1. Introduction
Zirconia-based ceramics can be used in a wide range of applications in energy, industry, and medicine [
1,
2]. Although fully stabilized zirconia ceramics are required for most applications, partially stabilized ceramics offer certain advantages that are useful in specific applications. For example, partially stabilized zirconia ceramics have increased resistance to mechanical stress, including higher crack resistance, since the presence of phases with different crystal structures effectively prevents the propagation of cracks [
3,
4]. In addition, partially stabilized zirconia ceramics effectively withstand thermal shocks, allowing them to be used in conditions of sudden temperature changes typical of high-temperature industrial processes.
However, zirconia ceramics can be susceptible to phase transformations during processing and subsequent operation [
5,
6]. For example, previous studies have shown that grinding of ceramics can lead to phase transformations in surface layers [
7,
8,
9,
10]. One study reported that grinding induced the formation of a monoclinic phase in the surface layer of zirconia ceramics initially composed entirely of the tetragonal phase and that a short 30 min annealing at 1000 °C led to a reverse transformation back to the tetragonal phase [
11].
It is also interesting to trace the phase and structural changes in ceramics after indentation. The stress concentrated in the center of the indentation can become high enough to destabilize the crystal structure, often reaching values comparable to the material’s hardness. As a result, amorphization, or the formation of metastable high-pressure phases, may occur beneath the indenter. One of the early investigations in this field explored the use of highly localized mechanical stresses to induce phase transformations and amorphization in semiconductors such as Si, Ge, GaAs, and InSb, as well as in ceramics including SiC and SiO
2. The results confirmed the appearance of amorphization and the formation of high-pressure phases, with these transformations shown to be strongly influenced by the unloading rate [
12]. A study combining indentation and Raman spectroscopy was also conducted on LaCoO
3, where significant stress-induced changes in the Raman spectra were observed. In particular, tensile stress was found to stabilize a structural state characterized by an increased average Co–O bond length [
13]. Raman spectroscopy was also used to construct residual stress maps for carbide ceramics [
14]. Similar studies performed on ZrO
2 revealed that indentation of stabilized Y-doped ZrO
2 ceramics (or single crystals) can induce tetragonal to monoclinic (t → m) transformations in the indentation area, driven by the high shear stresses generated during loading [
15,
16]. One study also noted that yttria-stabilized zirconia (YSZ) ceramics composed of nanosized grains are less susceptible to phase transformation during indentation compared to materials with submicron-sized grains [
17]. A detailed study on 3Y-TZP ceramics using piezospectroscopy analyzed stress-induced Raman shifts of two stress-sensitive zirconia modes (A
1 ≈ 468 cm
−1 and A
2 ≈ 648 cm
−1) to map residual stress. At low loads, the stress distribution around cracks matched the Yoffe model, while at high loads, unexpectedly high compressive stresses were observed, in contrast to model predictions, which is explained by stress-induced phase transformation [
18]. The results highlight the importance of experimental validation, as existing theoretical models may not accurately predict residual stress behavior after loading.
Studying the Raman spectra of indentation marks not only immediately after microhardness testing but especially after subsequent annealing can be useful for revealing the effects of recrystallization and phase transformations in ceramics. Similar investigations have previously been performed on transparent polycrystalline yttrium aluminum garnet. Indentations were introduced into the sample, followed by annealing at 1500 °C. As a result, grains within the indentation region exhibited recrystallization and were found to be approximately ten times smaller than those in the surrounding unstressed areas [
19]. Strain-induced recrystallization was also observed for La
2Zr
2O
7 ceramics. The authors found that during annealing, recrystallization initiated in regions with high dislocation density, such as shear bands and grain boundaries, and progressed through nucleation and grain growth. At temperatures below 1100 °C, recovery dominated by dislocation rearrangement and annihilation slowed recrystallization. Above 1100 °C, recrystallization became the prevailing process, driven by stored deformation energy. The study also proposed a mechanism in which new grains nucleate in shear bands, consuming dislocations and leading to an inhomogeneous recrystallization pattern, followed by subgrain and abnormal grain growth with prolonged annealing [
20].
When using ceramic products, various combinations of factors can affect the structure of the ceramic and its properties [
21]. Mechanical stresses and elevated temperatures are a fairly common combination of factors that can lead to significant changes, such as phase transitions or microstructural changes [
22]. It is important to investigate this phenomenon, especially in partially stabilized zirconia, as this material is of particular interest due to its crack resistance and ability to withstand thermal shocks. However, under certain conditions, it may exhibit insufficient phase stability and undergo significant changes in its properties, potentially altering its behavior during manufacturing and operation.
The present work focuses on the recrystallization of partially stabilized Ce-rO2 ceramics subjected to prior indentation. The ceramics used in this study were fabricated using the solid-state sintering method. Particular attention was given to the investigation of phase transformations occurring in local regions subjected to indentation using Raman spectroscopy.
2. Materials and Methods
Solid-state sintering was used for ceramic sample fabrication, which involves the following sequential steps: combined milling of the initial oxide components, pre-annealing of powders, preparation of green powder, pressing of the green powder into tablet form, and subsequent sintering at high temperature. ZrO
2 and CeO
2 powders (manufactured by Sigma-Aldrich, purity 99.9%, St. Louis, MO, USA) were used to produce polycrystalline ceramics. At the initial stage, the powders were milled in a Fritsch Pulverisette 6 planetary mill (Fritsch, Idar-Oberstein, Germany) with tungsten carbide grinding sets to mix CeO
2 and ZrO
2 oxides. The powder ratios were selected to provide a composition corresponding to 20% Ce-doped ZrO
2. Mixing in the planetary mill was carried out for 30 min at a rotation speed of 250 rpm. To ensure the formation of dense and homogeneous ceramics, the powders were subjected to pre-annealing at 1300 °C. After pre-annealing, sintering aids based on MgO-SiO
2-Al
2O
3 amounting to 1 wt.% were added to the powders to facilitate liquid-phase sintering and promote grain growth. Larger grains were preferred to minimize the influence of grain boundaries during indentation. This composition is also of interest due to its reduced susceptibility to cracking [
23]. After pre-annealing, powders were milled in the planetary mill. To obtain the green powder, the oxide mixture was gradually added to an aqueous solution of polyvinyl alcohol preheated to 90 °C while stirring intensively with a magnetic stirrer. After evaporation of water and drying at 50 °C in a drying oven, the resulting green powder was ground in an agate mortar. The next stage involved forming tablets using a single-axis hydraulic press at a pressure of 250 MPa. Sintering was performed in a Nabertherm LHT 08/18 resistive furnace (Nabertherm, Lilienthal, Germany) in air for 5 h at a temperature of 1700 °C.
The diffractograms of the samples were recorded using a Rigaku SmartLab X-ray diffractometer (Rigaku, Tokyo, Japan) equipped with a CuKα source. Raman spectroscopy studies on ceramics were performed using an EnSpectr Raman microscope (Spectr-M, Chernogolovka, Russia) with a laser wavelength of λ = 532 nm in backscattering geometry. A 100× objective lens was employed, providing a laser spot diameter of approximately 1.6 µm. For recording spectra, laser power of 15 mW was used. It was ensured that this value of laser power does not lead to heating of the sample or additional phase transformations. Each spectrum was acquired with an integration time of ~100 s. The spectral resolution of the spectrometer was 4–6 cm−1. Indentation marks and their micrographs were made on a Metkon DuroLine (Metkon Instruments Inc., Bursa, Turkey) device for studying the microhardness of materials using the Vickers method. In addition, an analysis of microstructural changes in the indentation marks was performed using a Phenom ProX G6 scanning electron microscope (Thermo Fisher Scientific, Eindhoven, The Netherlands).
To prepare the samples for indentation, they were ground and polished using a Struers Tegramin-30 machine (Struers, Ballerup, Denmark). After indentation marks were left on the polished surface of the samples and all initial information on structural, phase, and morphological characteristics was collected, the samples were annealed for 5 min at 1000, 1100, and 1200 °C. After annealing, changes in the samples were recorded using Raman spectroscopy, X-ray diffraction, and scanning electron microscopy.
To estimate the monoclinic phase content, we used the following formula, that contains relative intensities of tetragonal and monoclinic Raman modes of ZrO
2 [
24]:
where
k = 0.97 and
δ = 1 represent empirical factors.
It is important to emphasize that the calculated Cm value does not represent the real phase composition. Instead, it serves as a relative indicator for comparing different samples, where a higher relative intensity of tetragonal peaks corresponds to a higher proportion of the tetragonal phase.
3. Results and Discussion
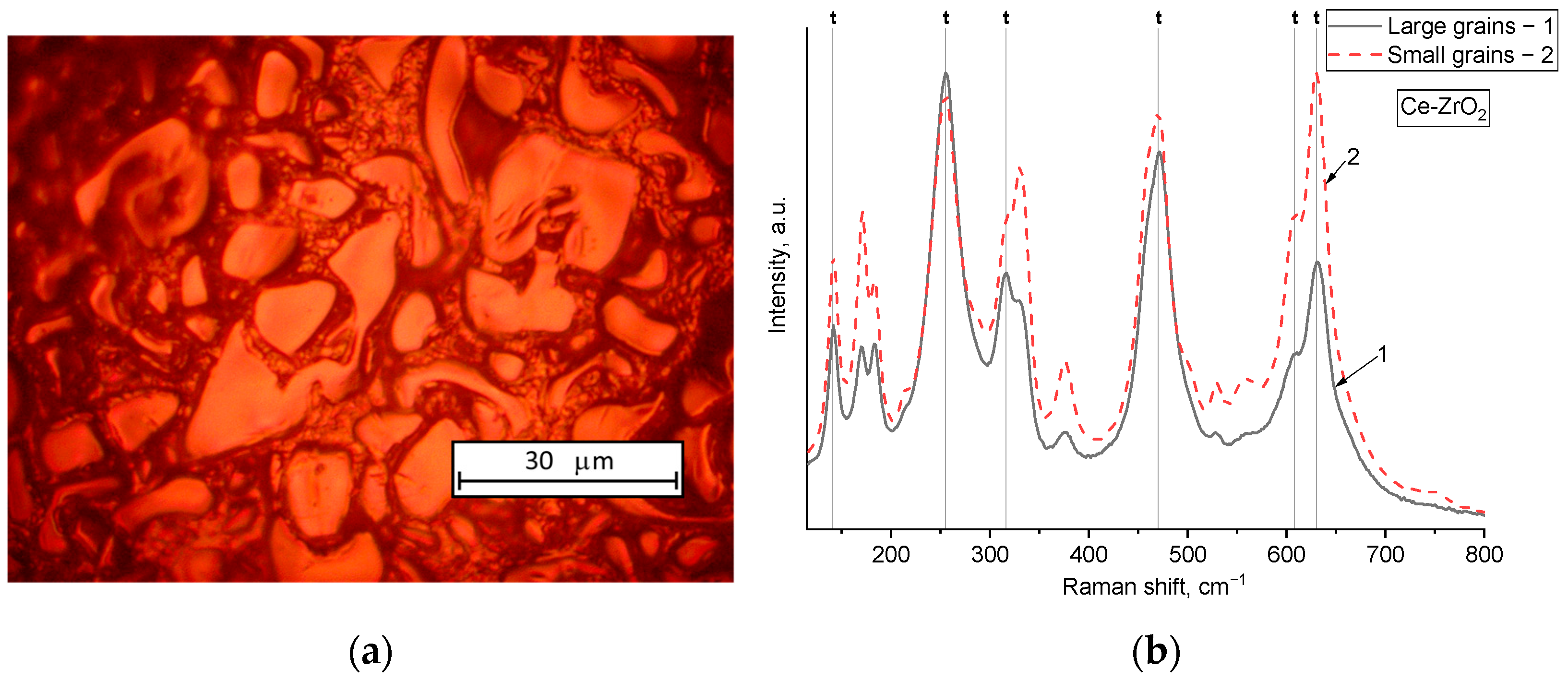
Figure 1a,c shows optical and SEM micrographs of the microstructure of Ce-doped ZrO
2 sample obtained after sintering, and
Figure 1b demonstrates the corresponding Raman spectra of this sample. The grains of this sample consist of a mixture of the monoclinic and tetragonal phases of zirconium oxide, which is reflected in the presence of peaks at 141, 255, 316, 470, 608, and 630 cm
−1 characteristic of the tetragonal phase (pointed out by the “t” letter) [
25], and peaks at 170, 184, 331, 376, 530, and 560 cm
−1 associated with monoclinic ZrO
2 [
26]. The microstructure of the sample is composed of large grains of complex shape separated by intergranular regions containing smaller grains. The Raman spectra of these two types of grains differ only slightly in relative peak intensities, indicating that both small and large grains have a similar phase composition, with the larger grains containing a slightly higher fraction of the tetragonal phase, as evidenced by the increased relative intensity of tetragonal peaks compared to monoclinic ones. It is important to take into account that the sample exhibits slight inhomogeneity in the distribution of tetragonal and monoclinic phases. To ensure consistency, we monitored changes at a precisely identified location on the sample, allowing us to track phase evolution during indentation and subsequent recrystallization.
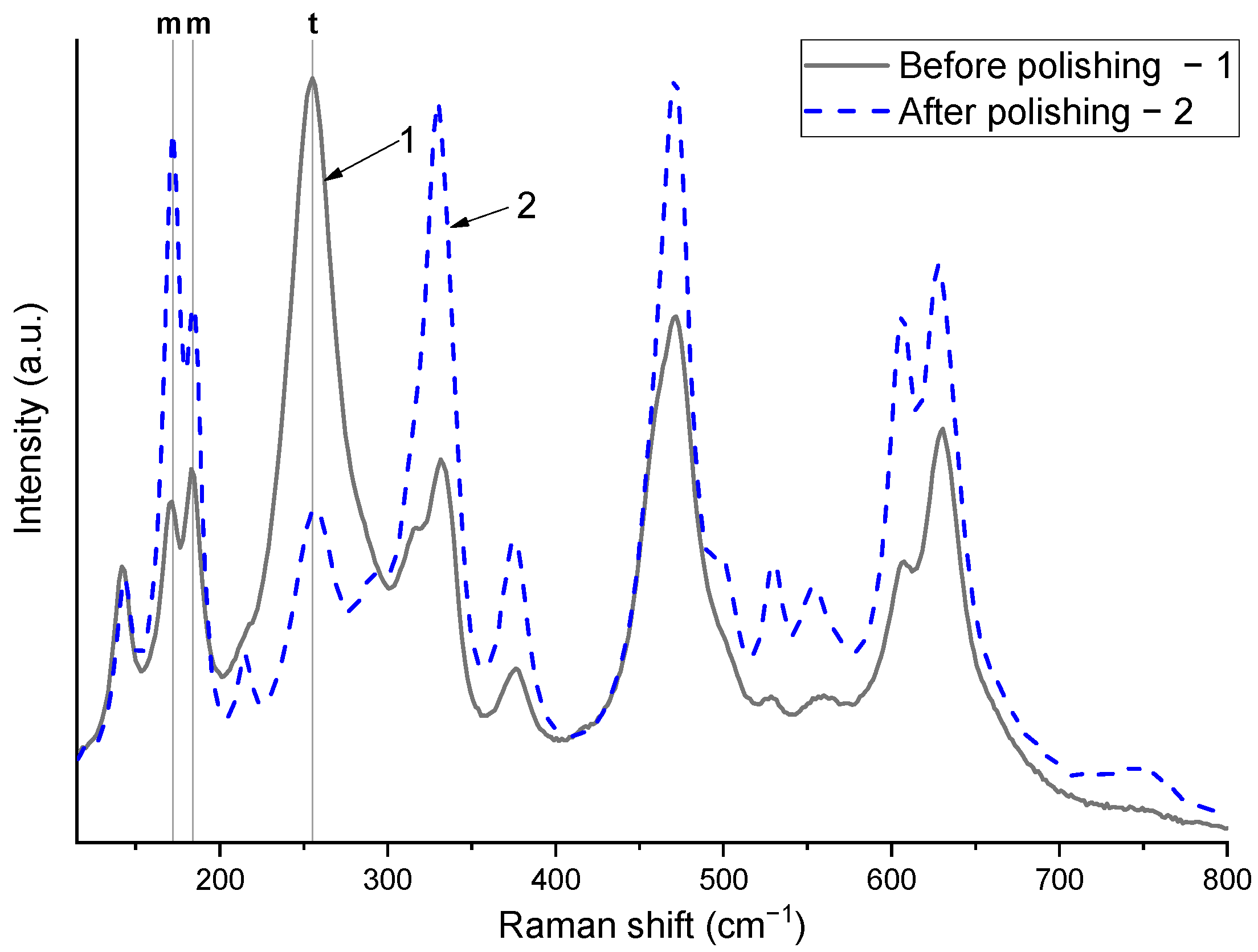
After grinding and polishing the samples, a second examination was performed using Raman spectroscopy.
Figure 2 presents a comparison of the Raman spectra of Ce-doped ZrO
2 ceramic samples before and after polishing. Before polishing, the peaks corresponding to the tetragonal phase were higher in relative intensity or comparable to those of the monoclinic phase. After polishing, a noticeable increase in the intensity of the monoclinic peaks is observed, indicating that t→m phase transformations have occurred in the surface layer of the CeO
2-doped ZrO
2 ceramics. It should be noted that the phase transition from the tetragonal to the monoclinic phase observed after polishing is a well-known phenomenon that has been studied in sufficient detail in previous research [
11].
After polishing, the samples were subjected to indentation with a 1 kgf load using a Vickers microhardness tester.
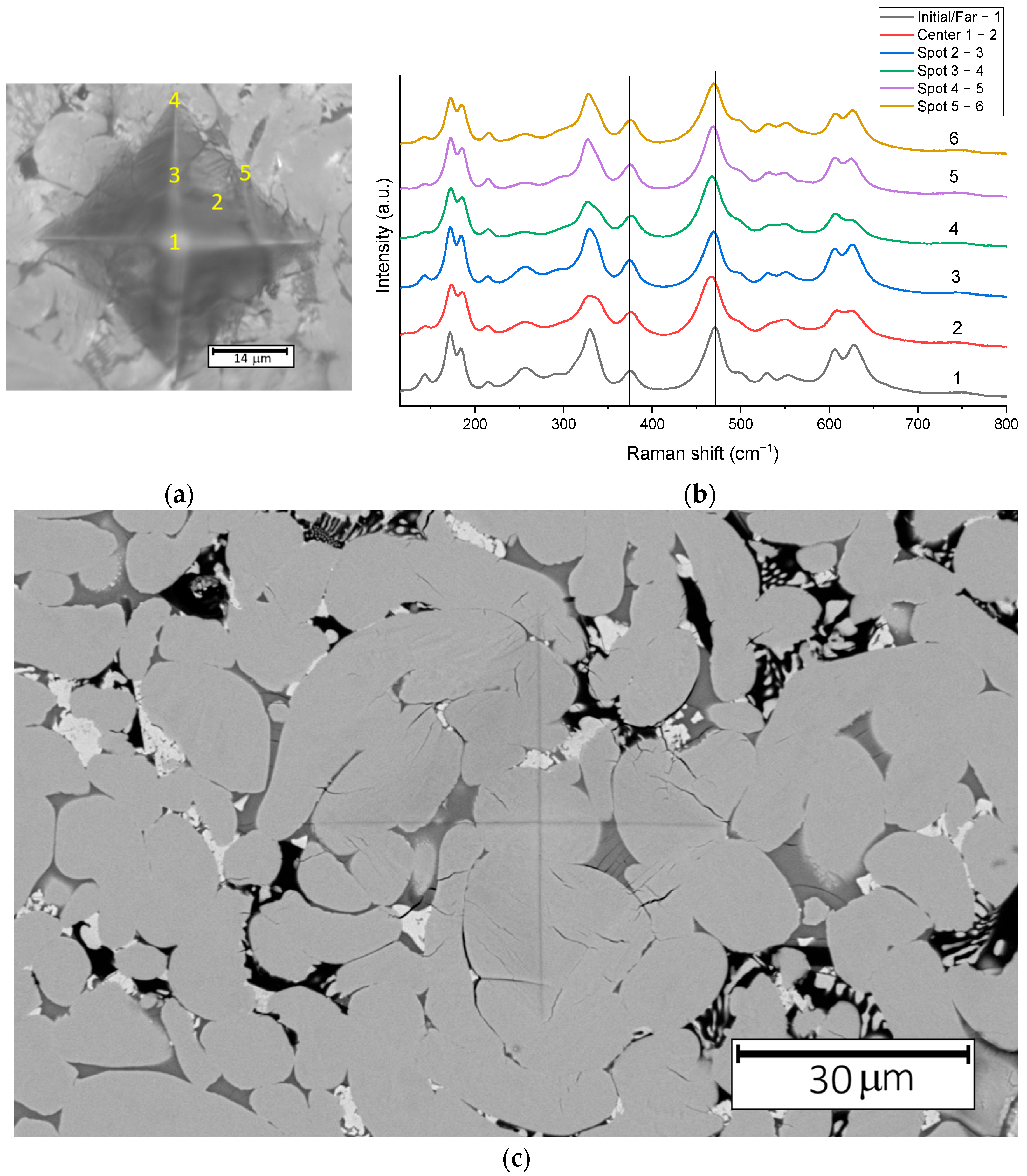
Figure 3a,c shows the indentation mark produced on Ce-doped ZrO
2 ceramics, captured using optical microscopy and scanning electron microscopy, respectively. No large cracks, typically observed at the corners of the indentation mark, were formed. Only small, short cracks are visible in the area of the indentation mark. This resistance to crack formation is attributed to the use of partially stabilized zirconia, known for its enhanced fracture toughness [
27,
28]. Crack propagation in such material is suppressed by transformational toughening. In partially stabilized zirconia containing a tetragonal phase, stresses near the crack tip trigger a transformation to the monoclinic phase, which is associated with a volume increase that acts to resist further crack growth [
29,
30].
Figure 3b depicts Raman spectra obtained from different regions of the indentation mark. The numbers correspond to specific spots: 1—center of the indentation, 2—inside the indentation, 3—midpoint of the distance from the vertex to the center, 4—vertex of the rhombus, 5—midpoint of the side of the rhombus. From the Raman spectra, it can be seen that there are no significant changes in the phase composition after indentation of the ceramics. However, shifts and broadening of certain peaks are observed compared to the non-indented area, which may indicate the presence of stress induced by the mechanical loading. In the following sections, we focus on two specific regions: the center of the indentation, where recrystallization is expected to be most pronounced due to the high localized stresses, and areas located sufficiently far from the indentation mark, where the material is assumed to be unaffected by indentation-induced stress.
To study recrystallization processes in Ce-ZrO
2 ceramics, annealing of pre-indented samples was performed.
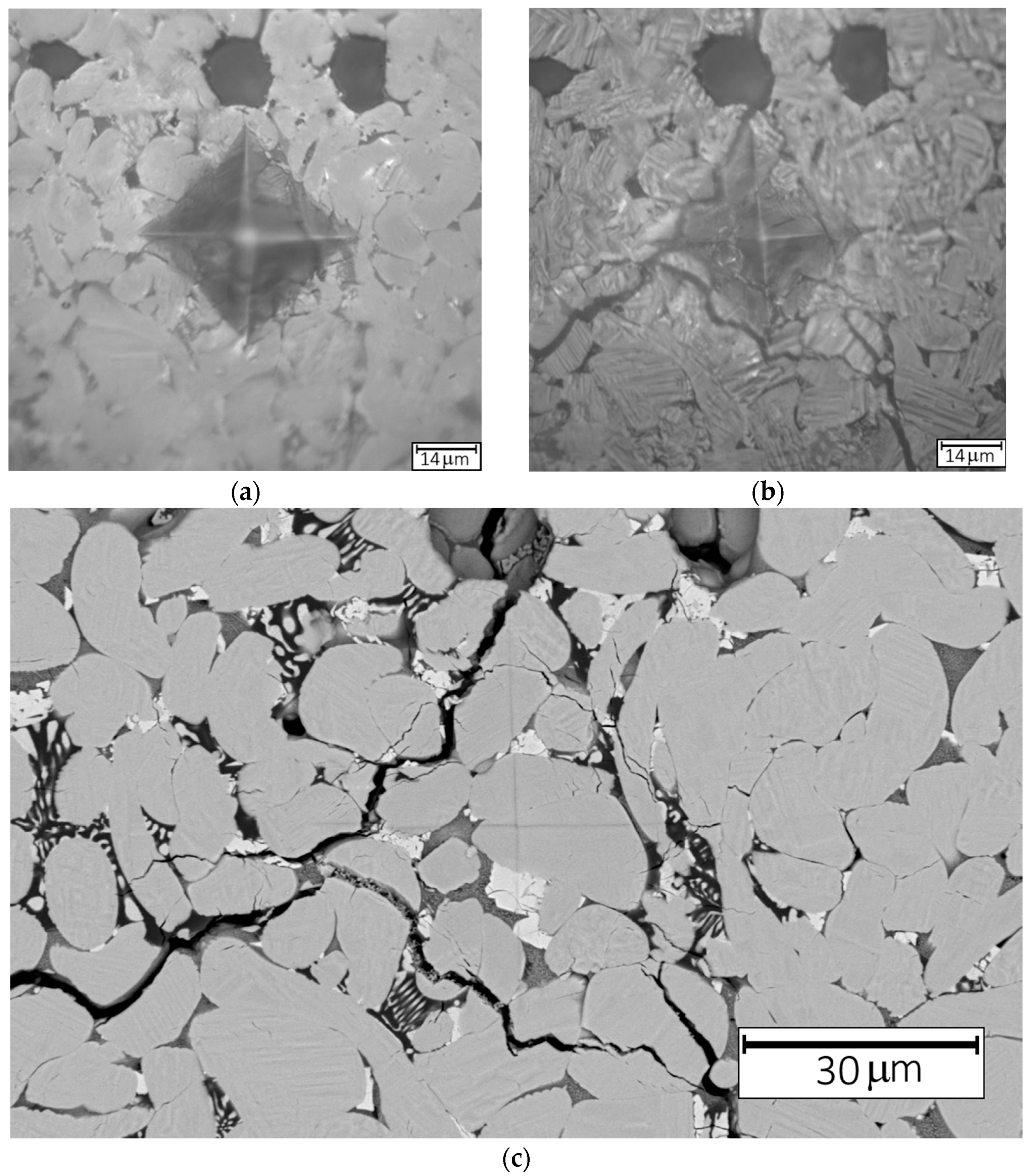
Figure 4a,b presents the microstructure of Ce-doped ZrO
2 ceramics before and after annealing at 1000 °C for 5 min, respectively.
Figure 4c shows SEM images of the indentation mark following the same annealing treatment. After annealing at 1000 °C, cracks were observed around the indentation marks in all samples. Notably, no such cracking was detected in the indentation areas of samples annealed at higher temperatures of 1100 °C and 1200 °C. This result can be explained by considering several contributing factors. First, the annealing temperature of 1000 °C may not have been sufficient to fully induce the monoclinic-to-tetragonal phase transformation. As a result, a high monoclinic phase content remained, reducing the mechanical stability of the material and increasing its brittleness. This likely led to post-annealing volume expansion due to residual transformation mismatch, ultimately causing microcracking around the indentation marks. In addition, at higher annealing temperatures, such as 1100 °C and 1200 °C, enhanced grain boundary mobility and slight creep can occur, allowing the material to accommodate strain more effectively. This can also lead to transient stress dissipation before cooling. In contrast, at 1000 °C, the material lacks sufficient mobility to relax these stresses within the short annealing duration, leading to residual stress buildup and subsequent cracking.
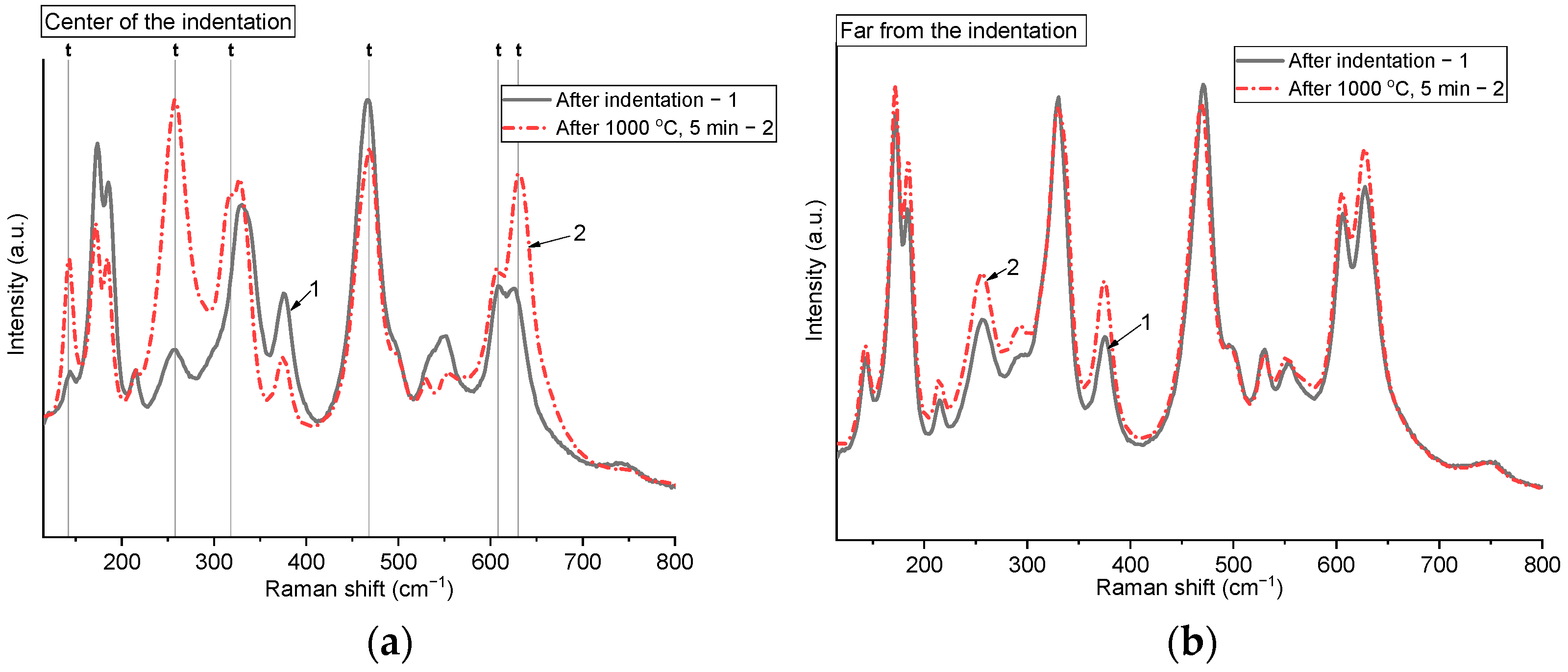
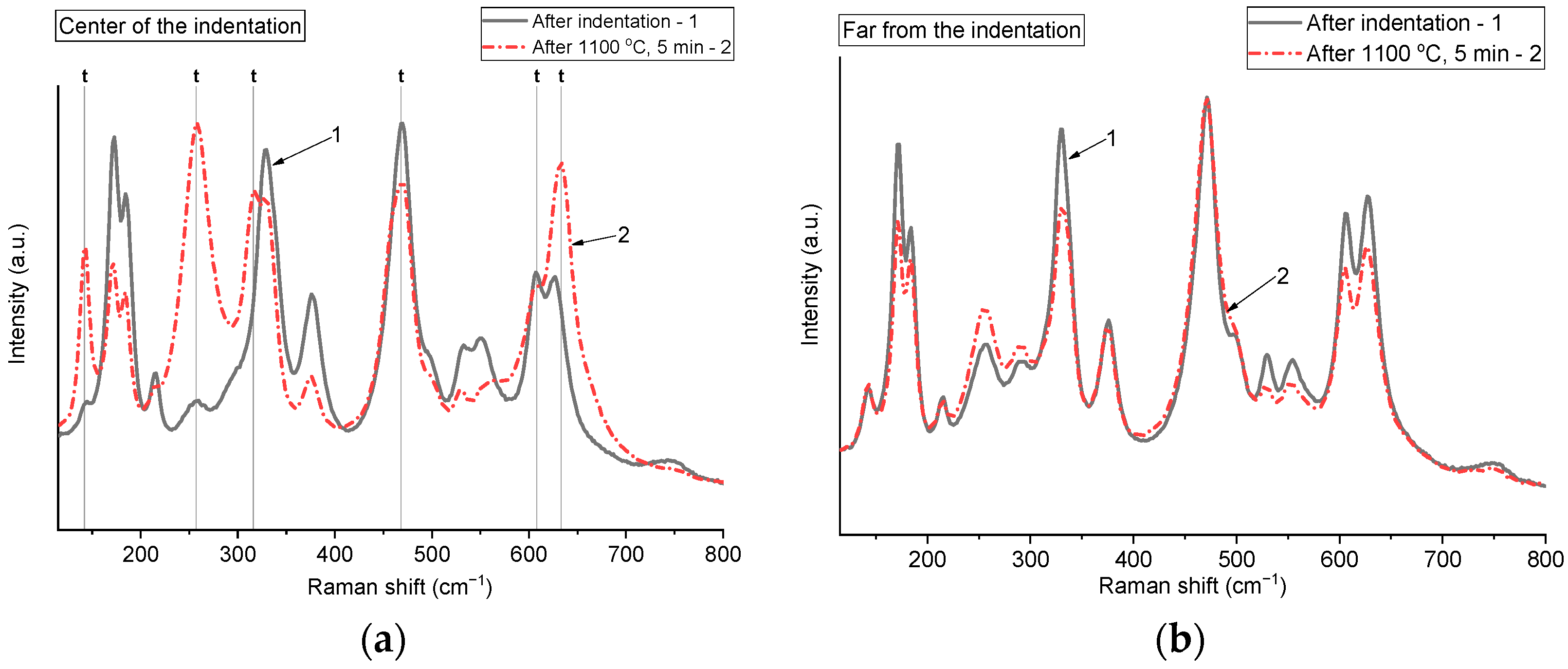
Figure 5 demonstrates a comparison of the Raman spectra collected from pre-indented samples before and after annealing, recorded at the center of the indentation and at a significant distance from the indentation mark. Raman spectra before and after annealing, obtained from spots located far from the indentation mark, are almost identical. By contrast, the spectra recorded before and after annealing from the indentation center show clear differences. Before annealing, the monoclinic phase was more prevalent in the center of the indentation mark, as evidenced by the significant intensity of the peaks at 178 and 191 cm
−1. After annealing for 5 min at 1000 °C, an increase in the relative intensity of the peak at 250 cm
−1 is observed, which characterizes the tetragonal phase. This indicates that annealing led to a phase transition m → t being triggered in the stressed area of the indentation center. Shifts in certain peaks after annealing are also evident, which may be connected to the formation of stress-free, relaxed grains during recrystallization.
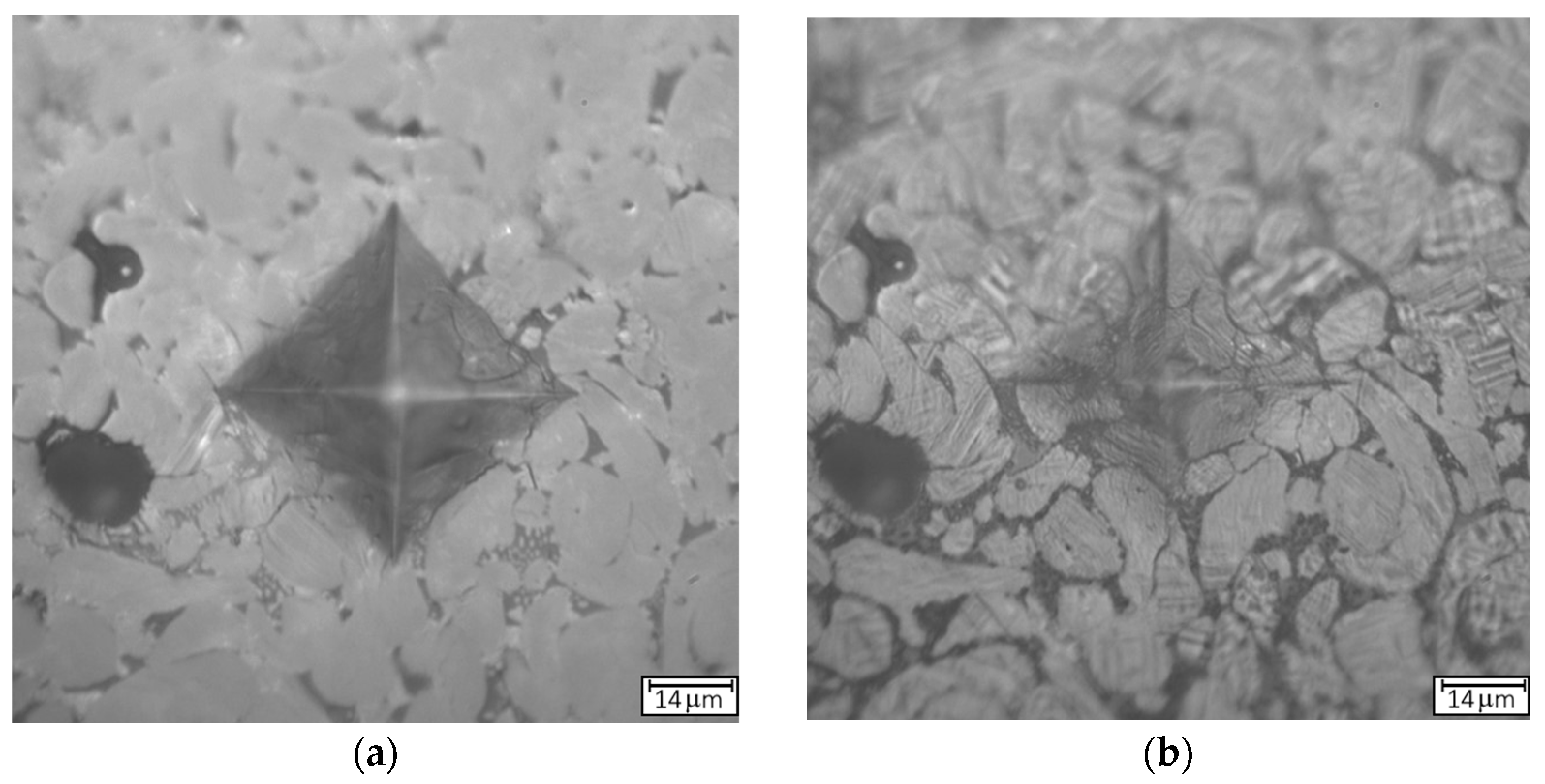
Figure 6a,b provides optical images illustrating the morphological changes in the indentation mark after annealing at 1100 °C for 5 min. In
Figure 6c, an SEM micrograph of the microstructure of the indentation after the annealing is shown. After annealing, the surface roughness increased, and partial recovery of the indentation mark was observed.
Figure 7 presents a comparison of Raman spectra of the ceramics before and after 5-min annealing at 1100 °C, collected at the center of the indentation and at a location distant from the indentation mark. These results are similar to those obtained at the lower annealing temperature of 1000 °C. An increase in the relative intensities of tetragonal peaks was also detected, indicating the formation of a tetragonal-rich zone in the center of the indentation mark. In the non-indented area, the relative intensity of the tetragonal phase peaks became slightly higher than before annealing. This may indicate that, as the temperature rises, phase transformations occur not only in the indentation area but also across the entire surface or volume of the ceramic.
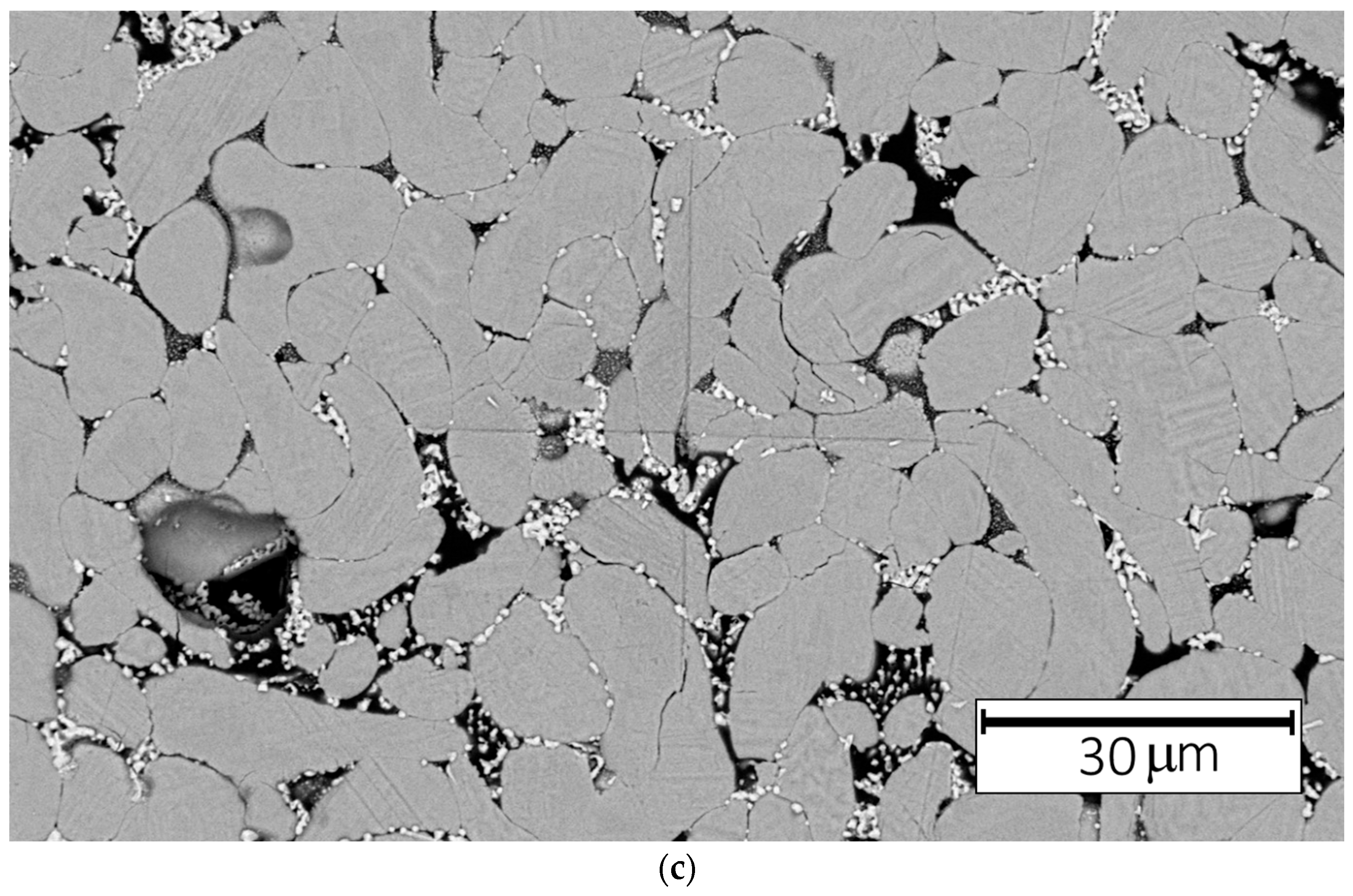
Figure 8a,b displays the microstructural changes in the indentation mark area after annealing at 1200 °C for 5 min.
Figure 8c illustrates an SEM micrograph of the indentation mark following the same annealing treatment. Following annealing, the surface of the indentation exhibits a pronounced grainy texture, attributed to the coarsening of grains that had previously experienced stress. In addition, an increase in the size of grains located within the intergranular regions between larger grains can also be observed.
Figure 9a presents a comparison of the Raman spectra of the ceramics before annealing, while
Figure 9b displays optical and SEM images, respectively, of the sample after annealing at 1200 °C for 5 min. In this case, the initially selected area contained a higher fraction of the tetragonal phase compared to the samples annealed at 1000 °C and 1100 °C. Nevertheless, a clear trend is still observed: the region subjected to the highest stress (the center of the indentation) underwent a more pronounced monoclinic-to-tetragonal phase transformation. The measurement spots were precisely identified, and Raman spectra before and after annealing were recorded at the exact same locations. Unlike lower annealing temperatures, in this case, only peaks of the tetragonal phase of ZrO
2 are observed in the Raman spectra of the indentation center after annealing, indicating that the phase transformation in this area has been completed. At the same time, peaks of the monoclinic phase were recorded on spectra obtained far from the indentation mark. After annealing, the intensity of the tetragonal phase also increased in the spectra recorded outside the indentation area, indicating that the tetragonal phase content increased not only in the stressed region but throughout the entire sample.
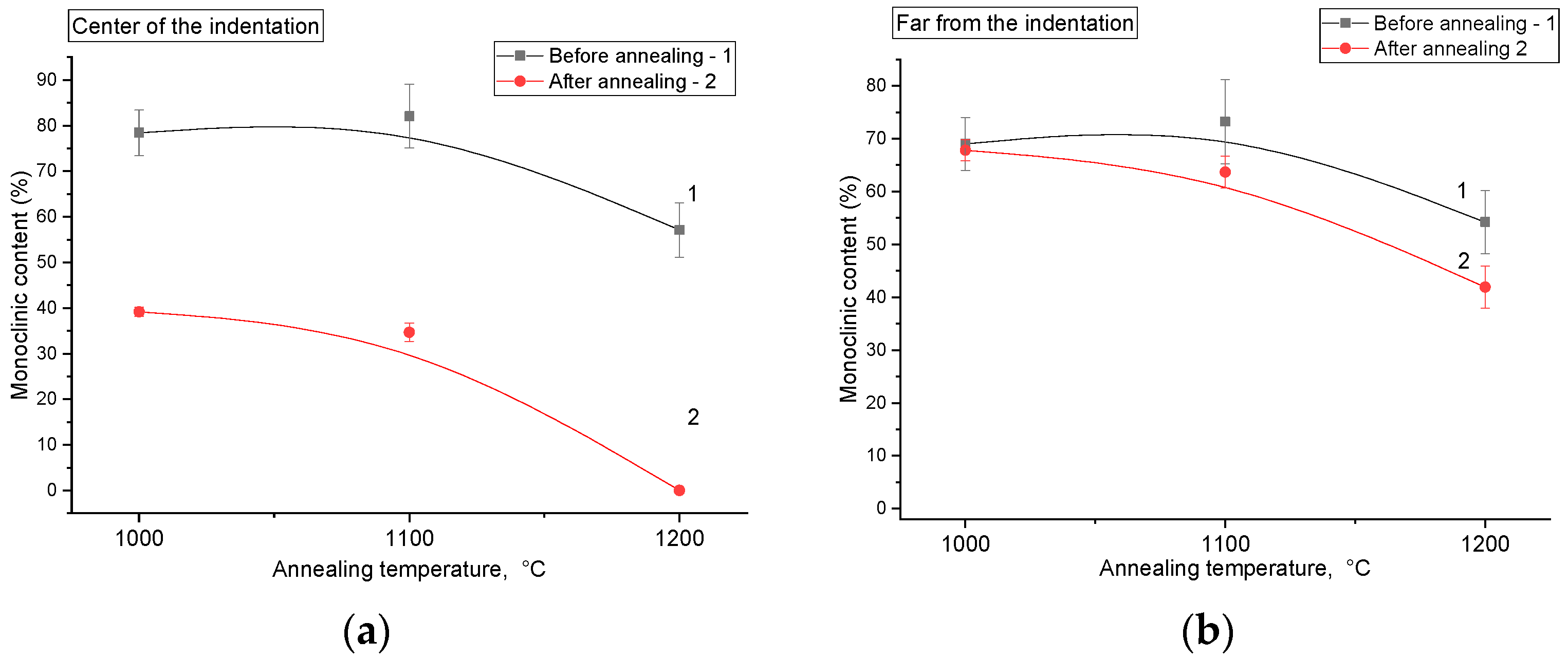
In
Figure 10, changes in monoclinic phase content based on Raman spectroscopy, calculated using Equation (1) are presented. It is important to emphasize that these values do not represent true phase compositions but rather serve as relative parameters based on the intensity ratio of monoclinic and tetragonal peaks, used to illustrate the overall trend. It can be clearly seen that the monoclinic phase content in the center of the indentation changes substantially (by several tens of percent) after annealing, whereas the monoclinic content in regions far from indentation undergoes only minor changes.
It was also important to track the phase composition not only in the surface layer but also at a certain depth in the samples. For this purpose, the X-ray diffraction method was used.
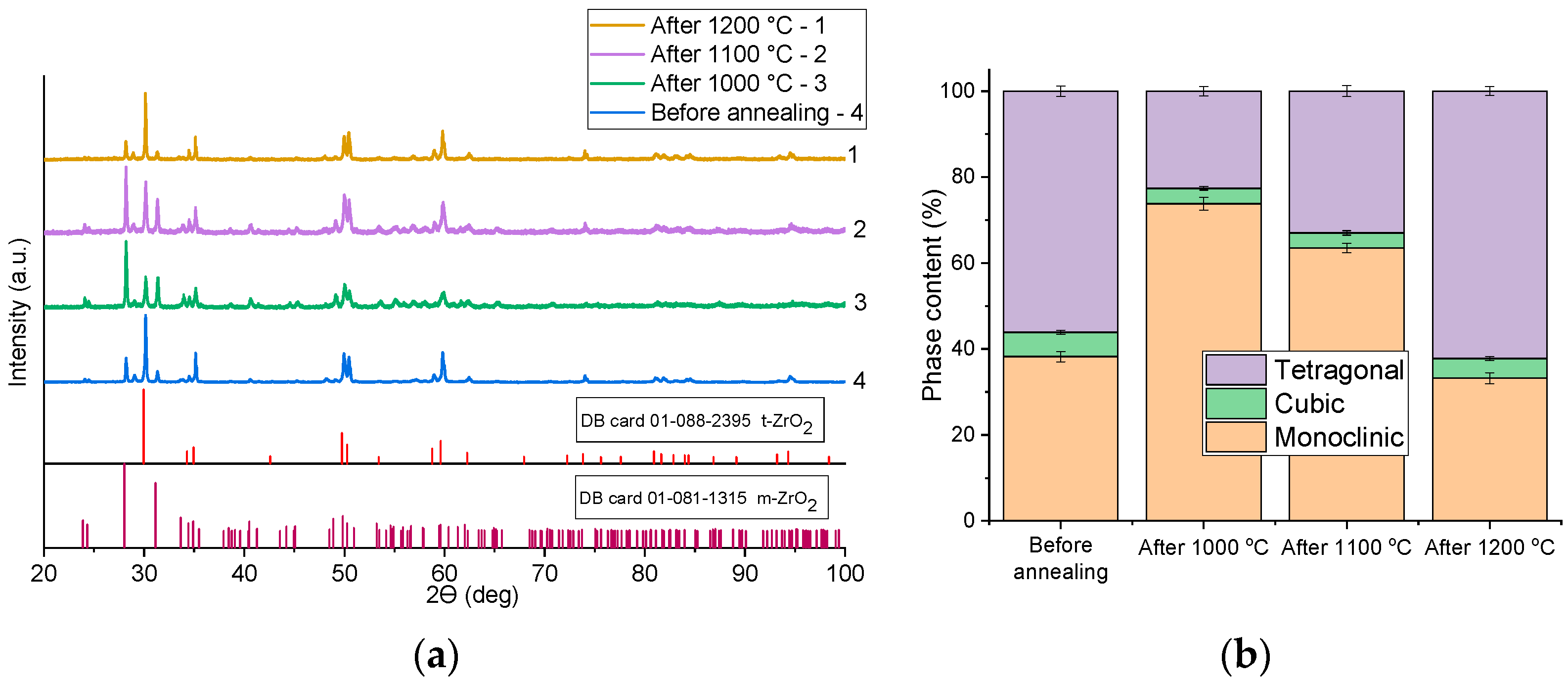
Figure 11a,b shows XRD patterns and comparisons of the phase composition after annealing at different temperatures of Ce-doped ZrO
2 ceramics. The samples in all experiments contained tetragonal, monoclinic, and cubic phases. Interestingly, after annealing at 1000 °C, the proportion of the tetragonal phase in the samples decreased from 56% to 22%, while the proportion of the monoclinic phase increased from 38% to 74%. At the same time, with an increase in annealing temperature, the proportion of the tetragonal phase increased from 22% to 62%. However, it should be noted that although the proportion of the tetragonal phase increased overall with increasing annealing temperature, Raman spectroscopy showed the most pronounced difference before/after annealing precisely in the center of the indentation mark, suggesting that the stresses created in the sample can have a significant effect on recrystallization processes in samples during repeated heat treatments after sintering. According to XRD analysis, only annealing at 1200 °C resulted in an increase in the tetragonal phase content, while annealing at 1000 °C and 1100 °C led to an increase in the monoclinic phase fraction. This can be explained as follows: after polishing, a monoclinic surface layer formed on the samples. During subsequent annealing, this monoclinic layer may continue to grow, leading to an overall increase in monoclinic phase content. At 1000–1100 °C, the surface is free to transform further to the monoclinic due to the absence of constraints, and the monoclinic phase is thermodynamically stable at these temperatures. This could result in a higher monoclinic fraction (mostly near the surface) after annealing. This observation is consistent with the XRD results, which indicated an increase in monoclinic content, while Raman spectroscopy (sensitive primarily to the thin surface layer) showed minimal change, likely because the monoclinic phase was already present on the polished surface prior to annealing. As for annealing at 1200 °C, in pure ZrO
2 the monoclinic phase transforms to the tetragonal phase at around 1170 °C. Therefore, in this case, the transformation barrier was achieved, and the fraction of the tetragonal phase increased.
The obtained results suggest that residual stresses induced after applying mechanical stress to the sample can stimulate a phase transition from the monoclinic phase to the tetragonal phase during annealing. This corresponds to an earlier finding that discovered that residual compressive strain in zirconia suppresses tetragonal to monoclinic transformation. In their study, the authors created compressive stress in a layer through As
+ bombardment, which opposed the lattice expansion required for the monoclinic phase formation, and this led to improved thermal stability [
31]. Additional evidence that high compressive strain promotes the stability of the tetragonal phase and can even trigger a reverse transformation from monoclinic to tetragonal was provided in another study. Notably, the same study also demonstrated that regions exposed to tensile or shear stresses were more likely to undergo the conventional transformation from tetragonal to monoclinic [
32].