Crystal Structure of Anthranilate Phosphoribosyltransferase from Methanocaldococcus jannaschii
Abstract
1. Introduction
2. Materials and Methods
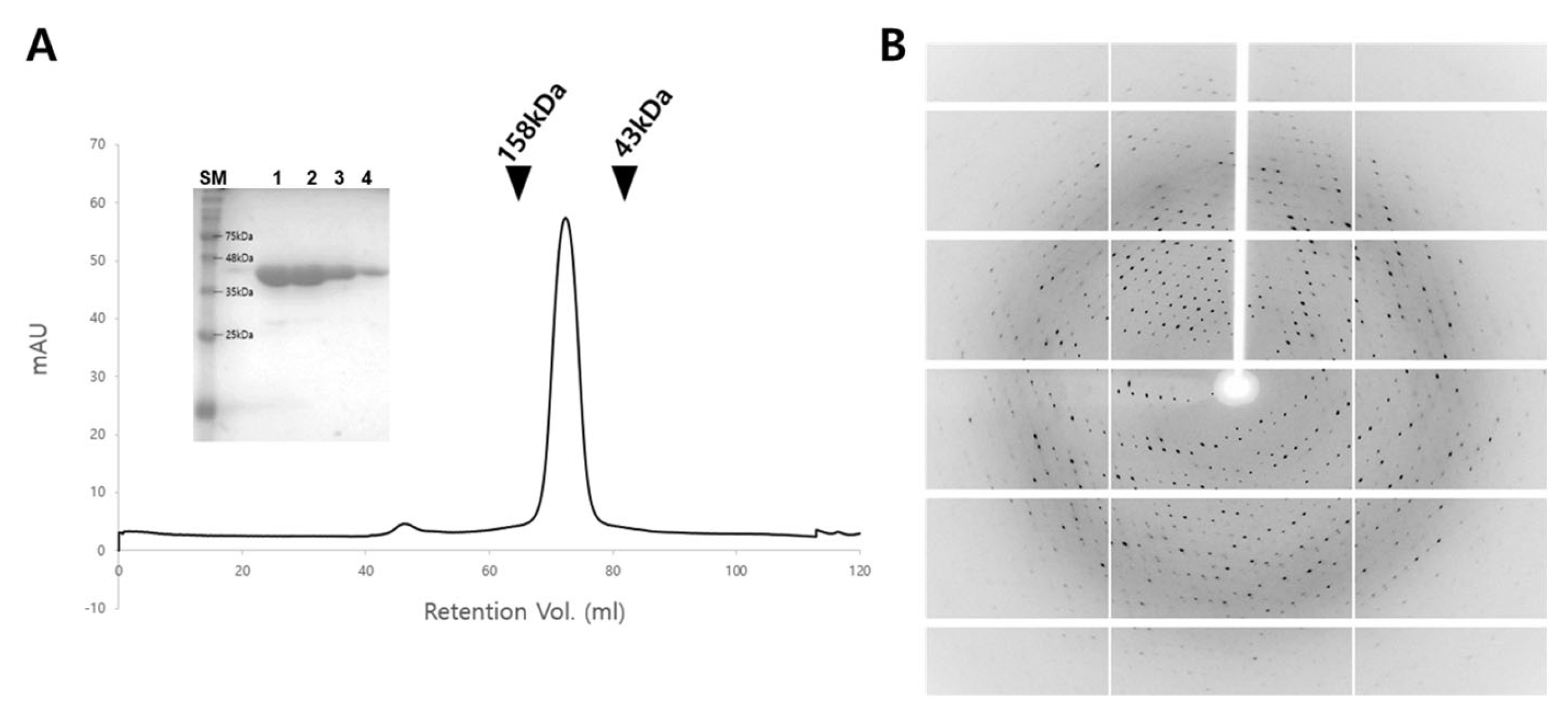
2.1. Cloning, Protein Expression and Purification
2.2. Crystallization
2.3. X-Ray Diffraction Data Collection
2.4. Structure Determination
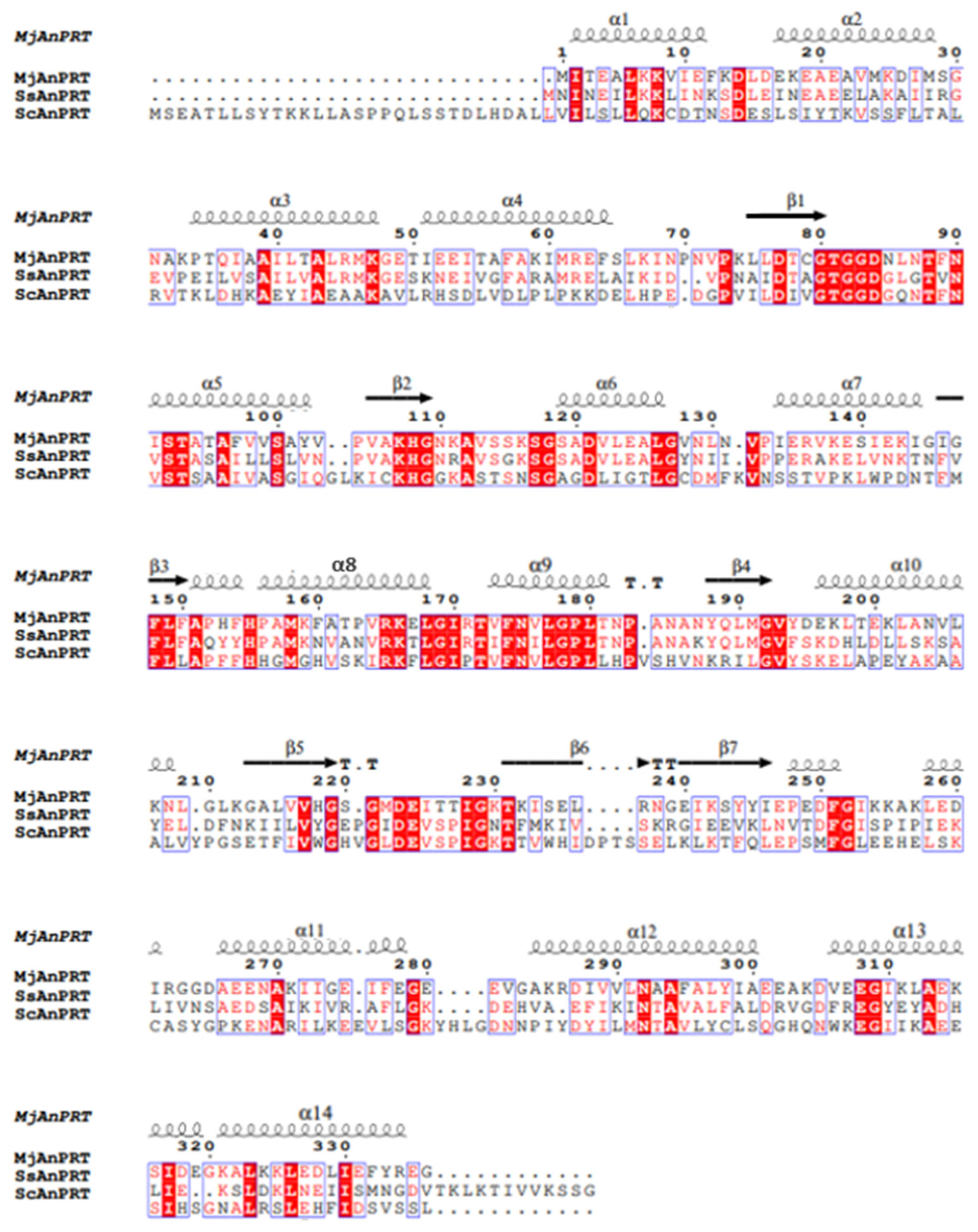
2.5. Bioinformatics and Structure Analysis
2.6. Preparation of B-Factor Analysis
3. Results
3.1. Structure Determination of MjAnPRT
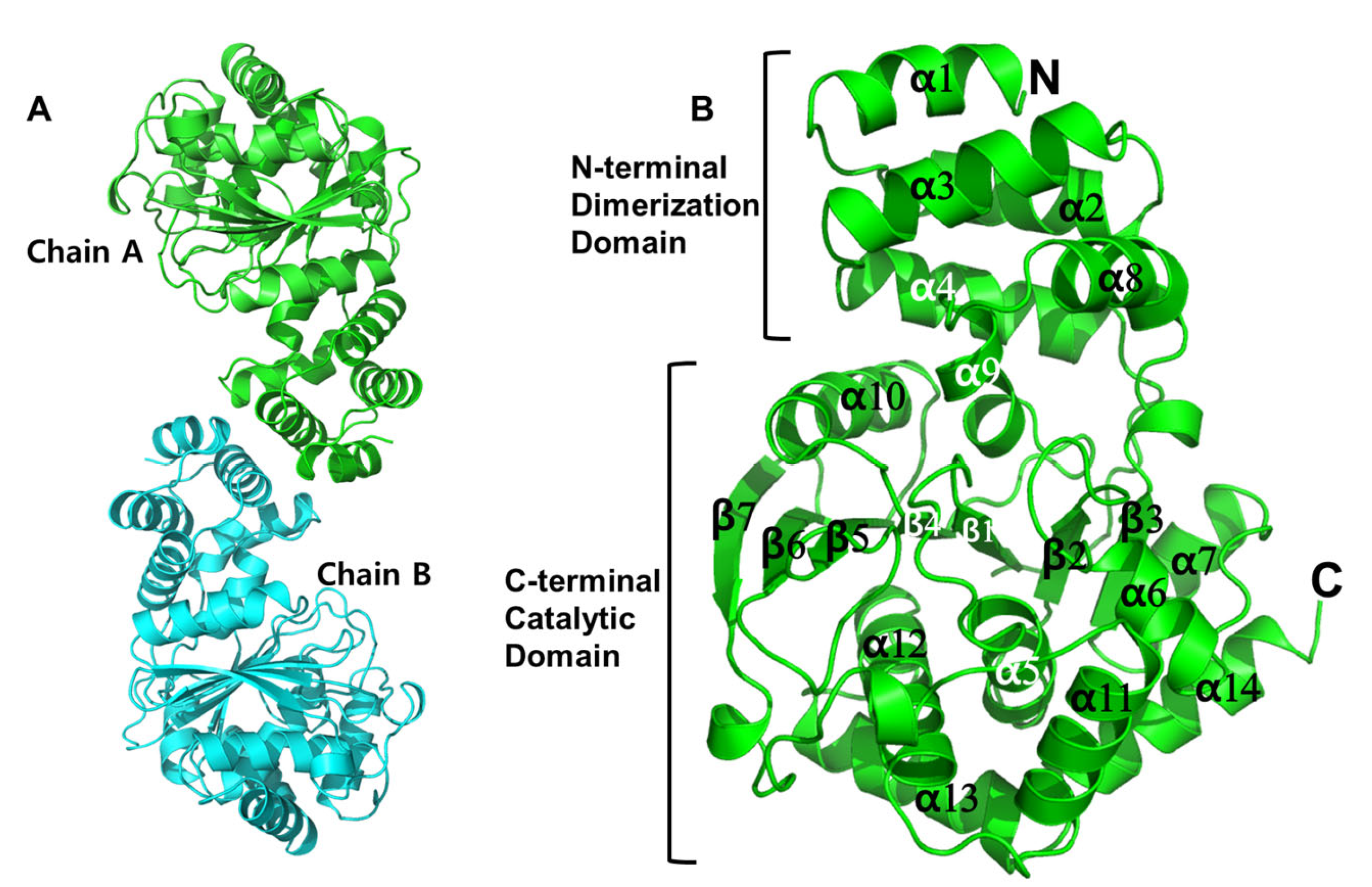
3.2. Crystal Structure of MjAnPRT
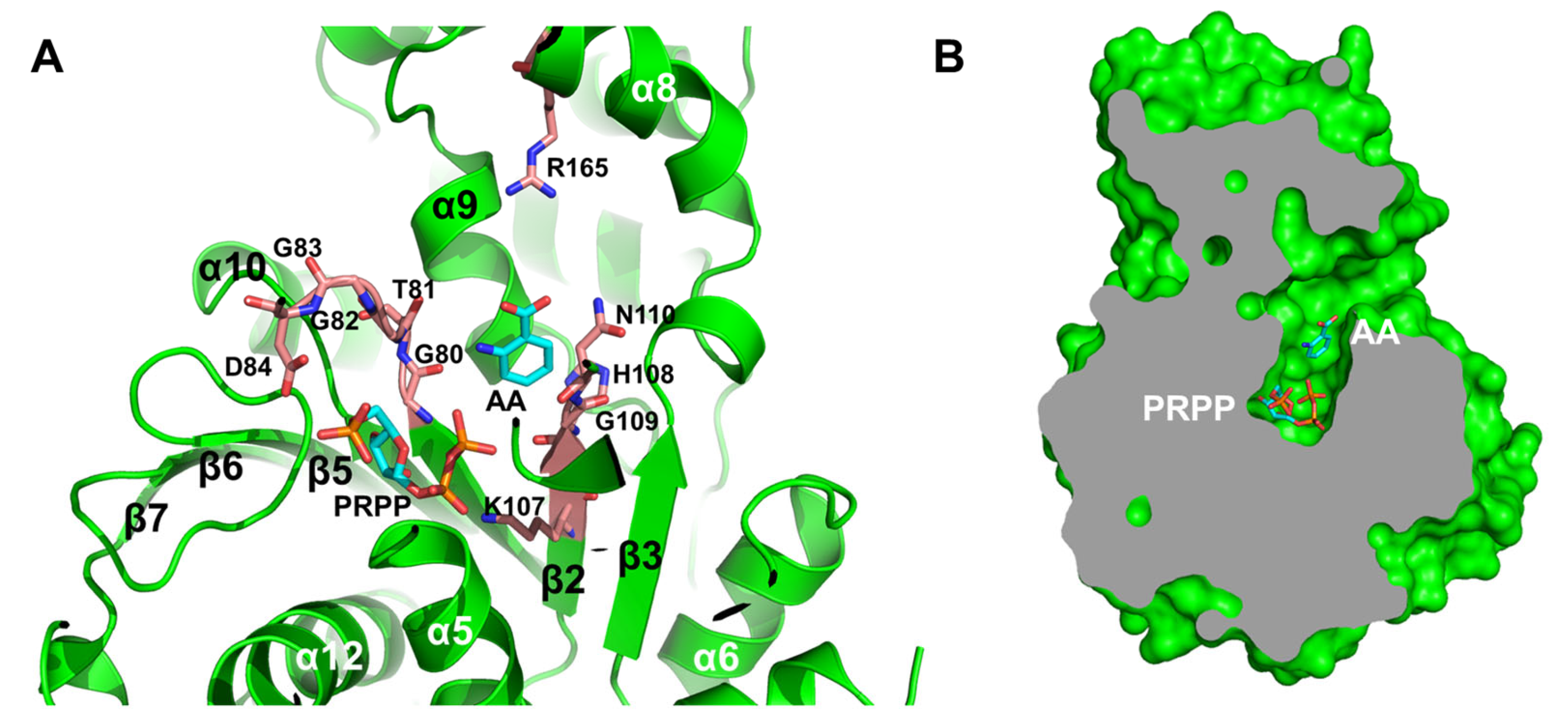
3.3. Active Site Structure of MjAnPRT
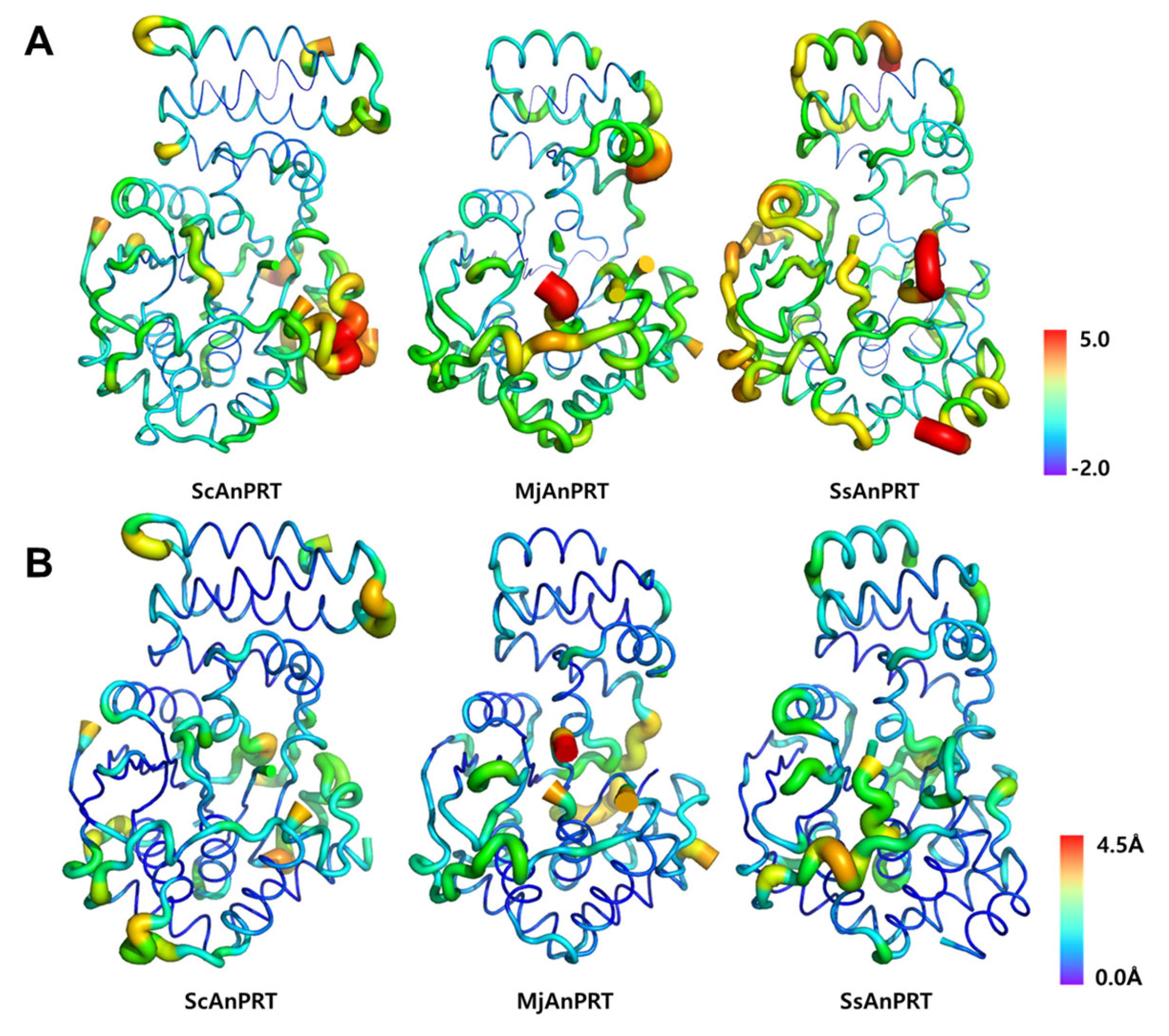
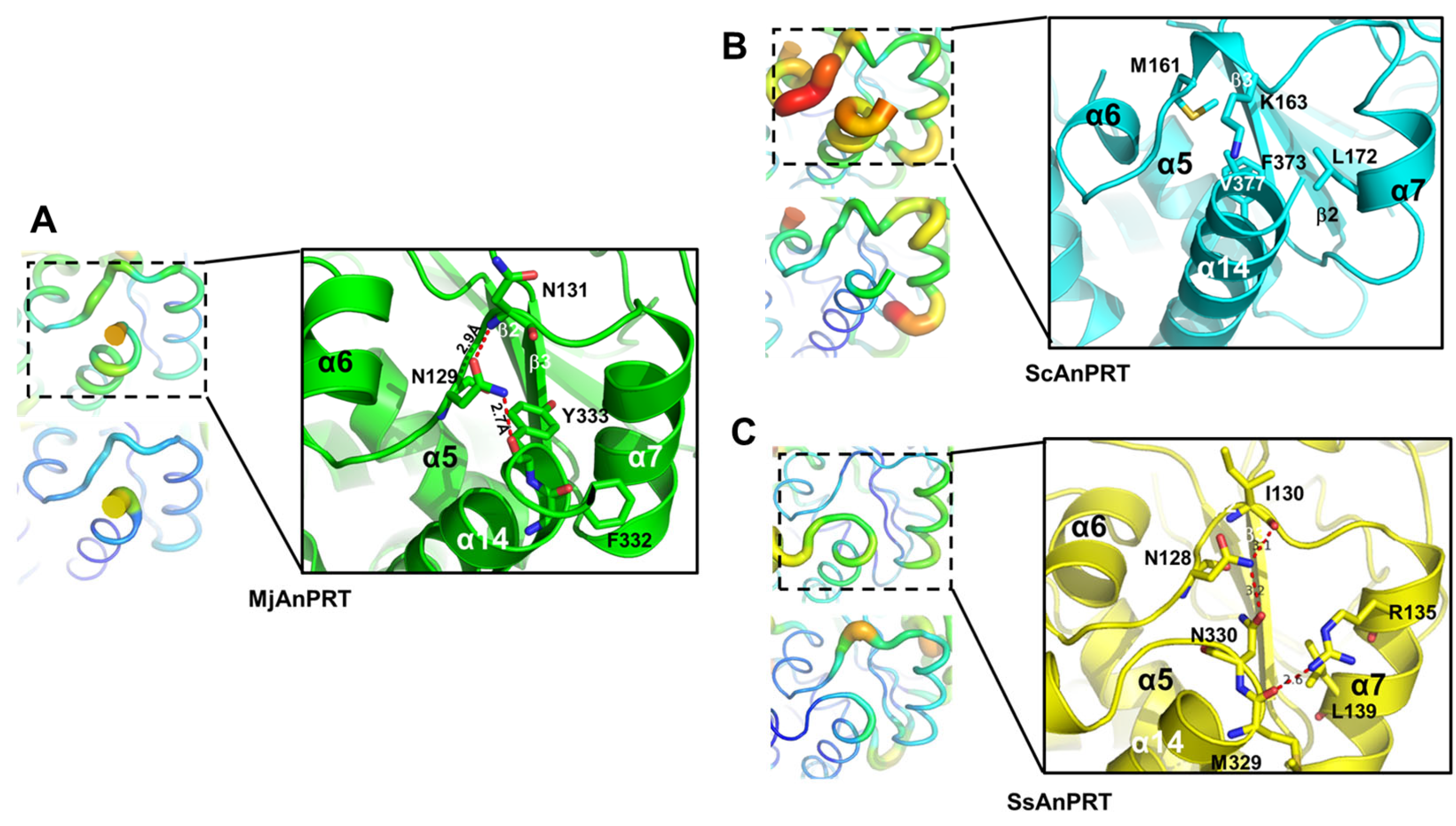
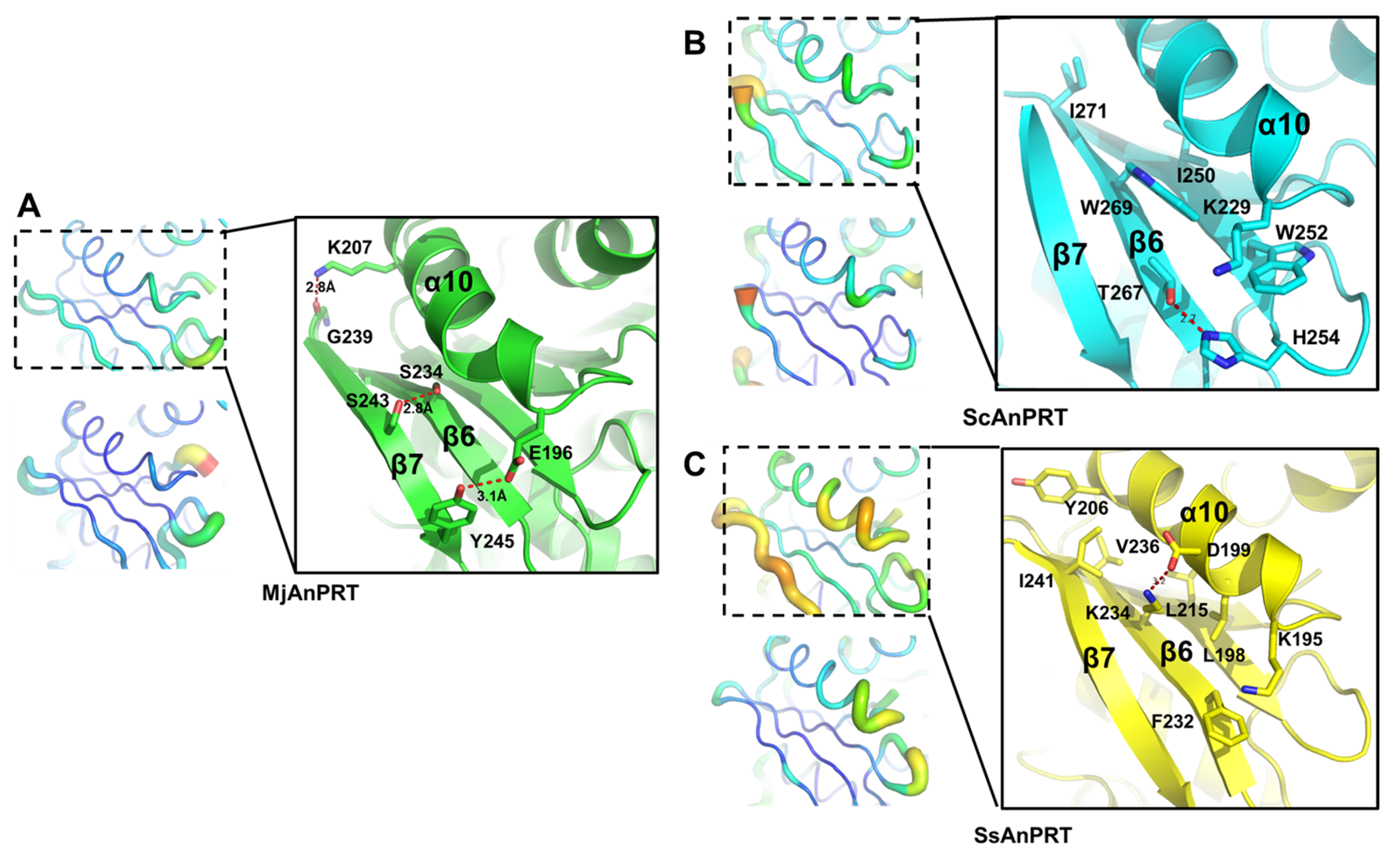
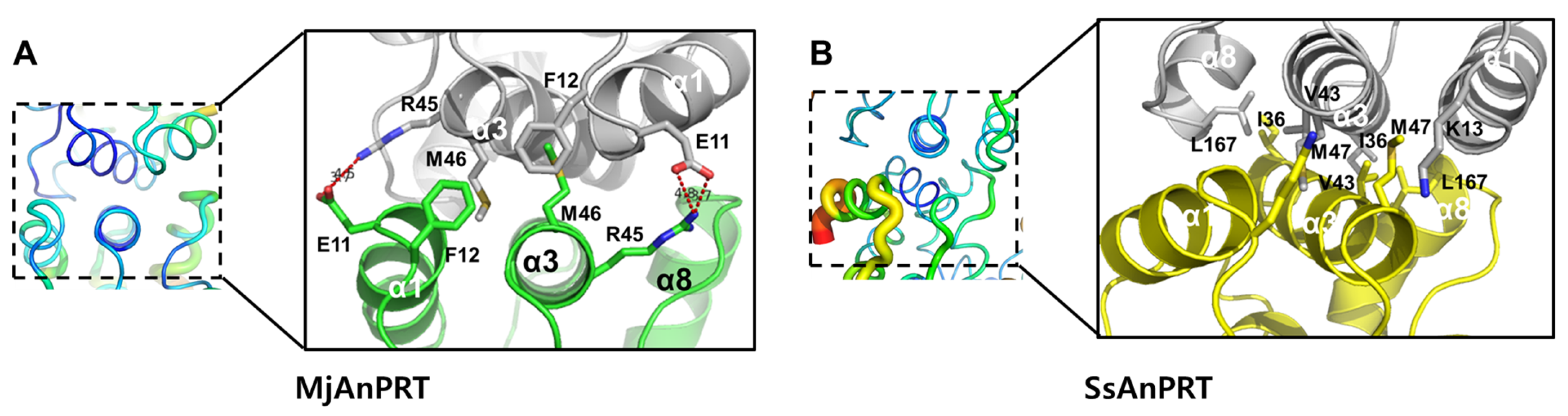
3.4. Structural Analysis of the Thermostability of MjAnPRT
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin and its relationship to plant hormones. Ann. Bot. 2018, 121, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Mano, Y.; Nemoto, K. The pathway of auxin biosynthesis in plants. J. Exp. Bot. 2012, 63, 2853–2872. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.; Cho, D.H.; Oh, S.J.; Hwang, J.H.; Kim, H.J.; Shin, N.; Ahn, J.; Choi, K.Y.; Bhatia, S.K.; Yang, Y.H. Enhanced production of bio-indigo in engineered Escherichia coli, reinforced by cyclopropane-fatty acid-acyl-phospholipid synthase from psychrophilic Pseudomonas sp. B14-6. J. Biotechnol. 2023, 366, 1–9. [Google Scholar] [CrossRef]
- Yin, H.; Chen, H.; Yan, M.; Li, Z.; Yang, R.; Li, Y.; Wang, Y.; Guan, J.; Mao, H.; Wang, Y. Efficient bioproduction of indigo and indirubin by optimizing a novel terpenoid cyclase XiaI in Escherichia coli. ACS Omega 2021, 6, 20569–20576. [Google Scholar] [CrossRef]
- Zhou, Y.; Fang, M.-Y.; Li, G.; Zhang, C.; Xing, X.-H. Enhanced production of crude violacein from glucose in Escherichia coli by overexpression of rate-limiting key enzyme(s) involved in violacein biosynthesis. Appl. Biochem. Biotechnol. 2018, 186, 909–916. [Google Scholar] [CrossRef]
- Pawar, S.; Chaudhari, A.; Prabha, R.; Shukla, R.; Singh, D.P. Microbial pyrrolnitrin: Natural metabolite with immense practical utility. Biomolecules 2019, 9, 443. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, Z. Systems metabolic engineering of Corynebacterium glutamicum for efficient l-tryptophan production. Synth. Syst. Biotechnol. 2025, 10, 511–522. [Google Scholar] [CrossRef]
- Ramos-Valdovinos, M.A.; Martínez-Antonio, A. Optimizing Fermentation Strategies for Enhanced Tryptophan Production in Escherichia coli: Integrating Genetic and Environmental Controls for Industrial Applications. Processes 2024, 12, 2422. [Google Scholar] [CrossRef]
- Guo, L.; Ding, S.; Liu, Y.; Gao, C.; Hu, G.; Song, W.; Liu, J.; Chen, X.; Liu, L. Enhancing tryptophan production by balancing precursors in Escherichia coli. Biotechnol. Bioeng. 2022, 119, 983–993. [Google Scholar] [CrossRef]
- Rodrigues, A.L.; Trachtmann, N.; Becker, J.; Lohanatha, A.F.; Blotenberg, J.; Bolten, C.J.; Korneli, C.; de Souza Lima, A.O.; Porto, L.M.; Sprenger, G.A. Systems metabolic engineering of Escherichia coli for production of the antitumor drugs violacein and deoxyviolacein. Metab. Eng. 2013, 20, 29–41. [Google Scholar] [CrossRef]
- Du, J.; Yang, D.; Luo, Z.W.; Lee, S.Y. Metabolic engineering of Escherichia coli for the production of indirubin from glucose. J. Biotechnol. 2018, 267, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Perveen, S.; Rashid, N.; Tang, X.F.; Imanaka, T.; Papageorgiou, A.C. Anthranilate phosphoribosyltransferase from the hyperthermophilic archaeon Thermococcus kodakarensis shows maximum activity with zinc and forms a unique dimeric structure. FEBS Open Bio 2017, 7, 1217–1230. [Google Scholar] [CrossRef] [PubMed]
- Schwab, T.; Skegro, D.; Mayans, O.; Sterner, R. A rationally designed monomeric variant of anthranilate phosphoribosyltransferase from Sulfolobus solfataricus is as active as the dimeric wild-type enzyme but less thermostable. J. Mol. Biol. 2008, 376, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Schramm, V.L.; Grubmeyer, C. Phosphoribosyltransferase mechanisms and roles in nucleic acid metabolism. Prog. Nucleic Acid Res. Mol. Biol. 2004, 78, 261–304. [Google Scholar] [CrossRef]
- Kim, C.; Xuong, N.H.; Edwards, S.; Madhusudan; Yee, M.C.; Spraggon, G.; Mills, S.E. The crystal structure of anthranilate phosphoribosyltransferase from the enterobacterium Pectobacterium carotovorum. FEBS Lett. 2002, 523, 239–246. [Google Scholar] [CrossRef]
- Marino, M.; Deuss, M.; Svergun, D.I.; Konarev, P.V.; Sterner, R.; Mayans, O. Structural and Mutational Analysis of Substrate Complexation by Anthranilate Phosphoribosyltransferase from Sulfolobus solfataricus. J. Biol. Chem. 2006, 281, 21410–21421. [Google Scholar] [CrossRef]
- Jones, W.J.; Leigh, J.A.; Mayer, F.; Woese, C.R.; Wolfe, R.S. Methanococcus jannaschii sp. nov., an extremely thermophilic methanogen from a submarine hydrothermal vent. Arch. Microbiol. 1983, 136, 254–261. [Google Scholar] [CrossRef]
- Park, S.-Y.; Ha, S.-C.; Kim, Y.-G. The protein crystallography beamlines at the pohang light source II. Biodesign 2017, 5, 30–34. [Google Scholar]
- Minor, W.; Cymborowski, M.; Otwinowski, Z.; Chruszcz, M. HKL-3000: The integration of data reduction and structure solution—from diffraction images to an initial model in minutes. Acta Crystallogr. Sect. D Biol. Crystallogr. 2006, 62 Pt 8, 859–866. [Google Scholar] [CrossRef]
- Liebschner, D.; Afonine, P.V.; Baker, M.L.; Bunkóczi, G.; Chen, V.B.; Croll, T.I.; Hintze, B.; Hung, L.-W.; Jain, S.; McCoy, A.J. Macromolecular structure determination using X-rays, neutrons and electrons: Recent developments in Phenix. Biol. Crystallogr. 2019, 75, 861–877. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Biol. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef]
- Holm, L. Dali server: Structural unification of protein families. Nucleic Acids Res. 2022, 50, W210–W215. [Google Scholar] [CrossRef]
- Sievers, F.; Higgins, D.G. Clustal Omega for making accurate alignments of many protein sequences. Protein Sci. 2018, 27, 135–145. [Google Scholar] [CrossRef]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef]
- Barthels, F.; Schirmeister, T.; Kersten, C. BANΔIT: B’-Factor Analysis for Drug Design and Structural Biology. Mol. Inform. 2021, 40, 2000144. [Google Scholar] [CrossRef]
- Kuriata, A.; Gierut, A.M.; Oleniecki, T.; Ciemny, M.P.; Kolinski, A.; Kurcinski, M.; Kmiecik, S. CABS-flex 2.0: A web server for fast simulations of flexibility of protein structures. Nucleic Acids Res. 2018, 46, W338–W343. [Google Scholar] [CrossRef]
- Jamroz, M.; Orozco, M.; Kolinski, A.; Kmiecik, S. Consistent View of Protein Fluctuations from All-Atom Molecular Dynamics and Coarse-Grained Dynamics with Knowledge-Based Force-Field. J. Chem. Theory Comput. 2013, 9, 119–125. [Google Scholar] [CrossRef]
- Scully, T.W.; Jiao, W.; Mittelstädt, G.; Parker, E.J. Structure, mechanism and inhibition of anthranilate phosphoribosyltransferase. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2023, 378, 20220039. [Google Scholar] [CrossRef]
- Cookson, T.V.M. Probing the Active Site of Anthranilate Phosphoribosyltransferase from Mycobacterium tuberculosis to Facilitate Novel Drug Development; University of Canterbury Chemistry: Christchurch, New Zealand, 2013. [Google Scholar]
- Leuenberger, P.; Ganscha, S.; Kahraman, A.; Cappelletti, V.; Boersema, P.J.; von Mering, C.; Claassen, M.; Picotti, P. Cell-wide analysis of protein thermal unfolding reveals determinants of thermostability. Science 2017, 355, eaai7825. [Google Scholar] [CrossRef]
- Caspeta, L.; Nielsen, J. Thermotolerant Yeast Strains Adapted by Laboratory Evolution Show Trade-Off at Ancestral Temperatures and Preadaptation to Other Stresses. mBio 2015, 6, e00431-15. [Google Scholar] [CrossRef]
- Schwab, T.; Sterner, R. Stabilization of a metabolic enzyme by library selection in Thermus thermophilus. ChemBioChem 2011, 12, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Liu, Q.; Qu, G.; Feng, Y.; Reetz, M.T. Utility of B-factors in protein science: Interpreting rigidity, flexibility, and internal motion and engineering thermostability. Chem. Rev. 2019, 119, 1626–1665. [Google Scholar] [CrossRef]
- Mlynek, G.; Djinović-Carugo, K.; Carugo, O. B-Factor Rescaling for Protein Crystal Structure Analyses. Crystals 2024, 14, 443. [Google Scholar] [CrossRef]
- Shelley, K.L.; Garman, E.F. Identifying and avoiding radiation damage in macromolecular crystallography. Acta Crystallogr. Sect. D 2024, 80, 314–327. [Google Scholar] [CrossRef]
- Ribeiro, F.d.S.; Lima, L.M.T.R. Temperature-Resolved Crystallography Reveals Rigid-Body Dominance Over Local Flexibility in B-Factors. bioRxiv 2025. [Google Scholar] [CrossRef]
- Hung, C.-L.; Kuo, Y.-H.; Lee, S.W.; Chiang, Y.-W. Protein stability depends critically on the surface hydrogen-bonding network: A case study of Bid protein. J. Phys. Chem. B 2021, 125, 8373–8382. [Google Scholar] [CrossRef]
- Xu, D.; Tsai, C.-J.; Nussinov, R. Hydrogen bonds and salt bridges across protein-protein interfaces. Protein Eng. 1997, 10, 999–1012. [Google Scholar] [CrossRef]
| Data Collection | MjanPRT (PDB ID: 9UCW) |
|---|---|
| X-ray source | 7A beamline, PLS-II |
| Wavelength (Å) | 0.9793 |
| Space group | P21 |
| Cell dimension | |
| a, b, c (Å) | 60.99, 82.66, 73.87 |
| α, β, γ (◦) | 90, 112.74, 90 |
| Resolution (Å) | 30.00–2.16 (2.23–2.16) |
| Unique reflections | 35,499 (3417) |
| Completeness (%) | 97.40 (88.20) |
| Redundancy | 6.7 (6.0) |
| I/σ | 13.9 (2.1) |
| Rmerge | 0.007 (0.087) |
| Rmeas | 0.008 (0.095) |
| CC1/2 | 0.998 (0.734) |
| Refinement | |
| Resolution (Å) | 28.12–2.16 |
| Rwork a | 0.2144 |
| Rfree b | 0.2580 |
| R.m.s. deviations | |
| Bonds (Å) | 0.009 |
| Angles (◦) | 1.037 |
| No. macromolecules | 2 |
| protein residues | 614 |
| water | 96 |
| B factors (Å2) | |
| Protein | 54.25 |
| water | 54.58 |
| Rotamer outliers (%) | 1.55 |
| Clashcore | 9.37 |
| Ramachandran plot | |
| Favored (%) | 97.72 |
| Allowed (%) | 2.28 |
| Disallowed (%) | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.-M. Crystal Structure of Anthranilate Phosphoribosyltransferase from Methanocaldococcus jannaschii. Crystals 2025, 15, 702. https://doi.org/10.3390/cryst15080702
Choi J-M. Crystal Structure of Anthranilate Phosphoribosyltransferase from Methanocaldococcus jannaschii. Crystals. 2025; 15(8):702. https://doi.org/10.3390/cryst15080702
Chicago/Turabian StyleChoi, Jung-Min. 2025. "Crystal Structure of Anthranilate Phosphoribosyltransferase from Methanocaldococcus jannaschii" Crystals 15, no. 8: 702. https://doi.org/10.3390/cryst15080702
APA StyleChoi, J.-M. (2025). Crystal Structure of Anthranilate Phosphoribosyltransferase from Methanocaldococcus jannaschii. Crystals, 15(8), 702. https://doi.org/10.3390/cryst15080702






