Dispersion of Sintered Mg-Ni-Ce Materials for Efficient Hydrogen Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Composition and Ratio of Alloying Components
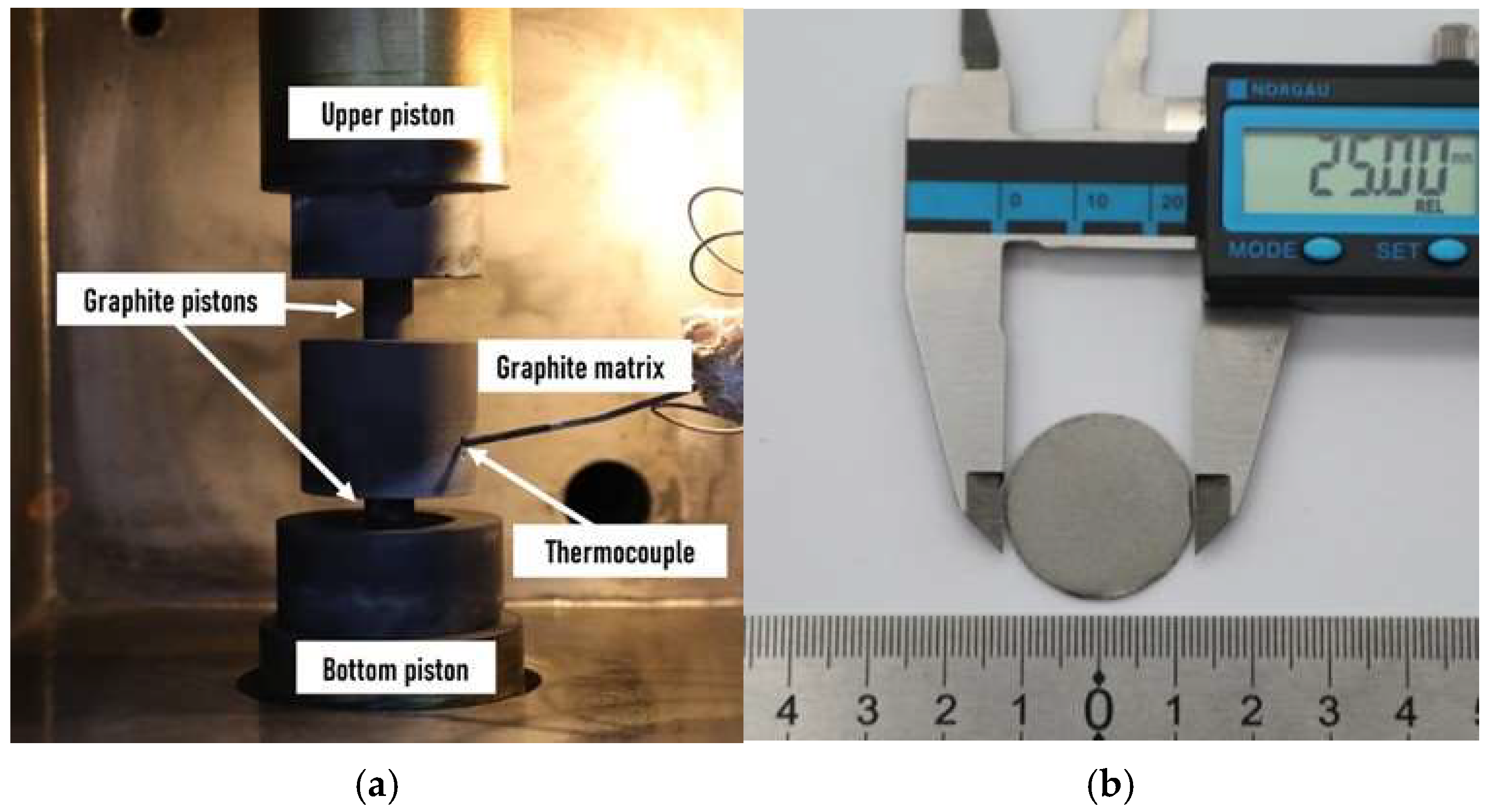
2.2. Synthesis, Sintering and Dispersion of Materials of the Mg-Ni-Ce System
2.3. Research Methods
3. Results and Discussion
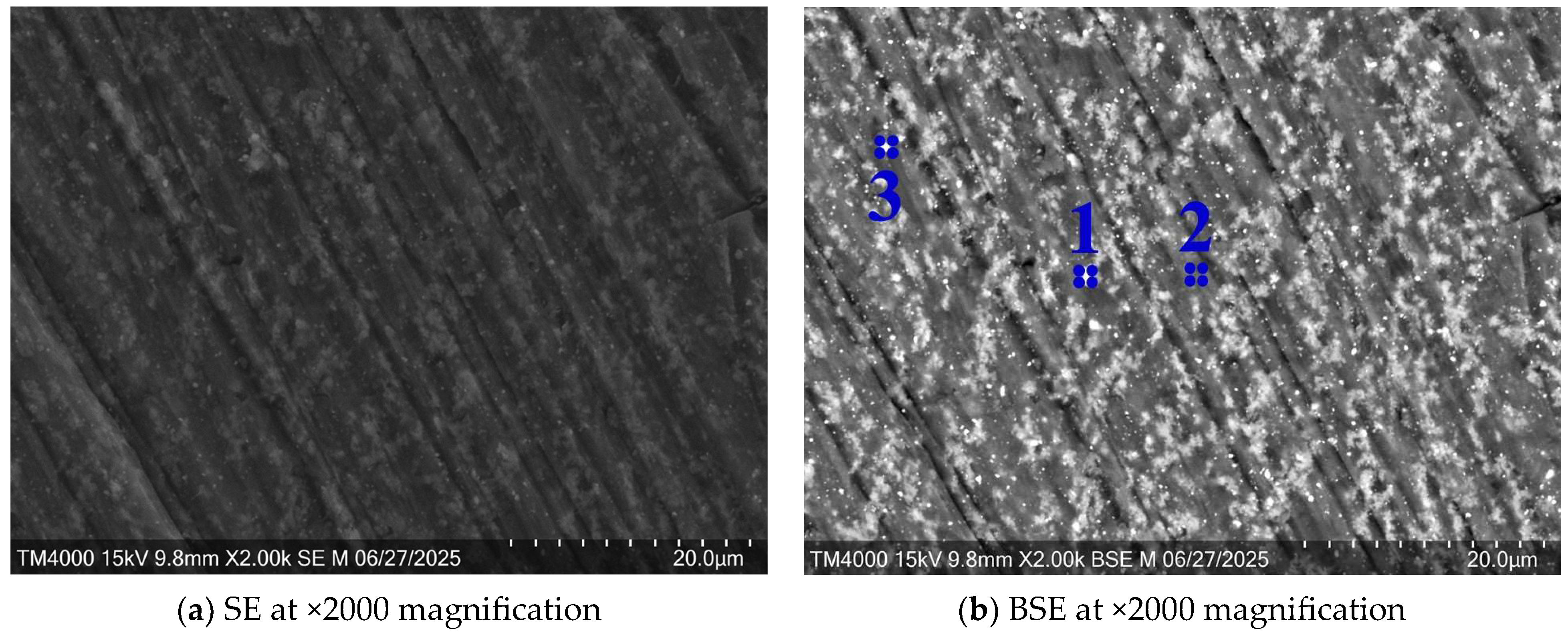
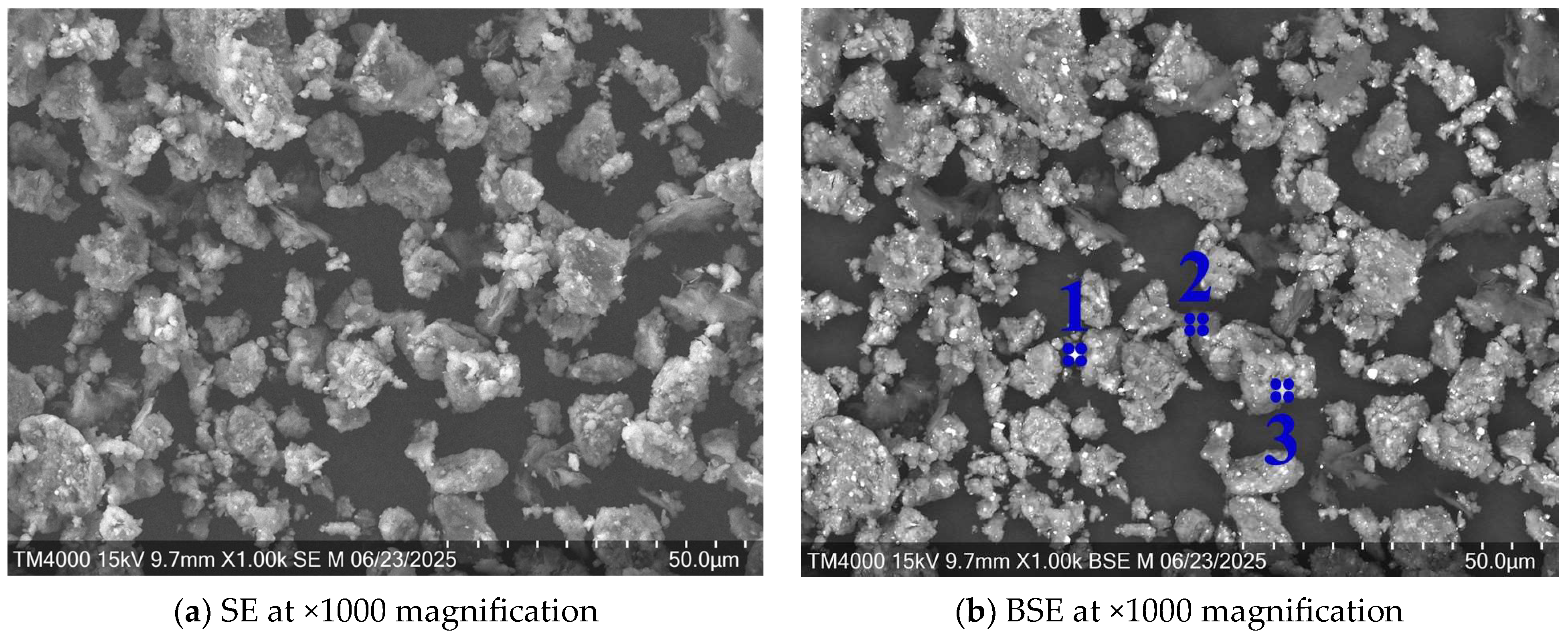
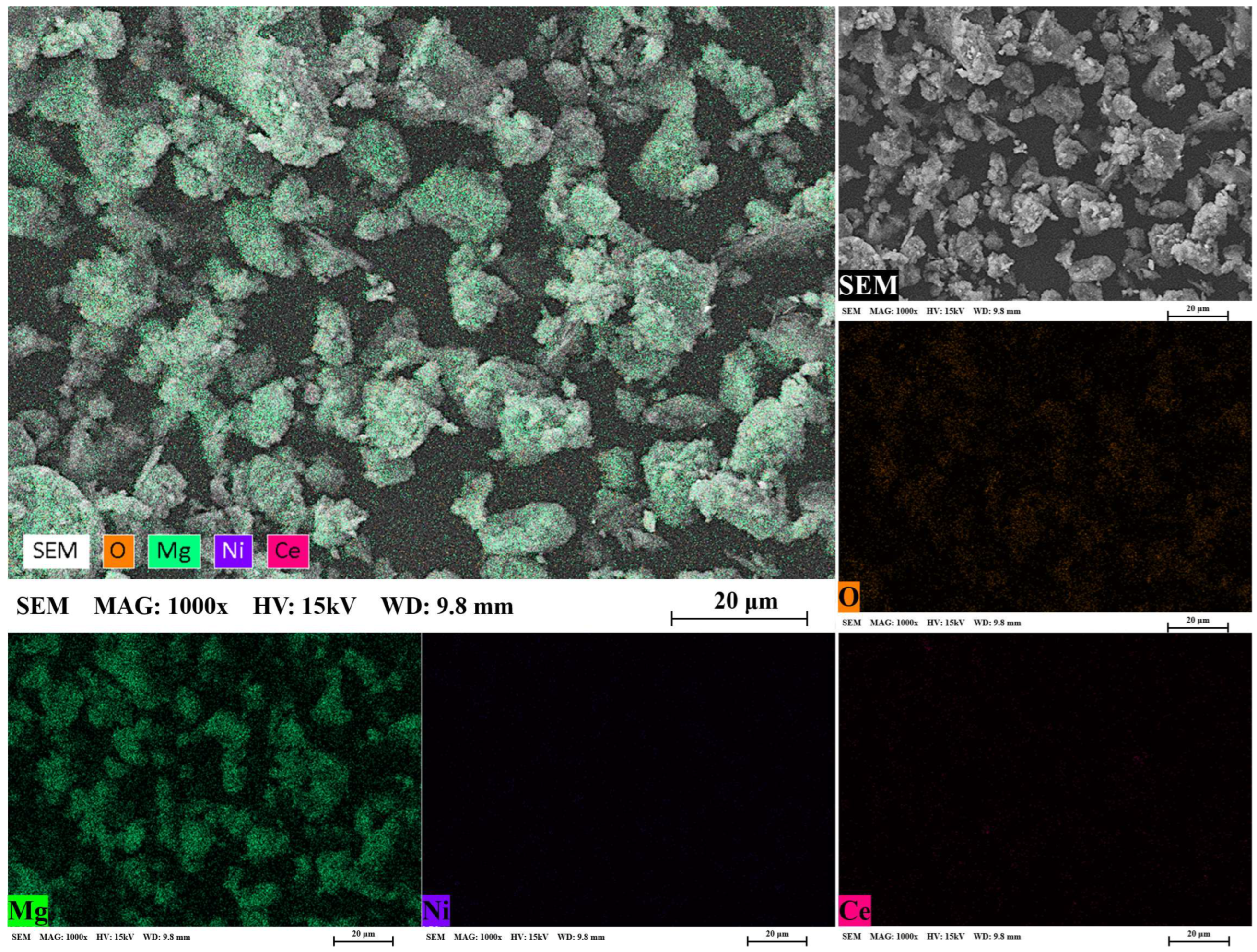
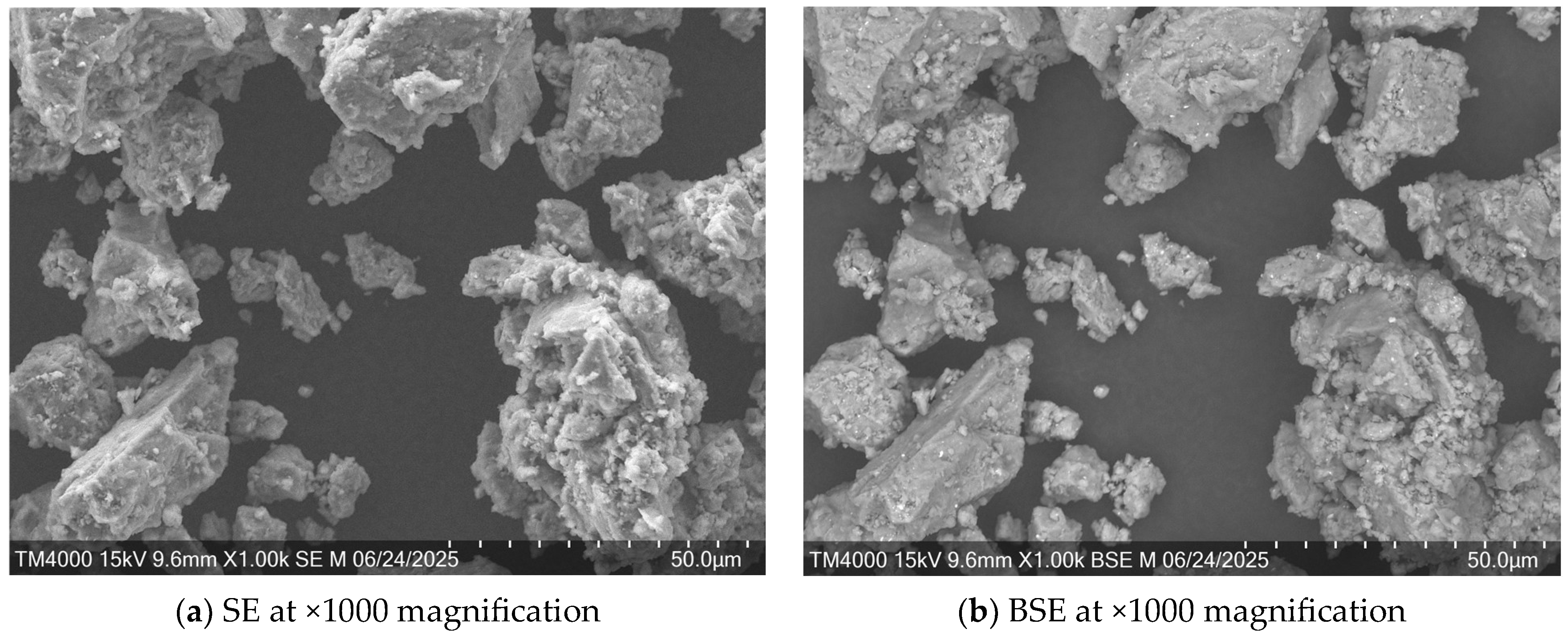
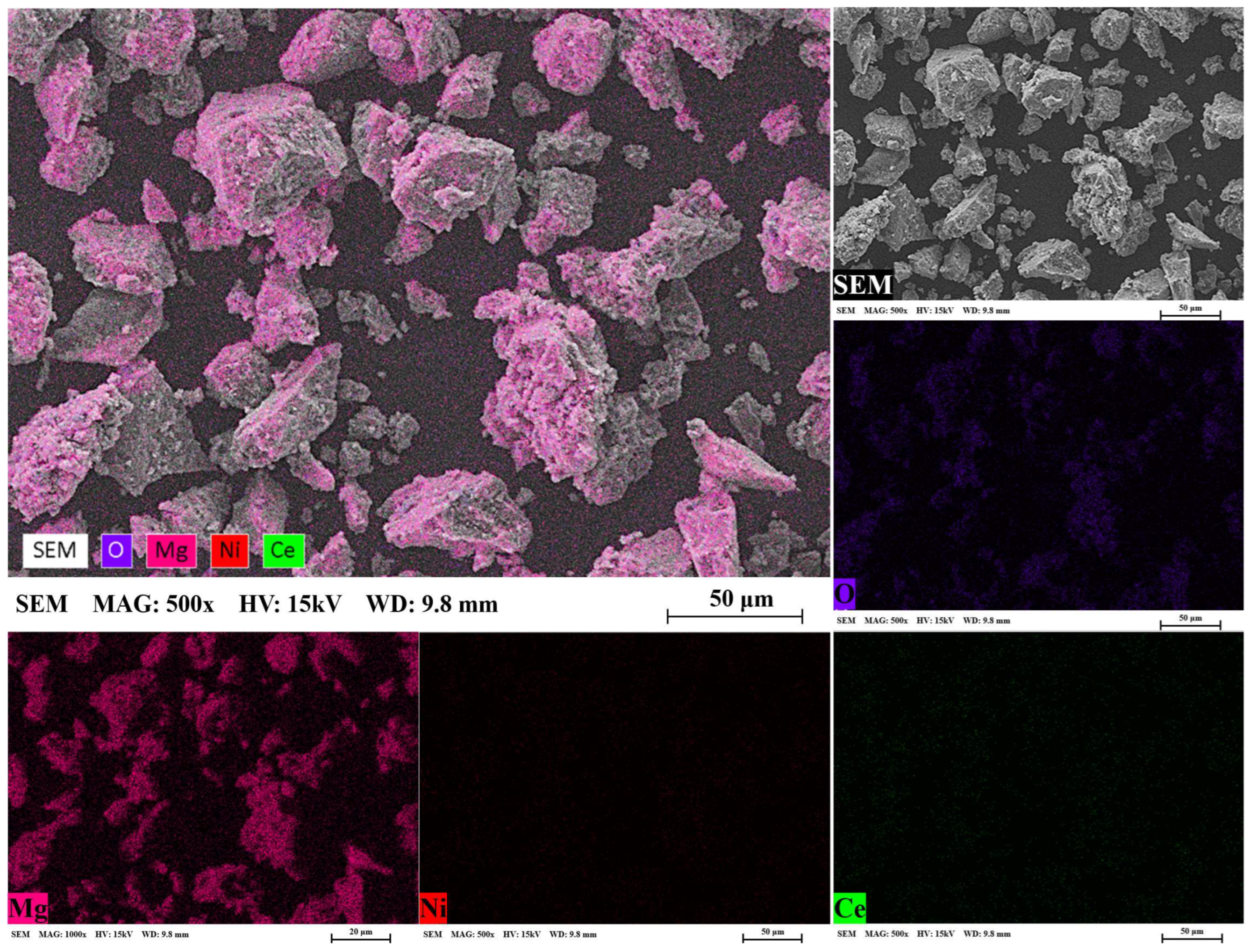
3.1. Microstructural Analysis
3.2. X-Ray Phase Analysis
3.3. Specific Surface Area Analysis
3.4. Hydrogen Sorption Kinetics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moradi, R.; Growth, K.M. Hydrogen storage and delivery: Review of the state of the art technologies and risk and reliability analysis. Int. J. Hydrogen Energy 2019, 44, 12254–12269. [Google Scholar] [CrossRef]
- Sadeq, A.M. Advances and Challenges in Hydrogen Energy: A Review. Chem. Eng. Process Tech. 2024, 9, 1087. [Google Scholar]
- Kyriakopoulos, G.L.; Aravossis, K.G. Literature Review of Hydrogen Energy Systems and Renewable Energy Sources. Energies 2023, 16, 7493. [Google Scholar] [CrossRef]
- Nyangon, J.; Darekar, A. Advancements in hydrogen energy systems: A review of levelized costs, financial incentives and technological innovations. Innov. Green Dev. 2024, 3, 100149. [Google Scholar] [CrossRef]
- Strategy for Achieving Carbon Neutrality of the Republic of Kazakhstan Until 2060. Available online: https://adilet.zan.kz/rus/docs/U2300000121 (accessed on 14 July 2025).
- Hwang, H.T.; Varma, A. Hydrogen storage for fuel cell vehicles. Curr. Opin. Chem. Eng. 2014, 5, 42–48. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. Review and evaluation of hydrogen production options for better environment. J. Clean. Prod. 2019, 218, 835–849. [Google Scholar] [CrossRef]
- Paul, A.R.; Mehla, S.; Bhargava, S. Intermetallic Compounds for Hydrogen Storage: Current Status and Future Perspectives. Small 2025, 21, e2408889. [Google Scholar] [CrossRef]
- Yartys, V.A.; Lototskyy, M.V. Laves type intermetallic compounds as hydrogen storage materials: A review. J. Alloys Compd. 2022, 916, 165219. [Google Scholar] [CrossRef]
- Lys, A.; Fadonougbo, J.O.; Faisal, M.; Suh, J.-Y.; Lee, Y.-S.; Shim, J.-H.; Cho, Y.W. Enhancing the Hydrogen Storage Properties of AxBy Intermetallic Compounds by Partial Substitution: A Short Review. Hydrogen 2020, 1, 38–63. [Google Scholar] [CrossRef]
- Liu, Y.; Chabane, D.; Elkedim, O. Intermetallic Compounds Synthesized by Mechanical Alloying for Solid-State Hydrogen Storage: A Review. Energies 2021, 14, 5758. [Google Scholar] [CrossRef]
- Ley, M.B.; Jepsen, L.H.; Lee, Y.S.; Cho, Y.W.; Von Colbe, J.M.B.; Dornheim, M.; Rokni, M.; Jensen, J.O.; Sloth, M.; Filinchuk, Y.; et al. Complex hydrides for hydrogen storage—New perspectives. Mater. Today 2014, 17, 122–128. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, Y.; Li, Y.; Zheng, Y. Bridging Materials and Analytics: A Comprehensive Review of Characterization Approaches in Metal-Based Solid-State Hydrogen Storage. Molecules 2023, 29, 5014. [Google Scholar] [CrossRef]
- Xie, Z.; Jin, Q.; Su, G.; Lu, W. A Review of Hydrogen Storage and Transportation: Progresses and Challenges. Energies 2024, 17, 4070. [Google Scholar] [CrossRef]
- Sun, Y.; Shen, C.; Lai, Q.; Liu, W.; Wang, D.-W.; Aguey-Zinsou, K.-F. Tailoring magnesium based materials for hydrogen storage through synthesis: Current state of the art. Energy Storage Mater. 2018, 10, 168–198. [Google Scholar] [CrossRef]
- Leiva, D.R.; Jorge, A.M., Jr.; Ishikawa, T.T.; Botta, W.J. Hydrogen Storage in Mg and Mg-Based Alloys and Composites Processed by Severe Plastic Deformation. Mater. Trans. 2019, 60, 1561–1570. [Google Scholar] [CrossRef]
- Yartys, V.A.; Lototskyy, M.V.; Akiba, E.; Albert, R.; Antonov, V.E.; Ares, J.R.; Baricco, M.; Bourgeois, N.; Buckley, C.E.; Bellosta von Colbe, J.M.; et al. Magnesium based materials for hydrogen based energy storage: Past, present and future. Int. J. Hydrogen Energy 2019, 44, 7809–7859. [Google Scholar] [CrossRef]
- Bastide, J.-P.; Bonnetot, B.; Létoffé, J.-M.; Claudy, P. Polymorphisme de l’hydrure de magnesium sous haute pression. Mater. Res. Bull. 1980, 15, 1215–1224. [Google Scholar] [CrossRef]
- Baran, A.; Jensen, T.R.; Polanski, M. High-temperature high-pressure reactive ball milling synthesis of Mg-Ni-based solid-state hydrogen storage materials. J. Energy Storage 2024, 103, 114271. [Google Scholar] [CrossRef]
- Au, M. Hydrogen storage properties of magnesium based nanostructured composite materials. Mater. Sci. Eng. B 2005, 117, 37–44. [Google Scholar] [CrossRef]
- Song, M.; Lee, J.Y. ИccлeдoвaHиe киHeтики гидpиpoвaHия Mg-(10–20 w/o) LaNi5. Int. J. Hydrogen Energy 1983, 8, 363–367. [Google Scholar] [CrossRef]
- Baraban, A.P.; Chernov, I.A.; Dmitriev, V.A.; Elets, D.I.; Gabis, I.E.; Kuznetsov, V.G.; Voyt, A.P. The Mg2NiH4 film on nickel substrate: Synthesis, properties and kinetics of formation. Thin Solid Films 2022, 762, 139556. [Google Scholar] [CrossRef]
- Floriano, R.; Leiva, D.R.; Deledda, S.; Hauback, B.C.; Botta, W.J. Cold rolling of MgH2 powders containing different additives. Int. J. Hydrogen Energy 2013, 38, 16193–16198. [Google Scholar] [CrossRef]
- Xie, L.; Li, J.; Zhang, T.; Kou, H. De/Hydrogenation Kinetics against Air Exposure and Microstructure Evolution during Hydrogen Absorption/Desorption of Mg-Ni-Ce Alloys. Renew. Energy 2017, 113, 1399–1407. [Google Scholar] [CrossRef]
- Xie, L.; Li, J.; Zhang, T.; Song, L.; Kou, H. Microstructure and Hydrogen Storage Properties of Mg-Ni-Ce Alloys with a Long-Period Stacking Ordered Phase. J. Power Sources 2017, 338, 91–102. [Google Scholar] [CrossRef]
- Qi, Y.; Sheng, P.; Sun, H.; Li, J.; Zhang, W.; Guo, S.; Zhao, D.; Zhang, Y. Hydrogen Storage Thermodynamics and Kinetics of the As-Cast and Milled Ce-Mg-Ni-Based Alloy. Mater. Today Commun. 2023, 35, 106217. [Google Scholar] [CrossRef]
- Hou, Z.; Hua, M.; Liu, Y.; Deng, J.; Zhou, X.; Feng, Y.; Li, Y.; Dai, H. Exploring Intermetallic Compounds: Properties and Applications in Catalysis. Catalysts 2024, 14, 538. [Google Scholar] [CrossRef]
- Sun, B.; Li, S.; Imai, H.; Umeda, J.; Kondoh, K. Synthesis kinetics of Mg2Si and solid-state formation of Mg–Mg2Si composite. Powder Technol. 2012, 217, 157–162. [Google Scholar] [CrossRef]
- Ralls, A.M.; Daroonparvar, M.; Menezes, P.L. Spark Plasma Sintering of Mg-Based Alloys: Microstructure, Mechanical Properties, Corrosion Behavior, and Tribological Performance. J. Magnes. Alloys 2024, 12, 405–442. [Google Scholar] [CrossRef]
- Filipović, S.; Obradović, N.; Fahrenholtz, W.G.; Smith, S.; Mirković, M.; Peles Tadić, A.; Petrović, J.; Đorđević, A. Spark Plasma Sintering of Magnesium Titanate Ceramics. Ceram. Int. 2024, 50, 15283–15291. [Google Scholar] [CrossRef]
- Li, G.; Meng, X.; Geng, C.; Wang, C.; Ren, H.; Guo, X.; Li, S.; Tao, Y. Microstructure and Properties of AlxCr1−xCoFeNi High-Entropy Alloys Prepared by Spark Plasma Sintering. Materials 2025, 18, 755. [Google Scholar] [CrossRef]
- Somasundaram, M.; Uttamchand, N.K.; Annamalai, A.R.; Jen, C.-P. Insights on Spark Plasma Sintering of Magnesium Composites: A Review. Nanomaterials 2022, 12, 2178. [Google Scholar] [CrossRef]
- Mukhamedova, N.M.; Miniyazov, A.Z.; Zhanbolatova, G.K.; Ospanova, Z.N.; Sabyrtayeva, A.A.; Shaikieva, K.S. Evolution of Phase Transformations in the Mg-Ni-Ce System After Mechanical Synthesis and Spark Plasma Sintering. Materials 2025, 18, 2131. [Google Scholar] [CrossRef]
- Zou, J.; Sun, H.; Zeng, X.; Ji, G.; Ding, W. Preparation and Hydrogen Storage Properties of Mg-Rich Mg-Ni Ultrafine Particles. J. Nanomater. 2012, 2012, 592147. [Google Scholar] [CrossRef]
- Jurczyk, M.; Smardz, L.; Okonska, I.; Jankowska, E.; Nowak, M.; Smardz, K. Nanoscale Mg-based materials for hydrogen storage. Int. J. Hydrogen Energy 2008, 33, 374–380. [Google Scholar] [CrossRef]
- Zaluski, L.; Zaluska, A.; Strom-Olsen, J.O. Nanocrystalline Metal Hydrides. J. Alloys Compd. 1997, 253–254, 70–79. [Google Scholar] [CrossRef]
- Huot, J.; Liang, G.; Boily, S.; Van Neste, A.; Schulz, R. Structural Study and Hydrogen Sorption Kinetics of Ball-Milled Magnesium Hydride. J. Alloys Compd. 1999, 293–295, 495–500. [Google Scholar] [CrossRef]
- Arf, A.N.; Kareem, F.A.; Gul, S.S. Dispersion and Homogeneity of MgO and Ag Nanoparticles Mixed with Polymethylmethacrylate. Polymers 2023, 15, 1479. [Google Scholar] [CrossRef]
- Lesz, S.; Karolus, M.; Gabryś, A.; Kremzer, M. Influence of Milling Time on Phase Composition and Product Structure of Mg-Zn-Ca-Ag Alloys Obtained by Mechanical Synthesis. Materials 2022, 15, 7333. [Google Scholar] [CrossRef]
- Rios, J.; Restrepo, A.; Zuleta, A.; Bolívar, F.; Castaño, J.; Correa, E.; Echeverria, F. Effect of Ball Size on the Microstructure and Morphology of Mg Powders Processed by High-Energy Ball Milling. Metals 2021, 11, 1621. [Google Scholar] [CrossRef]
- Skakov, M.; Kozhakhmetov, Y.; Mukhamedova, N.; Miniyazov, A.; Sokolov, I.; Urkunbay, A.; Zhanbolatova, G.; Tulenbergenov, T. Effect of a High-Temperature Treatment on Structural-Phase State and Mechanical Properties of IMC of the Ti-25Al-25Nb at.% System. Materials 2022, 15, 5560. [Google Scholar] [CrossRef]
- Mukhamedova, N.M.; Ospanova, J.N.; Oken, O.; Miniyazov, A.Z.H.; Shaikieva, K.S.; Sabyrtaeva, A.A.; Akhmedi, T.D. Assessment of the Influence of Mechanosynthesis Parameters on the Morphology of a Magnesium-Based Powder Mixture. Bull. NNC RK 2025, 1, 64–71. [Google Scholar] [CrossRef]
- Li, J.; Xie, L.; Zhang, T.; Song, L. Microstructure and Absorption/Desorption Kinetics Evolutions of Mg-Ni-Ce Alloys during Hydrogenation and Dehydrogenation Cycles. Int. J. Hydrogen Energy 2018, 43, 8404–8414. [Google Scholar] [CrossRef]
- Kwak, Y.J.; Park, H.R.; Song, M.Y. Changes in Microstructure, Phases, and Hydrogen Storage Characteristics of Metal Hydro-Borate and Nickel-Added Magnesium Hydride with Hydrogen Absorption and Release Reactions. Int. J. Hydrogen Energy 2017, 42, 1018–1026. [Google Scholar] [CrossRef]
- International Centre for Diffraction Data (ICDD). PDF-4 Axiom, 2025; ICDD: Newtown Square, PA, USA, 2025; Available online: https://www.icdd.com/pdf-4-axiom/ (accessed on 1 August 2025).
- Olivero, J.J.; Longbothum, R.L. Empirical fits to the Voigt line width: A brief review. J. Quant. Spectrosc. Radiat. Transf. 1977, 17, 233–236. [Google Scholar] [CrossRef]
- Hou, X.; Wang, Y.; Hou, K.; Yang, L.; Shi, H.; Feng, L.; Suo, G.; Ye, X.; Zhang, L.; Yang, Y. Outstanding Hydrogen Production Properties of Surface Catalysts Promoted Mg–Ni–Ce Composites at Room Temperature in Simulated Seawater. J. Mater. Sci. 2020, 55, 14675–14687. [Google Scholar] [CrossRef]
- Lin, H.J.; Ouyang, L.Z.; Wang, H.; Zhao, D.Q.; Wang, W.H.; Sun, D.L.; Zhu, M. Hydrogen Storage Properties of Mg–Ce–Ni Nanocomposite Induced from Amorphous Precursor with the Highest Mg Content. Int. J. Hydrogen Energy 2012, 37, 14329–14335. [Google Scholar] [CrossRef]
- Meng, J.; Wang, X.-L.; Chou, K.-C.; Li, Q. Hydrogen Storage Properties of Graphite-Modified Mg-Ni-Ce Composites Prepared by Mechanical Milling Followed by Microwave Sintering. Metall. Mater. Trans. A 2013, 44, 58–67. [Google Scholar] [CrossRef]
- Hwang, S.; Nishimura, C.; McCormick, P. Mechanical Milling of Magnesium Powder. Mater. Sci. Eng. A 2001, 318, 22–33. [Google Scholar] [CrossRef]
- Ou, K.-L.; Chen, C.-C.; Chiu, C. Production of Oxide Dispersion Strengthened Mg–Zn–Y Alloy by Equal Channel Angular Pressing of Mechanically Alloyed Powder. Metals 2020, 10, 679. [Google Scholar] [CrossRef]
- Pradeep, N.B.; Hegde, M.M.R.; Patel, G.C.M.; Giasin, K.; Pimenov, D.Y.; Wojciechowski, S. Synthesis and Characterization of Mechanically Alloyed Nanostructured Ternary Titanium-Based Alloy for Bio-Medical Applications. J. Mater. Res. Technol. 2021, 16, 88–101. [Google Scholar] [CrossRef]
- Lei, J.P.; Huang, H.; Dong, X.L.; Sun, J.P.; Lu, B.; Lei, M.K.; Cao, G.Z. Formation and Hydrogen Storage Properties of In Situ Prepared Mg–Cu Alloy Nanoparticles by Arc Discharge. Int. J. Hydrogen Energy 2009, 34, 8127–8134. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kim, H.-A.; Lee, A.-Y.; Kim, D.-H.; Lim, H.; Lee, M.-H. Synthesis of Bulk Nanostructured Mg-Based Alloy via Spark Plasma Sintering through In Situ Crystallization of an Amorphous Precursor. Mater. Res. Express 2021, 8, 055201. [Google Scholar] [CrossRef]
- Jain, I.P.; Lal, C.; Jain, A. Hydrogen Storage in Mg: A Most Promising Material. Int. J. Hydrogen Energy 2010, 35, 5133–5144. [Google Scholar] [CrossRef]
- Palacios-Lazcano, A.F.; Luna-Sanchez, J.L.; Cruz-Gandarilla, F.; Cabanas-Moreno, J.G. Microstructural Study of Mg-Zn Alloys Prepared by Mechanical Alloying. Rev. Mex. Fis. 2007, 53, 72–77. [Google Scholar]
- Kakade, P.M.; Kachere, A.R.; Mandlik, N.T.; Rondiya, S.R.; Jadkar, S.R.; Bhosale, S.V. Graphene Oxide Assisted Synthesis of Magnesium Oxide Nanorods. ES Mater. Manuf. 2021, 12, 63–71. [Google Scholar] [CrossRef]
- Huot, J.; Liang, G.; Schulz, R. Mechanically Alloyed Metal Hydride Systems. Appl. Phys. A 1999, 69, 403–408. [Google Scholar] [CrossRef]
- Yang, X.; Lu, X.; Zhang, J.; Hou, Q.; Zou, J. Progress in Improving Hydrogen Storage Properties of Mg-Based Materials. Mater. Today Adv. 2023, 19, 100387. [Google Scholar] [CrossRef]
- Bhat, V.; Rougier, A.; Aymard, L.; Nazri, G.; Tarascon, J. Enhanced Hydrogen Storage Property of Magnesium Hydride by High Surface Area Raney Nickel. Int. J. Hydrogen Energy 2007, 32, 4900–4906. [Google Scholar] [CrossRef]
- López Gómez, E.I.; Gonzalez, J.; Cubero-Sesin, J.M.; Huot, J. Synthesis of Nanostructured Mg2Ni for Hydrogen Storage by Mechanical Alloying via High-Pressure Torsion. Reactions 2024, 5, 651–663. [Google Scholar] [CrossRef]

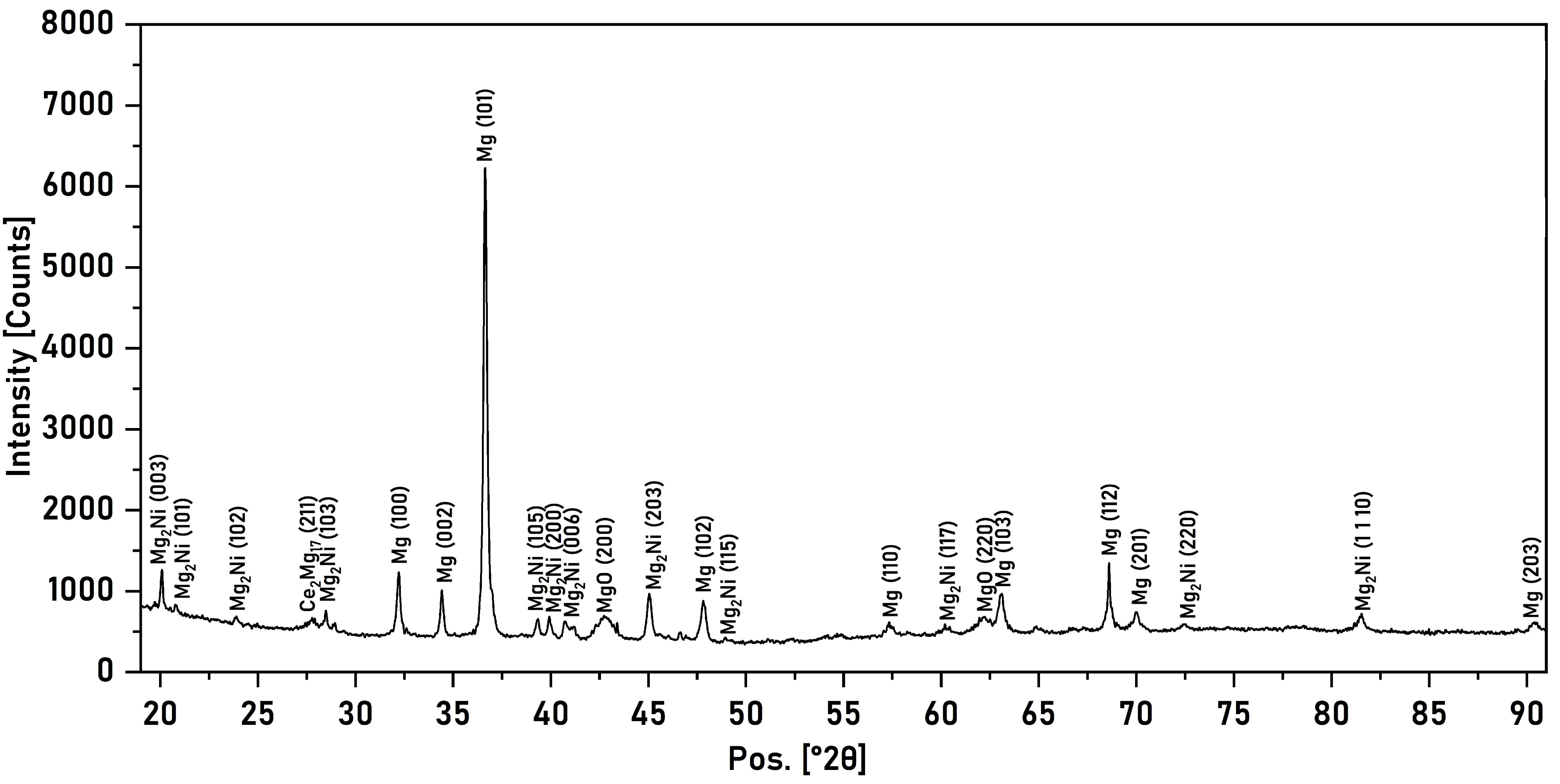
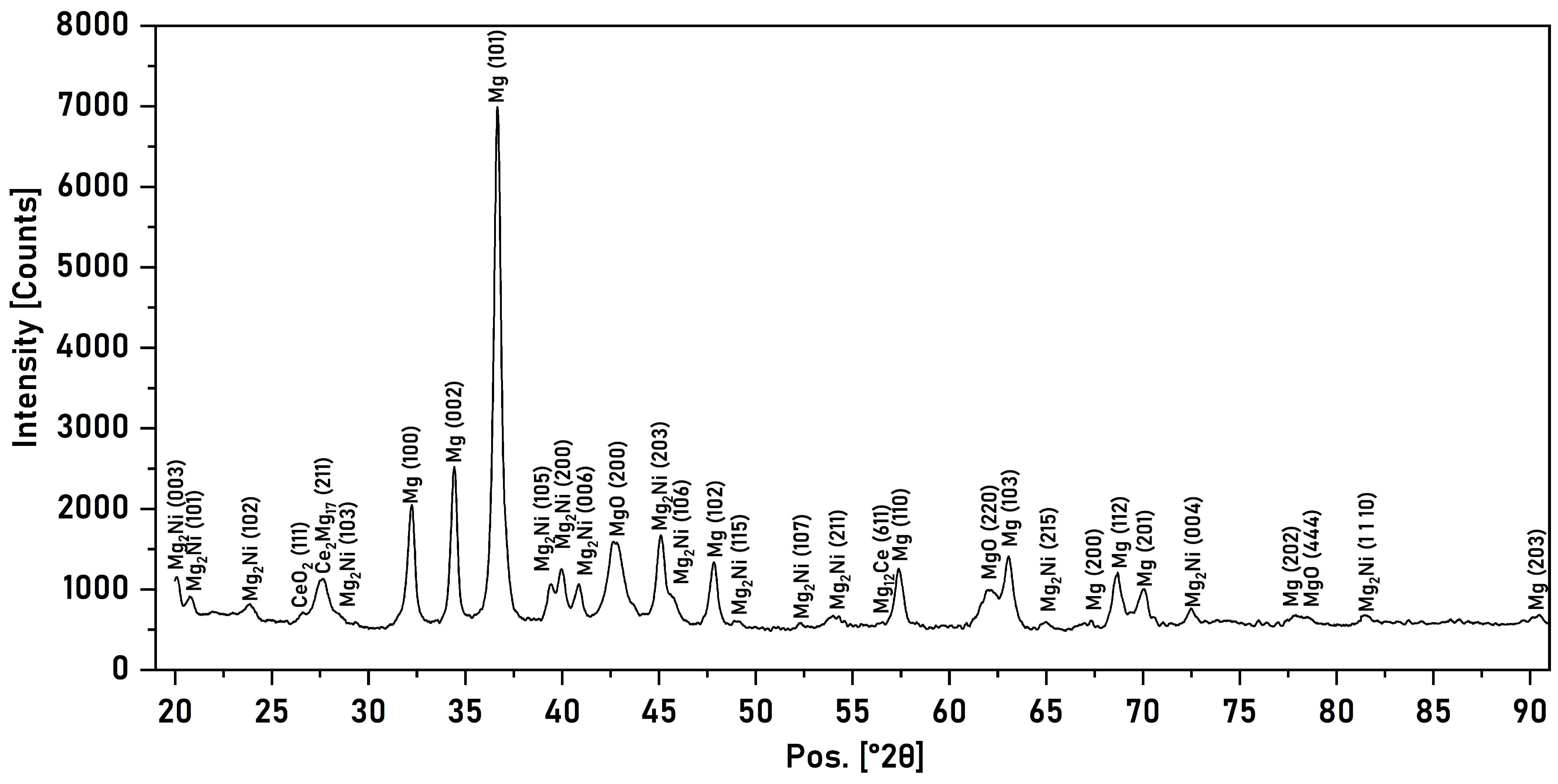
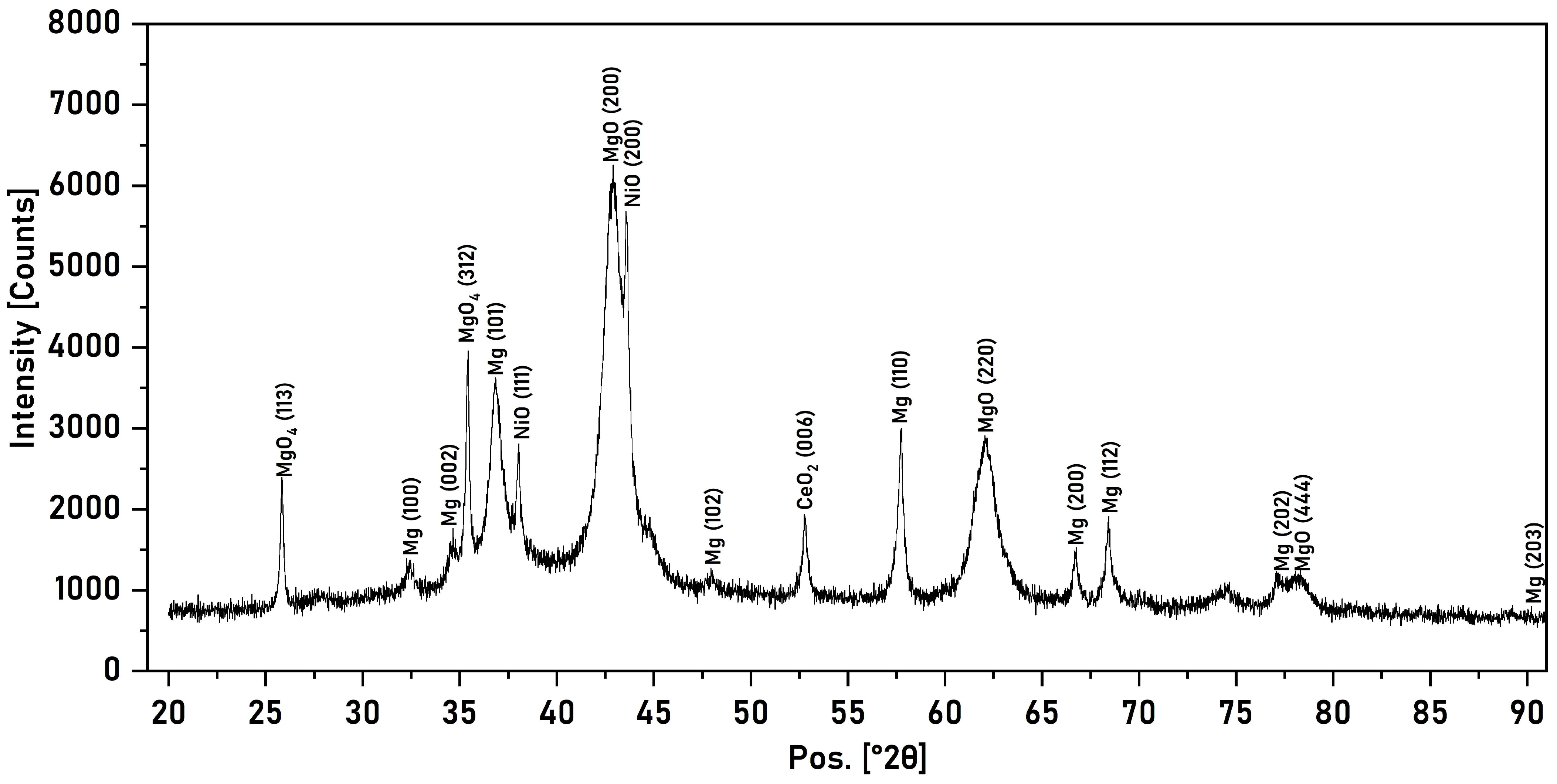
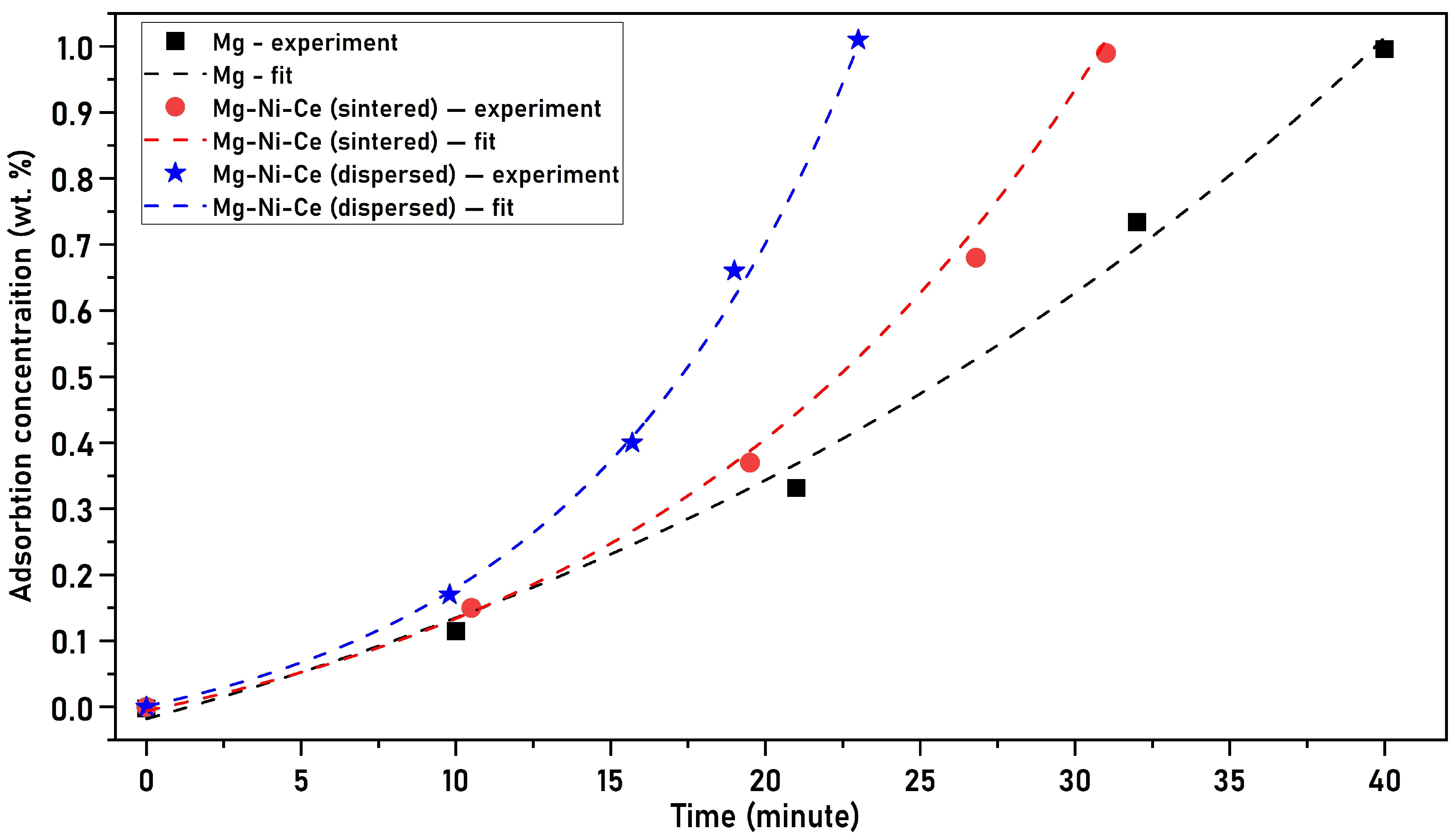
| Material | Phase | Lattice Parameter (a, Å) | Lattice Parameter (c, Å) | Microdeformation (ε) | Crystallite Size (D, HM) |
|---|---|---|---|---|---|
| Sintered by SPS method | Mg | 3.2159 ± 0.0002 | 5.2208 ± 0.00015 | 0.00073 ± 0.00021 | 48.28 ± 0.015 |
| Mg2Ni | 5.2284 ± 0.00015 | 13.3397 ± 0.00017 | 0.00262 ± 0.0002 | 61.34 ± 0.018 | |
| After dispersion | |||||
| 1 h | Mg | 3.2152 ± 0.00014 | 5.2234 ± 0.00015 | 0.00144 ± 0.00017 | 37.77 ± 03013 |
| Mg2Ni | 5.2368 ± 0.00021 | 13.3311 ± 0.00016 | 0.00298 ± 0.00018 | 33.67 ± 0.001 | |
| 2 h | Mg | 3.3571 ± 0.00023 | 5.4312 ± 0.00015 | 0.02365 ± 0.00023 | 32.24 ± 0.002 |
| Material | Specific Surface Area, m2/g |
|---|---|
| Sintered on SPS | 1.22 ± 0.007 |
| After dispersion | |
| 1 h | 6.34 ± 0.01 |
| 2 h | 0 (−0.115) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukhamedova, N.; Miniyazov, A.; Sabyrtayeva, A.; Tulenbergenov, T.; Oken, O. Dispersion of Sintered Mg-Ni-Ce Materials for Efficient Hydrogen Storage. Crystals 2025, 15, 743. https://doi.org/10.3390/cryst15080743
Mukhamedova N, Miniyazov A, Sabyrtayeva A, Tulenbergenov T, Oken O. Dispersion of Sintered Mg-Ni-Ce Materials for Efficient Hydrogen Storage. Crystals. 2025; 15(8):743. https://doi.org/10.3390/cryst15080743
Chicago/Turabian StyleMukhamedova, Nuriya, Arman Miniyazov, Aisara Sabyrtayeva, Timur Tulenbergenov, and Ospan Oken. 2025. "Dispersion of Sintered Mg-Ni-Ce Materials for Efficient Hydrogen Storage" Crystals 15, no. 8: 743. https://doi.org/10.3390/cryst15080743
APA StyleMukhamedova, N., Miniyazov, A., Sabyrtayeva, A., Tulenbergenov, T., & Oken, O. (2025). Dispersion of Sintered Mg-Ni-Ce Materials for Efficient Hydrogen Storage. Crystals, 15(8), 743. https://doi.org/10.3390/cryst15080743






