Structural Comparison of Three N-(4-Methoxyphenyl)-Nitrobenzenesulfonamide Derivatives
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Section
2.2. Synthesis and Crystallization
2.3. Data Collection and Refinement
3. Results and Discussion
3.1. Synthesis
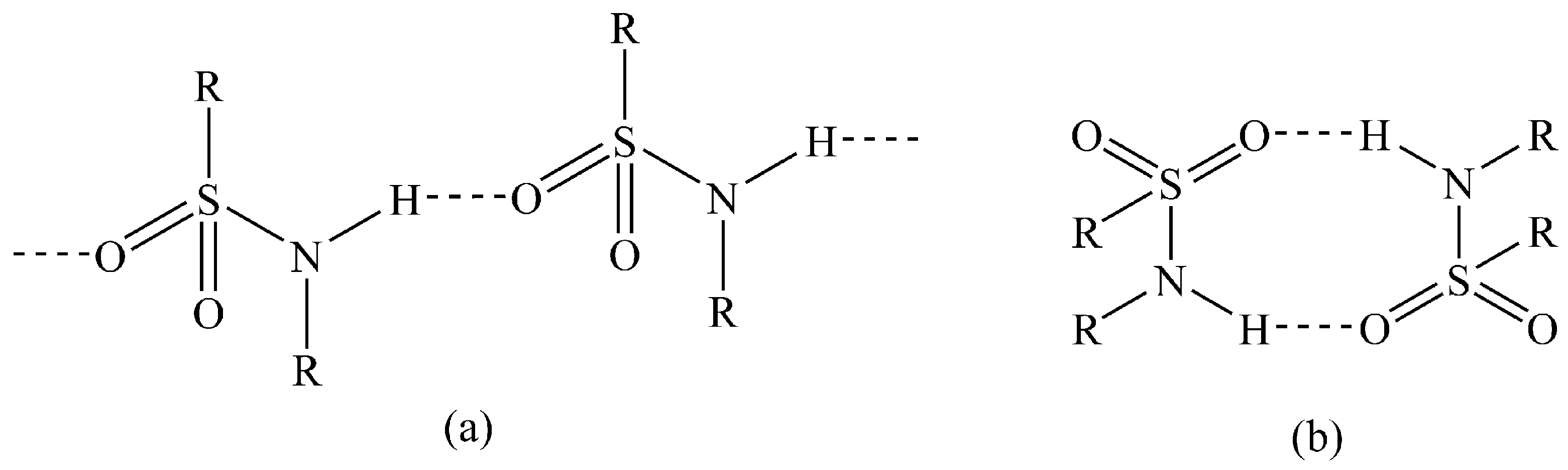
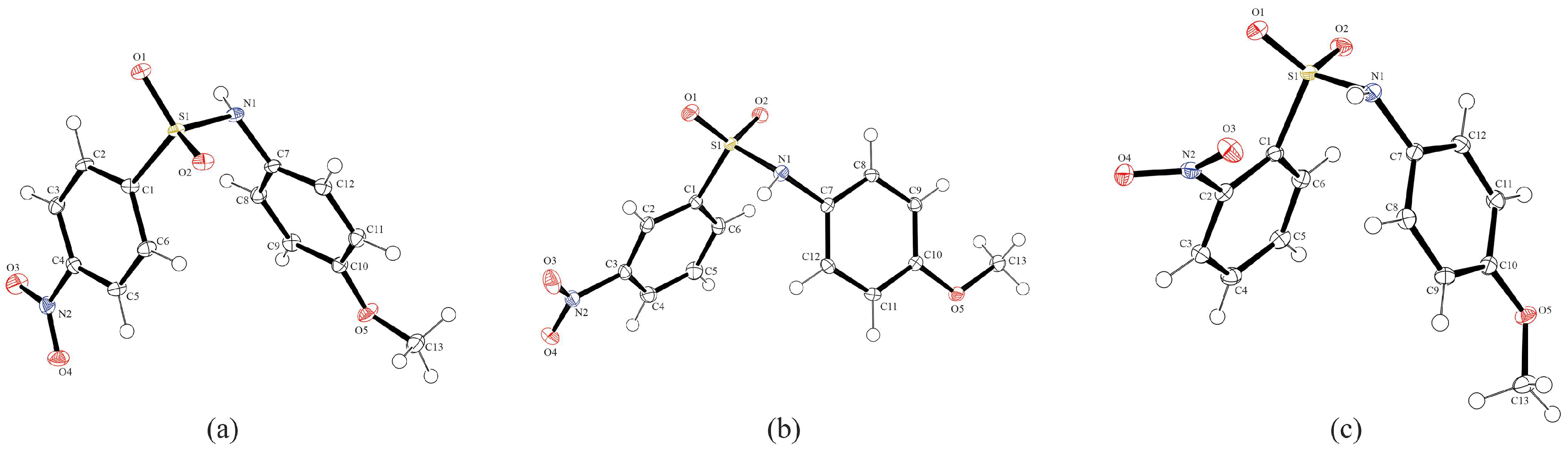
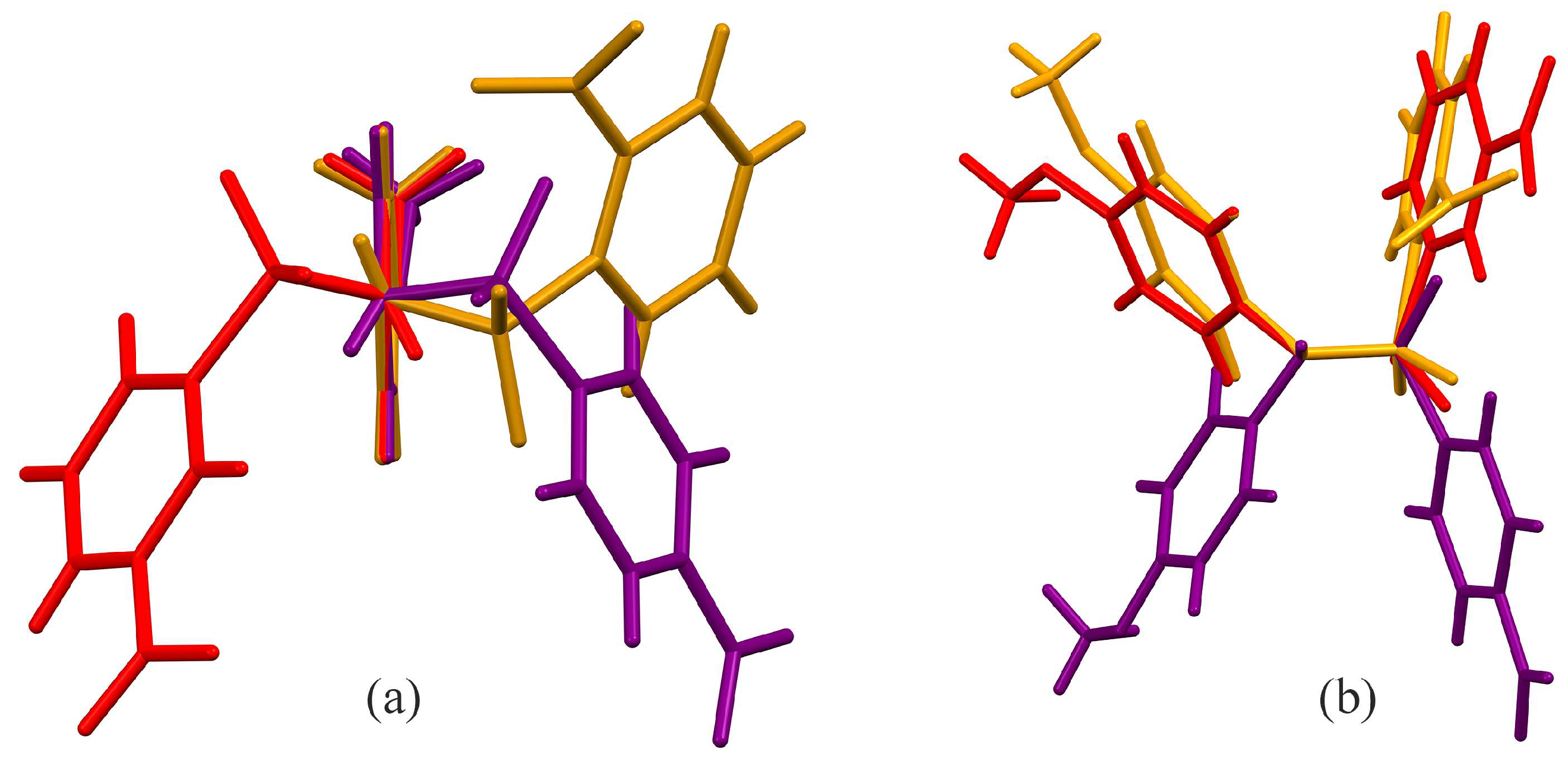
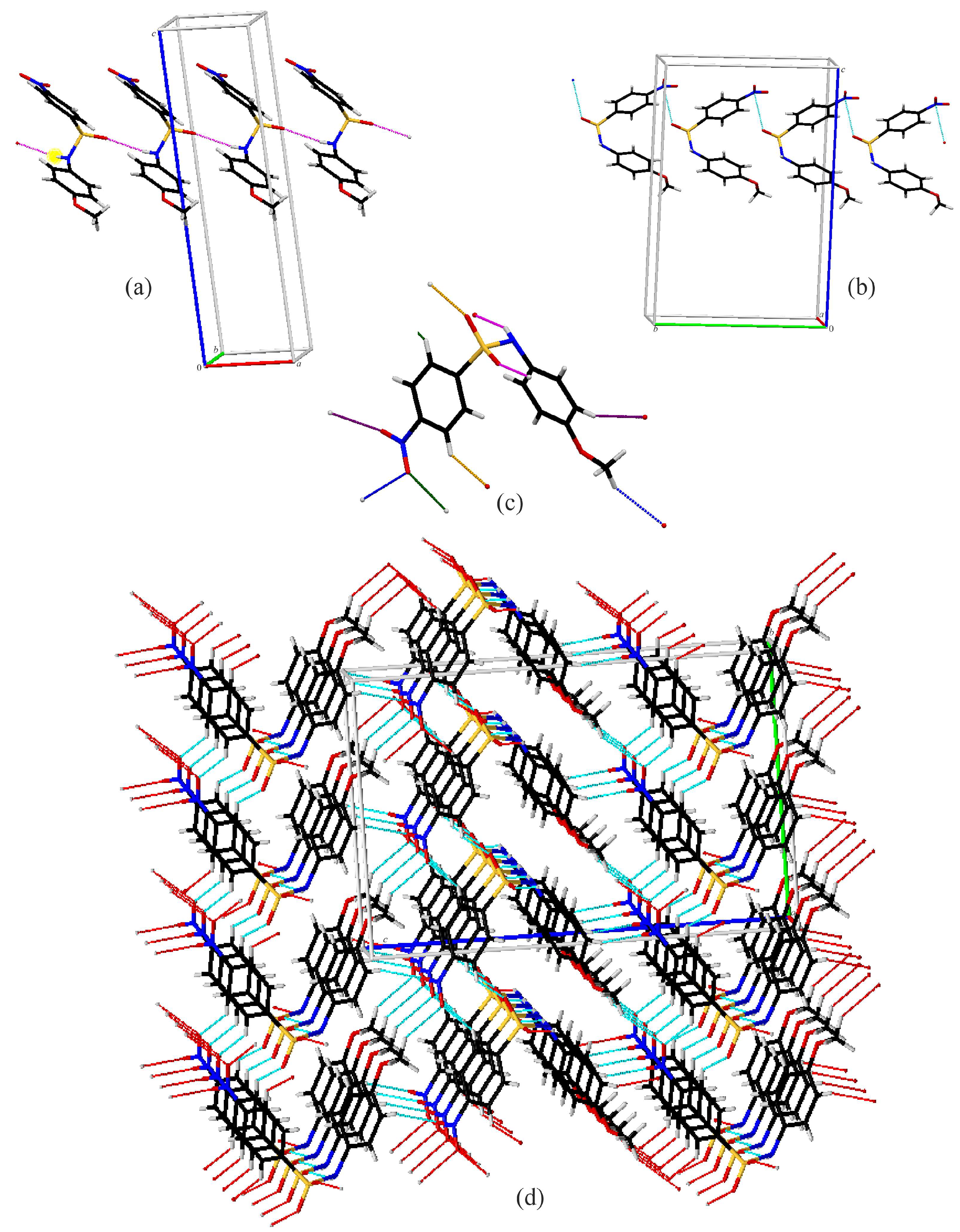
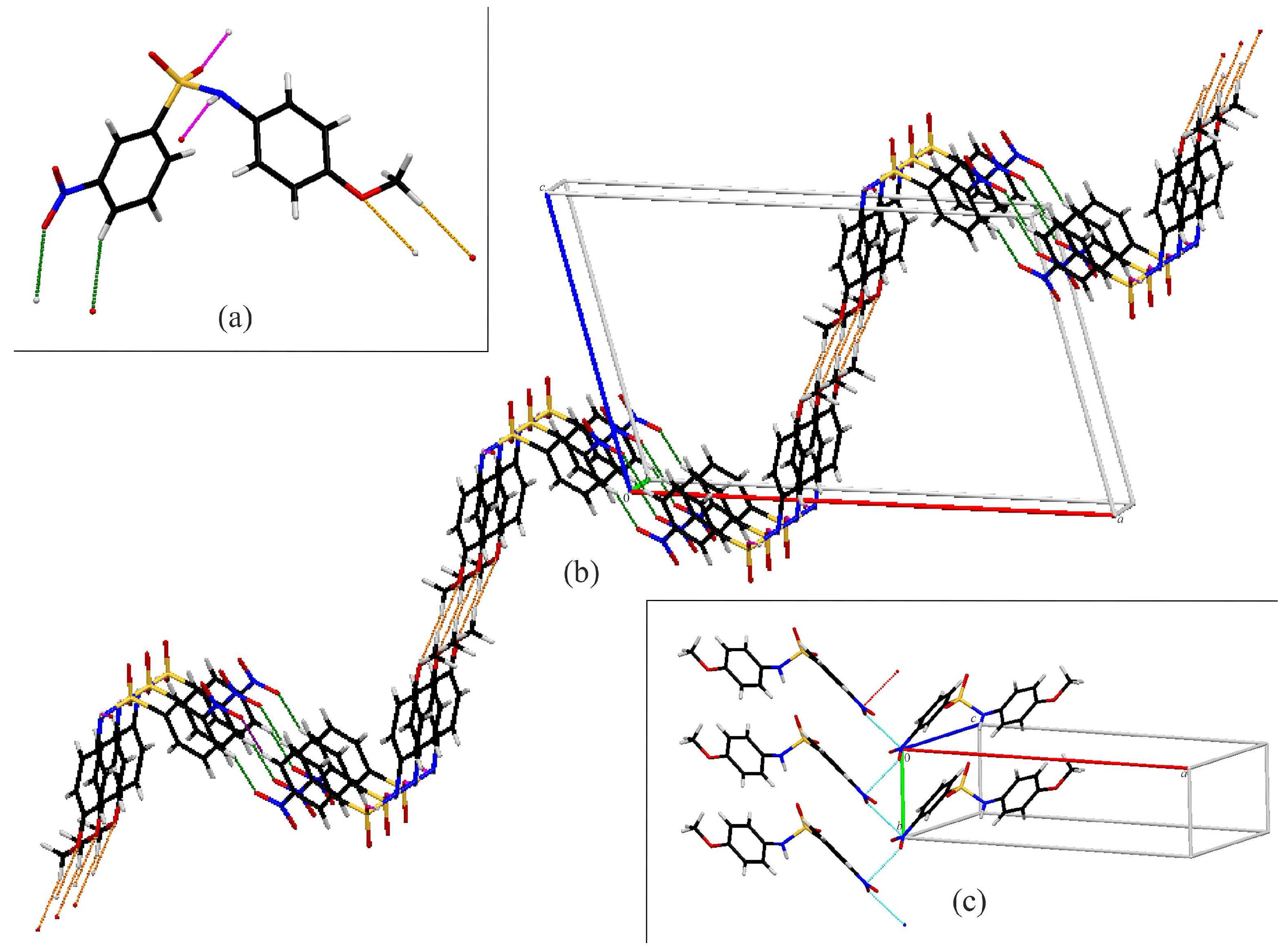
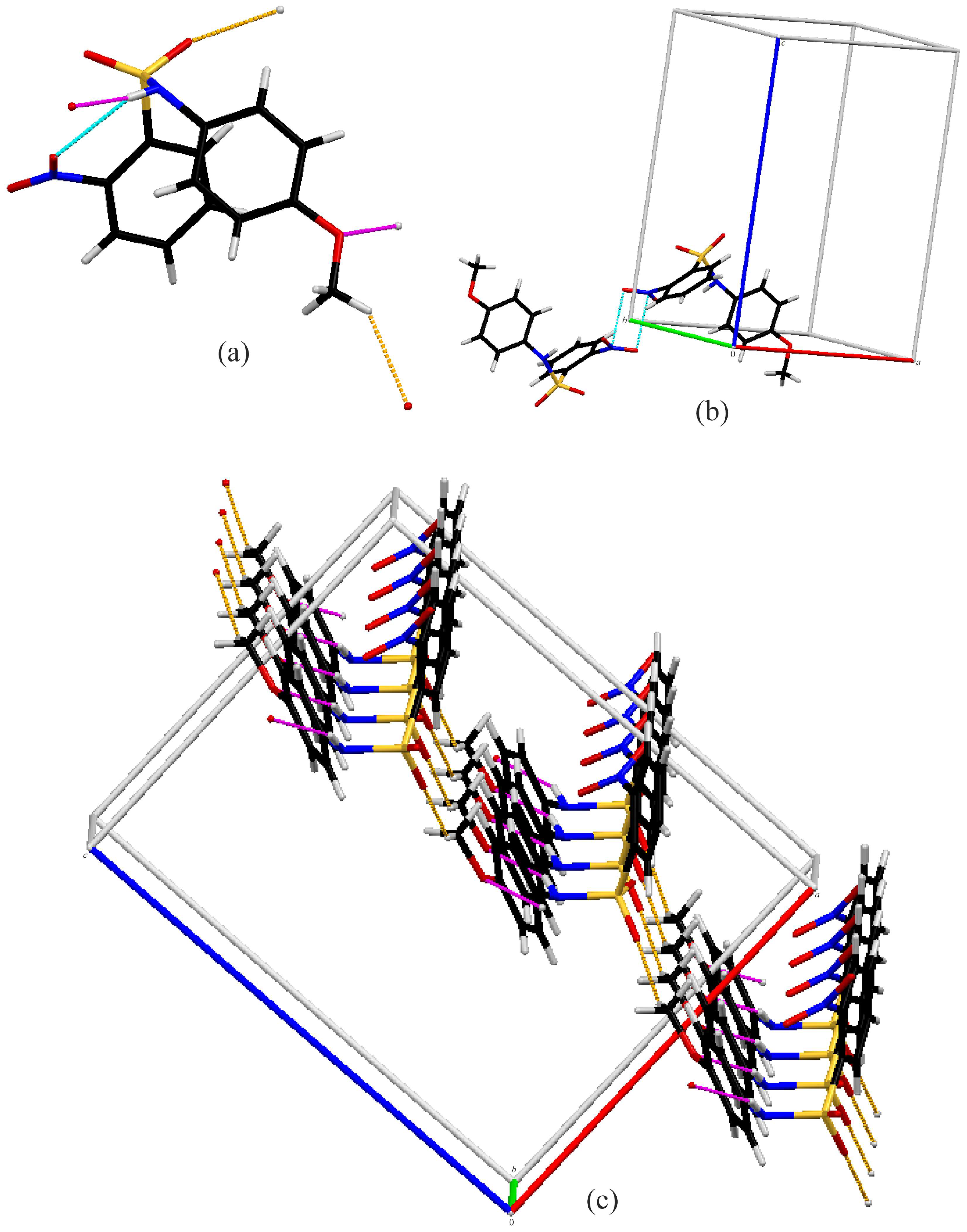
3.2. Crystal Structures
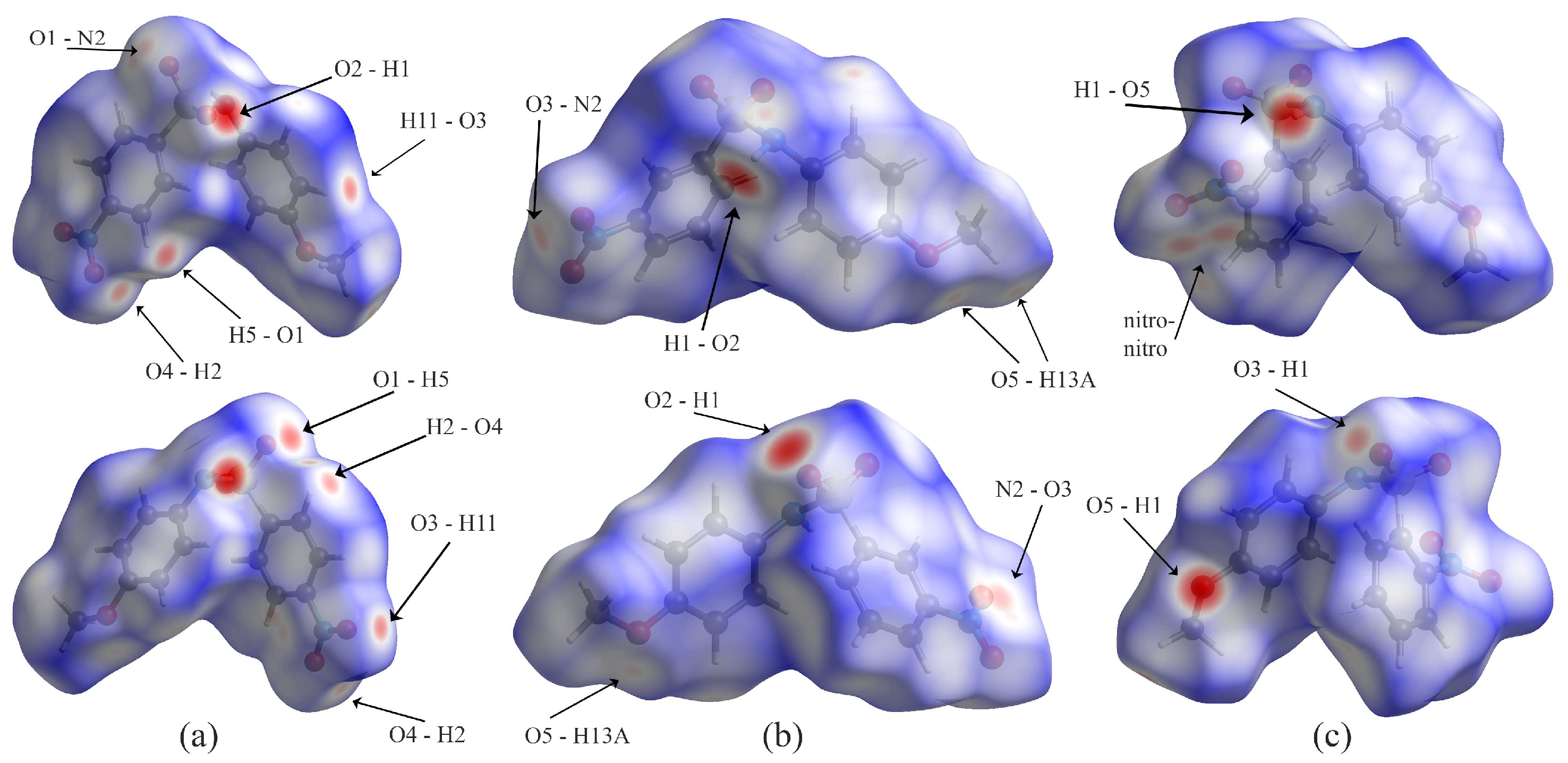
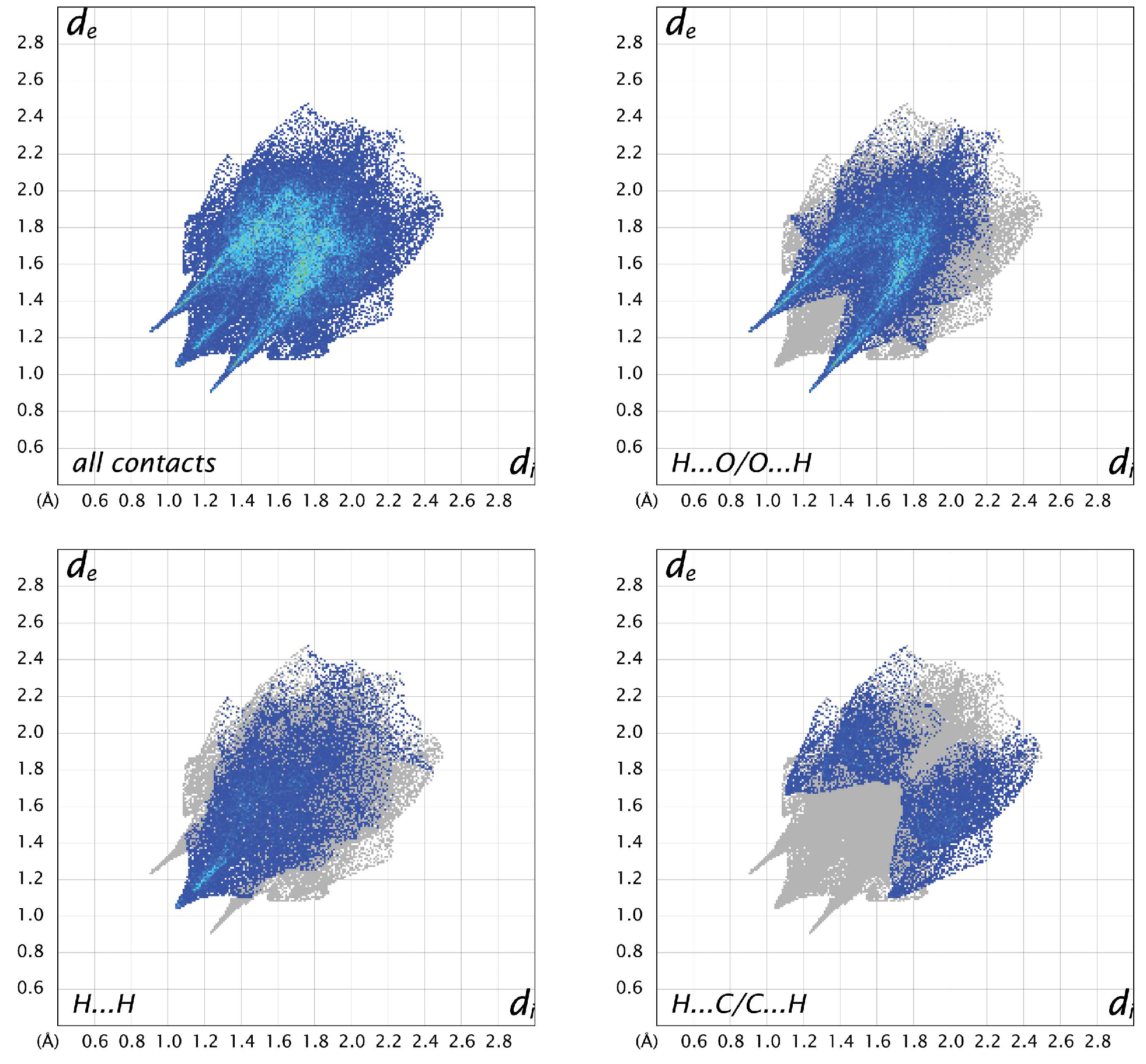
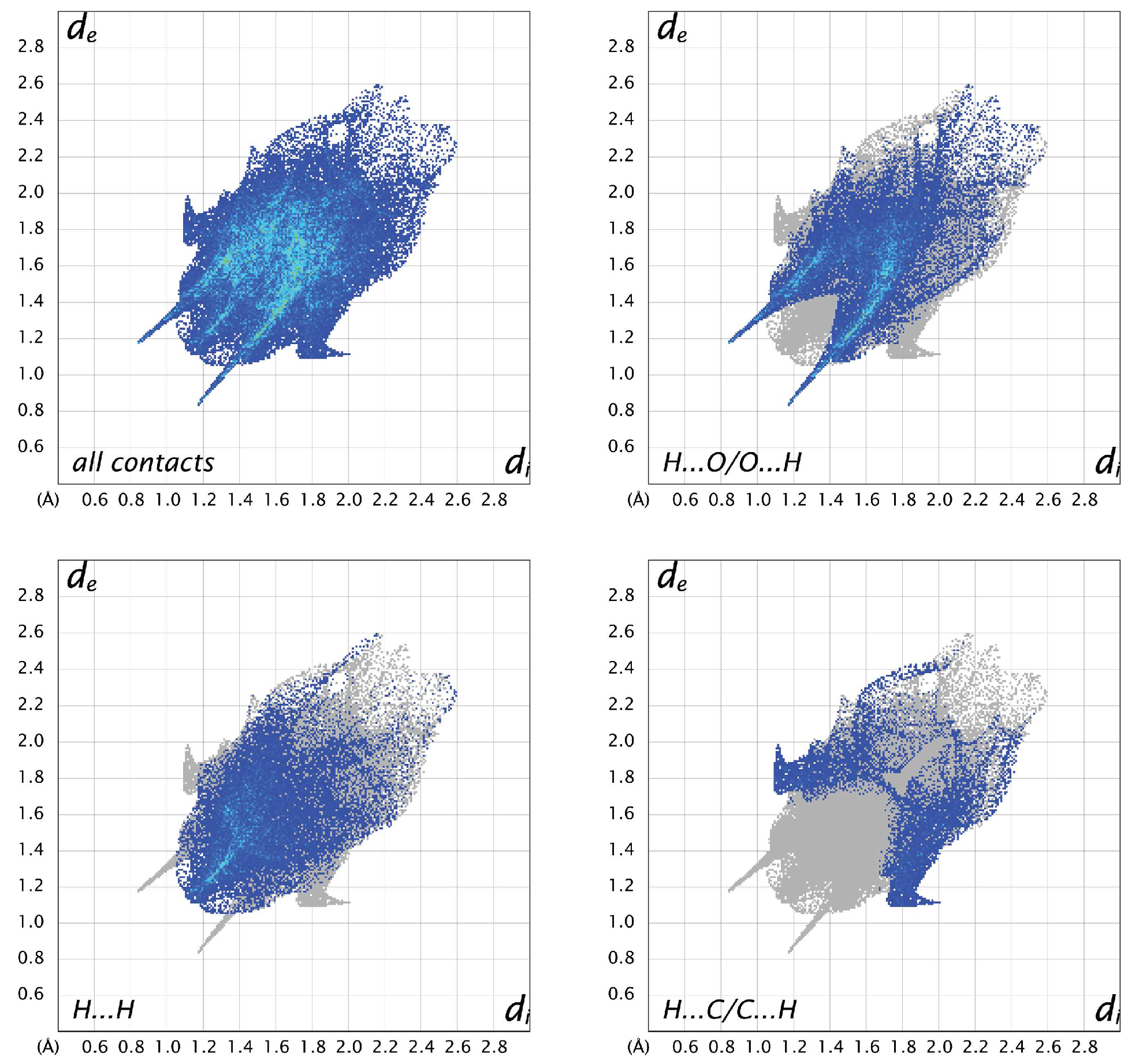
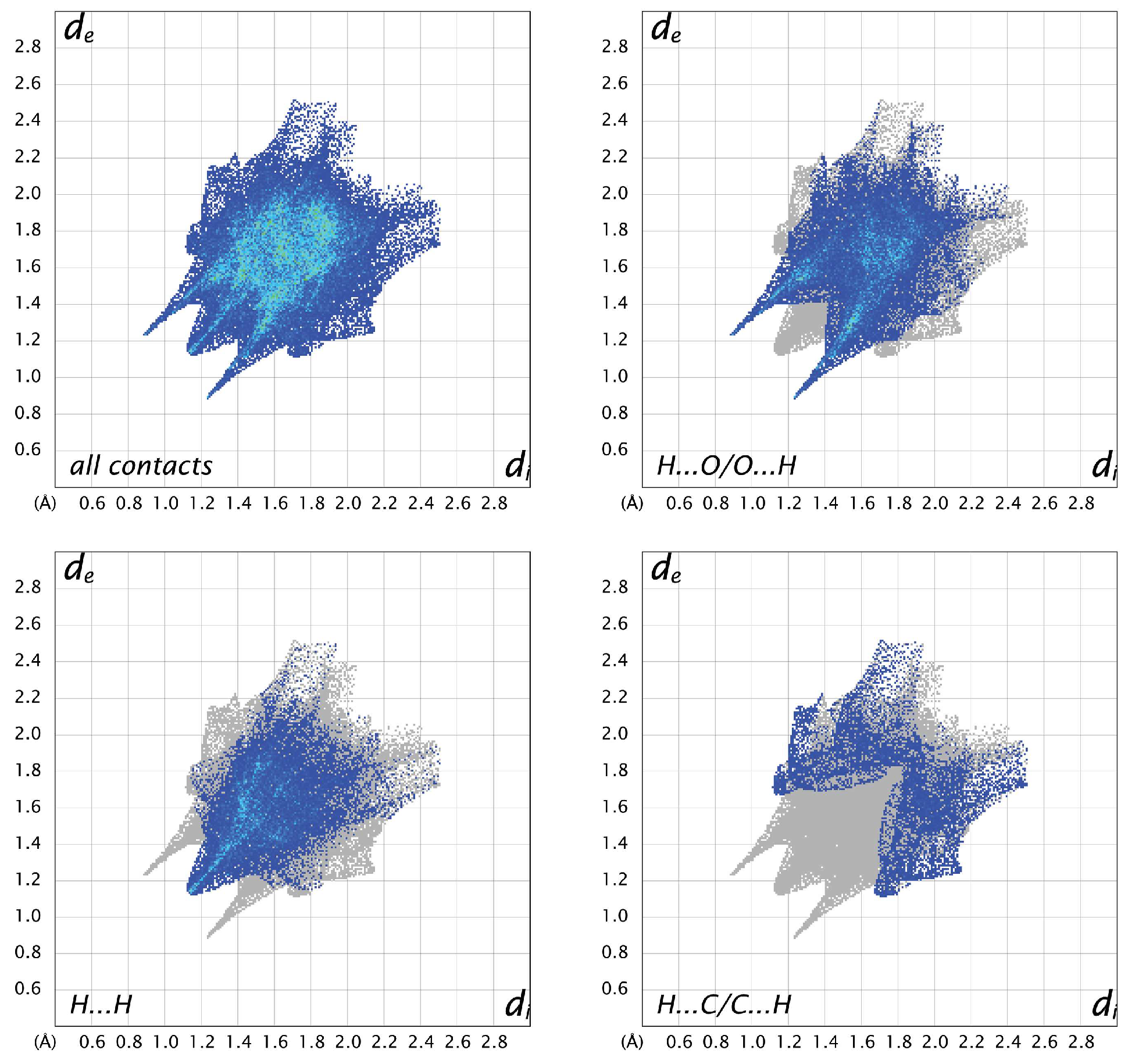
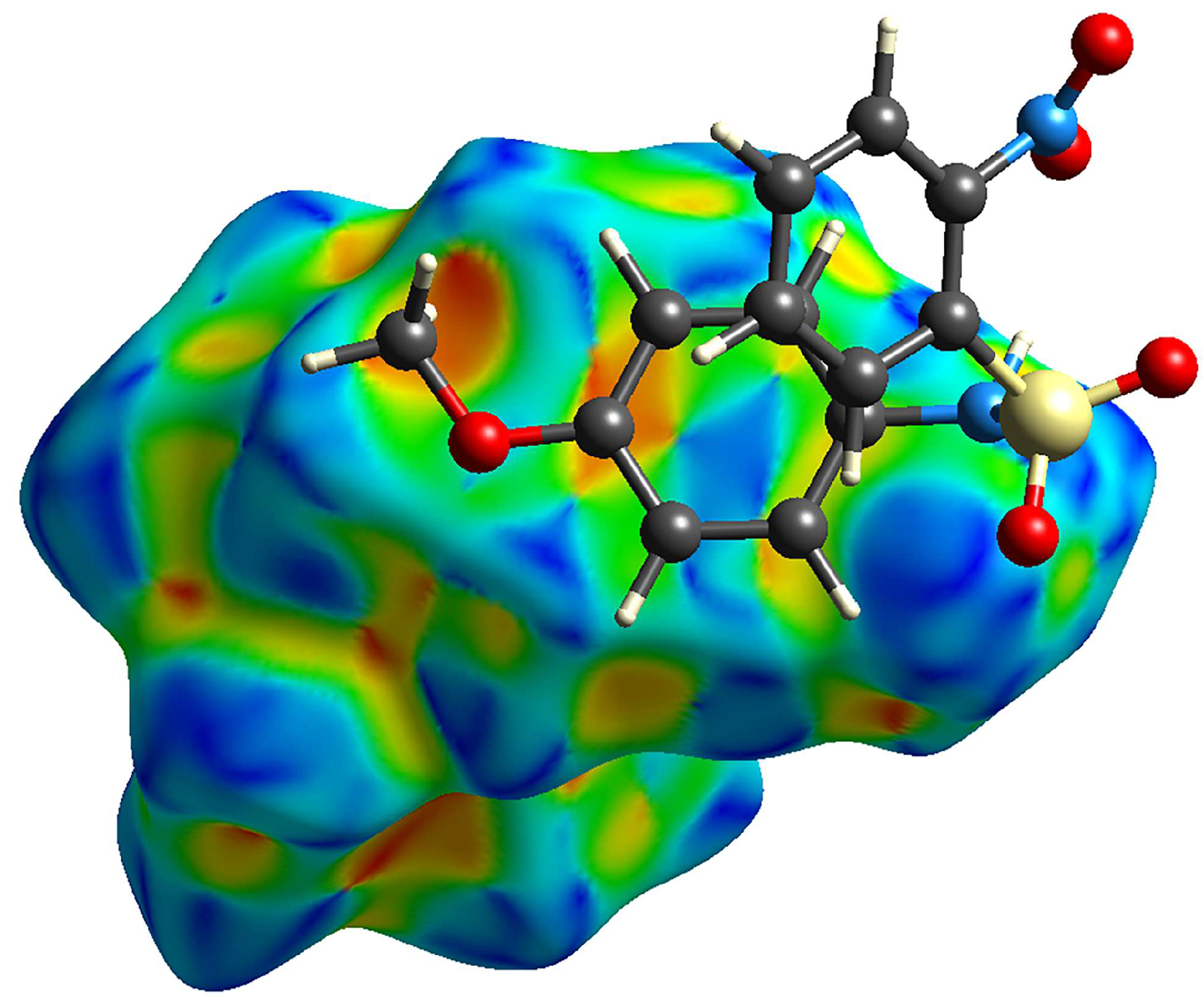
3.3. Hirshfeld Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lesch, J.E. The First Miricle Drugs How the Sulfa Drugs Transformed Medicine; Oxford University Press: Oxford, UK, 2007; pp. 51–67. [Google Scholar]
- Sulfonamides. Available online: https://my.clevelandclinic.org/health/treatments/sulfonamides (accessed on 18 June 2025).
- Zessel, K.; Mohring, S.; Hamscher, G.; Kietzmann, K.; Stahl, J. Biocompatibility and antibacterial activity of photolytic products of sulfonamides. Chemosphere 2014, 100, 167–174. [Google Scholar] [CrossRef]
- Liu, W.; Chen, J.; Su, W. Recent Advances in the Synthesis of Sulfonamides Intermediates. Pharmaceut. Fronts 2024, 6, e355–e381. [Google Scholar] [CrossRef]
- Elsayad, K.A.; Elmasry, G.F.; Mahmoud, S.T.; Awadullah, F.M. Sulfonamides as anticancer agents: A brief review on sulfonamide derivatives as inhibitors of various proteins overexpressed in cancer. Bioorg. Chem. 2024, 147, 107409. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Fang, G.; Chen, H.; Deng, X.; Tang, Z. Sulfonamide derivatives as potential anti-cancer agents and their SARs elucidation. Eur. J. Med. Chem. 2021, 226, 113837. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.A.; Michaels, H.; Molina, B.; Toenjes, S.; Davis, J.; Marconi, G.D.; Hecht, D.; Gustafson, J.L.; Piedrafita, F.J.; Nefzi, A. Discovery of cyclic guanidine-linked sulfonamides as inhibitors of LMTK3 kinase. Bioorg. Med. Chem. Let. 2020, 30, 127108. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, H.; Lit, L.C.; Grothey, A.; Athanasiadou, M.; Kiritsi, M.; Lombardo, Y.; Frampton, A.E.; Green, A.R.; Ellis, I.O.; et al. The kinase LMTK3 promotes invasion in breast cancer through GRB2-mediated induction of integrin β1. Sci. Signal. 2014, 7, ra58. [Google Scholar] [CrossRef]
- Szafrański, K.; Sławiński, J.; Kawiak, A.; Chojnacki, J.; Kosno, M.; Ammara, A.; Supuran, C.T. 4-Substituted Pyridine-3-Sulfonamides as Carbonic Anhydrase Inhibitors Modified by Click Tailing: Synthesis, Activity, and Docking Studies. Int. J. Mol. Sci. 2025, 26, 3817. [Google Scholar] [CrossRef]
- Fadaly, W.A.A.; Nemr, M.T.M.; Abd El-Hameed, A.M.; Giovannuzzi, S.; Alkabbani, M.A.; Hefina, M.M.; Nocentini, A.; Mohamed, M.F.A.; Supuran, C.T.; Eldehna, W.M.; et al. Novel benzenesulfonamide derivatives linked to diaryl pyrazole tail as potential carbonic anhydrase II/VII inhibitors with anti-epileptic activity. Eur. J. Med. Chem. 2025, 291, 117619. [Google Scholar] [CrossRef]
- Selvam, B.; Landagaray, E.; Cartereau, E.A.; Laurent, A.D.; Graton, J.; Lebreton, J.; Thany, S.H.; Mathé-Allainmat, M.; Le Questel, J.-Y. Identification of sulfonamide compounds active on the insect nervous system: Molecular modeling, synthesis and biological evaluation. Bioorg. Med. Chem. Lett. 2023, 80, 129124. [Google Scholar] [CrossRef]
- Gaffer, H.E.; Mahmoud, S.A.; El-Sedik, M.S.; Aysha, T.; Abdel-Rhman, M.H.; Abdel-Latif, E. Synthesis, molecular modelling, and antibacterial evaluation of new sulfonamide-dyes based pyrrole compounds. Sci. Rep. 2024, 14, 10973. [Google Scholar] [CrossRef]
- Mohamed-Ezzat, R.A.; Elgemeie, G.H. Novel synthesis of the first new class of triazine sulfonamide thioglycosides and the evaluation of their anti-tumor and anti-viral activities against human coronavirus. Nucleosides Nucleotides Nucleic 2024, 43, 1511–1528. [Google Scholar] [CrossRef]
- Li, E.W.; Katinas, J.; Jones, M.A.; Hamaker, C.G. Structural characterization of naphthalene sulfonamides and a sulfonate ester and their in vitro efficacy against Leishmania tarentolae promastigotes. New J. Chem. 2021, 45, 4791–4801. [Google Scholar] [CrossRef]
- Ochu, R.C.; Okoro, U.C.; Conradie, J.; Ugwu, D.I. New antibacterial, antifungal and antioxidant agents bearing sulfonamide. Eur. J. Med. Chem. Rep. 2024, 10, 100136. [Google Scholar] [CrossRef]
- You, T.; Li, J. Ni(cod)(duroquinone)-Catalyzed C−N Cross-Coupling for the Synthesis of N,N-Diarylsulfonamides. Org. Lett. 2022, 24, 6642–6646. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Song, J.; Dong, J.; Li, G.; Fan, J.; Xue, D. Ni-Catalyzed Photochemial Sulfamidation of Aryl Chlorides with Soluble Organic Amine as Base. Organometallics 2024, 43, 1706–1712. [Google Scholar] [CrossRef]
- Deratt, L.G.; Wang, C.Y.; Kuduk, S.D. Tandem Amination/Oxetane Ring Opening toward Benzomorpholines. J. Org. Chem. 2021, 86, 17482–17486. [Google Scholar] [CrossRef]
- Shen, L.; Wu, X.; Shi, L.; Xu, X.; Zhang, J.; Li, F. Selective N-Alkylation of Aminobenzenesulfonamides with Alcohols for the Synthesis of Amino-(N-Alkyl)benzenesulfonamides Catalyzed by a Metal-Ligand Bifunctional Ruthenium Catalyst. J. Org. Chem. 2024, 89, 8397–8406. [Google Scholar] [CrossRef] [PubMed]
- Anandaraj, P.; Ramesh, R. N-alkylation of benzamides/sulfonamides using alcohols via borrowing hydrogen approach by well-defined Pd(II) pincer complexes. Appl. Organomet. Chem. 2023, 37, e7228. [Google Scholar] [CrossRef]
- Yu, X.; Ma, Z.; Zhu, W.; Liu, H.; Zhang, Z.; Liu, Y.; Zhang, M.; Zhao, J.; Zhang, P.; Xia, C. Tandem Reduction, Ammonolysis, Condensation, and Deamination Reaction for Synthesis of Benzothiadiazines and 1-(Phenylsulfonyl)-1H-benzimidazoles. J. Org. Chem. 2022, 87, 14738–14752. [Google Scholar] [CrossRef]
- Etter, M.C.; MacDonald, J.C.; Bernstein, J. Graph-set analysis of hydrogen-bond patterns in organic crystals. Acta Crystallogr. 1990, B46, 256–262. [Google Scholar] [CrossRef]
- Asmond, D.A.; Grant, D.J.W. Hydrogen bonding in sulfonamides. J. Pharm. Sci. 2001, 90, 2058–2077. [Google Scholar] [CrossRef] [PubMed]
- Perlovich, G.L.; Tkachev, V.V.; Strakhova, N.N.; Kazachenko, V.P.; Volkova, T.V.; Surov, O.V.; Schaper, K.-J.; Raevsky, O.A. Thermodynamic and structural aspects of sulfonamide crystals and solutions. J. Pharm. Sci. 2009, 98, 4738–4755. [Google Scholar] [CrossRef] [PubMed]
- Bolla, G.; Nangia, A. Supramolecular synthon hierarchy in sulfonamide cocrystals with syn-amides and N-oxides. IUCrJ 2019, 6, 751–760. [Google Scholar] [CrossRef]
- Shaikh, S.R.; Gawade, R.L.; Dabke, N.B.; Dahs, S.R.; Vanka, K.; Gonnade, R.G. Crystal engineering for intramolecular π–π stacking: Effect of substitution of electron-donating and electron-withdrawing groups on the molecular geometry in conformationally flexible Sulfoesters and sulfonamides. CrystEngComm 2024, 26, 3557–3573. [Google Scholar] [CrossRef]
- Goettler, P.E.; Hamaker, C.G. Crystal structure analysis of two 4-nitro-N-methylaniline derivatives. J. Chem. Cryst. 2022, 52, 251–259. [Google Scholar] [CrossRef]
- Hamaker, C.G.; Oberts, B.P. Synthesis and crystal structures of the bis-Schiff bases of 2-(methylthio)aniline with isophthaldehyde, terephthaldehyde, and para-diacetylbenzene. J. Chem. Cryst. 2006, 36, 735–742. [Google Scholar] [CrossRef]
- Shang, H.H.; Zelaya, Z.Z.; Hamaker, C.G.; Jones, M.A. Inhibitory Effects of Sulfur Derivatives on Leishmania tarentolae Cell Viability and Secreted Acid Phosphatase In Vitro. Microorganisms 2024, 12, 2641. [Google Scholar] [CrossRef]
- SAINT; Bruker AXS Inc.: Madison, WI, USA, 2015.
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Farrugia, L.J. WinGX and ORTEP for Windows: An Update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer 17; The University of Western Australia: Perth, Australia, 2017. [Google Scholar]
- Mansfeld, M.; Pařík, P.; Ludwig, M. Effect of Para Substitution on Dissociation of N-Phenylbenzenesulfonamides. Collect. Czech. Chem. Commun. 2004, 69, 1479–1490. [Google Scholar] [CrossRef]
- Formen, J.S.S.K.; Wolf, C. Optical Relay Sensing of Cryptochiral Alcohols Displaying α-, β-, γ- and δ-Stereocenters or Chirality by Virtue of Isotopic Substitution. Angew. Chem. Int. Ed. 2024, 63, e202409790. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Gelbrich, T.; Threlfall, T.L.; Hursthouse, M.B. Eight isostructural 4,4′-disubstituted N-phenyl benzene sulfonamides. Acta Crystallogr. 2012, C68, o421–o426. [Google Scholar]
- Alvarez, S. A cartography of the van der Waals territories. Dalton Trans. 2013, 42, 8617–8636. [Google Scholar] [CrossRef]
- Maglic, J.B.; Levendomme, R. MoloVol: An easy-to-use program for analyzing cavities, volumes and surface areas of chemical structures. J. Appl. Crystallogr. 2022, 55, 1033–1044. [Google Scholar] [CrossRef]
| Compound | A (p-NO2) | B (m-NO2) | C (o-NO2) |
|---|---|---|---|
| CCDC Deposit No. | 2467049 | 2467050 | 2467051 |
| Chemical formula | C13H12N2O5S | C13H12N2O5S | C13H12N2O5S |
| Mr | 308.31 | 308.31 | 308.31 |
| Crystal system, space group | Monoclinic, Cc | Monoclinic, P21/c | Monoclinic, P21/c |
| Temperature (K) | 100 (2) | 100 (2) | 100 (2) |
| a, b, c (Å) | 5.1710 (4) | 20.5686 (7) | 12.0195 (7) |
| 12.9028 (10) | 5.1731 (2) | 7.2684 (4) | |
| 19.7324 (15) | 13.0803 (5) | 15.0297 (8) | |
| β (°) | 94.420 (4) | 107.7598 (16) | 91.529 (3) |
| V (Å3) | 1312.64 (8) | 1325.46 (9) | 1312.27 (13) |
| Z | 4 | 4 | 4 |
| Radiation type | Mo Kα | Mo Kα | Mo Kα |
| μ (mm−1) | 0.271 | 0.269 | 0.271 |
| Crystal size (mm) | 0.28 × 0.20 × 0.13 | 0.39 × 0.32 × 0.20 | 0.31 × 0.18 × 0.10 |
| Diffractometer | Bruker APEX-II CCD | ||
| Absorption correction | Multi-scan SADABS | ||
| Tmin, Tmax | 0.70, 0.75 | 0.90, 0.95 | 0.92, 0.97 |
| No. measured, independent, and observed [I > 2σ(I)] reflections | 17529, 2667, 2661 | 36710, 2815, 2749 | 38370, 2880, 2687 |
| Rint | 0.020 | 0.017 | 0.024 |
| (sin θ/λ)max (Å−1) | 0.625 | 0.633 | 0.641 |
| R[F2 > 2σ(F2)], wR(F2), S | 0.0291, 0.0874, 1.173 | 0.0277, 0.0750, 1.052 | 0.0277, 0.0729, 1.065 |
| No. of reflections | 2667 | 2815 | 2880 |
| No. of parameters | 194 | 194 | 194 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement | ||
| Δrmax, Δrmin (e Å−3) | 0.50, −0.32 | 0.48, −0.45 | 0.41, −0.41 |
| Bond | A (p-NO2) | B (m-NO2) | C (o-NO2) |
|---|---|---|---|
| S1–C1 | 1.770 (3) | 1.7664 (12) | 1.7871 (12) |
| S1–N1 | 1.646 (3) | 1.6450 (10) | 1.6426 (11) |
| N1–C7 | 1.450 (4) | 1.4415 (14) | 1.4467 (15) |
| Bond | A (p-NO2) | B (m-NO2) | C (o-NO2) |
|---|---|---|---|
| C1–S1–N1 | 105.43 (15) | 105.00 (5) | 104.97 (5) |
| S1–N1–C7 | 116.9 (2) | 119.00 (8) | 115.49 (8) |
| O1–S1–O2 | 120.45 (14) | 120.66 (5) | 118.98 (6) |
| C1–S1–N1–C7 | –58.6 (3) | 66.56 (3) | 41.78 (10) |
| D–H…A | D–H | H…A | D…A | D–H…A |
|---|---|---|---|---|
| C5–H5…O1 i | 0.95 | 2.43 | 3.144 (4) | 132 |
| C2–H2…O4 ii | 0.95 | 2.52 | 3.294 (4) | 139 |
| C11–H11…O3 iii | 0.95 | 2.50 | 3.414 (4) | 161 |
| N1–H1…O2 iv | 0.89 (6) | 2.25 (6) | 3.089 (4) | 156 (5) |
| C13–H13A…O4 v | 0.98 | 2.56 | 3.352 (5) | 138 |
| D–H…A | D–H | H…A | D…A | D–H…A |
|---|---|---|---|---|
| C13–H13A…O5 i | 0.98 | 2.60 | 34703 (15) | 148 |
| C4–H4…O4 ii | 0.95 | 2.42 | 3.2965 (16) | 153 |
| N1–H1…O2 iii | 0.856 (18) | 2.176 (19) | 3.0127 (14) | 165.5 (16) |
| D–H…A | D–H | H…A | D…A | D–H…A |
|---|---|---|---|---|
| N1–H1…O5 i | 0.826 (19) | 2.281 (19) | 3.0534 (14) | 155.8 (16) |
| N1–H1…O3 | 0.826 (19) | 2.524 (18) | 3.0612 (14) | 123.8 (15) |
| C13–H13A…O2 ii | 0.98 | 2.47 | 3.2891 (16) | 141 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oblazny, M.; Hamaker, C.G. Structural Comparison of Three N-(4-Methoxyphenyl)-Nitrobenzenesulfonamide Derivatives. Crystals 2025, 15, 673. https://doi.org/10.3390/cryst15080673
Oblazny M, Hamaker CG. Structural Comparison of Three N-(4-Methoxyphenyl)-Nitrobenzenesulfonamide Derivatives. Crystals. 2025; 15(8):673. https://doi.org/10.3390/cryst15080673
Chicago/Turabian StyleOblazny, Mark, and Christhoper G. Hamaker. 2025. "Structural Comparison of Three N-(4-Methoxyphenyl)-Nitrobenzenesulfonamide Derivatives" Crystals 15, no. 8: 673. https://doi.org/10.3390/cryst15080673
APA StyleOblazny, M., & Hamaker, C. G. (2025). Structural Comparison of Three N-(4-Methoxyphenyl)-Nitrobenzenesulfonamide Derivatives. Crystals, 15(8), 673. https://doi.org/10.3390/cryst15080673








