High-Pressure Behavior and Thermal Stability of Water-Bearing TiO2-II Formed by Phase Transition of Natural Rutile
Abstract
1. Introduction
2. Sample Preparation and Experimental Methods
3. Results and Discussion
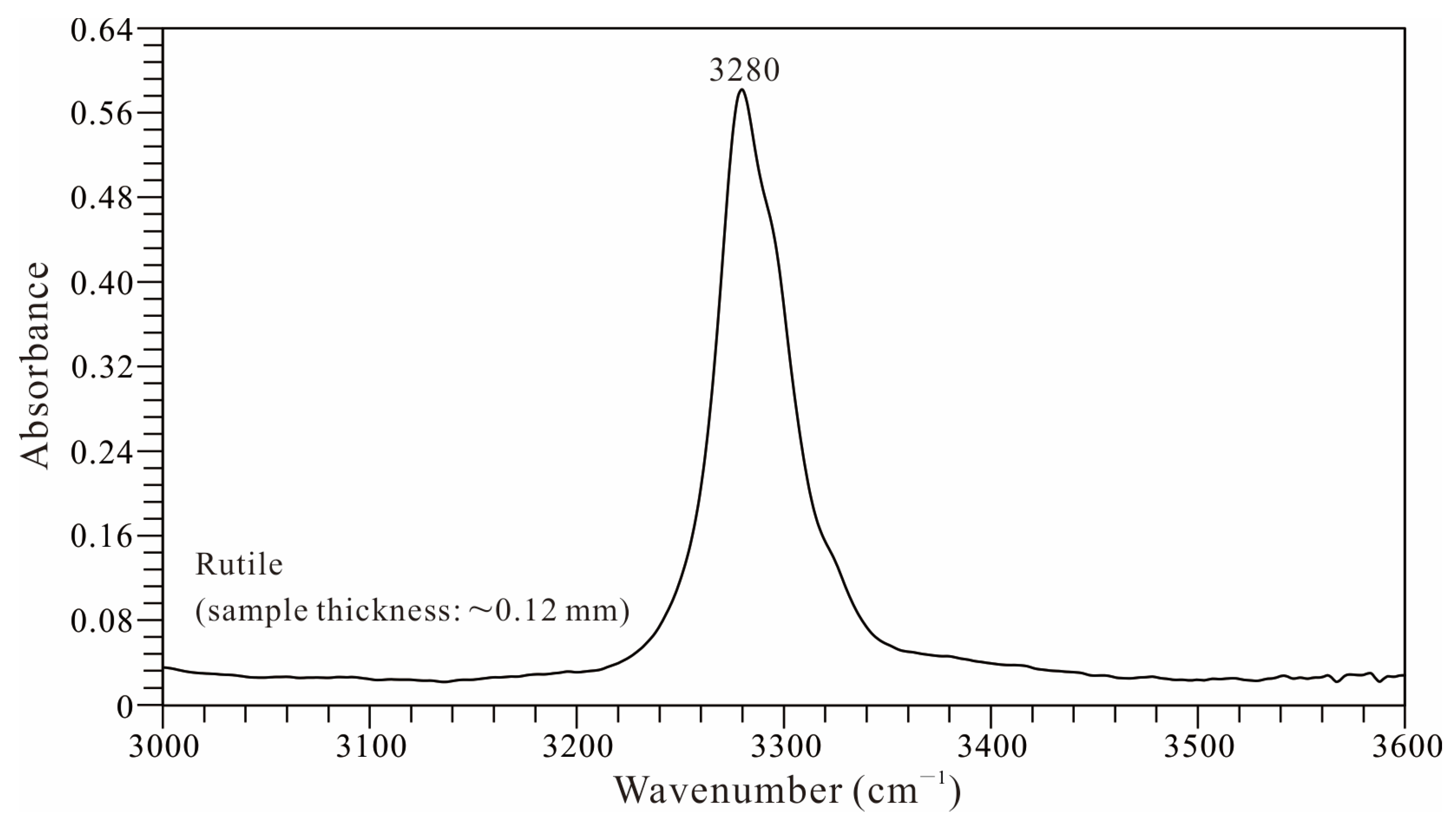
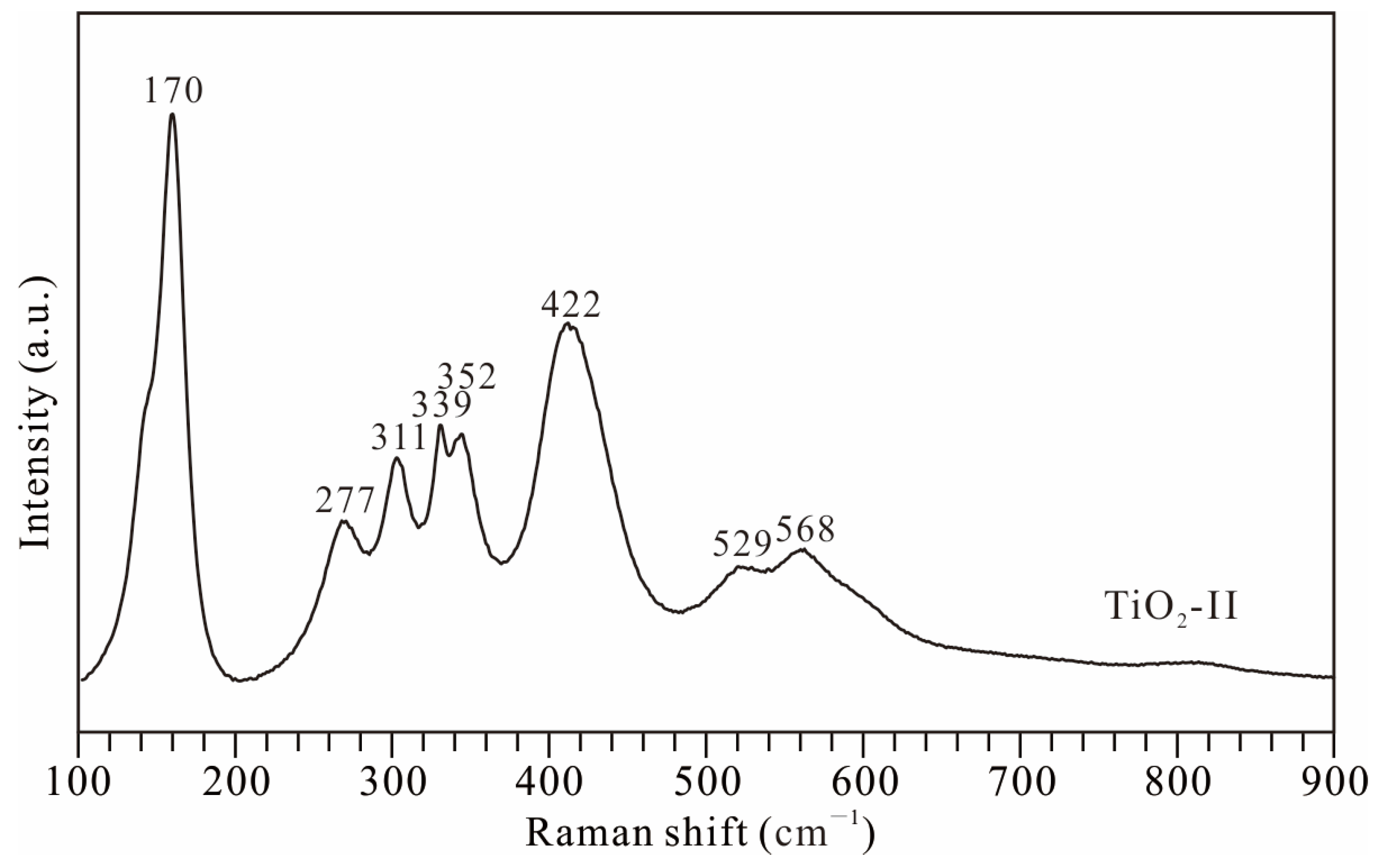
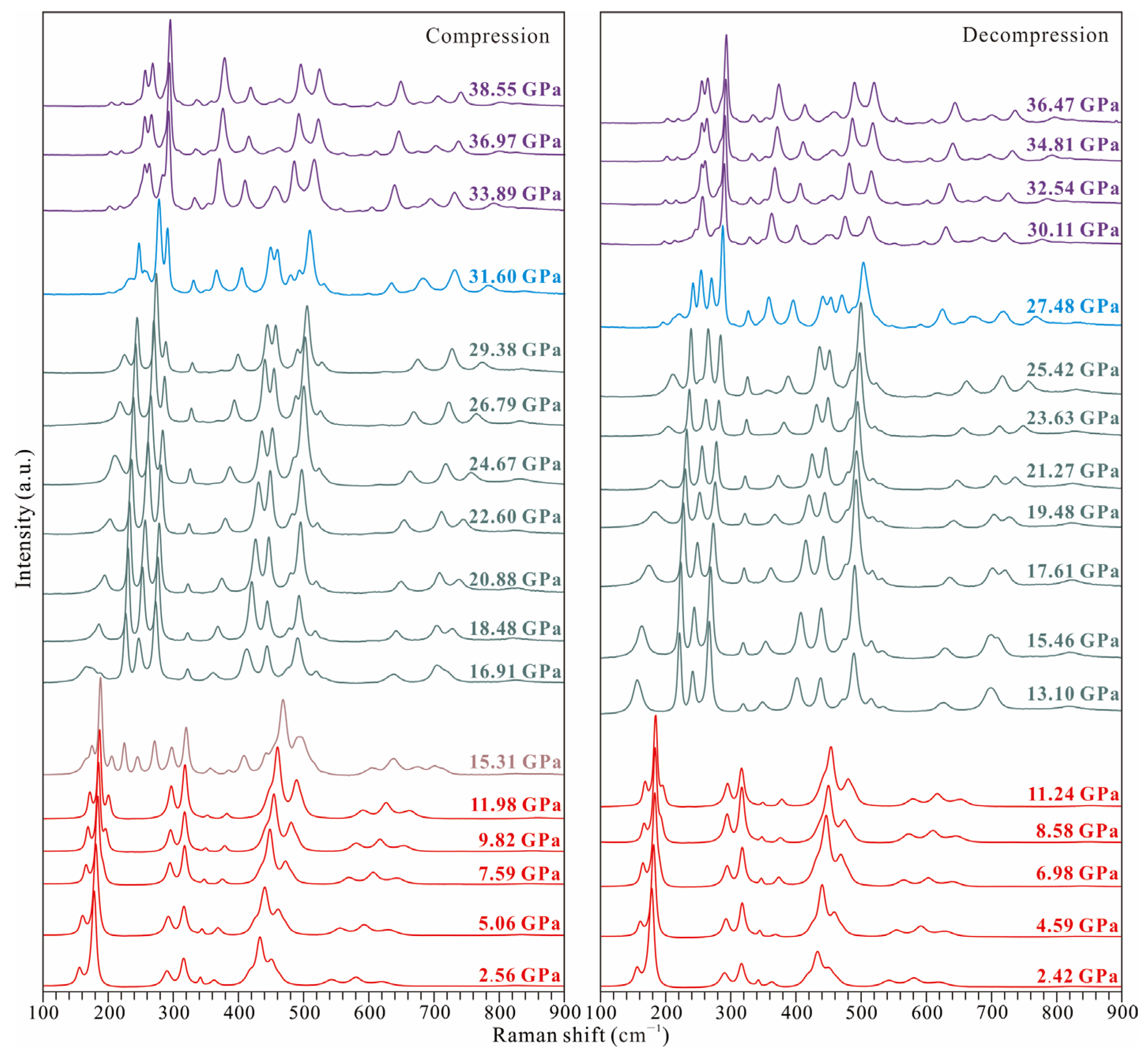
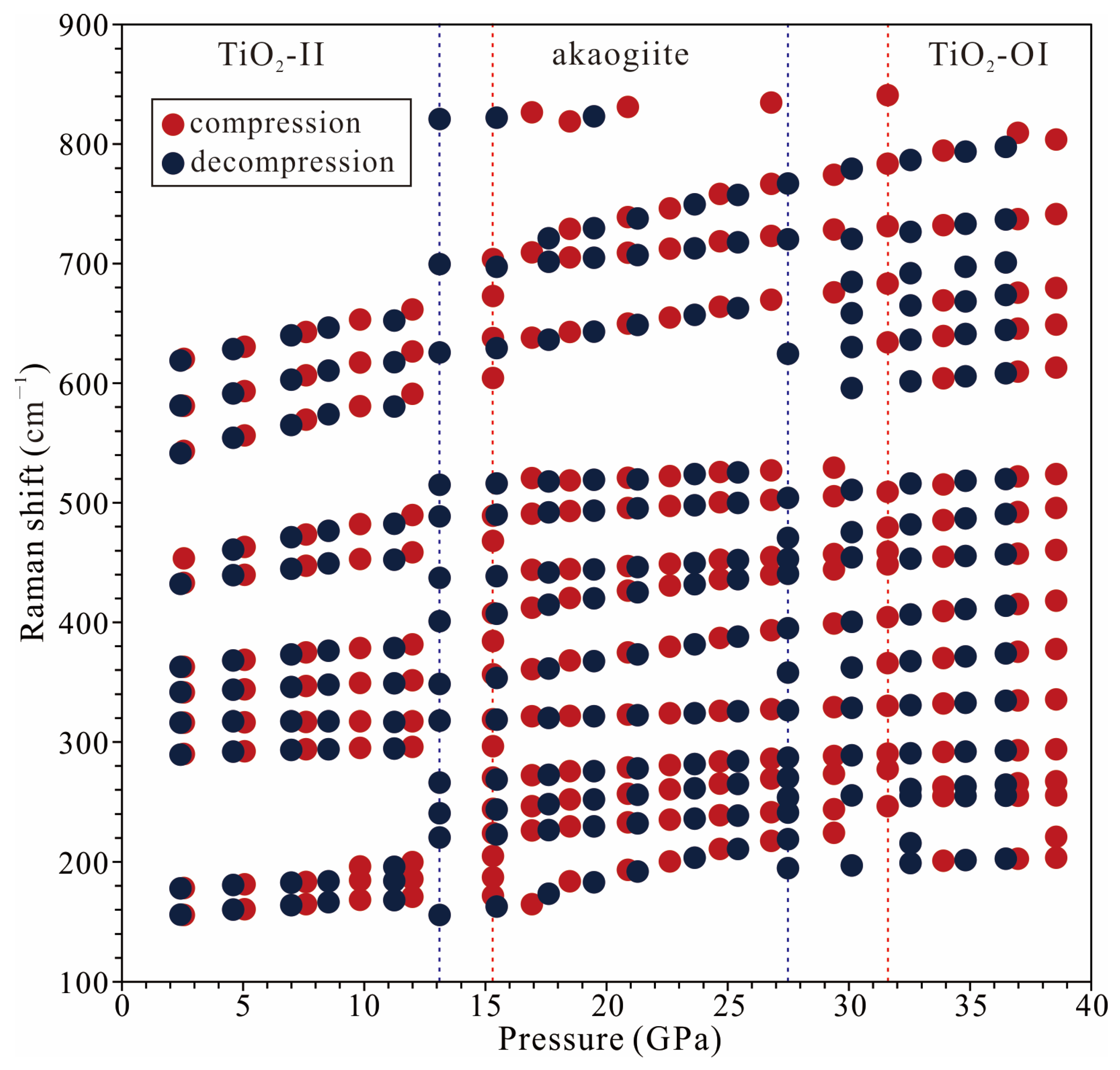
3.1. High-Pressure Behavior of TiO2-II
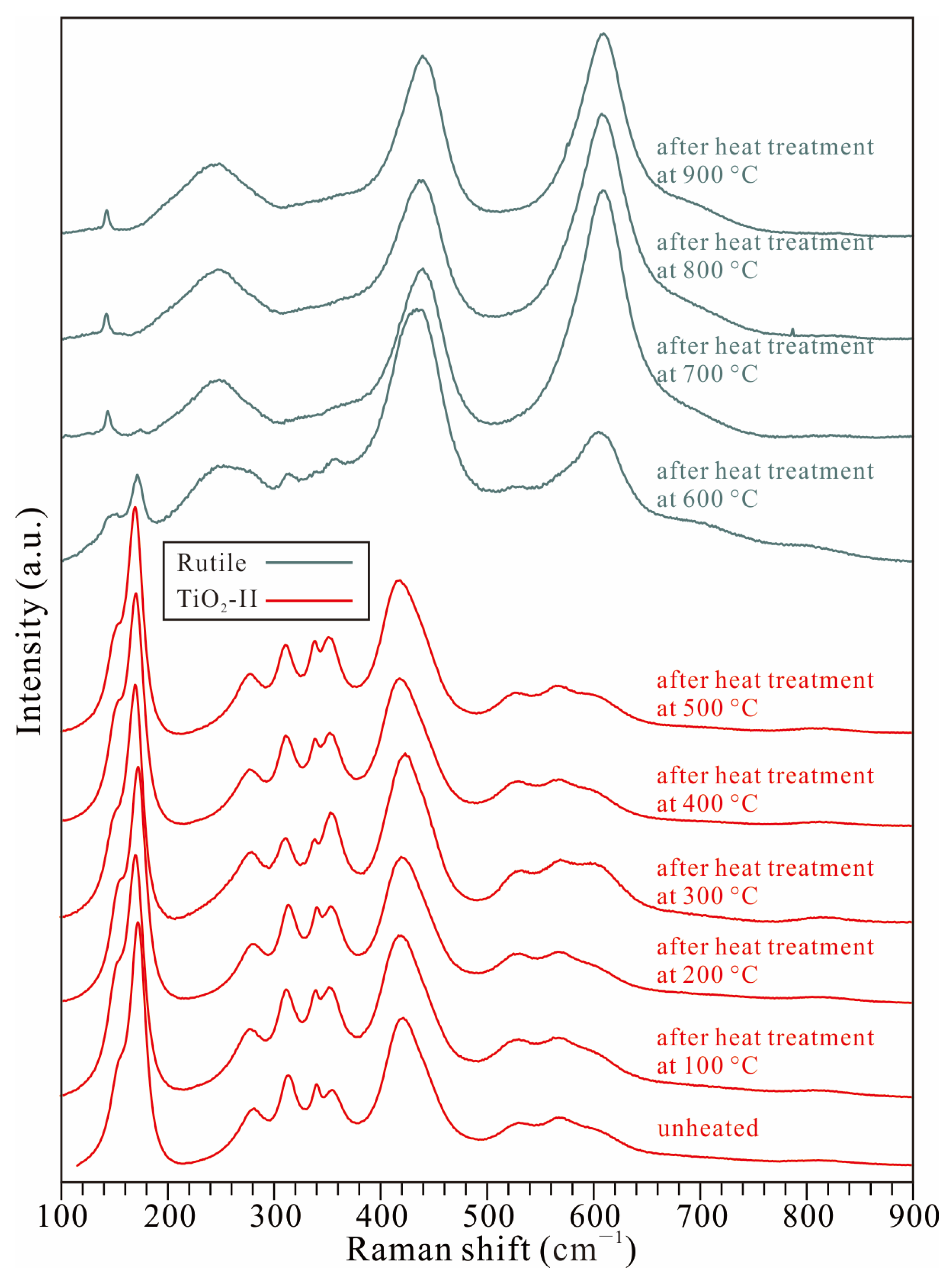
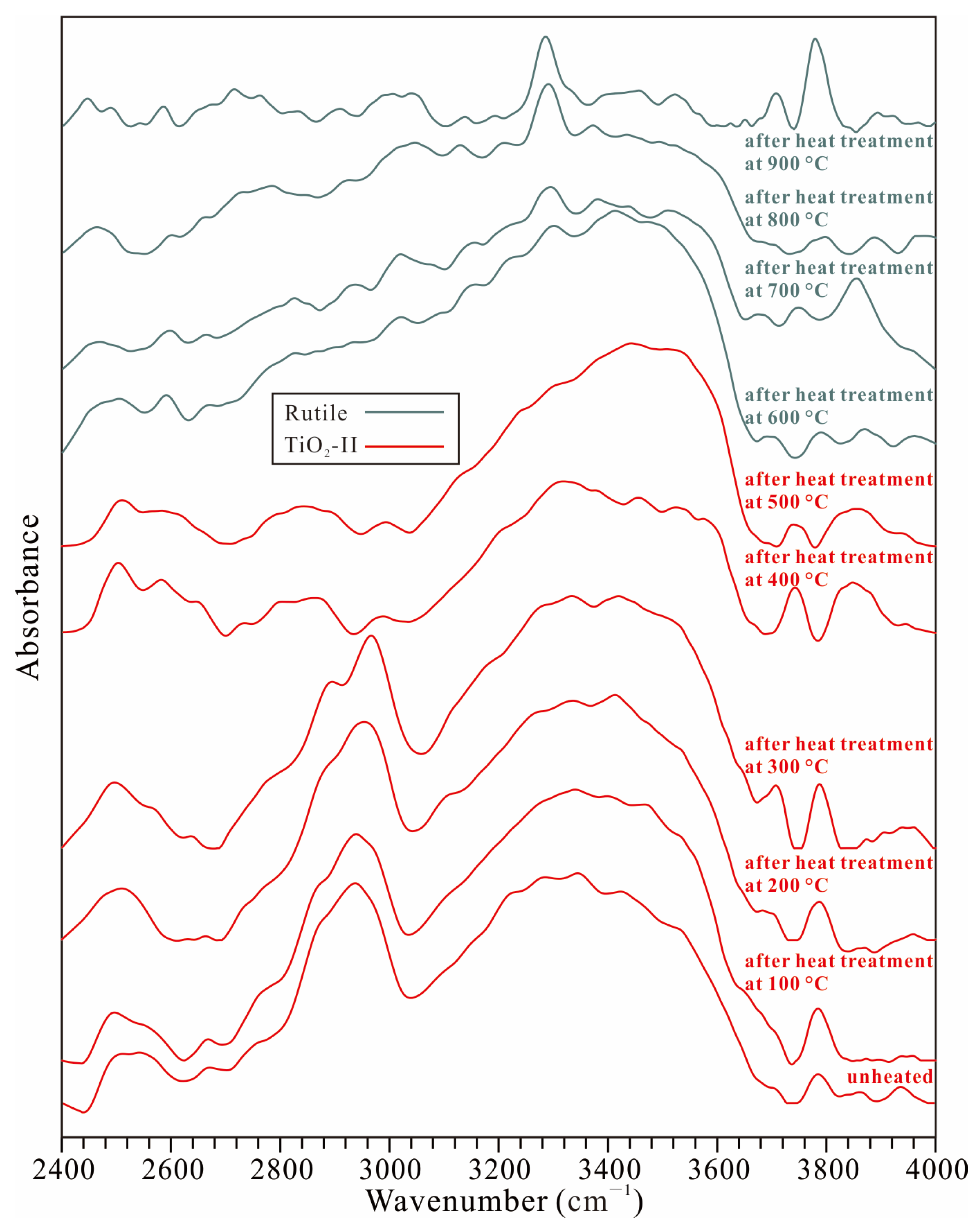
3.2. Thermal Stability of TiO2-II
4. Conclusions
- (1)
- At room temperature, water-bearing TiO2-II (with water content of up to 0.12 wt% H2O) transitions to akaogiite at pressures of approximately 12–15 GPa. Although this transition pressure is lower than the values previously reported for hydrous Al-bearing samples (>19 GPa), it is higher than those reported for anhydrous pure TiO2 samples (10 GPa). This suggests that water (hydrogen) can affect the pressure stability of TiO2-II.
- (2)
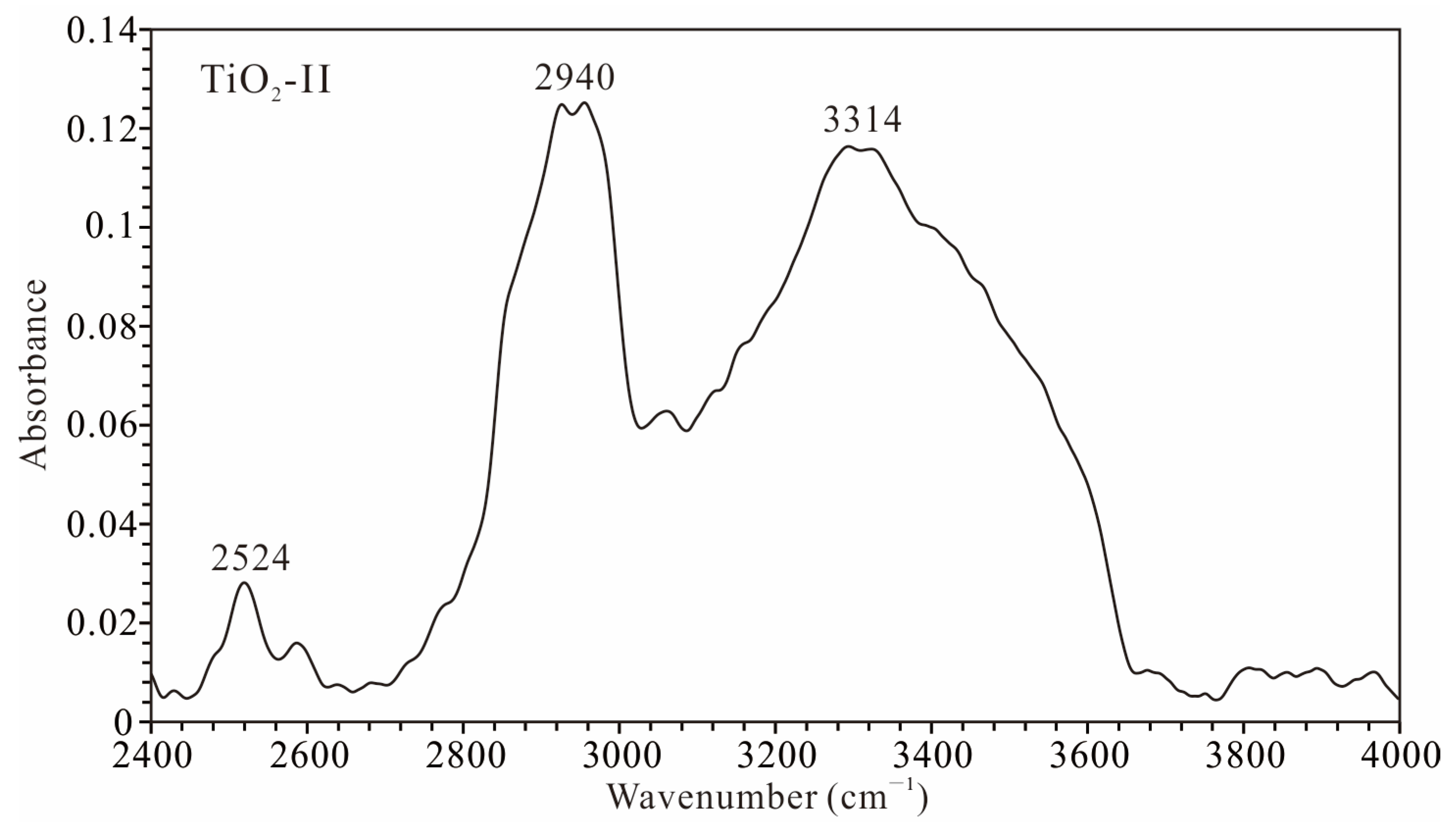
- Hydroxyl groups at distinct sites within the crystal structure of the TiO2-II sample display differing thermal stabilities. Although the sample underwent partial dehydration between 300 and 400 °C under atmospheric pressure, marked by the disappearance of the ~2900–3000 cm−1 OH band, the majority of the hydroxyl content persisted at higher temperatures (even after the phase transition from TiO2-II to rutile).
- (3)
- The transition temperature from water-bearing TiO2-II to rutile observed in this study is at least 130 °C lower than that reported for anhydrous pure TiO2-II and markedly lower than that for hydrous Al-bearing TiO2-II. The dehydration or partial dehydration of the water-bearing TiO2-II during heating may be the underlying reason for the phase transition from TiO2-II to rutile occurring at a lower temperature.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Al-Khatatbeh, Y.; Lee, K.K.M.; Kiefer, B. High-pressure behavior of TiO2 as determined by experiment and theory. Phys. Rev. B 2009, 79, 134114. [Google Scholar] [CrossRef]
- Liu, W.; Chen, J.; Zhang, X.; Yan, J.; Hou, M.; Kunz, M.; Zhang, D.; Zhang, H. Pressure-induced phase transitions of natural brookite. ACS Earth Space Chem. 2019, 3, 844–853. [Google Scholar] [CrossRef]
- Dachille, F.; Simons, P.Y.; Roy, R. Pressure-temperature studies of anatase, brookite, rutile and TiO2-II. Am. Mineral. 1968, 53, 1929–1939. [Google Scholar]
- Vlassopoulos, D.; Rossman, G.R.; Haggerty, S.E. Coupled substitution of H and minor elements in rutile and the implications of high OH contents in Nb- and Cr-rich rutile from the upper mantle. Am. Mineral. 1993, 78, 1181–1191. [Google Scholar]
- Johnson, E.A. Water in nominally anhydrous crustal minerals: Speciation, concentration, and geologic significance. Rev. Mineral. Geochem. 2006, 62, 117–154. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, Q.; Feng, M.; Gu, X. In situ FTIR investigations at varying temperatures on hydrous components in rutile. Am. Mineral. 2011, 96, 1851–1855. [Google Scholar] [CrossRef]
- Jamieson, J.C.; Olinger, B. High-pressure polymorphism of titanium dioxide. Science 1968, 161, 893–895. [Google Scholar] [CrossRef]
- Sato, H.; Endo, S.; Sugiyama, M.; Kikegawa, T.; Shimomura, O.; Kusaba, K. Baddeleyite-type high-pressure phase of TiO2. Science 1991, 251, 786–788. [Google Scholar] [CrossRef]
- Dubrovinskaia, N.A.; Dubrovinsky, L.S.; Ahuja, R.; Prokopenko, V.B.; Dmitriev, V.; Weber, H.-P.; Osorio-Guillen, J.M.; Johansson, B. Experimental and theoretical identification of a new high-pressure TiO2 polymorph. Phys. Rev. Lett. 2001, 87, 275501. [Google Scholar] [CrossRef] [PubMed]
- El Goresy, A.; Chen, M.; Gillet, P.; Dubrovinsky, L.; Graup, G.; Ahuja, R. A natural shock-induced dense polymorph of rutile with α-PbO2 structure in the suevite from the Ries crater in Germany. Earth Planet. Sci. Lett. 2001, 192, 485–495. [Google Scholar] [CrossRef]
- Dawei, M.; Xiuling, W.; Xiaoyu, F.; Zhengjie, Z.; Hong, C.; Xin, M.; Jianping, Z. High pressure response of rutile polymorphs and its significance for indicating the subduction depth of continental crust. Acta Geol. Sin. 2008, 82, 371–376. [Google Scholar] [CrossRef]
- Wu, X.; Meng, D.; Han, Y. α-PbO2-Type Nanophase of TiO2 from Coesite-bearing eclogite in the dabie mountains, China. Am. Mineral. 2005, 90, 1458–1461. [Google Scholar] [CrossRef]
- Gerward, L.; Staun Olsen, J. Post-rutile high-pressure phases in TiO2. J. Appl. Cryst. 1997, 30, 259–264. [Google Scholar] [CrossRef]
- Swamy, V.; Dubrovinskaia, N.A.; Dubrovinsky, L.S. Compressibility of baddeleyite-type TiO2 from static compression to 40 GPa. J. Alloys Compd. 2002, 340, 46–48. [Google Scholar] [CrossRef]
- Lu, X.; Gao, S.; Wu, P.; Zhang, Z.; Zhang, L.; Li, X.; Qin, X. In situ high-pressure raman spectroscopic, single-crystal X-ray diffraction, and FTIR investigations of rutile and TiO2II. Minerals 2023, 13, 703. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.; Ye, Y.; Liu, D.; Zhu, X.; Hu, Y.; Miao, Y.; Wu, Z.; Pan, Y. Al3+ and H+ substitutions in TiO2 polymorphs: Structural and vibrational investigations. Am. Mineral. 2025, 110, 241–254. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, Y.; Hoefs, J.; Liou, J.G.; Simon, K. Ultrahigh pressure metamorphic rocks from the Chinese Continental Scientific Drilling Project: I. Petrology and geochemistry of the main hole (0–2050 m). Contrib. Mineral. Petrol. 2006, 152, 421–441. [Google Scholar] [CrossRef]
- Maldener, J.; Rauch, F.; Gavranic, M.; Beran, A. OH absorption coefficients of rutile and cassiterite deduced from nuclear reaction analysis and FTIR spectroscopy. Miner. Petrol. 2001, 71, 21–29. [Google Scholar] [CrossRef]
- Libowitzky, E.; Rossman, G.R. An IR absorption calibration for water in minerals. Am. Mineral. 1997, 82, 1111–1115. [Google Scholar] [CrossRef]
- Purevjav, N.; Fei, H.; Ishii, T.; Criniti, G.; Lin, Y.; Mao, H.-K.; Katsura, T. Temperature dependence of H2O solubility in Al-free stishovite. Geophys. Res. Lett. 2024, 51, e2023GL104029. [Google Scholar] [CrossRef]
- Mao, H.K.; Xu, J.; Bell, P.M. Calibration of the ruby pressure gauge to 800 kbar under quasi-hydrostatic conditions. J. Geophys. Res. 1986, 91, 4673–4676. [Google Scholar] [CrossRef]
- Escudero, A.; Langenhorst, F. Aluminum incorporation in α-PbO2 type TiO2 at pressures up to 20 GPa. Phys. Earth Planet. Inter. 2012, 190–191, 87–94. [Google Scholar] [CrossRef]
- Guo, H. In-situ infrared spectra of OH in rutile up to 1000 °C. Phys. Chem. Miner. 2017, 44, 547–552. [Google Scholar] [CrossRef]
- Liu, M.; Wang, J.; Hu, J.; Liu, P.; Niu, H.; Yan, X.; Li, J.; Yan, H.; Yang, B.; Sun, Y.; et al. Layer-by-layer phase transformation in Ti3O5 revealed by machine-learning molecular dynamics simulations. Nat. Commun. 2024, 15, 3079. [Google Scholar] [CrossRef]
- Libowitzky, E. Correlation of O-H stretching frequencies and O-H…O hydrogen bond lengths in minerals. Monatsh. Chem. 1999, 130, 1047–1059. [Google Scholar] [CrossRef]
- Bromiley, G.D.; Hilairet, N. Hydrogen and minor element incorporation in synthetic rutile. Mineral. Mag. 2005, 69, 345–358. [Google Scholar] [CrossRef]
- Hirose, K.; Takafuji, N.; Sata, N.; Ohishi, Y. Phase Transition and density of subducted MORB crust in the lower mantle. Earth Planet. Sci. Lett. 2005, 237, 239–251. [Google Scholar] [CrossRef]
- Lin, Y.; Mao, H.-K. Dense hydrous silica carrying water to the deep Earth and promotion of oxygen fugacity heterogeneity. Matter Radiat. Extremes 2022, 7, 068101. [Google Scholar] [CrossRef]
- Nomura, R.; Hirose, K.; Sata, N.; Ohishi, Y. Precise determination of post-stishovite phase transition boundary and implications for seismic heterogeneities in the mid-lower mantle. Phys. Earth Planet. Inter. 2010, 183, 104–109. [Google Scholar] [CrossRef]
- Tsutsumi, Y.; Sakamoto, N.; Hirose, K.; Tagawa, S.; Umemoto, K.; Ohishi, Y.; Yurimoto, H. Retention of water in subducted slabs under core–mantle boundary conditions. Nat. Geosci. 2024, 17, 697–704. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Qu, S.; Ouyang, Y.; Cao, Y.; Fu, M.; Liu, J.; Zhang, L. High-Pressure Behavior and Thermal Stability of Water-Bearing TiO2-II Formed by Phase Transition of Natural Rutile. Crystals 2025, 15, 720. https://doi.org/10.3390/cryst15080720
Lu X, Qu S, Ouyang Y, Cao Y, Fu M, Liu J, Zhang L. High-Pressure Behavior and Thermal Stability of Water-Bearing TiO2-II Formed by Phase Transition of Natural Rutile. Crystals. 2025; 15(8):720. https://doi.org/10.3390/cryst15080720
Chicago/Turabian StyleLu, Xiaofeng, Shuo Qu, Yuanze Ouyang, Yifan Cao, Meiting Fu, Jinpu Liu, and Li Zhang. 2025. "High-Pressure Behavior and Thermal Stability of Water-Bearing TiO2-II Formed by Phase Transition of Natural Rutile" Crystals 15, no. 8: 720. https://doi.org/10.3390/cryst15080720
APA StyleLu, X., Qu, S., Ouyang, Y., Cao, Y., Fu, M., Liu, J., & Zhang, L. (2025). High-Pressure Behavior and Thermal Stability of Water-Bearing TiO2-II Formed by Phase Transition of Natural Rutile. Crystals, 15(8), 720. https://doi.org/10.3390/cryst15080720






