Abstract
Protein packing within crystal lattices plays a critical role in determining molecular flexibility; therefore, the observed conformation and flexibility of protein side chains can vary depending on the crystal space group. Protein crystal dehydration affects crystal lattice mosaicity, which can either reduce crystal quality or enhance X-ray diffraction intensity. It also often alters the crystal lattice, leading to space group transition. Accordingly, dehydration-induced space group transitions could theoretically offer an alternative when there are experimental limitations obstructing the obtainment of diverse crystal forms. However, this remains underexplored experimentally. Here, a dehydration-induced space group transition was explored to observe different conformations and flexibilities of the protein structure. Xylanase GH11 crystals from Thermoanaerobacterium saccharolyticum (TsaGH11) were air-dehydrated, and their structure at room temperature was determined. Upon dehydration, the space group of the TsaGH11 crystal changed from tetragonal to orthorhombic, affecting the protein–protein interfaces within the crystal lattice. The dehydrated crystal structure of TsaGH11 revealed multiple conformations of residues involved in substrate binding and recognition within the substrate-binding cleft. These diverse molecular conformations and flexibilities provide significant and previously unrevealed structural information for TsaGH11. This approach demonstrates the potential of dehydration-induced space group transitions to reveal diverse protein conformations, offering valuable insights into molecular properties and functions.
1. Introduction
Macromolecular crystallography (MX) is a powerful technique for understanding molecular functions at atomic resolution [,,]. The structural information obtained by this approach has been widely applied in drug design and in enzyme engineering for industrial applications [,,,]. In MX, diffraction data are typically collected using single crystals, which are formed by the assembly of macromolecules into an ordered, periodic lattice arrangement [,]. Crystal packing, which arises from macromolecular interactions, can potentially affect protein structure [,]. Crystal contacts may induce structural changes by burying a significant portion of the protein surface that is normally accessible to the solvent [,]. Accordingly, the structural features of macromolecules—such as their conformation and flexibility—can vary depending on the crystal form, which is defined by the space group [,,]. As such, different crystal forms can provide alternative structural properties of the macromolecule [,,]. Observing variations in the macromolecular conformation and flexibility between different crystal forms is important for accurately understanding molecular functions [,]. Different crystal forms can generally be obtained by altering the crystallization conditions, including the composition of the crystallization solution, temperature, and other environmental factors.
Crystal dehydration is defined as any process that removes available water molecules from the crystal lattice []. Dehydration can physically affect the crystal lattice. Therefore, macromolecular crystals are typically maintained in a hydrated state during the collection of data, either in crystallization solution or in a cryoprotectant, to preserve the integrity of the lattice. In contrast, crystal dehydration techniques have often been used to improve the diffraction quality, particularly by extending the resolution limit and reducing the mosaicity [,]. To date, various dehydration techniques, including air-drying dehydration, salt-based methods, and humidity control systems, have been developed and applied in the collection of MX data [,,].
Meanwhile, in a previous study using the HC1b humidity controller, the space group of glucose isomerase (GI) crystals was observed to change in response to controlled humidity conditions []. Specifically, at final humidities of 96% and 90%, the GI crystals belonged to the I222 space group, whereas at 85%, 80%, 70%, and 64% humidity they transitioned to P222. Interestingly, at 88% humidity, the crystals exhibited features of both of these space groups. The hydrated GI structure, collected at a cryogenic temperature (PDB code: 4ZB0), was reported to belong to the I222 space group with unit cell dimensions of a = 92.904, b = 98.152, and c = 102.704 Å. In contrast, the dehydrated GI structure (PDB code: 4ZBC) belonged to the P21212 space group, with corresponding dimensions of 81.629, 93.641, and 97.640 Å, respectively []. Similarly, controlled dehydration of cytomegalovirus immediate-early 1 (IE1) protein crystals triggered a space group transition from monoclinic (P21), with unit cell dimensions of a = 58.0, b = 278.6, and c = 61.6 Å, to tetragonal (P43), with unit cell dimensions of a = b = 56.5 and c = 276.9 Å, which induced changes in the intrinsic flexibility of the molecule. These studies indicated that space group transitions can be induced by crystal dehydration. Theoretically, such dehydration-induced changes in the space group have the potential to reveal alternative protein conformations and flexibility by altering the crystal form. To the best of our knowledge, such systematic demonstrations or applications of dehydration-induced space group transitions remain rare in the exploration of conformational diversity in protein structures.
Here, we directly demonstrate that dehydration-induced space group transitions lead to structural changes in macromolecules, using xylanase GH11 from the hemicellulose-degrading bacterium Thermoanaerobacterium saccharolyticum (TsaGH11) as a model system. TsaGH11 exhibits high catalytic efficiency, with reported Km and kcat values of 12.9 mg·mL−1 and 34,015.3 s−1, respectively []. The crystal structure of TsaGH11 was determined by both serial synchrotron crystallography and conventional macromolecular crystallography [,,]. All previously reported TsaGH11 crystals belonged to the tetragonal space group P43212, containing two molecules in the asymmetric unit. Interestingly, these two TsaGH11 molecules exhibited distinct conformations and flexibility in the substrate-binding cleft and active site. One molecule adopted a rigid conformation stabilized by crystal packing, with an open conformation between the thumb and finger domains. In contrast, the other molecule, which was more exposed to the solvent channels, adopted a closed conformation at the substrate-binding cleft. This suggests that crystal packing effects were a factor involved in the diversity in the observed conformations. These findings imply that changes in the crystal packing, such as transitions in space group, may reveal additional structural features.
We hypothesized that dehydration-induced changes in crystal packing could unmask conformational states not observed in the native hydrated form. In this study, the TsaGH11 crystal was air-dehydrated, and the diffraction data were collected at room temperature. Dehydration induced a change in space group from a tetragonal to an orthorhombic crystal form. The dehydrated TsaGH11 structure exhibited distinct conformations of residues at the substrate-binding cleft, as well as altered molecular flexibility, compared with the previously reported TsaGH11 structures. This dehydration-induced transition in crystal space group provides a useful strategy for exploring the conformational landscape and flexibility of macromolecules, which could deepen our understanding of their molecular properties and functions.
2. Materials and Methods
2.1. Preparation of TsaGH11
The protein preparation of TsaGH11 was performed as previously described []. In brief, the pBT7 vector (Bioneer, Daejeon, Republic of Korea) containing the TsaGH11 gene was transformed into Escherichia coli BL21 (DE3) cells. These cells were grown in LB broth medium supplemented with 50 μg/mL ampicillin at 37 °C in a shaking incubator at 180 rpm. When the OD600 reached 0.4–0.8, protein expression was induced by adding 0.5 mM isopropyl-β-D-1-thiogalactopyranoside (IPTG), and the culture was incubated overnight at 18 °C with shaking at 180 rpm. Cells were harvested by centrifugation at 4000 rpm for 20 min and resuspended in a lysis buffer containing 50 mM Tris-HCl (pH 8.0), 200 mM NaCl, and 20 mM imidazole. The cells were disrupted on ice by sonication, after which the cell debris was removed by centrifugation at 14,000 rpm for 30 min. The supernatant was filtered through a 0.22 μm syringe filter and loaded onto a Ni–NTA resin (Qiagen, Valencia, CA, USA) column. This resin was then washed with the lysis buffer, and the protein was eluted using that buffer supplemented with 300 mM imidazole. Next, the eluted protein was concentrated using a Centricon filter and incubated with thrombin (Sigma-Aldrich, St. Louis, MO, USA) at room temperature for 18 h to remove the N-terminal hexahistidine tag. The concentrated protein was further purified using a Sephacryl S-100 column (GE Healthcare, Chicago, IL, USA) equilibrated with 10 mM Tris-HCl (pH 8.0) and 200 mM NaCl. The purity of the protein was verified by SDS-PAGE. The purified protein was concentrated to 20 mg/mL for crystallization, and the protein concentration was measured using a Bradford assay.
2.2. Protein Crystallization
The crystallization of TsaGH11 was performed using the hanging-drop vapor diffusion method at 20 °C. Equal volumes (2 μL) of the TsaGH11 solution and the crystallization solution [0.1 M sodium acetate (pH 4.6) and 4.0 M ammonium acetate] were mixed on a cover glass and equilibrated against 500 μL of the crystallization solution in a 24-well VDX plate (Hampton Research). Suitable crystals for X-ray diffraction appeared after 3 weeks. All crystals grown in the crystallization drop exhibited the same bipyramidal morphology. The crystal size was approximately 130 × 130 × 270 μm3.
2.3. X-Ray Diffraction Data Collection
Diffraction data were collected at Beamline 11C of Pohang Light Source II (PLS-II; Pohang, Republic of Korea) []. The X-ray wavelength and beam size at the sample position were 0.9796 Å and 4 × 8.5 μm (FWHM), respectively. The TsaGH11 crystal was fished from the crystallization drop using a nylon loop attached to a magnetic base, which was then mounted on the goniometer. During air exposure, the solution within the nylon loop and around the crystal gradually evaporated, leaving the crystal attached to the loop at ambient temperature (299 K). During data collection, the crystal was oscillated by 1° per image with an exposure time of 100 ms. The predicted average diffraction-weighted dose and the average dose in the exposed region of the crystal were calculated using RADDOSE-3D []. The diffraction data were recorded with a PILATUS 6M detector (DECTRIS, Baden, Switzerland) at a crystal-to-detector distance of 400 mm. The diffraction images were indexed, integrated, and scaled using HKL2000 [].
2.4. Crystal Structure Determination
The structure of TsaGH11 was determined using the molecular replacement method with MOLREP [] from the CCP4 package. The room-temperature structure of TsaGH11 determined by serial synchrotron crystallography (PDB code: 8IH0) [] was used as the search model. Manual model building was performed based on the electron density map using COOT []. Structural refinement was carried out with phenix.refine in PHENIX []. Model rebuilding and refinement were repeated iteratively. The quality of the final TsaGH11 structure was validated using MolProbity []. The structural Figures were prepared using PyMOL (DeLano Scientific LLC, San Carlos, CA, USA).
2.5. Bioinformatics
The secondary structures of TsaGH11 were analyzed using 2StrucCompare [] with the DSSP [] method. Structural similarity was analyzed using PDBeFold []. The values of B-factors and normalized B-factors were obtained using PHENIX [].
3. Results
3.1. Crystal Dehydration and Data Collection
To demonstrate the dehydration-induced transitions in space groups, TsaGH11 crystals were employed as a model system. Among the various methods of crystal dehydration, including simple air-drying, vapor diffusion using salts, immersion with dehydrating compounds, and humidity control [], air dehydration was applied to the TsaGH11 crystal to induce a change in space group from its original tetragonal form. Specifically, a TsaGH11 crystal was mounted on a goniometer and allowed to dehydrate under ambient conditions for 5 min prior to the collection of X-ray data. Processing of the diffraction data revealed that the crystal maintained the tetragonal space group P43212, which is identical to the structure of TsaGH11 at room temperature as previously determined []. This suggests that air-drying under these conditions was insufficient to induce a transition in space group, probably due to the dehydration rate or exposure time being inadequate.
To investigate whether dehydration time is a critical factor in triggering a space group transition, a new crystal was prepared, and the air-drying time was extended to 10 min. The diffraction data were subsequently collected at room temperature using a short exposure time of 100 ms per 1° oscillation to minimize damage caused by radiation. Data processing revealed that the crystal had successfully transitioned from the tetragonal space group to an orthorhombic C2221 space group. To confirm that this change in the space group had been caused by air-drying dehydration, the diffraction dataset was indexed using the original tetragonal space group P43212. As a result, the Rmerge and CC1/2 values were shown to be significantly high, indicating incorrect assignment of the space group. This demonstrates that air-drying dehydration can trigger a change in space group in TsaGH11 crystals, and that dehydration time is a key factor influencing this transition.
The diffraction data from the dehydrated TsaGH11 (TsaGH11Dehyd) crystal were processed to a resolution of 2.7 Å (Table 1). The TsaGH11Dehyd crystal belongs to the orthorhombic space group C2221, with unit cell parameters of a = 100.150 Å, b = 102.898 Å, and c = 174.636 Å. These dimensions differ significantly from those of the previously reported tetragonal form (space group P43212) of hydrated TsaGH11 (TsaGH11Hyd) (PDB code: 8YEA), for which the corresponding values are 69.89, 69.89, and 170.42 Å, respectively []. In the orthorhombic C2221 crystal form, four TsaGH11 molecules occupy the asymmetric unit, whereas only two molecules were present in the asymmetric unit of the previously reported tetragonal form []. To date, six different crystal structures of TsaGH11 have been reported (PDB code: 8IH0, 8IH1, 8X1D, 8YEA, 8YYN, and 8YYO) [,,,]. Among them, only the data collection environment of TsaGH11-8YEA [] is identical to that used in this study, whereas the other structures were determined under different conditions, such as different data collection methods or sample preparations. Accordingly, the differences in structure between the dehydrated orthorhombic crystal form and the native tetragonal form (PDB code: 8YEA) [] were further analyzed to explore how molecular packing and protein conformation are influenced by dehydration (Table S1).

Table 1.
Data collection and refinement statistics of TsaGH11Dehyd.
The crystal packing of TsaGH11Dehyd similarly exhibited large solvent channels along the a and b axes, whereas TsaGH11Hyd displayed relatively tighter packing with smaller solvent channels (Figure 1A). Matthews coefficient analysis showed that the Vm values of the TsaGH11Dehyd and TsaGH11Hyd crystals were 2.79 and 2.58 Å3/Da, respectively. Meanwhile, the estimated solvent contents of TsaGH11Dehyd and TsaGH11Hyd were approximately 52% and 55%, respectively. Analysis of the protein interface revealed that the buried area involved in crystal packing ranged from 270.4 to 528 Å2 in TsaGH11Dehyd and from 326.6 to 523.1 Å2 in TsaGH11Hyd. Moreover, the number of hydrogen bonds at the packing interface ranged from 2 to 13 in TsaGH11Dehyd and from 4 to 10 in TsaGH11Hyd (Tables S2–S4).
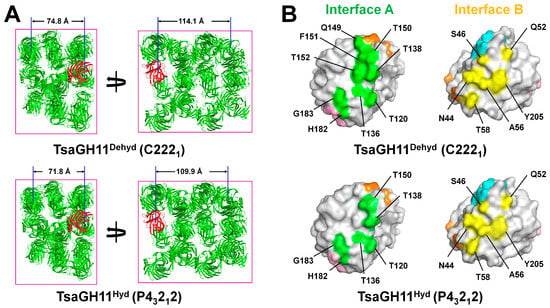
Figure 1.
Crystal packing and interface residues of TsaGH11Dehyd and TsaGH11Hyd crystals. (A) Crystal packing of TsaGH11Dehyd TsaGH11 (space group C2221) and TsaGH11Hyd (space group P43212). The superimposed TsaGH11 molecules from TsaGH11Dehyd and TsaGH11Hyd are shown in red. The distance between the TsaGH11 molecules was measured between the Cα atoms of the Glu105 residues. (B) Surface representation of residues in Molecule A located at interfaces A (green) and B (yellow), which are involved in crystal packing. Interfaces C, D, and E in Molecule A are colored orange, cyan, and pink, respectively.
Differences in the crystal space group suggest that the TsaGH11Dehyd and TsaGH11Hyd molecules interact differently with the neighboring molecules within their respective crystal lattices (Supplementary Figure S1). Analysis of the crystal lattices revealed that both TsaGH11Dehyd and TsaGH11Hyd commonly interact with five neighboring molecules. Among the five interfaces involved in crystal packing, two interfaces exhibited differences in the interaction surface areas. At interface A, nine residues (Thr120, Thr136, Thr138, Gln149, Thr150, Phe151, Thr152, His182, and Gly183) contribute to the interaction in TsaGH11Dehyd, whereas only six residues (Thr120, Thr136, Thr138, Gln149, Thr150, and Phe151) are involved in TsaGH11Hyd (Figure 1B). This indicated that more of the surface of TsaGH11Dehyd interacts at interface A than for TsaGH11Hyd following dehydration. At interface B, six residues (Asn44, Ser46, Gln52, Ala56, Thr58, and Tyr205) from both TsaGH11Dehyd and TsaGH11Hyd are involved in interactions with neighboring molecules; however, conformational differences, particularly at Ala56, result in variations in the buried surface area (Figure 1B). In contrast, the remaining interfaces (C–E) were maintained by the same residues in a similar manner in the two structures (Supplementary Figure S2). These results indicate that, in the TsaGH11 crystal, air-drying dehydration increases the size of surface that interacts at specific interfaces, facilitating a transition in the crystal packing from a tetragonal to an orthorhombic space group.
3.2. Overall Structure
The structure of TsaGH11Dehyd at room temperature was determined at a resolution of 2.70 Å, with Rwork and Rfree values of 17.59 and 22.78, respectively. TsaGH11Dehyd adopts a β-jelly roll fold comprising the thumb, finger, and palm domains, containing the six subsites (−3 to +3) (Figure 2A). This overall structure is almost identical to those observed in the previously reported TsaGH11 structures []. However, the conformations of the residues involved in substrate binding in TsaGH11Dehyd differed from those observed in the previously determined crystal structure of TsaGH11 obtained under hydrated conditions (see below). The secondary structures of the four TsaGH11Dehyd molecules within the asymmetric unit were not identical upon visualization in PyMOL (Supplementary Figure S3), indicating structural flexibility of the nonidentical regions. To understand the structural flexibility of TsaGH11, the secondary structures of the four TsaGH11 molecules in the asymmetric unit were analyzed using DSSP (Supplementary Figure S3). The results revealed that the secondary structures of 10 residues (Val40, Ser86, Pro87, Trp112, Pro143, Ser144, Gly147, Thr148, Asn167, and Ser181) varied among the TsaGH11Dehyd molecules (Figure 2B). Among these, Val40, Trp112, Pro143, Ser144, Gly147, and Thr148 were located near the substrate-binding cleft, the thumb domain, or above the finger domain, suggesting their potential influence of substrate recognition. Meanwhile, Pro140 was buried in the hydrophobic region of the β-sandwich fold in the finger domain and is thus unlikely to directly affect substrate recognition. In addition, Ser86, Asn167, and Ser181 were located on the opposite side of the substrate-binding cleft or active site and were unlikely to directly participate in substrate recognition. These findings indicate that all of these residues are relatively flexible in the absence of the substrate.
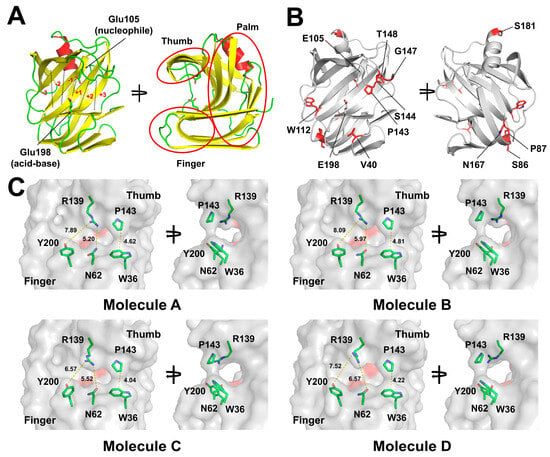
Figure 2.
Room temperature structures of TsaGH11Dehyd. (A) Cartoon representation of the TsaGH11Dehyd structure. The catalytic residues Glu105 (acid-base) and Glu198 (nucleophile) are shown as sticks. The six subsites (−3 to +3) are labeled in red font. α-helices, β-strands, and loops are colored in red, yellow, and green, respectively. (B) Cartoon and stick representations highlighting regions with distinct secondary structures among the TsaGH11Dehyd molecules, illustrated using Molecule A. (C) Surface structures of TsaGH11Dehyd. The catalytic residues Glu105 and Glu198 are indicated by the red surface.
In the structure of TsaGH11Hyd that was previously determined, the closed and open conformations of the enzyme between the thumb and finger domains were clearly observed in the two molecules present in the asymmetric unit []. In particular, in the open conformation, the side chains of Trp36 and Pro143 were separated by approximately 5.6 Å []. In contrast, in the TsaGH11Dehyd structure, the surfaces of Trp36 and Pro143 at subsite −1 were positioned close together, forming a narrow channel between the substrate-binding cleft (Figure 2C). The width of the channel formed beneath the interaction between Trp36 and Pro143 was approximately 4–5 Å (Figure 2C). The distances between the CZ atom of Trp36 and the CB atom of Pro143 from Molecules A, B, C, and D were 4.62, 4.81, 4.04, and 4.22 Å, respectively. These values differ from those for the previously reported open and closed conformations of TsaGH11 determined by serial synchrotron crystallography, measured as 5.6 and 3.8 Å between Trp36 and Pro143, respectively [].
The positions of Arg139 in the thumb domain and Asn62 and Tyr200 in the finger domain of TsaGH11 determine the width of subsite −1 within the substrate-binding cleft. The distances between the OD1 (or ND2) atom of Asn62 and the NH2 atom of Arg139 in molecules A, B, C, and D were 5.20, 5.97, 5.52, and 6.57 Å, respectively (Figure 2C). Similarly, the distances between the OH atom of Tyr200 and the NH1 atom of Arg139 were 7.89, 8.09, 6.57, and 7.52 Å, respectively. These variations are attributed to the differences in the conformation of Arg139 among the four molecules. These analyses showed that the dehydration-induced transition of space group enables alternative conformations in the substrate recognition region of TsaGH11 to be observed.
3.3. Molecular Flexibility of TsaGH11Dehyd
To obtain a deeper understanding of the molecular flexibility of TsaGH11Dehyd, four TsaGH11 molecules from the asymmetric unit were compared. The superimposition of these molecules showed a root-mean-square deviation (RMSD) of 0.155–0.266 Å. In the superimposed structures, the amino acid main chain exhibited the largest structural changes in the thumb domain (Figure 3A and Supplementary Figure S4). Upon superimposition of the thumb domain (Thr138–Thr152) of the four molecules, an RMSD of 0.124–0.334 Å was determined. There was variation in the main chain positions in the thumb domain, with Arg139, Val140, Asn141, Thr148, and Gln149 displaying conformational diversity in their side chains (Figure 3A). Among these, the side chains of Arg139 exhibited the most significant structural changes, as this residue is involved in determining the width of the substrate-binding cleft. Meanwhile, the side chains of Asp146 showed minimal conformational changes, as this amino acid interacts with neighboring Arg100 residues. Next, the structural flexibility of the substrate-binding cleft and catalytic residues was analyzed. The conserved amino acids located within the substrate-binding cleft exhibited relatively rigid conformations (Figure 3B). In particular, the amino acids Pro117, Tyr107, Tyr96, Tyr192, and Trp98 in the palm domain, where the substrate binds, displayed almost identical conformations in the main and side chains. In contrast, the side chains of Tyr92 and Gln153 exhibited positional shifts, showing relative flexibility within 1.07 Å. The conformational changes in the side chains of the catalytic residues Glu105 and Glu198 were observed to be within <1 Å. In the finger domain, the side chains of Tyr115 and Tyr200 in the thumb domain exhibited relatively large changes in conformation, with the most significant positional change in the side chain being <1.07 Å. Meanwhile, the amino acids in the solvent-exposed regions of the thumb and finger domains showed greater molecular flexibility than those in the palm domain (Figure 3C). The side chain of Arg139 in the thumb domain adopted distinct conformations with a rotational shift of over 43°, demonstrating conformational flexibility in the solvent-exposed region. The position of the main chain of Pro143 in the thumb domain was shifted by approximately 0.7 Å. In the finger domain, positional shifts in the side chains of Trp36 and Tyr115 were observed, which peaked at 2.84 Å and 0.66 Å, respectively. Meanwhile, the side chain of Tyr200 was rotated by up to 60°. Conversely, the conserved Asn62 and Asn90 in the finger domain maintained relatively rigid conformations. These results indicate that the conserved residues on the substrate-binding cleft were rigid in the dehydrated environment, whereas the solvent channel-exposed residues were relatively flexible.
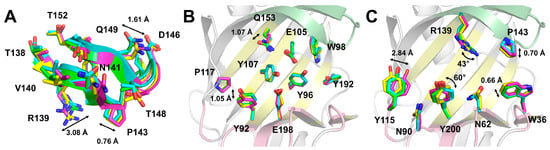
Figure 3.
Structural analysis of the molecular flexibility of TsaGH11Dehyd. (A) Close-up view of the thumb domain of four superimposed TsaGH11Dehyd molecules from the asymmetric unit. Molecules A, B, C, and D are colored green, cyan, purple, and yellow, respectively. (B) Superimposition of the conserved residues located on the palm domain of TsaGH11Dehyd. (C) Superimposition of the residues located on the finger and thumb domains of TsaGH11Dehyd. The palm, finger, and thumb domains of TsaGH11Dehyd are represented as cartoons colored in yellow, pink, and green, respectively.
To further understand the molecular flexibility of TsaGH11, B-factor analysis was performed. The B-factor putty representation revealed the relative rigidity of Molecules A and B, but high flexibility of Molecules C and D (Figure 4A). Molecules A and B were surrounded by neighboring molecules within the crystal packing, which likely contributed to the rigidity of their conformations. In contrast, the thumb and finger domains of Molecules C and D were exposed to solvent channels in the crystal packing, increasing their flexibility (Supplementary Figure S5). B-factor analysis of individual amino acid residues revealed that Molecules A and B exhibited B-factor values below 40 Å2 across the finger, palm, and thumb domains, and no significant molecular flexibility was observed (Figure 4B). In contrast, Molecules C and D showed relatively high molecular flexibility in these three domains, particularly in the thumb domain (Figure 4B). Although there were similar B-factor trends of Molecules A and B and Molecules C and D overall, notable differences were observed in the flexibility of specific regions. For example, in the region around Gly40 in the finger domain, Molecule C displayed higher B-factor values than Molecule D, whereas in the thumb domain the opposite pattern was exhibited (Figure 4B). Therefore, the TsaGH11Dehyd crystal allowed determination of a greater number of molecules within the asymmetric unit compared with previous structures, enabling the structural flexibility to be more comprehensively analyzed. Because the thumb and finger domains of Molecules C and D, which are associated with the substrate-binding cleft, do not interact with neighboring molecules, the observed molecular flexibility in these regions is considered to be more biologically relevant.
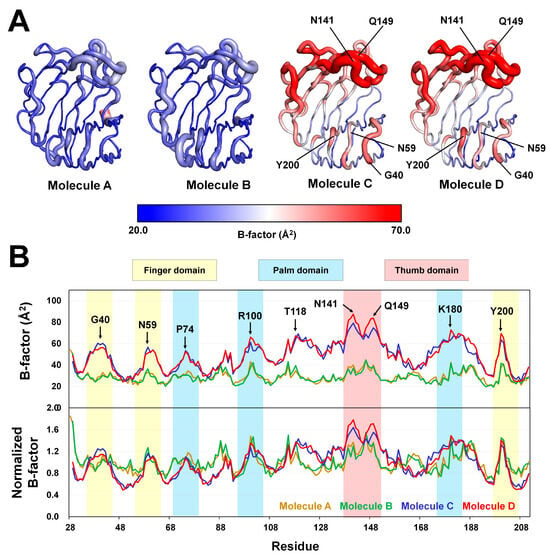
Figure 4.
B-factor analysis of TsaGH11Dehyd. (A) B-factor putty representation of the four TsaGH11Dehyd molecules in the asymmetric unit. (B) B-factor and normalized B-factor plots of the four TsaGH11Dehyd molecules. The finger, palm, and thumb domains are highlighted in yellow, blue, and red, respectively.
3.4. Structural Comparison of TsaGH11Dehyd and TsaGH11Hyd
The structure of TsaGH11Dehyd has a different crystal form compared with that of TsaGH11Hyd as previously determined, indicating a potential difference in the molecular flexibility of the surface residues involved in the crystal packing. This provides valuable information regarding the surface molecular flexibility of TsaGH11. Meanwhile, protein structures can be influenced not only by crystal packing but also by the experimental environment and methods of data collection. To specifically examine the effects of crystal packing, the TsaGH11Dehyd structure was compared with the TsaGH11Hyd structure (PDB code: 8YEA), both of which were determined at room temperature using conventional MX techniques. The TsaGH11Hyd structure with a tetragonal crystal form contains two molecules in the asymmetric unit, which show subtle differences in conformation between the finger and palm domains as well as slight movements of the side chains around the active site, likely due to the crystal packing effects. Molecules A and B of TsaGH11Hyd exhibited relatively open and closed conformations, respectively, between the finger and palm domains. The four molecules of TsaGH11Dehyd were individually compared to the open and closed conformations of TsaGH11Hyd. Superimposition of the TsaGH11Dehyd molecules onto the open and closed conformations of TsaGH11Hyd yielded RMSD values of 0.29–0.39 Å and 0.35–0.39 Å, respectively (Figure 5A). The structural alignment P-/Z-scores between TsaGH11Dehyd and TsaGH11Hyd were 16.0–25.6/13.2–15.3 for Molecule A and 28.6–33.2/16.4–17.2 for Molecule B, indicating that the crystal packing had influenced the overall main chain structures of TsaGH11 (Table S5). Notably, the thumb domain of TsaGH11Dehyd was shifted more toward the solvent region than that of TsaGH11Hyd (Figure 5A). Furthermore, the distances between the CB atom of Pro143 and the CZ2 atom of Trp36 were in the ranges of 4.04–4.86 Å in TsaGH11Dehyd and 4.37–4.77 Å in TsaGH11Hyd, suggesting a greater range of conformations between the thumb and finger domains in TsaGH11Dehyd. Detailed structural comparison revealed that the surface-exposed residues such as asparagine, tyrosine, and threonine exhibited significant conformational diversity, while small residues such as valine or serine showed relatively rigid conformations (Figure 5B). In addition, these conformational and positional differences in side chains may be influenced not only by intrinsic structural variability but also by possible main chain shifts induced by changes in crystal packing during dehydration. These results consequently influence the distribution of charge on the protein surface (Figure 5C). In particular, the conformational diversity of the residues in the thumb domain leads to variations in the distribution of surface charge. Furthermore, the conformational variability of the amino acids even on the opposite side of the substrate-binding site indicates that the surface charge distribution can also be influenced by the conformation of the side chain (Figure 5C). These results suggested that TsaGH11 can exhibit diverse structural charge properties on its surface depending on environmental conditions such as crystal packing and the dehydration effect, independent of substrate recognition. In addition, the superimposition of TsaGH11Dehyd and TsaGH11Hyd revealed molecular flexibility in the residues located at the substrate-binding cleft and in the substrate recognition regions above the thumb and finger domains. On the palm domain, Tyr107, Tyr96, Trp98, and Pro117 exhibited rigid conformations, whereas Tyr92 exhibited relatively flexible conformations (Figure 5D). In the catalytic residues Glu105 and Glu198, subtle movements of their side chains were observed (Figure 5D). Moreover, residues located above the thumb and finger domains, such as Arg139, Tyr115, and Tyr200, exhibited significant changes in conformation (Figure 5E). These results indicate that crystal packing and local solvent environments, such as dehydration, can induce structural variations in the active site and substrate-binding residues of TsaGH11.
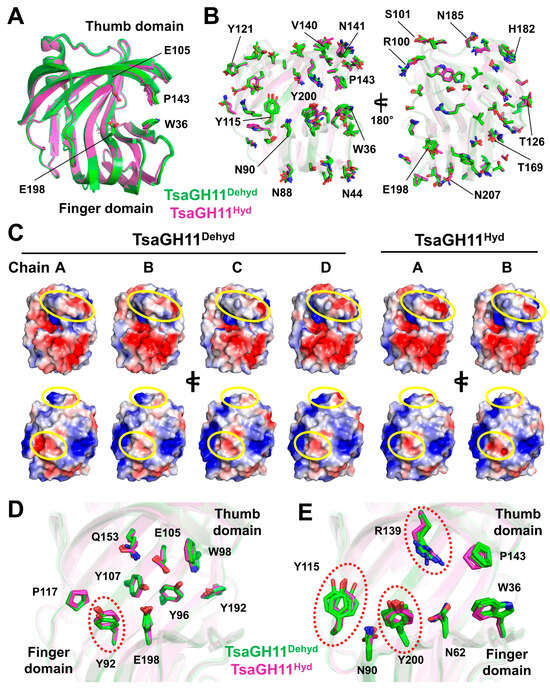
Figure 5.
Structural comparison of TsaGH11Dehyd and TsaGH11Hyd. (A) Surface view of the superimposed structures of TsaGH11Dehyd (cyan) and TsaGH11Hyd (PDB code: 8YEA, pink). (B) Close-up view of the superimposed thumb domains of TsaGH11Dehyd and TsaGH11Hyd. (C) Electrostatic surfaces of TsaGH11Dehyd and TsaGH11Hyd. Significantly different regions are indicated by yellow dotted circles. Differences in charge distribution are indicated by yellow circles. (D) Superimposition of residues involved in the active site and substrate-binding cleft on the palm domain of TsaGH11Dehyd and TsaGH11Hyd. (E) Superimposition of residues located above the substrate-binding cleft of TsaGH11Dehyd and TsaGH11Hyd. The flexible residues are marked by red dotted circles.
4. Discussion
In X-ray crystallography, the crystal space group provides information about how protein molecules are packed within the crystal lattice. Protein regions involved in intermolecular contacts through crystal packing tend to adopt more rigid conformations, whereas regions that are exposed to the solvent without such interactions can exhibit greater molecular flexibility. There is thus variation in the structural information obtained from the protein crystals depending on the crystal form, particularly in terms of molecular flexibility. Although different space groups can be obtained by altering the crystallization conditions, it is often challenging to achieve such variation when the crystallization conditions are limited.
Here, a crystal dehydration strategy was employed to induce a change in the crystal space group. Air-drying dehydration was carried out by exposing the TsaGH11 crystals to ambient air. After 5 min of such exposure, no change in the crystal space group was observed, with it remaining in its original tetragonal form. However, after 10 min of air-drying dehydration, the space group transitioned to the orthorhombic form, indicating that the length of exposure to air is critical for inducing a change in space group in the case of TsaGH11. Although this transition was observed after approximately 10 min of dehydration for TsaGH11, the timing and occurrence of changes in space group may be influenced by factors such as crystal size, solvent content, and solution composition. Therefore, this air exposure time may not match that for other crystals. Meanwhile, owing to the limited number of crystals available in this study, only the timepoints of 5 and 10 min were tested. For more precise evaluation, future investigations should monitor dehydration-induced changes in space group in relation to various factors such as crystal size and crystallization solution. In this study, due to software limitations at the beamline, diffraction data were collected by rotating the crystal at a single position. To minimize radiation damage and improve diffraction quality, future studies may benefit from using advanced data collection strategies such as helical scanning. Meanwhile, although the observed space group transition appeared consistent under identical dehydration conditions, this finding is based on a limited number of crystals. Only one dataset was successfully collected due to poor diffraction from other crystals. To confirm the reproducibility and generality of this transition, future studies involving a larger number of crystals and controlled dehydration experiments will be essential.
Meanwhile, crystal dehydration was also applied to other protein crystals to evaluate whether this method is more broadly applicable. However, in these cases, air dehydration either resulted in a significant reduction in the diffraction quality or failed to induce any change in the space group. These observations indicate that crystal lattice damage or resistance to space group transition may occur in a manner dependent on the specific protein crystal. Therefore, preliminary diffraction testing is essential to determine the suitability of each crystal for the dehydration-based change in space group.
In many crystallographic structures, the asymmetric unit contains a single molecule or a small number of molecules, typically representing a single conformation of the protein after structural refinement. Although such structures allow evaluation of the protein flexibility based on B-factors, they may offer limited structural information when molecular packing significantly constrains protein conformation. Because it is critical to understand protein conformation and flexibility in order to elucidate structural characteristics and biological functions, it is important to obtain structural information from crystals with different space groups. The dehydration-induced change in space group introduced in this study potentially provides valuable insights into the molecular conformation and flexibility of proteins by enabling alternative packing environments to be explored.
The crystal lattice analysis showed that more of the surface area of the TsaGH11Dehyd molecule interacted with neighboring molecules within the crystal lattice following the dehydration process, when compared with the TsaGH11Hyd crystal. This increase in molecular contacts induced changes in the crystal packing and led to alterations in the size of the solvent channels. Such changes in crystal packing can potentially influence the molecular flexibility of the protein structure. A structural comparison of the four TsaGH11Dehyd molecules revealed diverse conformations around the thumb domain and the substrate-binding cleft. Through these comparisons, residues displaying either rigid or flexible conformations within the substrate-binding cleft were identified. The observed structural variations and molecular flexibility in TsaGH11Dehyd provide broader insights into the structural characteristics of TsaGH11 than those provided by previously reported TsaGH11 structures. Comprehensive analysis of the current and previously obtained structural data suggests that the substrate-binding cleft of TsaGH11 can adopt multiple conformations, highlighting its inherent structural versatility in substrate recognition.
Although the crystal space group was successfully altered through crystal dehydration in this study, it should be noted that the dehydration environment may influence the conformational state and flexibility of the protein structure. Accordingly, direct structural comparison between TsaGH11Dehyd and previously determined TsaGH11Hyd structures may not be appropriate due to the differences in the conditions under which the data were collected and the crystal packing effects resulting from the distinct space groups. In addition, the resolution of the crystal structures is relatively moderate. Therefore, to more accurately characterize conformational changes at the amino acid level, future studies should aim to determine higher-resolution structures under similar conditions. Therefore, the structural insights derived from TsaGH11Dehyd should not be interpreted solely in terms of its biological function, but rather as a representation of the conformational flexibility that TsaGH11 may exhibit under varying environmental conditions. Nevertheless, the observation of transitions in space group and associated structural changes through crystal dehydration provides valuable insights into the intrinsic molecular flexibility of proteins. Such structural information not only enhances our understanding of the native conformational landscape of protein molecules, but also contributes to downstream applications, including molecular docking and molecular dynamics study.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cryst15080674/s1; Figure S1: Overlay of the crystal packing of TsaGH11Dehyd and TsaGH11Hyd; Figure S2: Surface representation of residues in Molecule A located at interfaces C, D, and E; Figure S3: Secondary structure analysis of the four TsaGH11Dehyd molecules; Figure S4: Superimposition of four TsaGH11Dehyd molecules; Figure S5: Crystal packing environment of the TsaGH11Dehyd structure; Table S1: Analysis of the interface of the crystal packing; Table S2: Structural alignment of TsaGH11Dehyd and TsaGH11Hyd; Table S3: Hydrogen-bond interactions at the interfaces between TsaGH11Dehyd molecules in the crystal lattice; Table S4: Hydrogen-bond interactions at the interfaces between TsaGH11Hyd molecules in the crystal lattice; Table S5. Structural alignment of TsaGH11Dehyd and TsaGH11Hyd.
Funding
This work was funded by the National Research Foundation of Korea (NRF) (NRF-2021R1I1A1A01050838). Experiments at PAL were supported in part by MSIT and POSTECH (XFEL2025-01).
Data Availability Statement
The structure factor and coordinates are deposited in the protein data bank (www.rcsb.org) with PDB code 8YPX.
Acknowledgments
I would like to thank the beamline staff at the 11C beamline at the Pohang Accelerator Laboratory for their assistance with data collection. The author thanks the Global Science experimental Data hub Center (GSDC) at the Korea Institute of Science and Technology Information (KISTI) for providing computing resources and technical support.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Bose, K.; Rathore, I.; Mishra, V.; Bhaumik, P. Advancements in macromolecular crystallography: From past to present. Emerg. Top. Life Sci. 2021, 5, 127–149. [Google Scholar] [CrossRef]
- Förster, A.; Schulze-Briese, C. A shared vision for macromolecular crystallography over the next five years. Struct. Dyn. 2019, 6, 064302. [Google Scholar] [CrossRef] [PubMed]
- Jaskolski, M.; Dauter, Z.; Wlodawer, A. A brief history of macromolecular crystallography, illustrated by a family tree and its Nobel fruits. FEBS J. 2014, 281, 3985–4009. [Google Scholar] [CrossRef] [PubMed]
- Śledź, P.; Caflisch, A. Protein structure-based drug design: From docking to molecular dynamics. Curr. Opin. Struc. Biol. 2018, 48, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Maveyraud, L.; Mourey, L. Protein X-ray Crystallography and Drug Discovery. Molecules 2020, 25, 1030. [Google Scholar] [CrossRef]
- Kim, I.J.; Kim, S.R.; Bornscheuer, U.T.; Nam, K.H. Engineering of GH11 Xylanases for Optimal pH Shifting for Industrial Applications. Catalysts 2023, 13, 1405. [Google Scholar] [CrossRef]
- Nam, K.H. Engineering Xylose Isomerase for Industrial Applications. Catalysts 2024, 14, 597. [Google Scholar] [CrossRef]
- McPherson, A.; Gavira, J.A. Introduction to protein crystallization. Struct. Biol. Cryst. Communications. 2013, 70, 2–20. [Google Scholar] [CrossRef]
- Russo Krauss, I.; Merlino, A.; Vergara, A.; Sica, F. An Overview of Biological Macromolecule Crystallization. Int. J. Mol. Sci. 2013, 14, 11643–11691. [Google Scholar] [CrossRef]
- Eyal, E.; Gerzon, S.; Potapov, V.; Edelman, M.; Sobolev, V. The Limit of Accuracy of Protein Modeling: Influence of Crystal Packing on Protein Structure. J. Mol. Biol. 2005, 351, 431–442. [Google Scholar] [CrossRef]
- Bala, S.; Shinya, S.; Srivastava, A.; Ishikawa, M.; Shimada, A.; Kobayashi, N.; Kojima, C.; Tama, F.; Miyashita, O.; Kohda, D. Crystal contact-free conformation of an intrinsically flexible loop in protein crystal: Tim21 as the case study. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2020, 1864, 129418. [Google Scholar] [CrossRef]
- Jacobson, M.P.; Friesner, R.A.; Xiang, Z.; Honig, B. On the Role of the Crystal Environment in Determining Protein Side-chain Conformations. J. Mol. Biol. 2002, 320, 597–608. [Google Scholar] [CrossRef]
- Lombardo, A.; Wang, Y.; Ni, C.Z.; Dai, X.; Kodandapani, R.; Chiang, S.; White, C.A.; Pio, F.; Ruoslahti, E.; Ely, K.R.; et al. Conformational flexibility and crystallization of tandemly linked type III modules of human fibronectin. Protein Sci. 2008, 5, 1934–1938. [Google Scholar] [CrossRef] [PubMed]
- Van Aalten, D.M.F.; Joshua-Tor, L.; Crielaard, W.; Hellingwerf, K.J. Conformational substates in different crystal forms of the photoactive yellow protein—Correlation with theoretical and experimental flexibility. Protein Sci. 2008, 9, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Kursula, P.; Jiang, Y.; Lu, G.; Trescott, L.R.; Hou, Y.; Guan, X.; Wang, S.; Stamenkovich, A.; Brunzelle, J.; Sirinupong, N.; et al. New Conformational State of NHERF1-CXCR2 Signaling Complex Captured by Crystal Lattice Trapping. PLoS ONE 2013, 8, e81904. [Google Scholar] [CrossRef]
- Salinas-Garcia, M.C.; Plaza-Garrido, M.; Alba-Elena, D.; Camara-Artigas, A. Major conformational changes in the structure of lysozyme obtained from a crystal with a very low solvent content. Struct. Biol. Cryst. Commun. 2019, 75, 687–696. [Google Scholar] [CrossRef]
- Hinsen, K. Structural flexibility in proteins: Impact of the crystal environment. Bioinformatics 2008, 24, 521–528. [Google Scholar] [CrossRef]
- Masuda, T.; Suzuki, M.; Yamasaki, M.; Mikami, B. Subatomic structure of orthorhombic thaumatin at 0.89 Å reveals that highly flexible conformations are crucial for thaumatin sweetness. Biochem. Biophys. Res. Commun. 2024, 703, 149601. [Google Scholar] [CrossRef]
- Sanchez-Weatherby, J.; Moraes, I. Crystal Dehydration in Membrane Protein Crystallography. In The Next Generation in Membrane Protein Structure Determination; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2016; pp. 73–89. [Google Scholar]
- Heras, B.; Edeling, M.A.; Byriel, K.A.; Jones, A.; Raina, S.; Martin, J.L. Dehydration Converts DsbG Crystal Diffraction from Low to High Resolution. Structure 2003, 11, 139–145. [Google Scholar] [CrossRef]
- Park, H.; Tran, T.; Lee, J.H.; Park, H.; Disney, M.D. Controlled dehydration improves the diffraction quality of two RNA crystals. BMC Struct. Biol. 2016, 16, 19. [Google Scholar] [CrossRef]
- Russo Krauss, I.; Sica, F.; Mattia, C.A.; Merlino, A. Increasing the X-ray Diffraction Power of Protein Crystals by Dehydration: The Case of Bovine Serum Albumin and a Survey of Literature Data. Int. J. Mol. Sci. 2012, 13, 3782–3800. [Google Scholar] [CrossRef]
- Lobley, C.M.; Sandy, J.; Sanchez-Weatherby, J.; Mazzorana, M.; Krojer, T.; Nowak, R.P.; Sorensen, T.L. A generic protocol for protein crystal dehydration using the HC1b humidity controller. Acta Crystallogr. D Struct. Biol. 2016, 72, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Kiefersauer, R.; Than, M.E.; Dobbek, H.; Gremer, L.; Melero, M.; Strobl, S.; Dias, J.M.; Soulimane, T.; Huber, R. A novel free-mounting system for protein crystals: Transformation and improvement of diffraction power by accurately controlled humidity changes. J. Appl. Crystallogr. 2000, 33, 1223–1230. [Google Scholar] [CrossRef]
- Kim, I.J.; Kim, S.R.; Kim, K.H.; Bornscheuer, U.T.; Nam, K.H. Characterization and structural analysis of the endo-1,4-β-xylanase GH11 from the hemicellulose-degrading Thermoanaerobacterium saccharolyticum useful for lignocellulose saccharification. Sci. Rep. 2023, 13, 17332. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.H. Comparative Analysis of Room Temperature Structures Determined by Macromolecular and Serial Crystallography. Crystals 2024, 14, 276. [Google Scholar] [CrossRef]
- Nam, K.H. Recognition of a Single β-D-Xylopyranose Molecule by Xylanase GH11 from Thermoanaerobacterium saccharolyticum. Crystals 2024, 14, 402. [Google Scholar] [CrossRef]
- Park, S.Y.; Ha, S.C.; Kim, Y.G. The Protein Crystallography Beamlines at the Pohang Light Source II. Biodesign 2017, 5, 30–34. [Google Scholar]
- Bury, C.S.; Brooks-Bartlett, J.C.; Walsh, S.P.; Garman, E.F. Estimate your dose: RADDOSE-3D. Protein Sci. 2018, 27, 217–228. [Google Scholar] [CrossRef]
- Otwinowski, Z.; Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997, 276, 307–326. [Google Scholar] [CrossRef]
- Vagin, A.; Teplyakov, A. Molecular replacement with MOLREP. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 22–25. [Google Scholar] [CrossRef]
- Casañal, A.; Lohkamp, B.; Emsley, P. Current developments in Coot for macromolecular model building of Electron Cryo-microscopy and Crystallographic Data. Protein Sci. 2020, 29, 1055–1064. [Google Scholar] [CrossRef]
- Liebschner, D.; Afonine, P.V.; Baker, M.L.; Bunkoczi, G.; Chen, V.B.; Croll, T.I.; Hintze, B.; Hung, L.W.; Jain, S.; McCoy, A.J.; et al. Macromolecular structure determination using X-rays, neutrons and electrons: Recent developments in Phenix. Acta Crystallogr. D Struct. Biol. 2019, 75, 861–877. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.J.; Headd, J.J.; Moriarty, N.W.; Prisant, M.G.; Videau, L.L.; Deis, L.N.; Verma, V.; Keedy, D.A.; Hintze, B.J.; Chen, V.B.; et al. MolProbity: More and better reference data for improved all-atom structure validation. Protein Sci. 2018, 27, 293–315. [Google Scholar] [CrossRef] [PubMed]
- Drew, E.D.; Janes, R.W. 2StrucCompare: A webserver for visualizing small but noteworthy differences between protein tertiary structures through interrogation of the secondary structure content. Nucleic Acids Res. 2019, 47, W477–W481. [Google Scholar] [CrossRef] [PubMed]
- Kabsch, W.; Sander, C. Dictionary of protein secondary structure: Pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 2004, 22, 2577–2637. [Google Scholar] [CrossRef]
- Krissinel, E.; Henrick, K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr. D Biol. 2004, 60, 2256–2268. [Google Scholar] [CrossRef]
- Nam, K.H. pH-Induced structural changes in xylanase GH11 from Thermoanaerobacterium saccharolyticum. F1000Research 2024, 13, 242. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).