Graphene Oxide-Constructed 2 nm Pore Anion Exchange Membrane for High Purity Hydrogen Production
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Chemicals
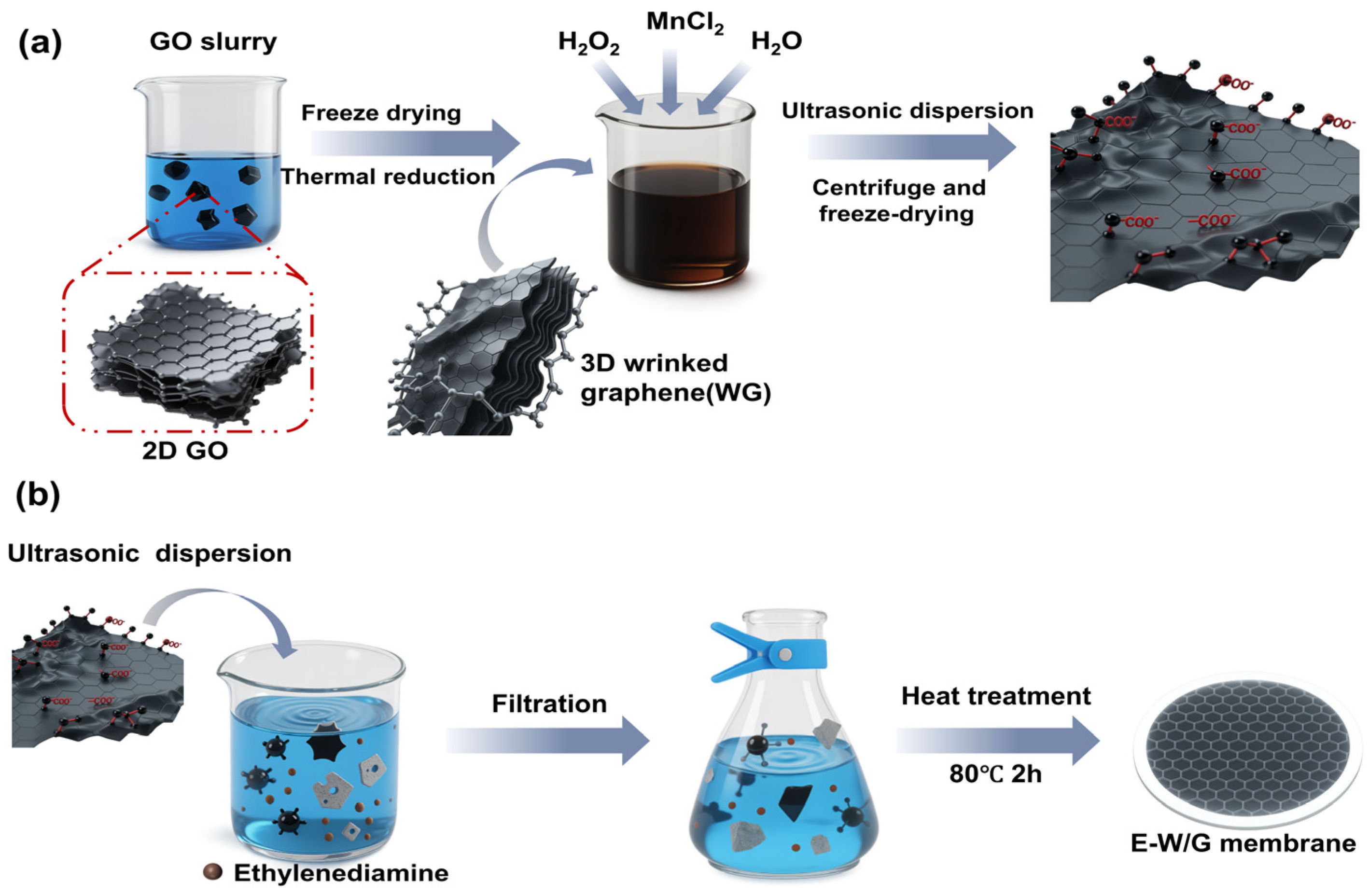
2.2. Preparation of WG
2.3. Preparation of E-W/G Membrane
2.4. Characterization of E-W/G Membrane
2.5. Properties Test of E-W/G Membrane Electrolytic Water and Gas Cross-Testing
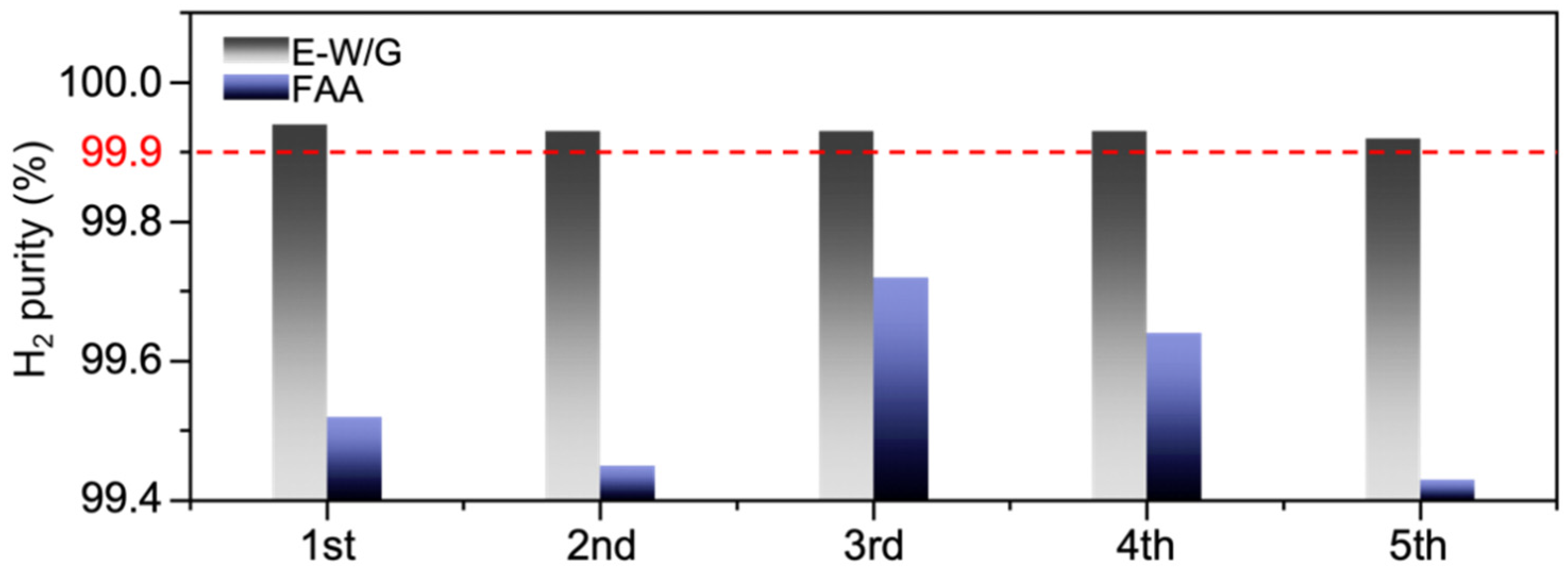
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hirscher, M.; Yartys, V.A.; Baricco, M.; Colbe, J.B.V.; Blanchard, D.; Bowman Jr, R.C.; Broom, D.P.; Buckley, C.E.; Chen, P.; Cho, Y.W. Materials for hydrogen-based energy storage: Past, recent progress and future outlook. J. Alloys Compd. 2024, 10, 827. [Google Scholar] [CrossRef]
- Ogden, J.M. Hydrogen: The Fuel of The Future. Phys. Today 2002, 55, 69–75. [Google Scholar] [CrossRef]
- Mansour-Saatloo, A.; Agabalaye-Rahvar, M.; Mirzaei, M.A.; Mohammadi-Ivatloo, B.; Abapour, M.; Zare, K. Robust scheduling of hydrogen based smart micro energy hub with integrated demand response. J. Clean. Prod. 2020, 267, 122041. [Google Scholar] [CrossRef]
- Kreuer, K.D.; Adams, S.; Münch, W.; Fuchs, A.; Klock, U.; Maier, J. Proton conducting alkaline earth zirconates and titanates for high drain electrochemical applications. Solid State Ion. 2001, 145, 295–306. [Google Scholar] [CrossRef]
- Schouten, J.A.; Rosmalen, J.V.; Michels, J.P.J. Modeling hydrogen production for injection into the natural gas grid: Balance between production, demand and storage. Int. J. Hydrogen Energy 2006, 31, 1698–1706. [Google Scholar] [CrossRef]
- Kim, D.; Oh, L.S.; Park, J.H.; Kim, H.J.; Lee, S.; Lim, E. Perovskite-based electrocatalysts for oxygen evolution reaction in alkaline media: A mini review. Front. Chem. 2022, 10, 1024865. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fang, Z.; Zhang, M.; Xie, S.; Xie, D.; Liu, P.; Wang, S.; Cheng, F.; Xu, T. Macromolecular crosslinked poly(aryl piperidinium)-based anion exchange membranes with enhanced ion conduction for water electrolysis. J. Membr. Sci. 2024, 700, 122717. [Google Scholar] [CrossRef]
- Li, D.; Park, E.J.; Zhu, W.; Shi, Q.; Zhou, Y.; Tian, H.; Lin, Y.; Serov, A.; Zulevi, B.; Baca, E.D. Highly quaternized polystyrene ionomers for high performance anion exchange membrane water electrolysers. Nat. Energy 2020, 5, 378–385. [Google Scholar] [CrossRef]
- Naqvi, S.A.H.; Taner, T.; Ozkaymak, M.; Ali, H.M. Hydrogen production through alkaline electrolyzers: A techno-economic and enviro-economic analysis. Chem. Eng. Technol. 2023, 46, 474–481. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, X.; Wen, H.; Pei, A. Hydrogen production from offshore wind power in South China. Int. J. Hydrogen Energy 2022, 47, 24558–24568. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, Q.L.; Zhang, Q.G.; Zhu, A.M. Synthesis and characterization of cross-linked quaternized poly(vinyl alcohol)/chitosan composite anion exchange membranes for fuel cells. J. Power Sources 2008, 183, 447–453. [Google Scholar] [CrossRef]
- Gopi, K.H.; Peera, S.G.; Bhat, S.D.; Sridhar, P.; Pitchumani, S. 3-Methyltrimethylammonium poly(2, 6-dimethyl-1, 4-phenylene oxide) based anion exchange membrane for alkaline polymer electrolyte fuel cells. Bull. Mater. Sci. 2014, 37, 877–881. [Google Scholar] [CrossRef]
- Vona, M.L.D.; Narducci, R.; Pasquini, L.; Pelzer, K.; Knauth, P. Anion-conducting ionomers: Study of type of functionalizing amine and macromolecular cross-linking. Int. J. Hydrogen Energy 2014, 39, 14039–14049. [Google Scholar] [CrossRef]
- Pasquini, L.; Vona, M.L.D.; Knauth, P. Effects of anion substitution on hydration, ionic conductivity and mechanical properties of anion-exchange membranes. New J. Chem. 2016, 40, 3671–3676. [Google Scholar] [CrossRef]
- Su, X.; Gao, L.; Hu, L.; Qaisrani, N.A.; Yan, X.; Zhang, W.; Jiang, X.; Ruan, X.; He, G. Novel piperidinium functionalized anionic membrane for alkaline polymer electrolysis with excellent electrochemical properties. J. Membr. Sci. 2019, 581, 283–292. [Google Scholar] [CrossRef]
- Lee, A.S. Poly(pyrrolidinium)-based anion exchange membranes and ionomers for anion exchange membrane water electrolyzers. ECS Meet. Abstr. 2024, MA2024-02, 2874. [Google Scholar] [CrossRef]
- Cao, Y.C.; Wu, X.; Scott, K. A quaternary ammonium grafted poly vinyl benzyl chloride membrane for alkaline anion exchange membrane water electrolysers with no-noble-metal catalysts. Int. J. Hydrogen Energy 2012, 37, 9524–9528. [Google Scholar] [CrossRef]
- Konovalova, A.; Kim, H.; Kim, S.; Lim, A.; Park, H.S.; Kraglund, M.R.; Aili, D.; Jang, J.H.; Kim, H.-J.; Henkensmeier, D. Blend membranes of polybenzimidazole and an anion exchange ionomer (FAA3) for alkaline water electrolysis: Improved alkaline stability and conductivity. J. Membr. Sci. 2018, 564, 653–662. [Google Scholar] [CrossRef]
- Merle, G.; Wessling, M.; Nijmeijer, K. Anion exchange membranes for alkaline fuel cells: A review. J. Membr. Sci. 2011, 377, 1–35. [Google Scholar] [CrossRef]
- Tetsuya, K.; Yuta, K.; Azumi, M.; Nur, L.H.; Kazuto, H.; Armando, T.Q.; Mitsuru, S.; Atsushi, U. Water Vapor Electrolysis with Proton-Conducting Graphene Oxide Nanosheets. ACS Sustain. Chem. Eng. 2018, 6, 11753–11758. [Google Scholar] [CrossRef]
- Liaw, D.J.; Huang, C.C.; Ju, J.Y. Novel Functional Norbornenes as Initiators for Radical Polymerization, Their Polymeric Derivatives and a Process for Producing the Same. U.S. Patent 20050182220 A1, 6 October 2024. [Google Scholar]
- Bailey, S.E.; Zink, J.I.; Nelsen, S.F. Contributions of symmetric and asymmetric normal coordinates to the intervalence electronic absorption and resonance Raman spectra of a strongly coupled p-phenylenediamine radical cation. J. Am. Chem. Soc. 2003, 125, 5939–5947. [Google Scholar] [CrossRef]
- Burress, J.W.; Gadipelli, S.; Ford, J.; Simmons, J.M.; Zhou, W.; Yildirim, T. Graphene Oxide Framework Materials: Theoretical Predictions and Experimental Results. Angew. Chem. Int. Ed. 2010, 49, 8902–8904. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tang, K.; Ruan, H.; Xue, L.; Bruggen, B.V.; Gao, C.; Shen, J. Sulfonated reduced graphene oxide modification layers to improve monovalent anions selectivity and controllable resistance of anion exchange membrane. J. Membr. Sci. 2017, 536, 167–175. [Google Scholar] [CrossRef]
- Guan, K.; Zhao, D.; Zhang, M.; Shen, J.; Zhou, G.; Liu, G.; Jin, W. 3D nanoporous crystals enabled 2D channels in graphene membrane with enhanced water purification performance. J. Membr. Sci. 2017, 542, 41–51. [Google Scholar] [CrossRef]
- Jang, S.; Min, H.; Cho, S.B.; Kim, H.W.; Son, W.; Choi, C.; Chun, S.; Pang, C. A Hierarchically Tailored Wrinkled Three-Dimensional Foam for Enhanced Elastic Supercapacitor Electrodes. Nano Lett. 2021, 21, 7079–7085. [Google Scholar] [CrossRef]
- Gao, X.; Lu, F.; Liu, Y.; Sun, N. The facile construction of an anion exchange membrane with 3D interconnected ionic nano-channels. Chem. Commun. 2016, 53, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zou, Q.; Niu, Z. Graphene Oxide Prompted Double-Crosslinked Poly(Vinyl Alcohol)/Poly(-Diallyldimethylammonium Chloride) Anion-Exchange Membrane for Superior CO2 Electrochemical Reduction. SSRN Electron. J. 2023. [Google Scholar] [CrossRef]
- Meyer, J.C.; Geim, A.K.; Katsnelson, M.I.; Novoselov, K.S.; Booth, T.J.; Roth, S. The structure of suspended graphene sheets. Nature 2007, 446, 60–63. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, X.; Yuan, Q.; Duan, X.; Liang, L.; Gao, Y.; Li, X.; Zou, M.; Ma, R.; Yuan, Q. Large-area graphene-nanomesh/carbon-nanotube hybrid membranes for ionic and molecular nanofiltration. Science 2019, 364, 1057–1062. [Google Scholar] [CrossRef]
- Hung, W.S.; Tsou, C.H.; De Guzman, M.; An, Q.F.; Liu, Y.L.; Zhang, Y.M.; Hu, C.C.; Lee, K.R.; Lai, J.Y. Cross-Linking with Diamine Monomers To Prepare Composite Graphene Oxide-Framework Membranes with Varying d-Spacing. Chem. Mater. 2014, 26, 2983–2990. [Google Scholar] [CrossRef]
- Tao, R.; Shao, M.; Kim, Y. Polyarylene-Based Anion Exchange Membranes for Fuel Cells. Chem.—A Eur. J. 2024, 30, e202401208. [Google Scholar] [CrossRef]
- Yu, R.; Yang, H.; Cheng, J.; Tan, Y.; Wang, X.; Yu, X. Preparation and research progress of anion exchange membranes. Int. J. Hydrogen Energy 2024, 50 Pt A, 582–604. [Google Scholar] [CrossRef]
- Wu, X.; Hu, X.I. Anion Exchange Membranes for Hydrogen Technologies: Challenges and Progress. Chim. Chem. Rep. 2023, 77, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Al-Gaashani, R.; Najjar, A.; Zakaria, Y.; Mansour, S.; Atieh, M.A. XPS and structural studies of high quality graphene oxide and reduced graphene oxide prepared by different chemical oxidation methods. Ceram. Int. 2019, 45, 14439–14448. [Google Scholar] [CrossRef]
- Takagi, K.; Takao, H.; Nakagawa, T. Synthesis and characterization of nitrogen-linked carbazole-containing fluorescent polymers. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 3729–3735. [Google Scholar] [CrossRef]
- Wirth, S.B.; Girardclos, S.; Rellstab, C.; Anselmettl, F.S. The sedimentary response to a pioneer geo-engineering project: Tracking the Kander River deviation in the sediments of Lake Thun (Switzerland). Sedimentology 2011, 58, 1737–1761. [Google Scholar] [CrossRef]
- Dai, J.; Wang, G.; Ma, L.; Wu, C. Study on the surface energies and dispersibility of graphene oxide and its derivatives. J. Mater. Sci. 2015, 50, 3895–3907. [Google Scholar] [CrossRef]
- Mungse, H.P.; Khatri, O.P. Chemically Functionalized Reduced Graphene Oxide as a Novel Material for Reduction of Friction and Wear. J. Phys. Chem. C 2014, 118, 14394–14402. [Google Scholar] [CrossRef]
- Guo, A.; Chen, E.; Wygant, B.R.; Heller, A.; Mullins, C.B. Lead Oxide Microparticles Coated by Ethylenediamine-Crosslinked Graphene Oxide for Lithium Ion Battery Anodes. ACS Appl. Energy Mater. 2019, 2, 3017–3020. [Google Scholar] [CrossRef]
- Park, S.; Dikin, D.A.; Nguyen, S.B.T.; Ruoff, R.S. Graphene Oxide Sheets Chemically Cross-Linked by Polyallylamine. J. Phys. Chem. C 2009, 113, 15801–15804. [Google Scholar] [CrossRef]
- Park, S.; Lee, K.S.; Bozoklu, G.; Cai, W.; Nguyen, S.B.T.; Ruoff, R.S. Graphene oxide papers modified by divalent ions-enhancing mechanical properties via chemical cross-linking. Acs Nano 2008, 2, 572–578. [Google Scholar] [CrossRef]
- Nahon, P.; Coffe, G.; Guyader, H. Identification of the epiplasmins, a new set of cortical proteins of the membrane cytoskeleton in Paramecium. J. Cell Sci. 1993, 104, 975–990. [Google Scholar] [CrossRef]
- Chen, S.; Wei, Z. Recent Advances of Electrocatalyst, Catalyst Utilization and Water Management in Polymer Electrolyte Membrane Fuel Cells. Sci. Adv. Mater. 2015, 7, 2053–2068. [Google Scholar] [CrossRef]
- Carbone, A.; Zignani, S.C.; Gatto, I.; Trocino, S.; Aricó, A.S. Assessment of the FAA3-50 polymer electrolyte in combination with a NiMn2O4 anode catalyst for anion exchange membrane water electrolysis—ScienceDirect. Int. J. Hydrogen Energy 2020, 45, 9285–9292. [Google Scholar] [CrossRef]
- Lto, H.; Maeda, T.; Nakano, A.; Takenaka, H. Properties of Nafion membranes under PEM water electrolysis conditions. Int. J. Hydrogen Energy 2011, 36, 10527–10540. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, L.; Xie, Q.; Shi, H.; Yu, H. Construction of national clinical research data center of Traditional Chinese medicine. In Proceedings of the 2012 IEEE 14th International Conference on e-Health Networking, Applications and Services (Healthcom), Beijing, China, 10–13 October 2012. [Google Scholar] [CrossRef]
- Oberli, L.; Caruso, D.; Hall, C.; Fabretto, M.; Murphy, P.J.; Evans, D. Condensation and freezing of droplets on superhydrophobic surfaces. Adv. Colloid Interface Sci. 2014, 210, 47–57. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, H.; Zhu, H.; Zhang, A.; Lv, K.; Wei, H.; Wang, Y.; Sun, H.; Zhang, L.; Liu, X.; Zhang, H. Graphene Oxide-Constructed 2 nm Pore Anion Exchange Membrane for High Purity Hydrogen Production. Crystals 2025, 15, 689. https://doi.org/10.3390/cryst15080689
Wan H, Zhu H, Zhang A, Lv K, Wei H, Wang Y, Sun H, Zhang L, Liu X, Zhang H. Graphene Oxide-Constructed 2 nm Pore Anion Exchange Membrane for High Purity Hydrogen Production. Crystals. 2025; 15(8):689. https://doi.org/10.3390/cryst15080689
Chicago/Turabian StyleWan, Hengcheng, Hongjie Zhu, Ailing Zhang, Kexin Lv, Hongsen Wei, Yumo Wang, Huijie Sun, Lei Zhang, Xiang Liu, and Haibin Zhang. 2025. "Graphene Oxide-Constructed 2 nm Pore Anion Exchange Membrane for High Purity Hydrogen Production" Crystals 15, no. 8: 689. https://doi.org/10.3390/cryst15080689
APA StyleWan, H., Zhu, H., Zhang, A., Lv, K., Wei, H., Wang, Y., Sun, H., Zhang, L., Liu, X., & Zhang, H. (2025). Graphene Oxide-Constructed 2 nm Pore Anion Exchange Membrane for High Purity Hydrogen Production. Crystals, 15(8), 689. https://doi.org/10.3390/cryst15080689





