Gone with the Wind—Adducts of Volatile Pyridine Derivatives and Copper(II) Acetylacetonate
Abstract
1. Introduction
2. Materials and Methods
2.1. General Aspects
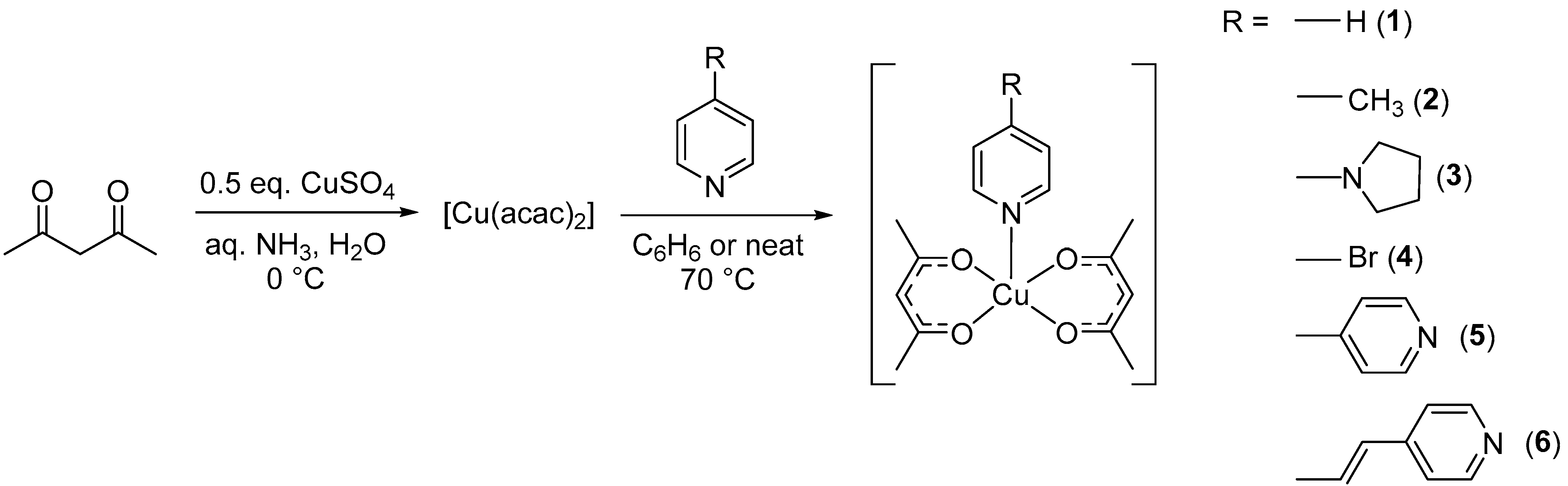
2.2. Synthesis
- General procedure A for the preparation of Cu(II) adducts using solid heterocycles
- General procedure B for the preparation of Cu(II) adducts using liquid heterocycles
2.2.1. Synthesis of [Cu(acac)2]
2.2.2. Synthesis of 4-Bromopyridine
2.2.3. Synthesis of [Cu(acac)2(py)] (1)
2.2.4. Synthesis of [Cu(acac)2(4-MePy)] (2)
2.2.5. [Cu(acac)2(4-PyrPy)] (3)
2.2.6. Synthesis of [Cu(acac)2(4-BrPy)] (4)
2.2.7. Synthesis of (5)
2.2.8. Synthesis of (6)
2.2.9. Synthesis of [(Cu(acac)2)3(TDPE)2] (7)
2.2.10. Synthesis of [(Cu(acac)2)2(TDPE)] · 3 CHCl3 (8)
2.3. X-Ray Crystallography
3. Results
3.1. Synthesis
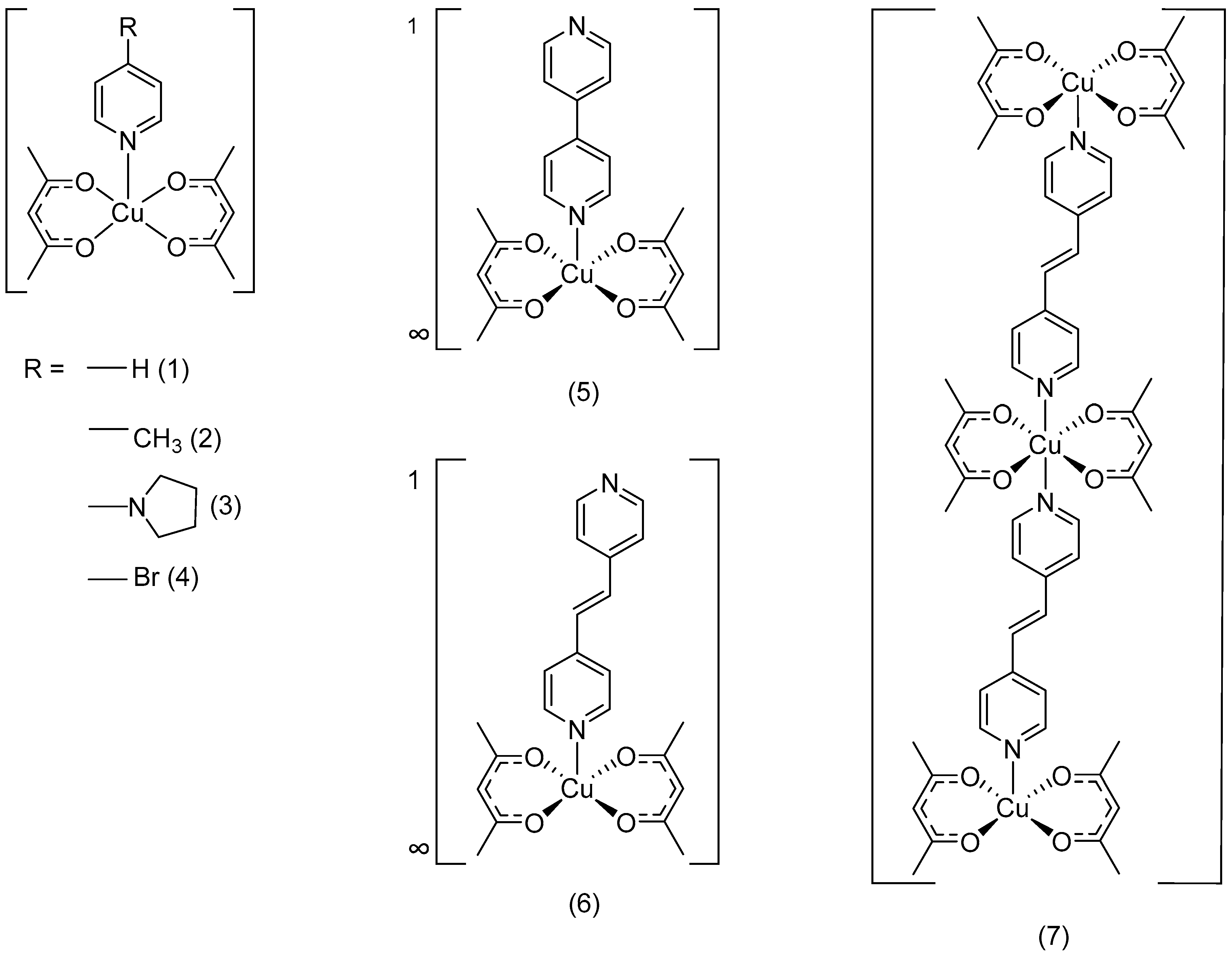
3.2. Structures of the Complexes
3.3. Structure of Complex 1 to 4
3.4. Structure of Coordination Polymer 5
3.5. Structure of Coordination Polymer 6
3.6. Structure of Oligomer 7
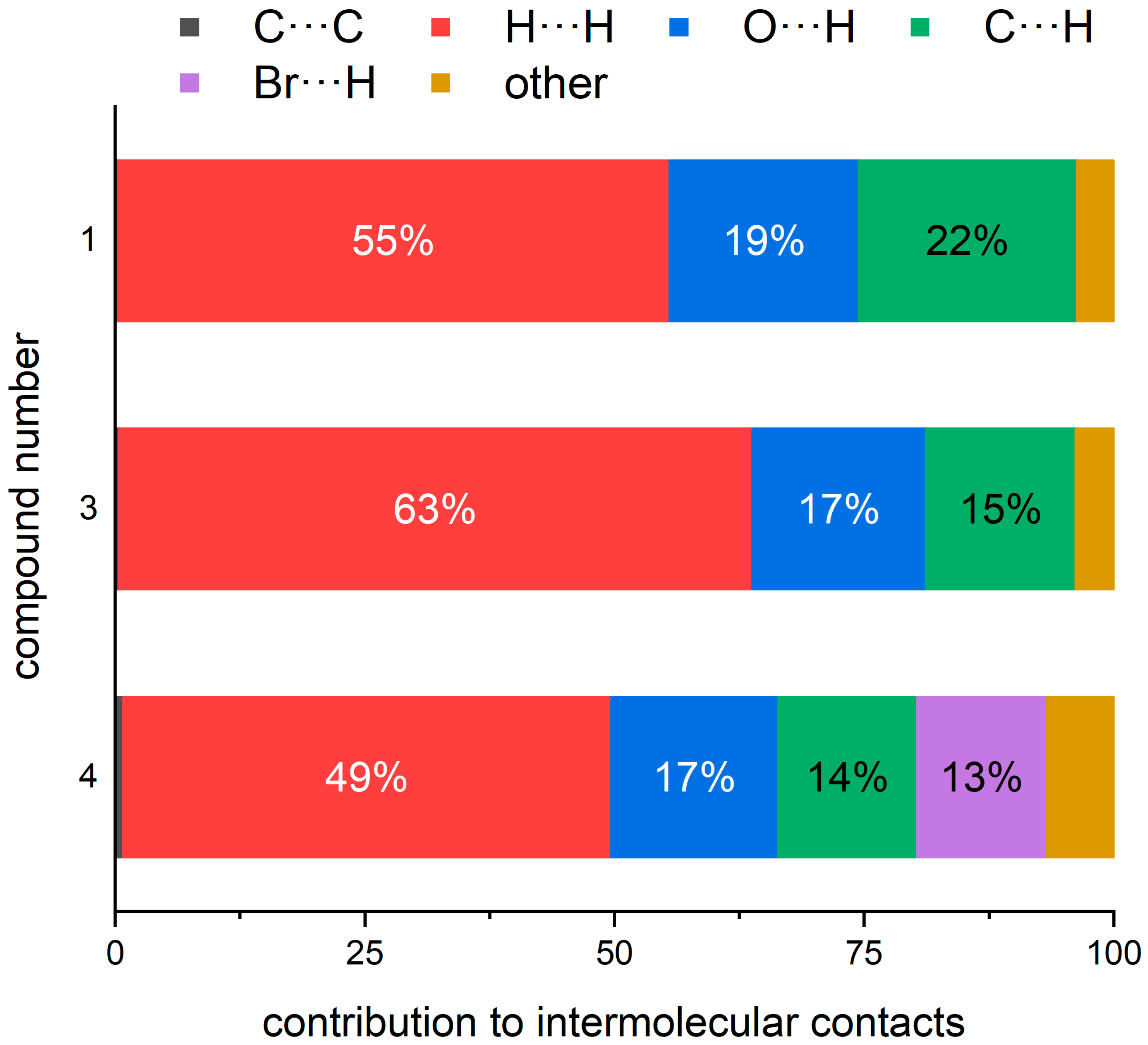
3.7. Hirshfeld Surface Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cox, E.G.; Webster, K.C. 162. The planar structure of quadricovalent cupric compounds. J. Chem. Soc. 1935, 731–733. [Google Scholar] [CrossRef]
- Shibata, S.; Sone, K. Study on the Molecular Structure of Bisacetylacetone-Copper(II) by Electron Diffraction. Bull. Chem. Soc. Jpn. 1956, 29, 852–856. [Google Scholar] [CrossRef]
- Starikova, Z.A.; Shugam, E.A. Crystal chemical data for inner complexes of β-diketones. J. Struct. Chem. 1969, 10, 267–269. [Google Scholar] [CrossRef]
- McGarvey, B.R. Paramagnetic Resonance in Copper Chelates. J. Phys. Chem. 1956, 60, 71–76. [Google Scholar] [CrossRef]
- Graddon, D.P. 5-Co-ordination of Divalent Copper. Nature 1959, 183, 1610–1611. [Google Scholar] [CrossRef]
- Graddon, D.P.; Watton, E.C. Adducts of copper(II) β-diketone chelates with heterocyclic bases. J. Inorg. Nucl. Chem. 1961, 21, 49–57. [Google Scholar] [CrossRef]
- Shigematsu, T.; Tabushi, M.; Matsui, M.; Munakata, M. Studies on Adduct Formation of β-Diketone Chelates with Heterocyclic Bases. Bull. Chem. Soc. Jpn. 1968, 41, 2656–2660. [Google Scholar] [CrossRef]
- Kwiatkowski, E.; Trojanowski, J. Spectroscopic study of the equilibrium of adduct formation by acetylacetonates of oxovanadium(IV) and copper(II) with nitrogen bases. J. Inorg. Nucl. Chem. 1976, 38, 131–135. [Google Scholar] [CrossRef]
- Walker, W.R.; Li, N.C. 4-methyl-pyridine adducts of copper (II) β-diketone chelates. J. Inorg. Nucl. Chem. 1965, 27, 2255–2261. [Google Scholar] [CrossRef]
- Traill, R.C. Addition Compounds between Copper Complexes and Organic Bases. Nature 1960, 186, 631. [Google Scholar] [CrossRef]
- Morgan, G.T.; Smith, J.D.M. CXXVII.—Researches on residual affinity and coordination. Part XXVI. A quadridentate group in combination with bivalent metals. J. Chem. Soc. 1926, 129, 912–921. [Google Scholar] [CrossRef]
- Jose, P.; Ooi, S.i.; Fernando, Q. The crystal and molecular structure of the pentacoordinated copper(II) complex, bis(acetylacetonato)quinolinecopper(II). J. Inorg. Nucl. Chem. 1969, 31, 1971–1981. [Google Scholar] [CrossRef]
- Jian, F.F.; Wang, J.; Zhang, J. {μ2-1,4-Bis[2-(4-pyridyl)ethenyl]benzene-κ2N:N’}bis[bis(acetylacetonato-κ2O,O’)copper(II)]. Acta Cryst. E 2009, 65, m1630. [Google Scholar] [CrossRef] [PubMed]
- Stabnikov, P.A.; Zharkova, G.I.; Alferova, N.I.; Zubareva, A.P.; Shusharina, E.A.; Pervukhina, N.V. Crystal structure of adducts of copper(II) acetylacetonate and hexafluoroacetylacetonate with 4-aminopyridine. J. Struct. Chem. 2011, 52, 371–375. [Google Scholar] [CrossRef]
- Perdih, F. Diversity of supramolecular aggregation in copper(II) pentane-2,4-dionato compounds with methyl substituted 2-aminopyridines. J. Coord. Chem. 2012, 65, 1580–1591. [Google Scholar] [CrossRef]
- Perdih, F. Different coordination modes and supramolecular aggregations in copper(II) pentane-2,4-dionato compounds with 2-pyridone and 3-hydroxypyridine. Monatsh. Chem. 2012, 143, 1011–1017. [Google Scholar] [CrossRef]
- Jia, L.-M.; Tong, J.; Yu, S.-Y. Neutral coordination polymers of Cobalt(II), Copper(II), Zinc(II) and Manganese(II) β-diketonate complexes with fluorescent anthracene dipyridine: Synthesis, structure and luminescence properties. J. Photochem. Photobiol. A 2018, 355, 84–93. [Google Scholar] [CrossRef]
- Germán-Acacio, J.M.; Hernández-Ortega, S.; Aakeröy, C.B.; Valdés-Martínez, J. Using Lewis acidity differences in chelating ligands to control molecular structure and supramolecular assembly of Cu(II) complexes. Inorg. Chim. Acta 2009, 362, 4087–4090. [Google Scholar] [CrossRef]
- Lindoy, L.F.; McMurtrie, J.C.; Schilter, D. [4-(Dimethylamino)pyridine-κN]bis(pentane-2,4-dionato-κ2O,O′)copper(II). Acta Cryst. E 2006, 62, m1142–m1143. [Google Scholar] [CrossRef]
- Perdih, F. (Pentane-2,4-dionato-κ2O,O’)(pyridin-2-amine-κN1)copper(II) and (pentane-2,4-dionato-κ2O,O’)(pyrimidin-2-amine-kappaN1)copper(II). Acta Cryst. C 2012, 68, m64–m68. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.H.; Lin, H.H.; Lee, C.J.; Liou, S.Y. Ligands Coordinated with Cu(II) Complexes in Rare Anti-Syn 2,2′-Dipyridylamine and Acetate-Bridged Forms: Preparations, Crystal Structures, Hydrogen Bonds and Magnetic Properties. J. Chin. Chem. 2013, 57, 864–875. [Google Scholar] [CrossRef]
- Sun, L.; Xiao, X.; He, Y.; Wang, G.; Shen, L.; Fang, J.; Yang, J.; Li, X. Syntheses, Crystal Structure, and Properties of Cu(II) and Zn(II) Complexes with EDO-TTF-3-py. Synth. React. Inorg. Met.-Org. Chem. 2013, 44, 65–69. [Google Scholar] [CrossRef]
- Bedeković, N.; Čavka, M.; Cinčić, D.; Stilinović, V. Influence of intramolecular hydrogen bonding on structures and thermal stability of Cu(II) and Zn(II) β-diketonate adducts. J. Mol. Struct. 2021, 1246, 131130. [Google Scholar] [CrossRef]
- Zhu, Q.Y.; Liu, Y.; Lu, W.; Zhang, Y.; Bian, G.Q.; Niu, G.Y.; Dai, J. Effects of protonation and metal coordination on intramolecular charge transfer of tetrathiafulvalene compound. Inorg. Chem. 2007, 46, 10065–10070. [Google Scholar] [CrossRef]
- Biswas, C.; Chattopadhyay, S.; Drew, M.G.B.; Ghosh, A. Synthesis, crystal structure and hydrolysis of a dinuclear copper(II) complex constructed by N2O donor Schiff base and 4,4′-bipyridine: Discrete supra-molecular ensembles vs. oligomers. Polyhedron 2007, 26, 4411–4418. [Google Scholar] [CrossRef]
- Shaabani, B.; Rad-Yousefnia, N.; Zahedi, M.; Ertan, Ş.; Blake, G.R.; Zakerhamidi, M.S. Construction of new 1D and 2D coordination polymers generated from rigid N,N′-bis(4-pyridylmethylene)-1,5-naphthalenediamine ligand: Syntheses, crystal structures and luminescence properties. Inorg. Chim. Acta 2017, 455, 158–165. [Google Scholar] [CrossRef]
- Zazouli, S.; Gruber, N.; Bulach, V.; Ferlay, S.; Jouaiti, A. Design of coordination polymers based on combinations of 1,2-diphenylethane-1,2-diyl diisonicotinate with Cu(ii), Zn(ii), Cd(ii) and Co(ii). CrystEngComm 2022, 24, 7632–7639. [Google Scholar] [CrossRef]
- Yuanzhi, X.; Shu, S. Study of spectra and crystal structure of bipyridine bridging Cu(II) acetylacetonate complex[µ-4,4-bipy-Cu(acac)2]. Acta Chim. Sin. 1986, 44, 336–342. [Google Scholar]
- Winter, S.; Weber, E.; Eriksson, L.; Csöregh, I. New coordination polymer networks based on copper(ii) hexafluoroacetylacetonate and pyridine containing building blocks: Synthesis and structural study. New J. Chem. 2006, 30, 1808–1819. [Google Scholar] [CrossRef]
- Walker, W.R. Five Coordination of Copper(II). Aust. J. Chem. 1961, 14, 161–162. [Google Scholar] [CrossRef]
- May, W.R.; Jones, M.M. Adducts of bis(2,4-pentanediono)-copper (II) with pyridine bases: Stability constants and heats of reaction. J. Inorg. Nucl. Chem. 1963, 25, 507–511. [Google Scholar] [CrossRef]
- Császár, J.; Bizony, N.M. Study of adduct formation of bis(2,4pentanediono)-copper (II) with pyridine bases. Acta Phys. Chem. 1978, 24, 487–490. [Google Scholar]
- Kokcam-Demir, U.; Goldman, A.; Esrafili, L.; Gharib, M.; Morsali, A.; Weingart, O.; Janiak, C. Coordinatively unsaturated metal sites (open metal sites) in metal-organic frameworks: Design and applications. Chem. Soc. Rev. 2020, 49, 2751–2798. [Google Scholar] [CrossRef] [PubMed]
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Podda, E.; Arca, M.; Pintus, A.; Lippolis, V.; Coles, S.J.; Orton, J.B.; Porcu, S.; Ricci, P.C.; Aragoni, M.C. Ultra-Low Optical Loss in Single Crystal Waveguides of Fluorene/Fluorenone Cd(II) Coordination Polymers. JACS Au 2025, 5, 727–739. [Google Scholar] [CrossRef]
- Kochetygov, I.; Bulut, S.; Asgari, M.; Queen, W.L. Selective CO2 adsorption by a new metal-organic framework: Synergy between open metal sites and a charged imidazolinium backbone. Dalton Trans. 2018, 47, 10527–10535. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Zhang, Q.; Wang, Y.; Duan, J. Unified meso-pores and dense Cu2+ sites in porous coordination polymers for highly efficient gas storage and separation. Dalton Trans. 2018, 47, 4424–4427. [Google Scholar] [CrossRef] [PubMed]
- Cepeda, J.; Pérez-Mendoza, M.; Calahorro, A.J.; Casati, N.; Seco, J.M.; Aragones-Anglada, M.; Moghadam, P.Z.; Fairen-Jimenez, D.; Rodríguez-Diéguez, A. Modulation of pore shape and adsorption selectivity by ligand functionalization in a series of “rob”-like flexible metal–organic frameworks. J. Mater. Chem. A 2018, 6, 17409–17416. [Google Scholar] [CrossRef]
- Yang, S.; Veerana, M.; Yu, N.; Ketya, W.; Park, G.; Kim, S.; Kim, Y. Copper(II)-MOF Containing Glutarate and 4,4′-Azopyridine and Its Antifungal Activity. Appl. Sci. 2021, 12, 260. [Google Scholar] [CrossRef]
- Ahamad, M.N.; Shahid, M.; Ahmad, M.; Sama, F. Cu(II) MOFs Based on Bipyridyls: Topology, Magnetism, and Exploring Sensing Ability toward Multiple Nitroaromatic Explosives. ACS Omega 2019, 4, 7738–7749. [Google Scholar] [CrossRef]
- Caputo, C.B.; Vukotic, V.N.; Sirizzotti, N.M.; Loeb, S.J. A tetrapyridine ligand with a rigid tetrahedral core forms metal-organic frameworks with PtS type architecture. Chem. Commun. 2011, 47, 8545–8547. [Google Scholar] [CrossRef]
- Mylonas-Margaritis, I.; Gerard, A.; Skordi, K.; Mayans, J.; Tasiopoulos, A.; McArdle, P.; Papatriantafyllopoulou, C. From 1D Coordination Polymers to Metal Organic Frameworks by the Use of 2-Pyridyl Oximes. Materials 2020, 13, 4084. [Google Scholar] [CrossRef]
- Mu, J.; Zhong, X.; Dai, W.; Pei, X.; Sun, J.; Zhang, J.; Luo, W.; Zhou, W. Metal-Organic Framework Assembled on Oriented Nanofiber Arrays for Field-Effect Transistor and Gas Sensor-Based Applications. Molecules 2022, 27, 2131. [Google Scholar] [CrossRef]
- Peacock, R.D. The preparation and investigation of bis(acetylacetonato)copper(II). J. Chem. Educ. 1971, 48, 133. [Google Scholar] [CrossRef]
- Mayer, N.; Schweiger, M.; Fuchs, E.; Migglautsch, A.K.; Doler, C.; Grabner, G.F.; Romauch, M.; Melcher, M.C.; Zechner, R.; Zimmermann, R.; et al. Structure-activity relationship studies for the development of inhibitors of murine adipose triglyceride lipase (ATGL). Bioorg. Med. Chem. 2020, 28, 115610. [Google Scholar] [CrossRef]
- Gu, J.G.; Wang, C.X.; Hu, G.Q.; Shen, K.; Zhang, H.H. K2CO3/18-Crown-6-Catalyzed Selective H/D Exchange of Heteroarenes with Bromide as a Removable Directing Group. Org. Lett. 2023, 25, 3055–3059. [Google Scholar] [CrossRef] [PubMed]
- APEX5, version 2023.9.2; Bruker AXS Inc.: Madison, WI, USA, 2023.
- SAINT, version 8.40B; Includes Xprep and SADABS, Eventuell TWINABS; Bruker AXS Inc.: Madison, WI, USA, 2001.
- Sheldrick, G.M. SADABS, Program for Area Detector Adsorption Correction; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Farrugia, L.J. WinGXandORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Burnett, M.N.; Johnson, C.K. ORTEP-III: Oak Ridge Thermal Ellipsoid Plot Program for Crystal Structure Illustrations; ORNL-6895; Oak Ridge National Laboratory Report: Oak Ridge, TN, USA, 1996. [Google Scholar]
- Chiyindiko, E.; Conradie, J. Redox behaviour of bis(β-diketonato)copper(II) complexes. J. Electroanal. Chem. 2019, 837, 76–85. [Google Scholar] [CrossRef]
- Graddon, D.P. The absorption spectra of complex salts—III cupric ethylacetoacetate. J. Inorg. Nucl. Chem. 1960, 14, 161–168. [Google Scholar] [CrossRef]
- Jones, M.M. A New Method of Preparing Some Acetylacetonate Complexes. J. Am. Chem. Soc. 1959, 81, 3188–3189. [Google Scholar] [CrossRef]
- Vreshch, V.D.; Yang, J.H.; Zhang, H.; Filatov, A.S.; Dikarev, E.V. Monomeric square-planar cobalt(II) acetylacetonate: Mystery or mistake? Inorg. Chem. 2010, 49, 8430–8434. [Google Scholar] [CrossRef] [PubMed]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, Structure, and Spectroscopic Properties of Copper(ii) Compounds containing Nitrogen-Sulphur Donor Ligands; the Crystal and Molecular Structure of Aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(ii) Perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Gillespie, R.J. Electron Correlation and Molecular Shape. Can. J. Chem. 1960, 38, 818–826. [Google Scholar] [CrossRef]
- Sacconi, L. Five coordination in 3d metal complexes. Pure Appl. Chem. 1968, 17, 95–128. [Google Scholar] [CrossRef]
- Gillespie, R.J. 895. The stereochemistry of five-co-ordination. Part I. Non-transition elements. J. Chem. Soc. 1963, 4672–4678. [Google Scholar] [CrossRef]
- Brotzel, F.; Kempf, B.; Singer, T.; Zipse, H.; Mayr, H. Nucleophilicities and carbon basicities of pyridines. Chem. Eur. J. 2007, 13, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.; Yuanzhi, X. Crystal structure of [4,4′-bipy-cu(acac)2]. Chin. J. Struct. Chem. 1985, 4, 38. [Google Scholar]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
 N,
N,  O,
O,  Cu,
Cu, 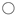 C.
C.
 N,
N,  O,
O,  Cu,
Cu,  C.
C.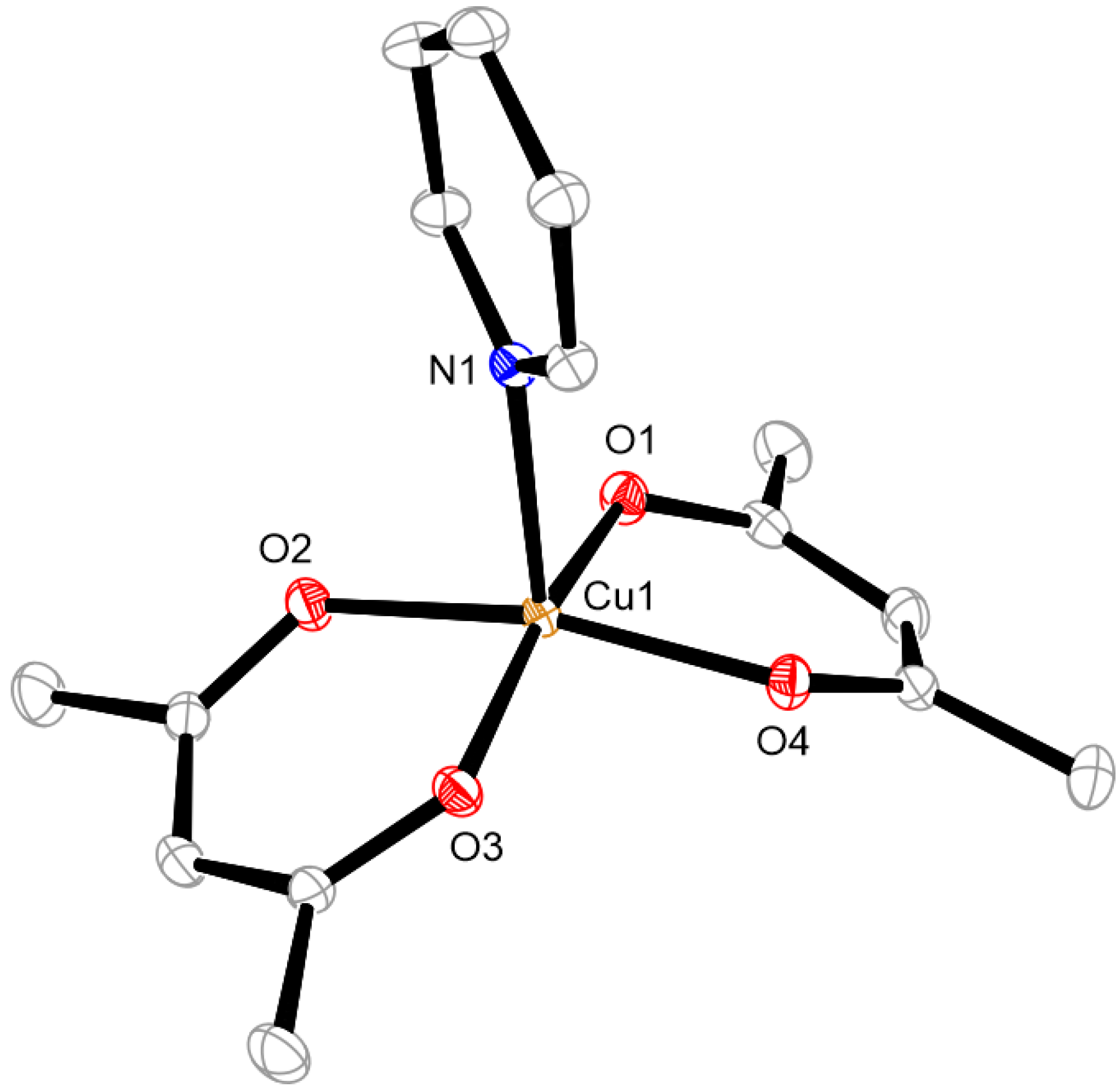
 N,
N,  O,
O,  Cu,
Cu,  C.
C.
 N,
N,  O,
O,  Cu,
Cu,  C.
C.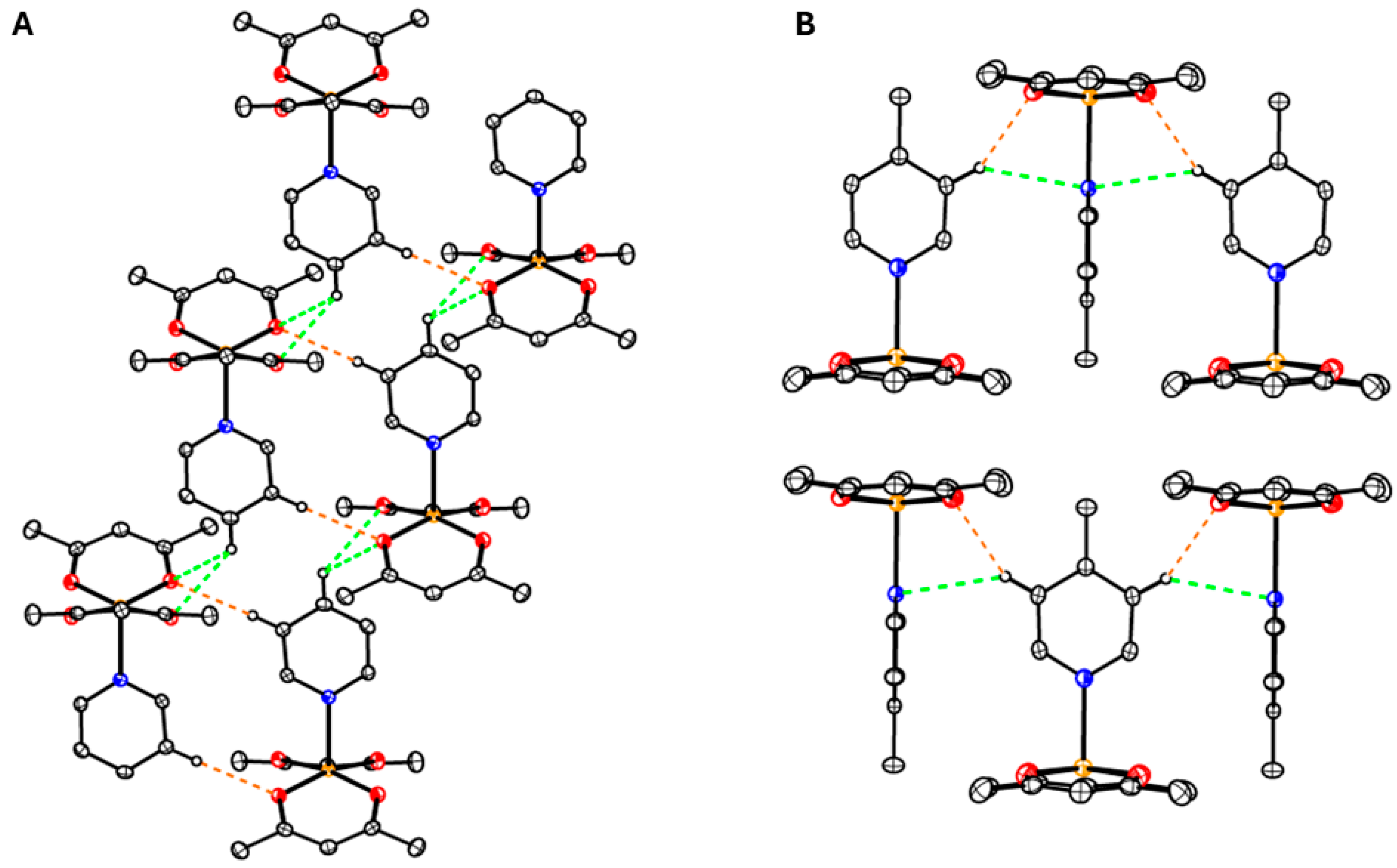
 N,
N,  O,
O,  Cu,
Cu,  C.
C.
 N,
N,  O,
O,  Cu,
Cu,  C.
C.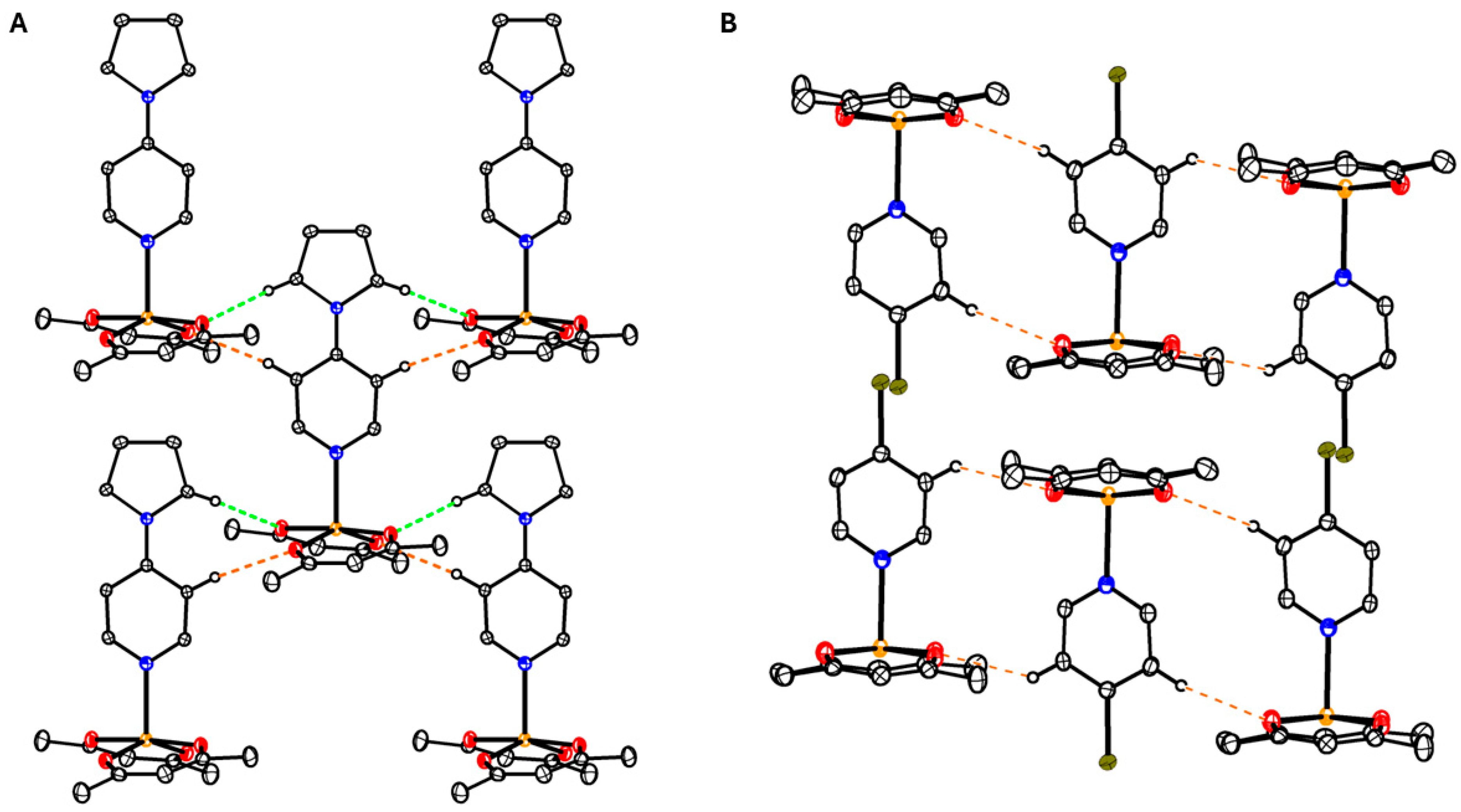
 N,
N,  O,
O,  Cu,
Cu,  C.
C.
 N,
N,  O,
O,  Cu,
Cu,  C.
C.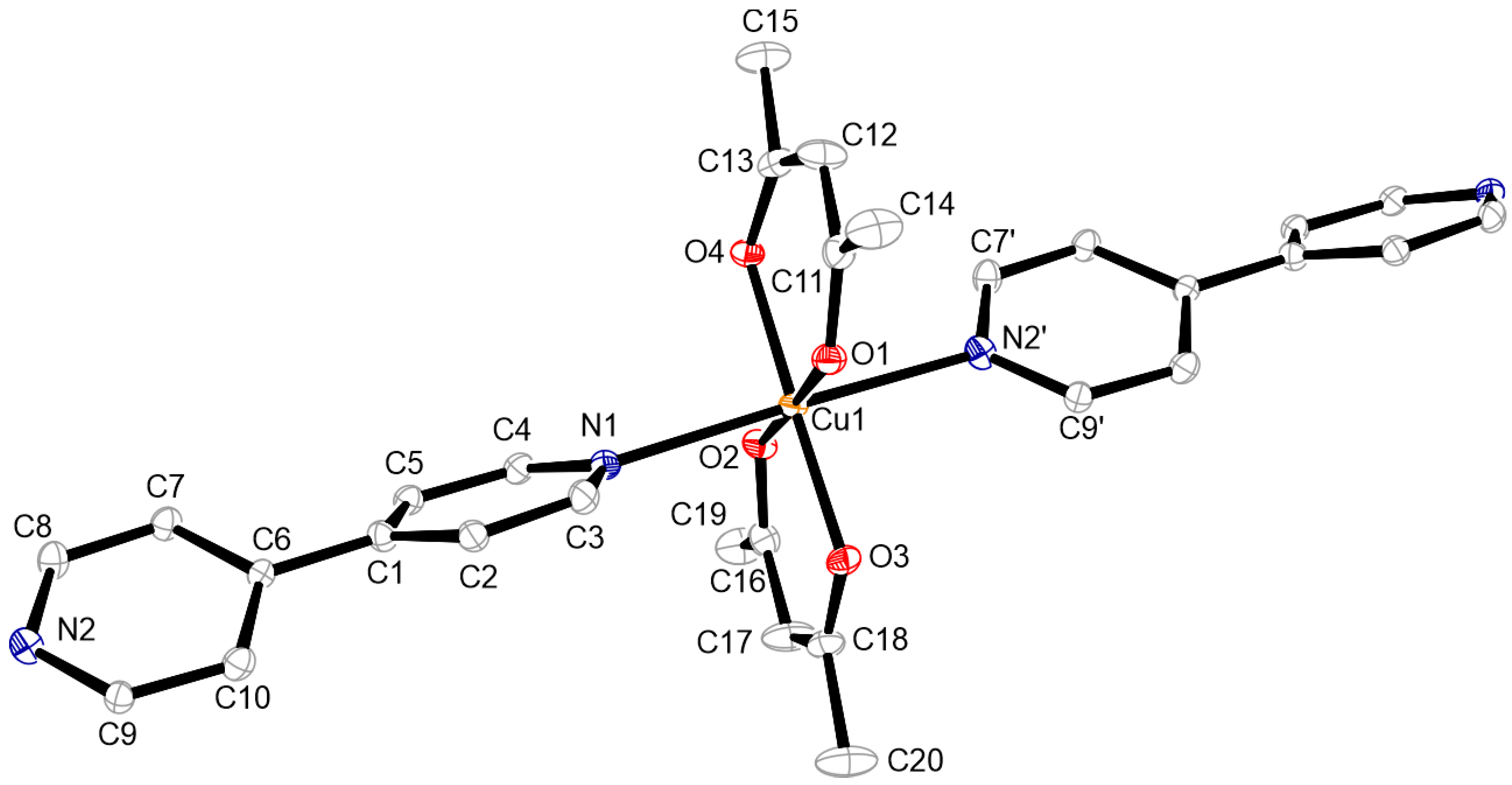
 N,
N,  O,
O,  Cu,
Cu,  C.
C.
 N,
N,  O,
O,  Cu,
Cu,  C.
C.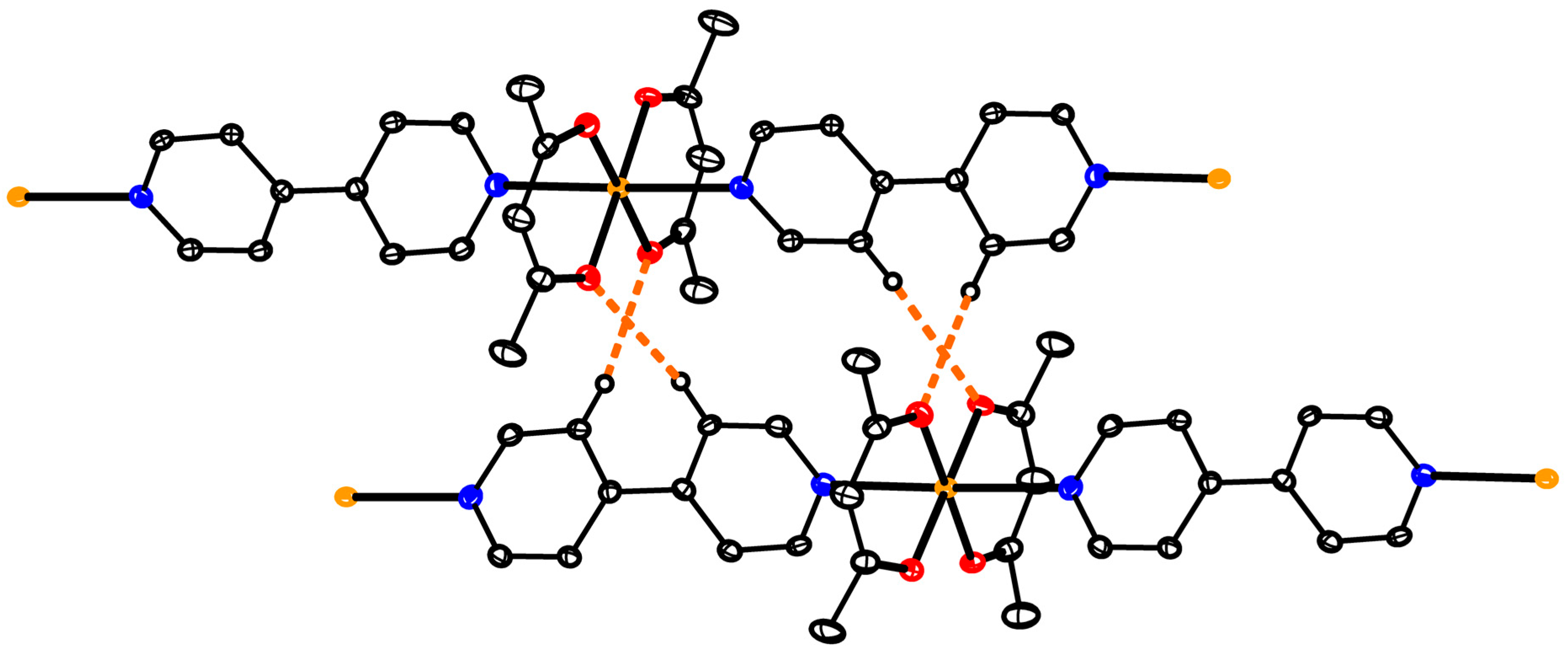
 N,
N,  O,
O,  Cu,
Cu,  C.
C.
 N,
N,  O,
O,  Cu,
Cu,  C.
C.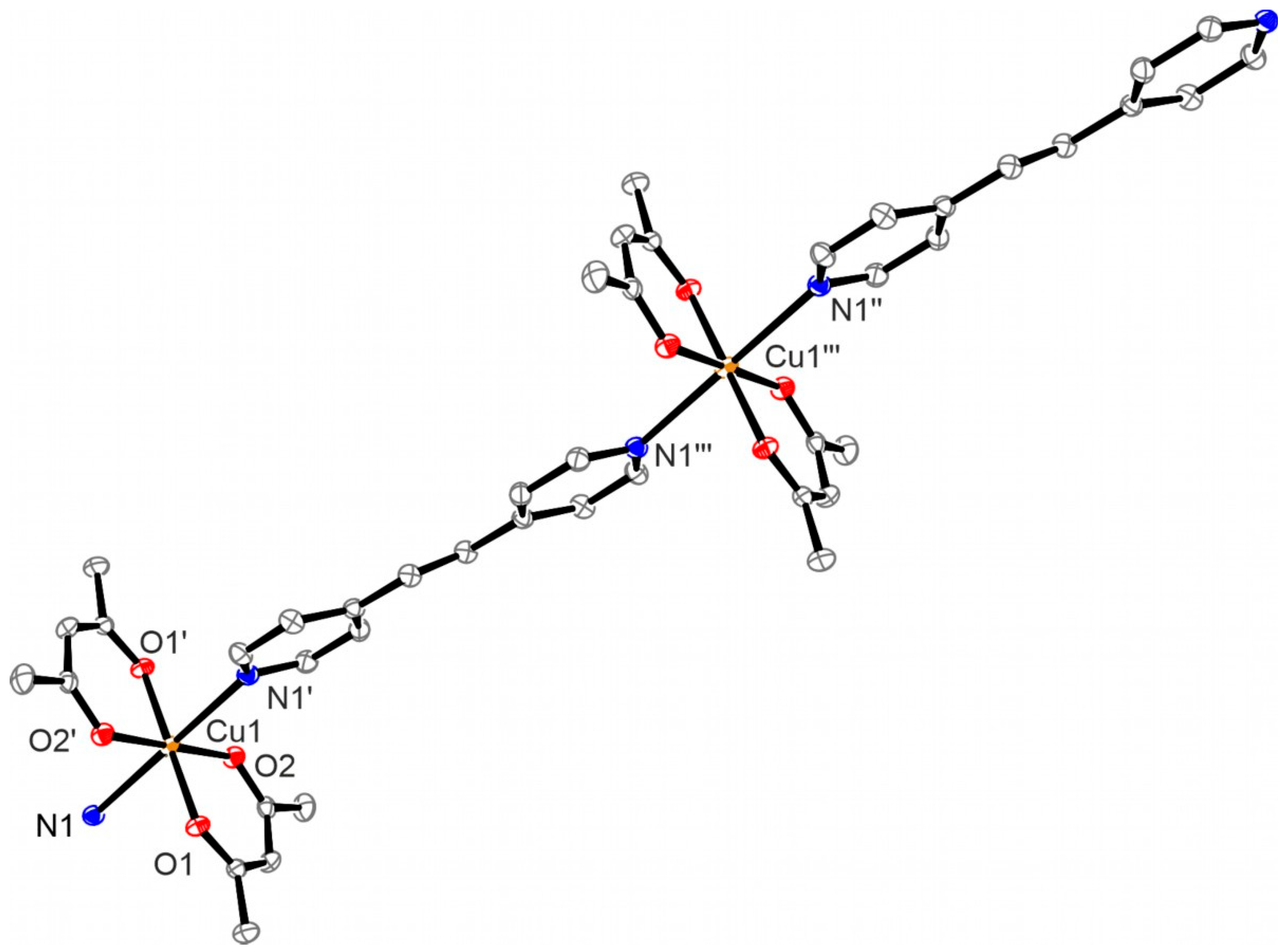
 N,
N,  O,
O,  Cu,
Cu,  C.
C.
 N,
N,  O,
O,  Cu,
Cu,  C.
C.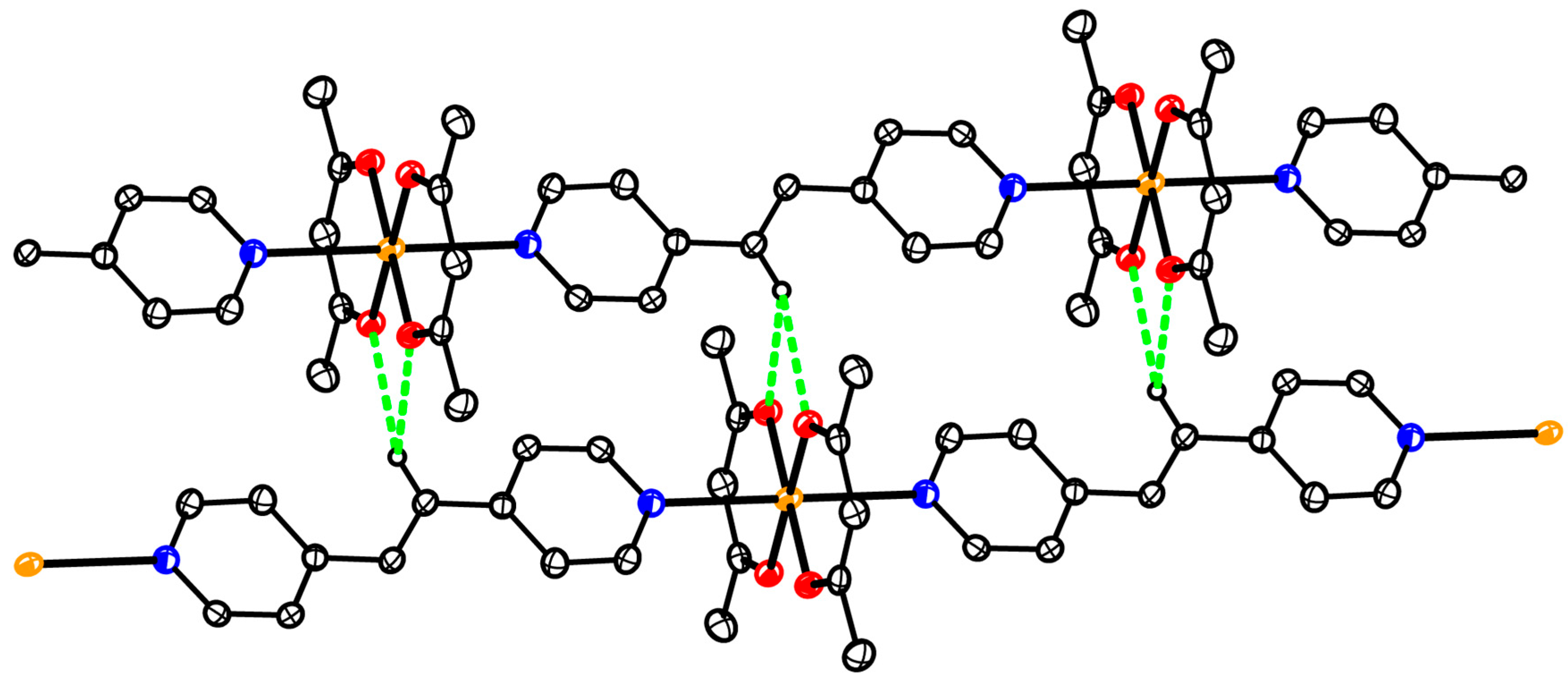
 N,
N,  O,
O,  Cu,
Cu,  C.
C.
 N,
N,  O,
O,  Cu,
Cu,  C.
C.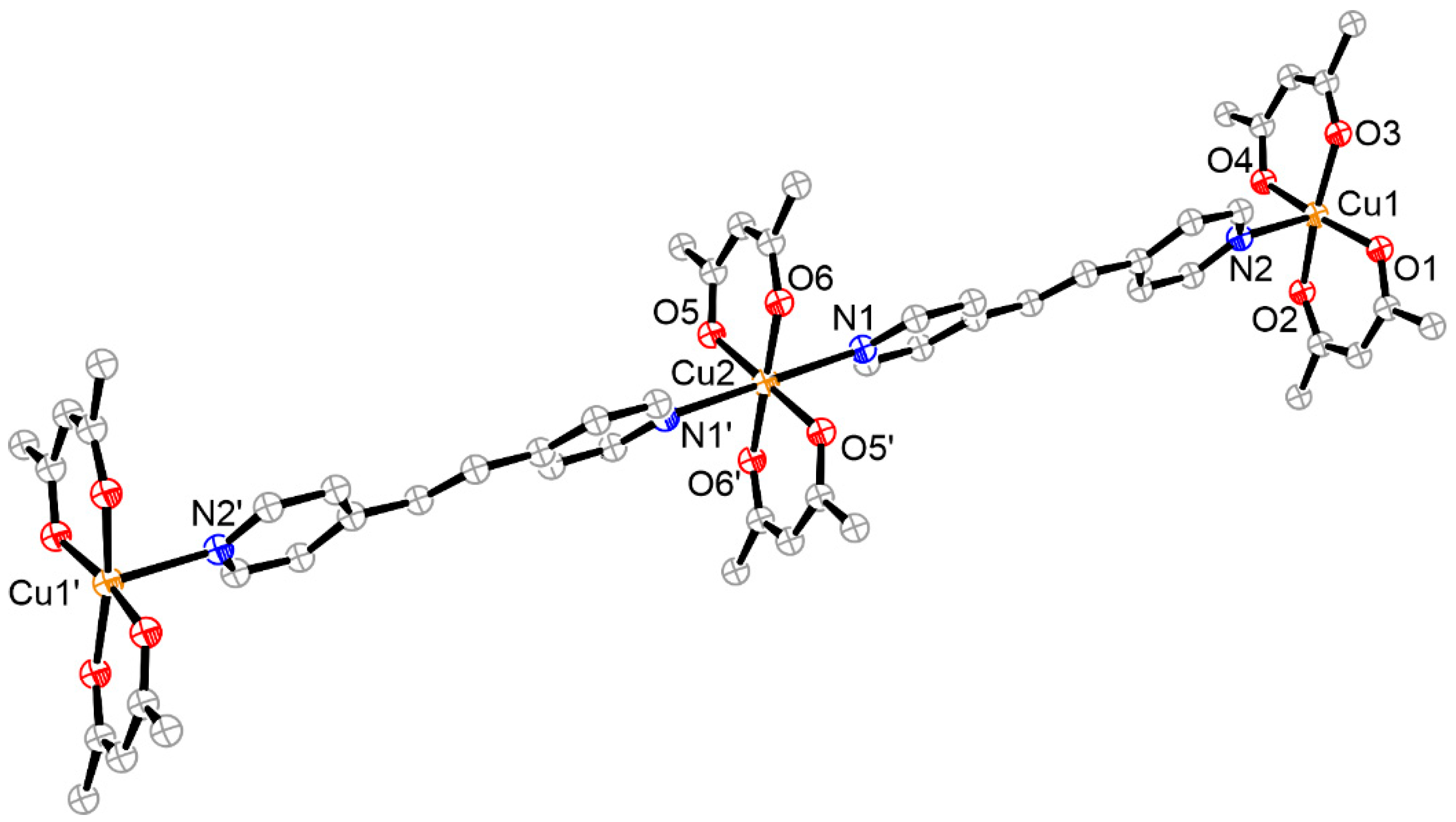
 N,
N,  O,
O,  Cu,
Cu,  C.
C.
 N,
N,  O,
O,  Cu,
Cu, 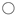 C.
C.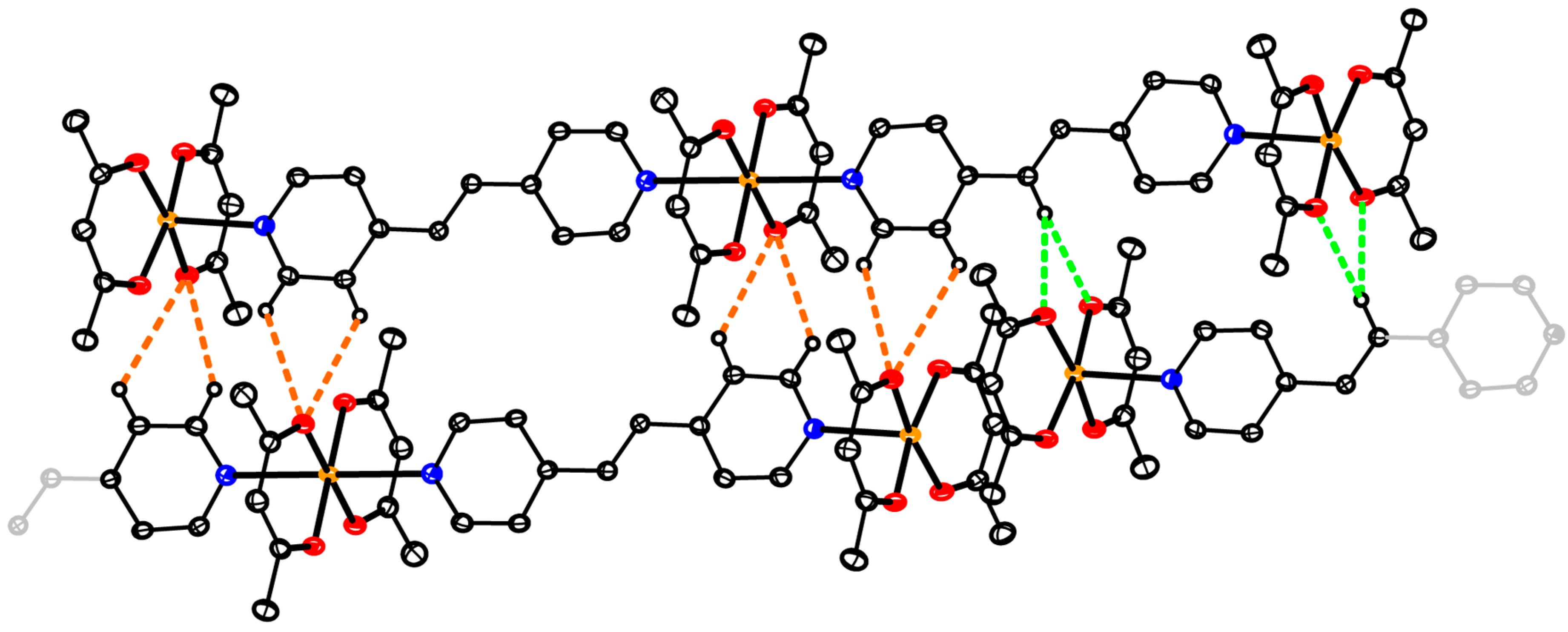
| Compound 1 | Compound 2 | Compound 3 | Compound 4 | Compound 5 | Compound 6 | Compound 7 | Compound 8 | |
|---|---|---|---|---|---|---|---|---|
| Empirical formula | C15H19CuNO4 | C16H24CuNO4 | Cu19H26CuN2O4 | C15H18BrCuNO4 | C20H22CuN2O4 | C22H24CuN2O4 | C54H62Cu3N4O12 | C37H43Cl15Cu2N2O8 |
| Formula weight | 340.85 | 354.88 | 409.96 | 419.75 | 417.93 | 443.97 | 1149.69 | 1302.56 |
| Crystal system | Triclinic | Monoclinic | Monoclinic | Triclinic | Monoclinic | Triclinic | Triclinic | Triclinic |
| Space group | P-1 | C2/m | C2/m | P-1 | P21 | P-1 | P-1 | P-1 |
| a, Å | 7.7444(3) | 21.7043(4) | 10.9402(3) | 8.6807(3) | 11.1929(4) | 8.2115(3) | 12.4530(10) | 9.1804(8) |
| b, Å | 8.1821(3) | 9.5368(2) | 12.2115(3) | 10.2923(5) | 14.2606(6) | 8.6888(3) | 14.0998(11) | 11.2336(10) |
| c, Å | 13.7360(5) | 17.9698(4) | 15.0631(4) | 11.4344(5) | 11.9295(5) | 8.7538(3) | 15.3886(12) | 13.2041(11) |
| α, deg. | 93.9659(10) | 90 | 90 | 112.0530(10) | 90 | 69.7339(10) | 88.207(3) | 78.857(3) |
| β, deg. | 98.1740(10) | 116.6720(10) | 107.4790(10) | 110.5830(10) | 92.6190(10) | 62.2510(10) | 74.608(3) | 86.404(2) |
| γ | 117.6440(10) | 90 | 90 | 96.890(2) | 90 | 76.4940(10) | 89.364(3) | 86.933(2) |
| V, Å3 | 754.10(5) | 3325.07(12) | 1919.46(9) | 848.21(6) | 1902.06(13) | 516.55(3) | 2603.8(4) | 1332.2(2) |
| Z | 2 | 8 | 4 | 2 | 4 | 1 | 2 | 1 |
| ρcalc, g cm−3 | 1.501 | 1.418 | 1.419 | 1.643 | 1.459 | 1.427 | 1.466 | 1.624 |
| μ (MoKα), mm−1 | 1.462 | 1.330 | 1.164 | 3.658 | 1.176 | 1.087 | 1.280 | 1.597 |
| Crystal size, mm3 | 0.44 × 0.17 × 0.12 | 0.18 × 0.17 × 0.12 | 0.09 × 0.08 × 0.04 | 0.27 × 0.20 × 0.18 | 0.09 × 0.05 × 0.02 | 0.18 × 0.12 × 0.07 | 0.13 × 0.12 × 0.11 | 0.45 × 0.11 × 0.09 |
| Temperature (K) | 100(2) | 100(2) | 100(2) | 100(2) | 100(2) | 100(2) | 100(2) | 100(2) |
| θ range, deg | 2.844–30.082 | 1.905–30.057 | 2.567–28.707 | 2.130–30.060 | 2.227–30.054 | 2.508–30.017 | 2.225–30.055 | 2.646–28.719 |
| hkl range, deg | −10:10, −11:11, −19:19 | −30:30, −13:13, −25:25 | −14:14, −16:16, −20:20 | −12:12, −14:14, −16:16 | −15:15, −20:20, −16:16 | −11:11, −12:12, −12:12 | −17:17, −19:19, −21:21 | −12:12, −15:15, −17:17 |
| Total/unique data/Rint | 11181/4425/0.0162 | 29205/5151/0.0190 | 14383/2482/0.0305 | 14932/4948/0.0174 | 32060/11140/0.0363 | 8874/3011/0.0369 | 44994/15126/0.0213 | 14488/6850/0.0258 |
| Observed data [I > 2σ(I)] | 4291 | 4783 | 2284 | 4668 | 9753 | 2790 | 12894 | 6140 |
| Nref/Npar | 4425/194 | 5151/229 | 2482/122 | 4948/203 | 11,140/496 | 8874/135 | 3011/673 | 6850/440 |
| R1/wR2 [I > 2σ(I)] | 0.0233/0.0651 | 0.0241/0.0731 | 0.0334/0.0808 | 0.0235/0.0607 | 0.0389/0.0931 | 0.0389/0.0887 | 0.0379/0.1102 | 0.0527/0.1529 |
| R1/wR2 [all data] | 0.0241/0.0656 | 0.0258/0.0743 | 0.0369/0.0826 | 0.0252/0.0615 | 0.0461/0.0968 | 0.0418/0.0912 | 0.0444/0.1143 | 0.0586/0.1600 |
| Flack parameter | n/a | n/a | n/a | n/a | 0.203(17) | n/a | n/a | n/a |
| S | 1.073 | 1.035 | 1.088 | 1.044 | 1.033 | 1.057 | 1.046 | 1.083 |
| Min./max. res. dens., eÅ−3 | 0.698/−0.577 | 0.440/−0.317 | 2.116/−0.733 | 1.342/−1.005 | 0.685/−0.693 | 0.584/−0.451 | 1.547/−0.634 | 1.848/−1.648 |
| Complex | Coordinating Pyridine Ligand | Cu-N [Å] [a] | Cu-O [Å] [a] | τ5 [a] |
|---|---|---|---|---|
| 1 | 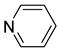 | 2.216(1) | 1.948(4) | 0.04 |
| 2 | 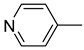 | 2.27(2) | 1.943(5) | 0 |
| 3 | 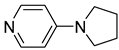 | 2.206(2) | 1.95(2) | 0.41 |
| 4 | 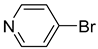 | 2.292(1) | 1.940(4) | 0.03 |
| 7 (CN=5) | 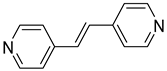 | 2.323(1) | 1.938(5) | 0.02 |
| 5 | 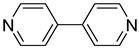 | 2.42(1) | 1.959(8) | / |
| 6 | 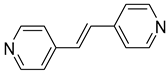 | 2.477(1) | 1.955(4) | / |
| 7 (CN=6) | 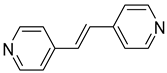 | 2.467(5) | 1.95(2) | / |
| Cu-N [Å] | T [K] | τ5 [a] | N [b] | Reference (CCDC Code) | |
|---|---|---|---|---|---|
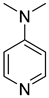 | 2.12 | 150 | 0.86 | 15.8 | [16] (608513) |
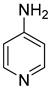 | 2.01 | 150 | 1.12 | 15.2 | [14] (769010) |
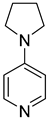 | 2.21 | 100 | 0.41 | 15.9 | this work |
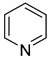 | 2.22 | 100 | 0.04 | 12.9 | this work |
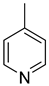 | 2.27 | 100 | 0 | 13.7 | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mensing, L.; Schäfer, T.; Layh, M.; Hebenbrock, M. Gone with the Wind—Adducts of Volatile Pyridine Derivatives and Copper(II) Acetylacetonate. Crystals 2025, 15, 690. https://doi.org/10.3390/cryst15080690
Mensing L, Schäfer T, Layh M, Hebenbrock M. Gone with the Wind—Adducts of Volatile Pyridine Derivatives and Copper(II) Acetylacetonate. Crystals. 2025; 15(8):690. https://doi.org/10.3390/cryst15080690
Chicago/Turabian StyleMensing, Luca, Tim Schäfer, Marcus Layh, and Marian Hebenbrock. 2025. "Gone with the Wind—Adducts of Volatile Pyridine Derivatives and Copper(II) Acetylacetonate" Crystals 15, no. 8: 690. https://doi.org/10.3390/cryst15080690
APA StyleMensing, L., Schäfer, T., Layh, M., & Hebenbrock, M. (2025). Gone with the Wind—Adducts of Volatile Pyridine Derivatives and Copper(II) Acetylacetonate. Crystals, 15(8), 690. https://doi.org/10.3390/cryst15080690







