillen, C.D.; Wagenknecht, P.S. Luminescent Arylalkynyltitanocenes: Effect of Modifying the Electron Density at the Arylalkyne Ligand, or Adding Steric Bulk or Constraint to the Cyclopentadienyl Ligand. Crystals 2025, 15, 745. https://doi.org/10.3390/cryst15080745
Barker M, Walter SC, McCallum EA, Golden RS, Zimmerman JH, McCarthy JS, McMillen CD, Wagenknecht PS. Luminescent Arylalkynyltitanocenes: Effect of Modifying the Electron Density at the Arylalkyne Ligand, or Adding Steric Bulk or Constraint to the Cyclopentadienyl Ligand. Crystals. 2025; 15(8):745. https://doi.org/10.3390/cryst15080745
Chicago/Turabian StyleBarker, Matilda, Samantha C. Walter, Elizabeth A. McCallum, River S. Golden, John H. Zimmerman, Jackson S. McCarthy, Colin D. McMillen, and Paul S. Wagenknecht. 2025. "Luminescent Arylalkynyltitanocenes: Effect of Modifying the Electron Density at the Arylalkyne Ligand, or Adding Steric Bulk or Constraint to the Cyclopentadienyl Ligand" Crystals 15, no. 8: 745. https://doi.org/10.3390/cryst15080745
APA StyleBarker, M., Walter, S. C., McCallum, E. A., Golden, R. S., Zimmerman, J. H., McCarthy, J. S., McMillen, C. D., & Wagenknecht, P. S. (2025). Luminescent Arylalkynyltitanocenes: Effect of Modifying the Electron Density at the Arylalkyne Ligand, or Adding Steric Bulk or Constraint to the Cyclopentadienyl Ligand. Crystals, 15(8), 745. https://doi.org/10.3390/cryst15080745





