Evaluation of Corrosion Behavior of Zn–Al–Mg-Coated Steel in Corrosive Heterogeneous Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials: Steel Specimens and Soil Samples
2.2. Corrosion Testing Method
2.3. Characterization
2.3.1. Gravimetric Determination of Corrosion Rates
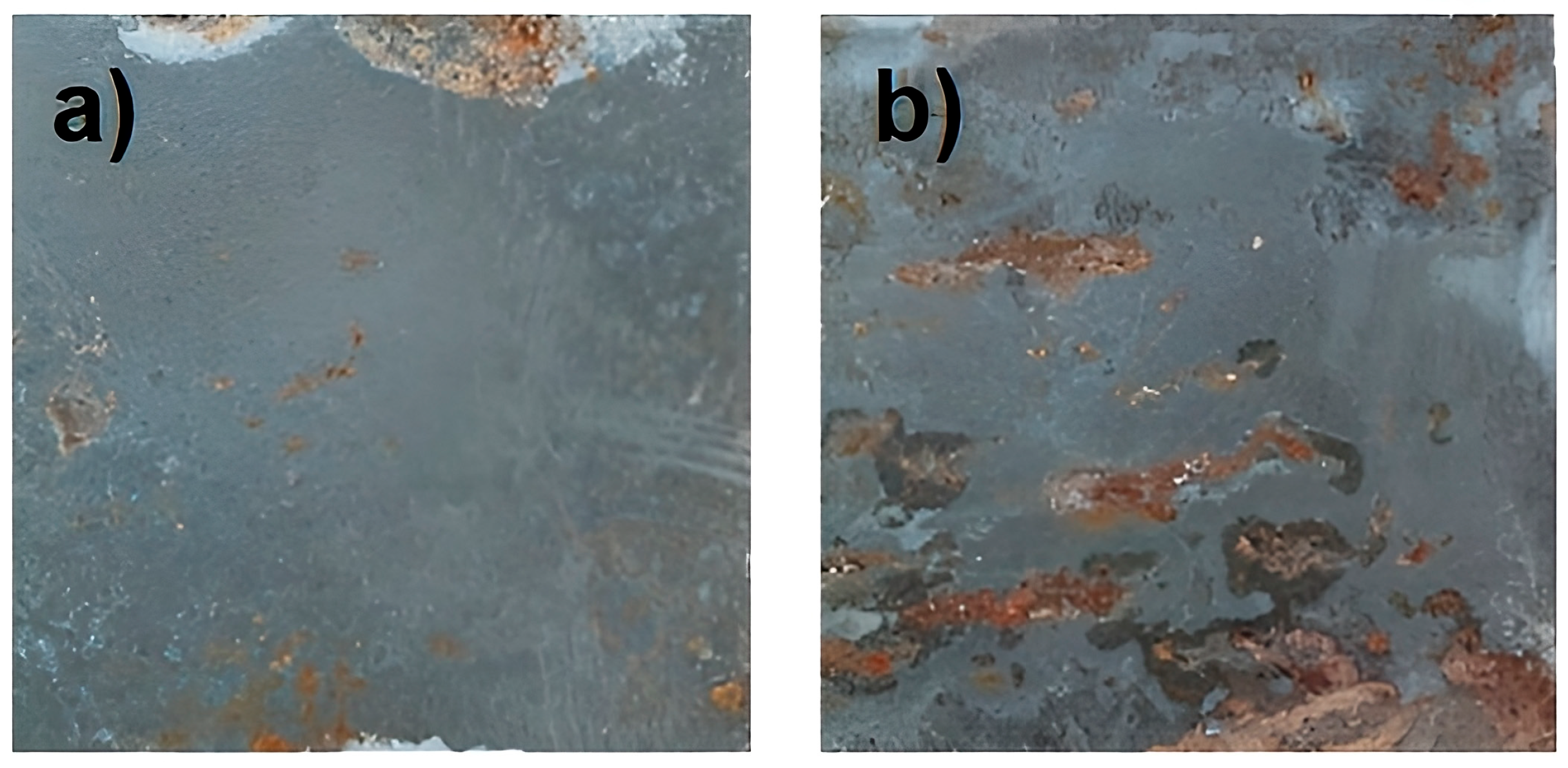
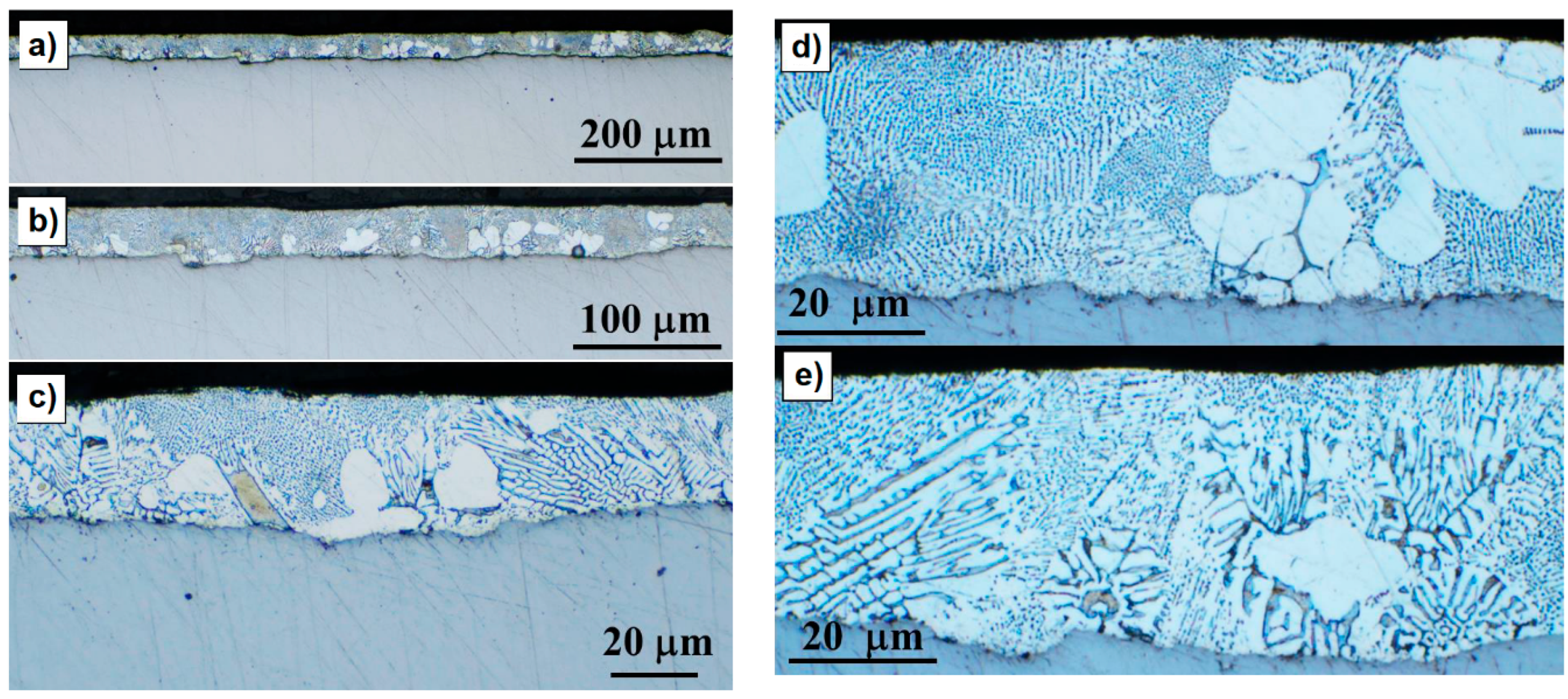
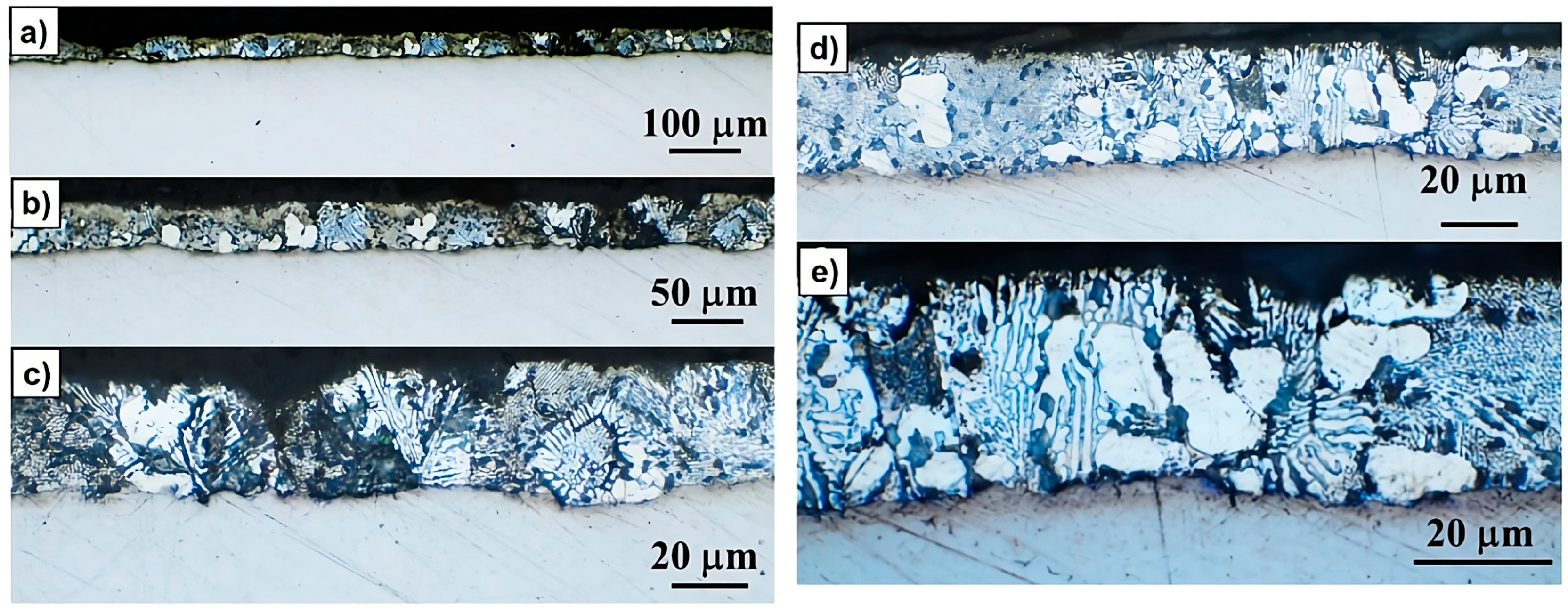
2.3.2. Direct Observation and Optical Microscopy
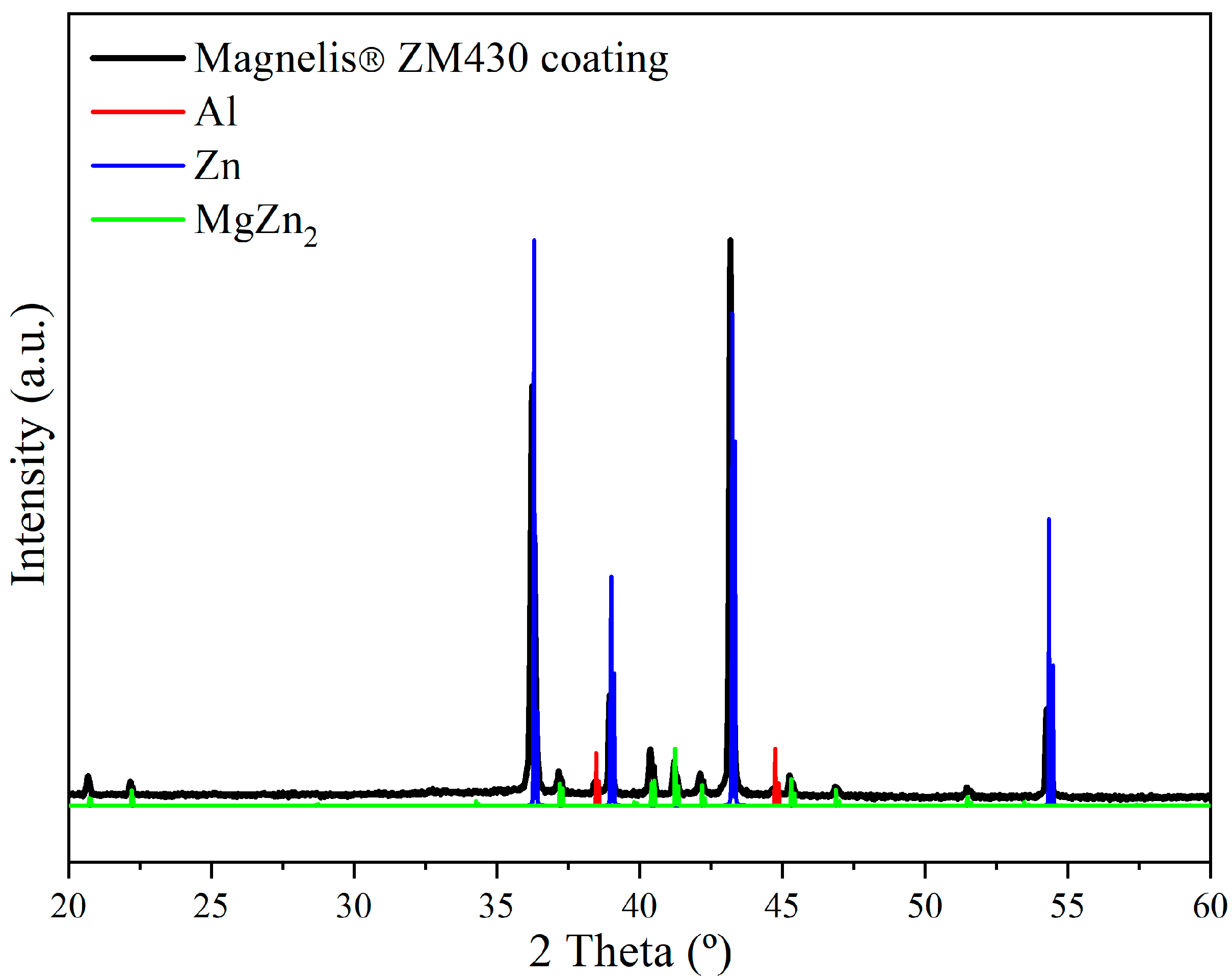
2.3.3. X-Ray Diffraction
2.3.4. Scanning Electron Microscopy and Energy Dispersive X-Ray Spectroscopy
3. Results and Discussion
3.1. Corrosion Rate in Soil
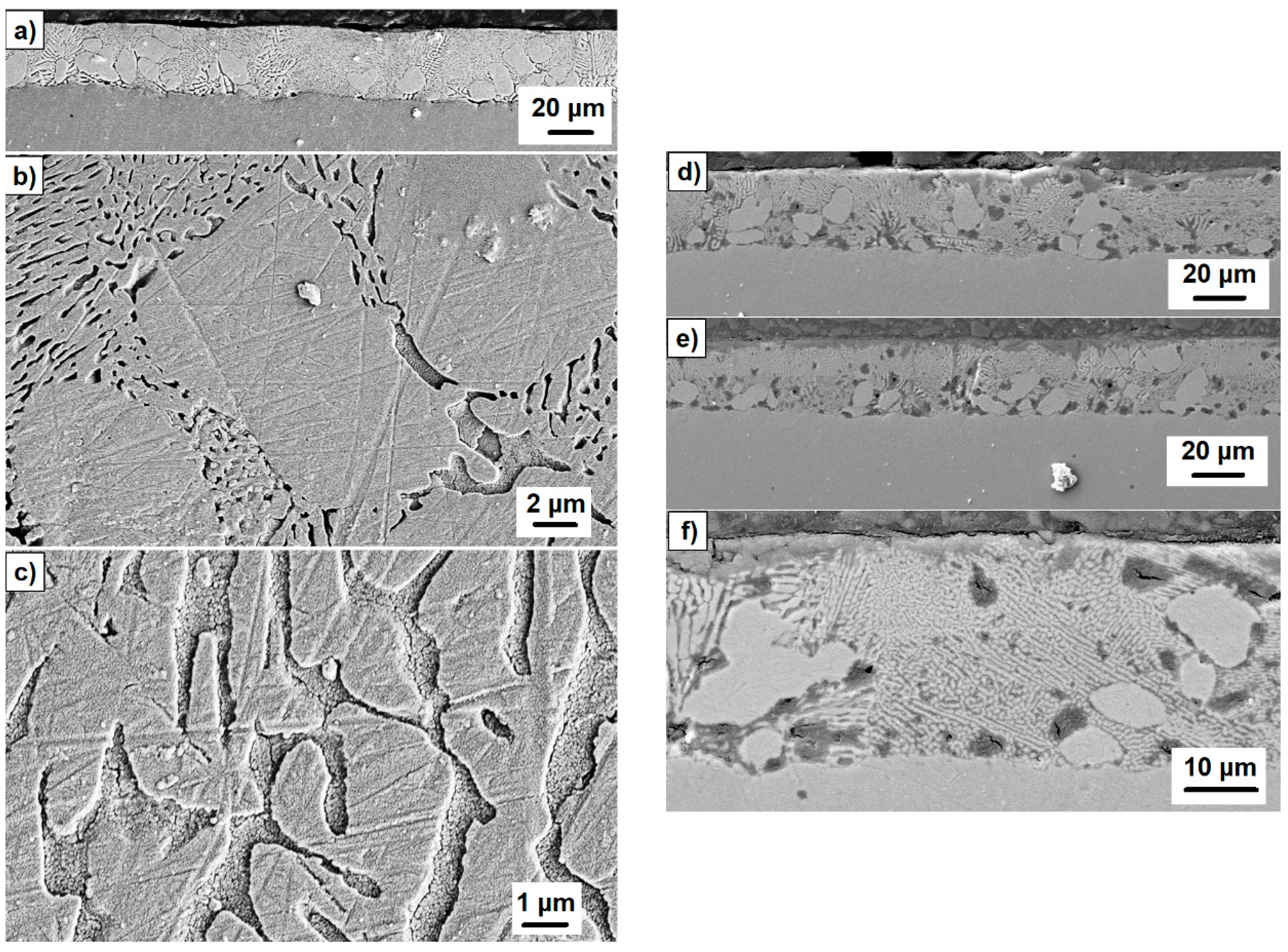
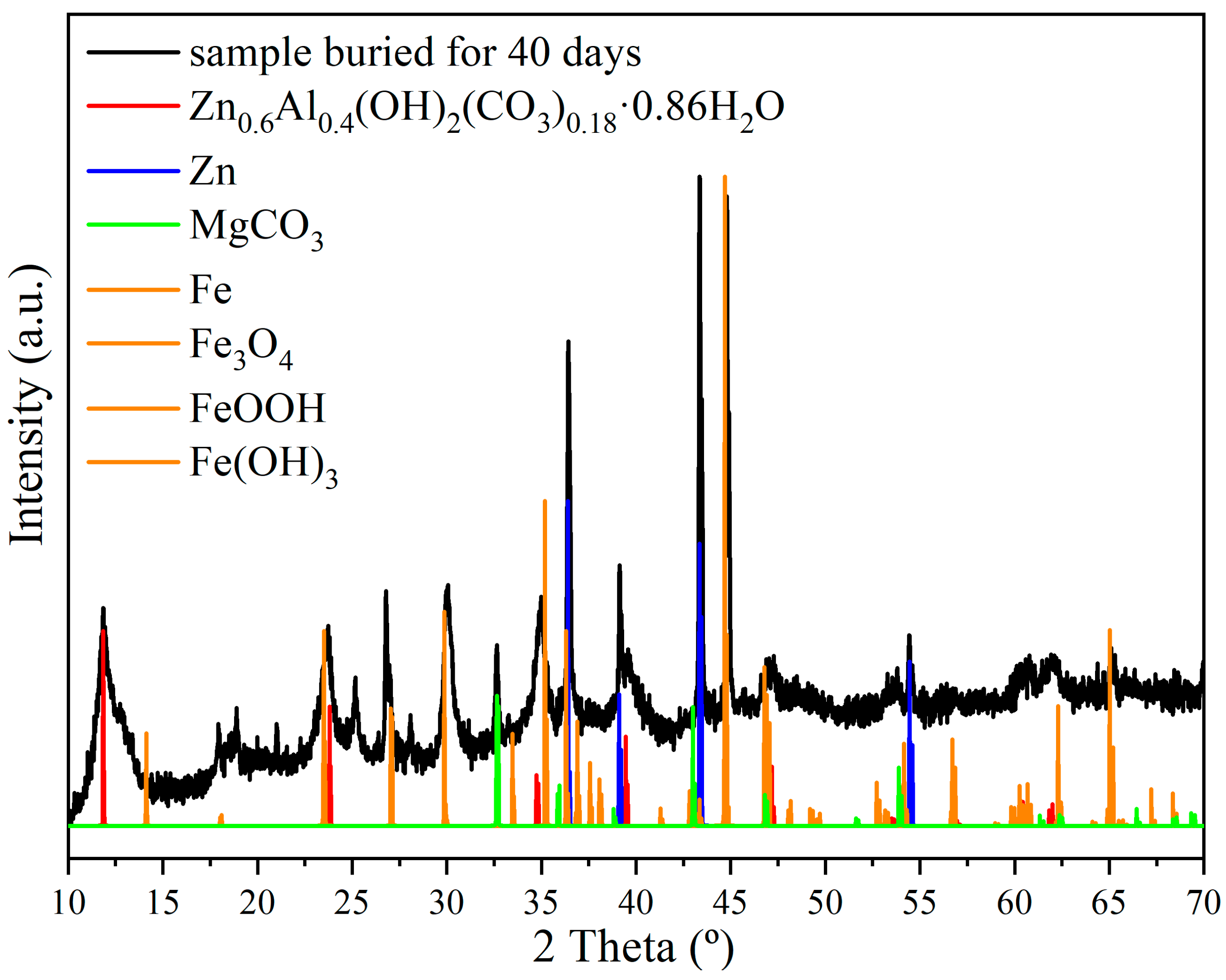
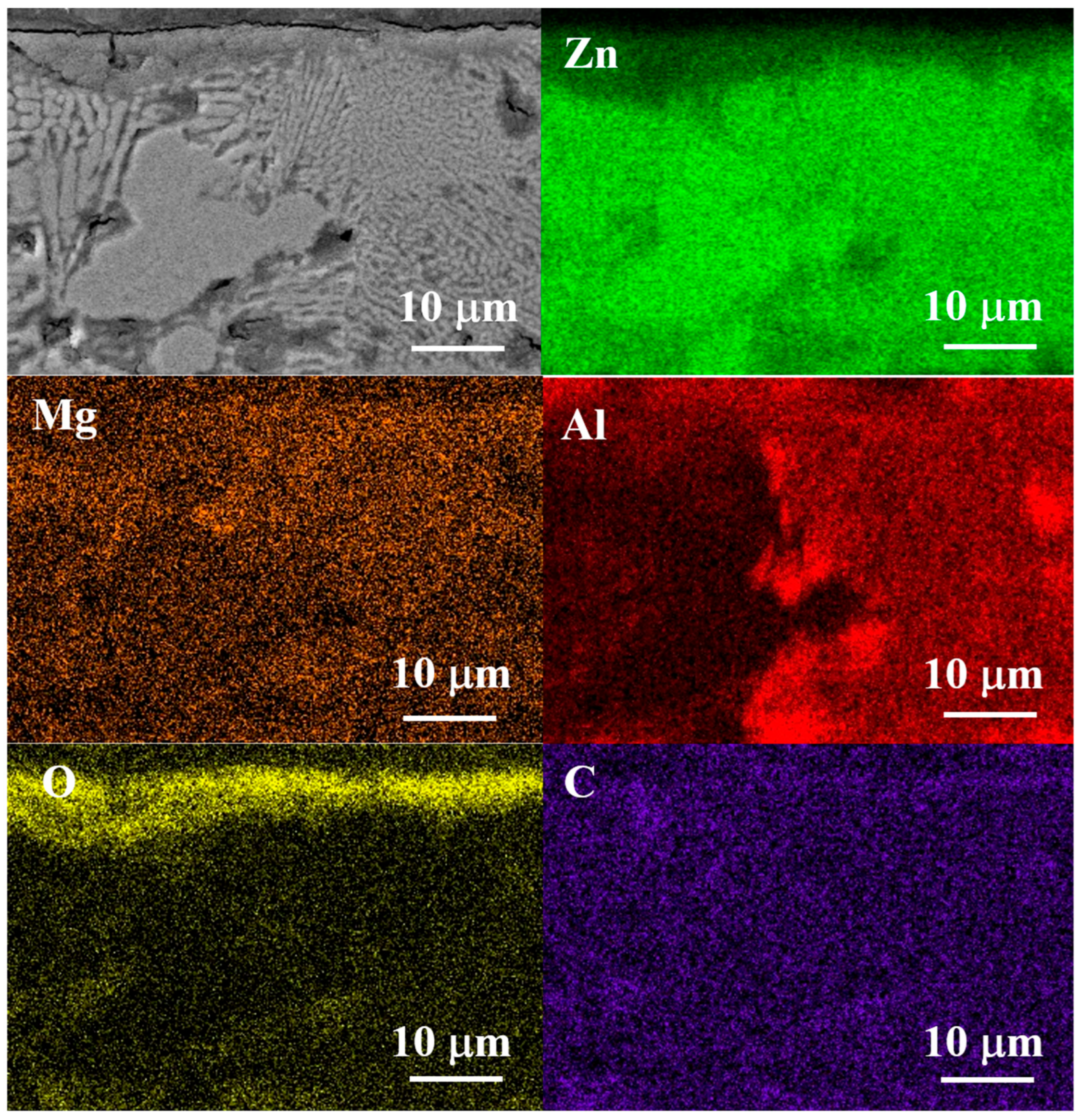
3.2. Microstructural and Phase Evolution
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morcillo, M.; Díaz, I.; Cano, H.; Chico, B.; De La Fuente, D. Atmospheric Corrosion of Weathering Steels. Overview for Engineers. Part I: Basic Concepts. Constr. Build. Mater. 2019, 213, 723–737. [Google Scholar] [CrossRef]
- Yang, X.; Li, Q.; Liu, S.; Hu, J.; Zhu, R.; Yang, G. Atmospheric Corrosion Prediction of Carbon Steel and Weathering Steel Based on Big Data Technology. Corros. Commun. 2025, 19, 63–75. [Google Scholar] [CrossRef]
- Corvo, F.; Betancourt, N.; Mendoza, A. The Influence of Airborne Salinity on the Atmospheric Corrosion of Steel. Corros. Sci. 1995, 37, 1889–1901. [Google Scholar] [CrossRef]
- Dalmora, G.P.V.; Borges Filho, E.P.; Maraschin Conterato, A.A.; Roso, W.S.; Pereira, C.E.; Dettmer, A. Methods of Corrosion Prevention for Steel in Marine Environments: A Review. Results Surf. Interfaces 2025, 18, 100430. [Google Scholar] [CrossRef]
- Santa, A.C.; Montoya, D.A.; Tamayo, J.A.; Gómez, M.A.; Castaño, J.G.; Baena, L.M. Atmospheric Corrosion of Carbon Steel: Results of One-Year Exposure in an Andean Tropical Atmosphere in Colombia. Heliyon 2024, 10, e29391. [Google Scholar] [CrossRef]
- Corcoran, P.; Jarvis, M.G.; Mackney, D.; Stevens, K.W. Soil Corrosiveness in South Oxfordshire. J. Soil Sci. 1977, 28, 473–484. [Google Scholar] [CrossRef]
- Ferreira, C.A.M.; Ponciano, J.A.C.; Vaitsman, D.S.; Pérez, D.V. Evaluation of the Corrosivity of the Soil through Its Chemical Composition. Sci. Total Environ. 2007, 388, 250–255. [Google Scholar] [CrossRef]
- Zhou, E.; Li, F.; Zhang, D.; Xu, D.; Li, Z.; Jia, R.; Jin, Y.; Song, H.; Li, H.; Wang, Q.; et al. Direct Microbial Electron Uptake as a Mechanism for Stainless Steel Corrosion in Aerobic Environments. Water Res. 2022, 219, 118553. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Dang, D.; Lanarde, L.; Jeannin, M.; Sabot, R.; Refait, P. Influence of Soil Moisture on the Residual Corrosion Rates of Buried Carbon Steel Structures under Cathodic Protection. Electrochim. Acta 2015, 176, 1410–1419. [Google Scholar] [CrossRef]
- Amadi, A.H.; Ajienka, J.A.; Akaranta, O.; Moses, P.R.; Achara, N.U.; Ola, V.D.; Udo, R.D. A Review of Soil Resistivity Testing for Enhanced Corrosion Control: Overcoming Limitations through Integrated Geophysical Approaches and Alternative Methodologies. Measurement 2025, 242, 116214. [Google Scholar] [CrossRef]
- Dong, Z.; Ding, L.; Meng, Z.; Xu, K.; Mao, Y.; Chen, X.; Ye, H.; Poursaee, A. Machine Learning-Based Corrosion Rate Prediction of Steel Embedded in Soil. Sci. Rep. 2024, 14, 18194. [Google Scholar] [CrossRef]
- Arriba-Rodriguez, L.; Villanueva-Balsera, J.; Ortega-Fernandez, F.; Rodriguez-Perez, F. Methods to Evaluate Corrosion in Buried Steel Structures: A Review. Metals 2018, 8, 334. [Google Scholar] [CrossRef]
- Ramesh Babu, J.; Karve, P.M.; Mahadevan, S. Probabilistic Prediction of External Corrosion Fatigue Life in Buried Steel Pipes. Int. J. Press. Vessel. Pip. 2025, 214, 105415. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, Y.; Liu, X.; Zhang, J.; Li, T.; Xu, B. Prediction of Interference Current of Buried Pipeline and Study on Corrosion of Q235A Steel. Constr. Build. Mater. 2023, 400, 132739. [Google Scholar] [CrossRef]
- Wang, T.; Xu, D.; Qu, L.; Fu, J.; Li, Z. An Extension Approach to Estimate Soil Corrosivity for Buried Pipelines. Int. J. Press. Vessel. Pip. 2021, 192, 104413. [Google Scholar] [CrossRef]
- Scheffer De Matos, T.; Franke Portella, K.; Luiz Henke, S.; D’Orey Gaivão Portella Bragança, M.; Medeiros De Almeida, L. Novel Approach to Detect Encasement Failures in the Anchorage System of Guyed Transmission Towers by Distribution of Relaxation Times. Eng. Fail. Anal. 2024, 165, 108788. [Google Scholar] [CrossRef]
- Matos, T.S.; Portella, K.F.; Henke, S.L.; Bragança, M.O.G.P.; Galvão, M.P.; Dias, B.G.; Lagoeiro, L.; Almeida, L.M. Analysis of Anchor Rod Failure in a Guyed Transmission Tower: Influence of Microstructures and Corrosion Mechanisms. Eng. Fail. Anal. 2021, 121, 105166. [Google Scholar] [CrossRef]
- Vivanco, J.R.; Breul, P.; Talon, A.; Benz-Navarrete, M.A.; Barbier, S.; Haddani, Y. Importance of Geotechnical Diagnosis in Railway Management: A Review. Transp. Eng. 2024, 18, 100293. [Google Scholar] [CrossRef]
- Sørensen, P.A.; Kiil, S.; Dam-Johansen, K.; Weinell, C.E. Anticorrosive Coatings: A Review. J. Coat. Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- Li, J.; Wang, D.; Hou, L.; Zhang, S.; Wu, P.; Zhang, Y.; Liu, B.; Wei, Y. Enhancing the Corrosion Resistance of the Epoxy Coating Using CaAl LDH Intercalated with L-Cysteine and Its Derivatives as a Pigment on Steel Substrate. Prog. Org. Coat. 2024, 193, 108527. [Google Scholar] [CrossRef]
- He, C.; Wang, Z.; You, Y.; Wang, X.; Zhao, P.; Yang, Z.; Zhou, L. Influence of Soil Variability on the Corrosion of Buried Hot-Dip Galvanized Steel. Int. J. Electrochem. Sci. 2025, 20, 100889. [Google Scholar] [CrossRef]
- Rossi, S.; Pinamonti, M.; Calovi, M. Influence of Soil Chemical Characteristics on Corrosion Behaviour of Galvanized Steel. Case Stud. Constr. Mater. 2022, 17, e01257. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, T.; Liu, M.; Wang, Z.; Cao, G.; Guo, Q.; Wang, C. Corrosion Mechanism of a High Corrosion-Resistance Zn–Al–Mg Coating in Typical Extremely Harsh Marine and Cold Environments. J. Mater. Res. Technol. 2024, 33, 4290–4302. [Google Scholar] [CrossRef]
- Volovitch, P.; Vu, T.N.; Allély, C.; Abdel Aal, A.; Ogle, K. Understanding Corrosion via Corrosion Product Characterization: II. Role of Alloying Elements in Improving the Corrosion Resistance of Zn–Al–Mg Coatings on Steel. Corros. Sci. 2011, 53, 2437–2445. [Google Scholar] [CrossRef]
- Thierry, D.; LeBozec, N.; Le Gac, A.; Persson, D. Long-term Atmospheric Corrosion Rates of Hot Dip Galvanised Steel and Zinc-aluminium-magnesium Coated Steel. Mater. Corros. 2019, 70, 2220–2227. [Google Scholar] [CrossRef]
- Kim, S.-H.; Jin, S.-Y.; Yang, J.-H.; Yang, J.-H.; Lee, M.-H.; Yun, Y.-S. Self-Healing Phenomenon at the Cut Edge of Zn-Al-Mg Alloy Coated Steel in Chloride Environments. Coatings 2024, 14, 485. [Google Scholar] [CrossRef]
- Zhang, C.; Liao, Y.; Gao, X.; Zhao, J.; Yuan, Y.; Liao, R. Research Advances of Soil Corrosion of Grounding Grids. Micromachines 2021, 12, 513. [Google Scholar] [CrossRef]
- DIN 50929-3:2018-03; Corrosion of Metals—Corrosion Likelihood of Metallic Materials When Subject to Corrosion from the Outside—Part 3: Buried and Underwater Pipelines and Structural Components. Beuth: Berlin, Germany, 2018. [CrossRef]
- ASTM G1-03(2017); Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. ASTM: West Conshohocken, PA, USA, 2017. [CrossRef]
- Iribarren, J.I.; Liesa, F.; Alemán, C.; Armelin, E. Corrosion Rate Evaluation by Gravimetric and Electrochemical Techniques Applied to the Metallic Reinforcing Structures of a Historic Building. J. Cult. Herit. 2017, 27, 153–163. [Google Scholar] [CrossRef]
- Romanoff, M. Underground Corrosion; National Bureau of Standards: Gaithersburg, MD, USA, 1957.
- Matsushima, I. Carbon Steel—Corrosion by Soils. In Uhlig’s Corrosion Handbook; Revie, R.W., Ed.; Wiley: Hoboken, NJ, USA, 2011; pp. 609–613. ISBN 978-0-470-08032-0. [Google Scholar]
- Itoh, M. Time-Dependent Power Laws in the Oxidation and Corrosion of Metals and Alloys. Sci. Rep. 2022, 12, 6944. [Google Scholar] [CrossRef] [PubMed]
- Salgueiro Azevedo, M.; Allély, C.; Ogle, K.; Volovitch, P. Corrosion Mechanisms of Zn(Mg,Al) Coated Steel: 2. The Effect of Mg and Al Alloying on the Formation and Properties of Corrosion Products in Different Electrolytes. Corros. Sci. 2015, 90, 482–490. [Google Scholar] [CrossRef]
- Sullivan, J.; Mehraban, S.; Elvins, J. In Situ Monitoring of the Microstructural Corrosion Mechanisms of Zinc–Magnesium–Aluminium Alloys Using Time Lapse Microscopy. Corros. Sci. 2011, 53, 2208–2215. [Google Scholar] [CrossRef]
- Ikeuba, A.I.; Kou, F.; Duan, H.; Zhang, B.; Wang, J.; Han, E.-H.; Ke, W. Understanding the Electrochemical Behavior of Bulk-Synthesized MgZn2 Intermetallic Compound in Aqueous NaCl Solutions as a Function of pH. J. Solid State Electrochem. 2019, 23, 1165–1177. [Google Scholar] [CrossRef]
- He, X.; Zhou, X.; Shang, T.; Liu, W.; Jiang, G.; Liu, C.; Cheng, X.; Zhang, X.; Li, X. Influence Mechanism of Different Elements and Alloy Phases on the Corrosion Resistance of Zn-Al-Mg Coated Steel in the Atmospheric Environment: A Review. Corros. Commun. 2024, 13, 49–59. [Google Scholar] [CrossRef]


| Sample | Corrosion Days | Non-Corroded Sample | Corroded Sample | |||||
|---|---|---|---|---|---|---|---|---|
| L1 (mm) | L2 (mm) | Thickness (mm) | m (g) | Density (g/mL) | m (g) | Corrosion Rate (μm/Year) | ||
| 1d10 | 10 | 39.94 | 49.46 | 2.63 | 41.1308 | 7.92 | 41.0866 | 53 |
| 2d10 | 10 | 40.00 | 49.97 | 2.71 | 41.5850 | 7.68 | 41.5446 | 48 |
| 3d10 | 10 | 39.94 | 50.39 | 2.70 | 41.8495 | 7.70 | 41.7626 | 102 |
| 4d10 | 10 | 39.95 | 49.57 | 2.70 | 41.3067 | 7.73 | 41.2652 | 49 |
| 5d10 | 10 | 40.06 | 49.67 | 2.74 | 41.4304 | 7.60 | 41.3908 | 47 |
| 6d10 | 10 | 39.36 | 49.57 | 2.71 | 41.2020 | 7.79 | 41.1508 | 62 |
| 7d10 | 10 | 39.73 | 40.27 | 2.71 | 42.2103 | 7.80 | 42.1433 | 79 |
| 8d10 | 10 | 40.08 | 49.91 | 2.73 | 42.3053 | 7.75 | 42.2188 | 102 |
| 1d20 | 20 | 40.43 | 49.90 | 2.70 | 42.0137 | 7.71 | 41.8636 | 88 |
| 2d20 | 20 | 40.17 | 49.45 | 2.71 | 41.4863 | 7.71 | 41.4307 | 33 |
| 3d20 | 20 | 39.91 | 49.27 | 2.74 | 40.9172 | 7.59 | 40.6254 | 175 |
| 4d20 | 20 | 40.02 | 49.86 | 2.71 | 41.6305 | 7.70 | 41.2645 | 217 |
| 5d20 | 20 | 39.8 | 49.39 | 2.67 | 41.0177 | 7.82 | 40.8936 | 75 |
| 6d20 | 20 | 39.48 | 50.20 | 2.68 | 41.2893 | 7.77 | 41.1480 | 84 |
| 7d20 | 20 | 40.43 | 50.00 | 2.68 | 42.0906 | 7.77 | 41.8823 | 122 |
| 8d20 | 20 | 40.40 | 50.23 | 2.70 | 42.3796 | 7.73 | 42.0820 | 173 |
| 1d30 | 30 | 39.81 | 49.81 | 2.70 | 41.3167 | 7.72 | 41.0825 | 93 |
| 2d30 | 30 | 40.45 | 49.81 | 2.71 | 41.8675 | 7.67 | 41.7523 | 45 |
| 3d30 | 30 | 40.08 | 50.67 | 2.71 | 41.9318 | 7.62 | 41.2881 | 249 |
| 4d30 | 30 | 39.98 | 50.09 | 2.69 | 41.5511 | 7.71 | 40.9136 | 251 |
| 5d30 | 30 | 39.72 | 50.26 | 2.70 | 41.4459 | 7.69 | 41.1886 | 101 |
| 6d30 | 30 | 39.87 | 49.86 | 2.72 | 42.1126 | 7.79 | 41.9163 | 78 |
| 7d30 | 30 | 39.85 | 50.27 | 2.72 | 42.3798 | 7.78 | 41.8225 | 219 |
| 8d30 | 30 | 40.36 | 50.36 | 2.69 | 42.2054 | 7.72 | 41.5805 | 242 |
| 1d40 | 40 | 40.20 | 49.98 | 2.70 | 41.7277 | 7.69 | 41.3118 | 122 |
| 2d40 | 40 | 40.11 | 49.80 | 2.70 | 41.5484 | 7.70 | 41.1879 | 107 |
| 3d40 | 40 | 40.29 | 49.82 | 2.71 | 41.8400 | 7.69 | 41.0005 | 234 |
| 4d40 | 40 | 40.41 | 49.47 | 2.71 | 41.8508 | 7.73 | 40.9799 | 198 |
| 5d40 | 40 | 40.49 | 50.15 | 2.67 | 42.2803 | 7.80 | 41.8701 | 119 |
| 6d40 | 40 | 39.67 | 49.51 | 2.72 | 41.7304 | 7.81 | 41.2106 | 156 |
| 7d40 | 40 | 39.76 | 49.96 | 2.73 | 41.9939 | 7.74 | 41.2263 | 198 |
| 8d40 | 40 | 40.34 | 50.15 | 2.70 | 42.1934 | 7.72 | 41.3657 | 183 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lloreda-Jurado, P.J.; Chicardi, E. Evaluation of Corrosion Behavior of Zn–Al–Mg-Coated Steel in Corrosive Heterogeneous Soil. Crystals 2025, 15, 738. https://doi.org/10.3390/cryst15080738
Lloreda-Jurado PJ, Chicardi E. Evaluation of Corrosion Behavior of Zn–Al–Mg-Coated Steel in Corrosive Heterogeneous Soil. Crystals. 2025; 15(8):738. https://doi.org/10.3390/cryst15080738
Chicago/Turabian StyleLloreda-Jurado, Pedro Javier, and Ernesto Chicardi. 2025. "Evaluation of Corrosion Behavior of Zn–Al–Mg-Coated Steel in Corrosive Heterogeneous Soil" Crystals 15, no. 8: 738. https://doi.org/10.3390/cryst15080738
APA StyleLloreda-Jurado, P. J., & Chicardi, E. (2025). Evaluation of Corrosion Behavior of Zn–Al–Mg-Coated Steel in Corrosive Heterogeneous Soil. Crystals, 15(8), 738. https://doi.org/10.3390/cryst15080738







