Abstract
With the continuous exploitation of bauxite mineral resources, Guangxi bauxite faces many difficulties in alumina production due to its characteristics of high silicon content, high iron content, and a low Al-Si ratio. In view of this, this study is closely related to the key link of bauxite pre-desiliconization and strives to break free from the status quo to improve the aluminum/silicon ratio and help optimize the subsequent alumina-refining process. In the work presented in this paper, the unique mineralogy of Guangxi bauxite was comprehensively considered, covering its complex mineral composition and fine distribution characteristics. The barium hydroxide pre-desilication technology was first used for in-depth experimental exploration, and the silicon removal efficiency under different working conditions was systematically compared. The system compared the silicon removal effect and the associated aluminum loss under different working conditions. The results of this study will lay a solid foundation for the rational and efficient development of bauxite in Guangxi, which is expected to reduce the cost of alumina production, improve the economic benefits for the Guangxi aluminum industry, simultaneously strengthen the efficiency of resource recycling, accelerate the sustainable development of the industry, and provide a useful reference example for subsequent similar studies.
1. Introduction
Bauxite is the main raw material for primary aluminum production. The essence of the whole chain of aluminum industrial production is to achieve the whole process conversion from mining bauxite, alumina, electrolytic aluminum, and aluminum primary products to deep aluminum processing according to the needs of the product terminal. Bauxite resources play a decisive role in the construction and development of aluminum industry. Guangxi is rich in bauxite resources, has good resource endowment, and has good geological conditions for bauxite mineralization. It is one of the four provinces and regions with the largest bauxite reserves in China and occupies an important position in the country. According to the mineral resources survey results, by the end of 2021, the total amount of bauxite resources in Guangxi, accounted for more than 25% of the country’s bauxite resources. More than 90% of these resources are accumulative bauxite, and less than 10% are sedimentary bauxite [1]. However, with the decrease in domestic bauxite reserves and grade, the import volume of bauxite is increasing year by year, and it is urgent to strengthen the utilization of low-grade bauxite [2].
Silicon has a significant impact on the utilization of bauxite resources. In the production process of primary aluminum, the silicon element exists in the form of silicate and reacts with an alkali solution to produce sodium silicate, which consumes a large amount of alkali, and increases production costs [3]. During the leaching process of bauxite, sodium silicate forms a co-precipitate with sodium aluminate, resulting in the loss of aluminum, reducing the leaching rate of alumina, and causing stuttering on the surface of the equipment, thus affecting its normal operation and increasing maintenance costs and energy consumption [4]. In addition, almost all silicon enters the red mud during the production process, resulting in an increase in the amount of red mud. At present, there are not many good treatments for red mud, only some methods of recycling and reusing parts of red mud such as oxides [5].
In the process of bauxite purification and primary aluminum production, desilication technology is one of the core links that determine the economic efficiency of the process. The current mainstream methods for desilication include physical [6], chemical [7], and biological [8] methods. Physical methods mainly utilize the differences in mineral density and surface properties to separate silicate impurities, such as flotation, gravity separation, and magnetic separation techniques [9]. These methods have certain advantages in processing high-silica bauxite, but usually require complex equipment and multi-step operations. Massola et al. [10] used reverse flotation (a physical method) with ether amine as the collector and corn starch as the inhibitor of desilication of bauxite under pH 10 conditions, and finally obtained a concentrate with an alumina content of 42.3% and an alumina/silica ratio of 12.6. After magnetic separation, the alumina content was further increased to 54.0%. Abroon et al. [11] tested the effects of cationic collectors such as DDA, CTAB, and DTAL on flotation efficiency within the pH range of 6–7, and found that DTAL performed the best in terms of the Al2O3 recovery rate and A/S ratio. Chemical methods use chemical reactions to remove silicon impurities, such as the alkaline leaching process in the Bayer process [12]. Zhengshuai J et al. [13] used lime for the desilication of the silicon-containing alkaline solution after alkaline leaching, achieving the recycling of the alkaline solution, and the aluminum/silicon ratio of the concentrate after recycling was still greater than 7. Sun Y et al. [14] successfully extracted Al2O3 and SiO2 from high-silica bauxite using a two-step process of sodium carbonate sintering combined with water leaching and acid leaching. The total recovery rate of Al2O3 was about 97% and that of SiO2 was about 86%, achieving efficient resource utilization. The biological method utilizes the reaction between microorganisms and minerals containing silicon, which exists in soluble substances to reduce the silicon content and has good environmental potential. Shah S S et al. [15] used organic acids produced by the filamentous fungus Penicillium simplicissimum to acid hydrolyze silicates in bauxite, leaching aluminum and achieving an environmentally friendly desilication pathway without the generation of red mud. Qi Wang et al. [16] used a biological method to remove silicon from high-silica bauxite. Through the action of two efficient silicon-solubilizing bacteria (Arthrobacter pascens H19 and Burkholderia anthena G21), the silicon content in the bauxite was significantly reduced, and its aluminum/silicon ratio was significantly increased to 9.15–9.64, meeting the requirements for producing alumina by the Bayer process.
The work of this article is to optimize the alkali leaching pre-desilication treatment in the Bayer process for producing primary aluminum. The Bayer process has the advantages of low energy consumption, simple process, and high product quality, and is currently the main method for producing aluminum from bauxite in the world [17]. Despite significant success in industrial production, the desilication process remains a complex and critical step. The presence of silicon can seriously affect the quality and production efficiency of alumina, so it is of great significance to study how to effectively remove silicon. Sodium hydroxide is commonly used as a desilication agent in the Bayer process [18]. Bauxite reacts with the sodium hydroxide solution under high-temperature and pressure conditions, and silicon dioxide reacts with sodium hydroxide to form sodium silicate, which then forms hydrated aluminum silicate precipitate to remove silicon impurities. However, this process has high requirements pertaining to the aluminum/silicon ratio of bauxite, and there are problems such as high alkali consumption and a large amount of red mud.
For this reason, barium hydroxide (Ba(OH)2) was selected as the desiliconization agent in this study due to its strong alkalinity and high reactivity with silicate.
2. Materials and Methods
2.1. Experimental Principle
Barium hydroxide, as the desilication agent in this study, exhibited the following core reaction during the experimental process:
Na2SiO3 + Ba(OH)2 = BaSiO3 + 2 NaOH
During the desilication process of barium hydroxide, the stable precipitation of barium silicate (BaSiO3) generated by the reaction can achieve the efficient separation of silicon, while regenerating NaOH further reduces alkali consumption. This reaction can also reduce the loss of aluminum to a lower level during the desilication process. After barium hydroxide treatment, the ratio of aluminum to silicon in the slurry is higher than the 6–7 control line required by the Bayer process, which reduces the burden of desilication in the subsequent Bayer process, is conducive to the subsequent Bayer process, and greatly reduces the cost of alumina production process. This method provides the possibility for the achievement of efficient desiliconization technology, which is more in line with the needs of Guangxi alumina production and promotes the rapid development of local economy.
2.2. Experimental Raw Materials and Reagents
The raw ore of bauxite used in the experiment was taken from a place in Guangxi. Its components are listed in Table 1.

Table 1.
The chemical composition of raw bauxite (wt. %).
During the experiment, bauxite and limestone were blended with a circulating mother liquor at a mass ratio of 6.85:1. This mixture was charged into an autoclave and maintained at 270 °C for 60 min. In the ore-blending stage, solids were added to achieve a target solid concentration of 370 g/L, utilizing 2 L of the circulating mother liquor. The composition of the mother liquor was as follows: total alkali (NT) = 261.7 g/L, caustic alkali (NK) = 250 g/L, and carbon alkali (Nc) = 4.5 g/L. Lime was incorporated according to a target CaO/SiO2 (C/S) molar ratio of 1.65. In the bauxite slurry prepared by the above operation, the solubility of the harmful substance TiO2 is relatively small, and the solubility of Al2O3 is sufficient [19]. The specific composition is shown in Table 2.

Table 2.
The chemical composition of raw slurry composition (wt. %).
The barium hydroxide powder used in the experiment is a pure reagent, the mass fraction of hydrochloric acid is 30%, and the carbon dioxide gas is a pure gas.
The equipment used in the test includes a constant-temperature water bath heating pot (to provide a stable-temperature environment for the experimental reaction), a vacuum drying oven (for drying the precipitate obtained during the experiment), and an electronic balance (for accurately weighing the mass of the experimental raw materials).
2.3. Experimental Methods
2.3.1. Pre-Desiliconization of Bauxite Slurry
The pre-desiliconization test of the slurry was carried out in an orderly manner in the constant-temperature water bath heating pot. Alumina slurry (100 mL) was transferred to a beaker. A measured quantity of barium hydroxide powder was added, and the mixture was homogenized using a magnetic stirrer to ensure complete contact. The beaker was sealed and immersed in a thermostatic water bath preheated to the target temperature. Stirring commenced immediately, and recording the reaction time was began. After the predetermined reaction time, the beaker was rapidly removed. The slurry underwent liquid–solid separation via filtration. The separated solids were vacuum-dried. Chemical composition analysis was performed on the dried solids, and their phase composition was characterized by XRD. The pre-desilication rate of the bauxite ore was calculated based on the chemical composition of the slurry following precipitation and acid dissolution, using the formula below:
Pre-desiliconization rate = M Si removed/M Si in original solution × 100%
2.3.2. Dissolution of Aluminum Hydroxide
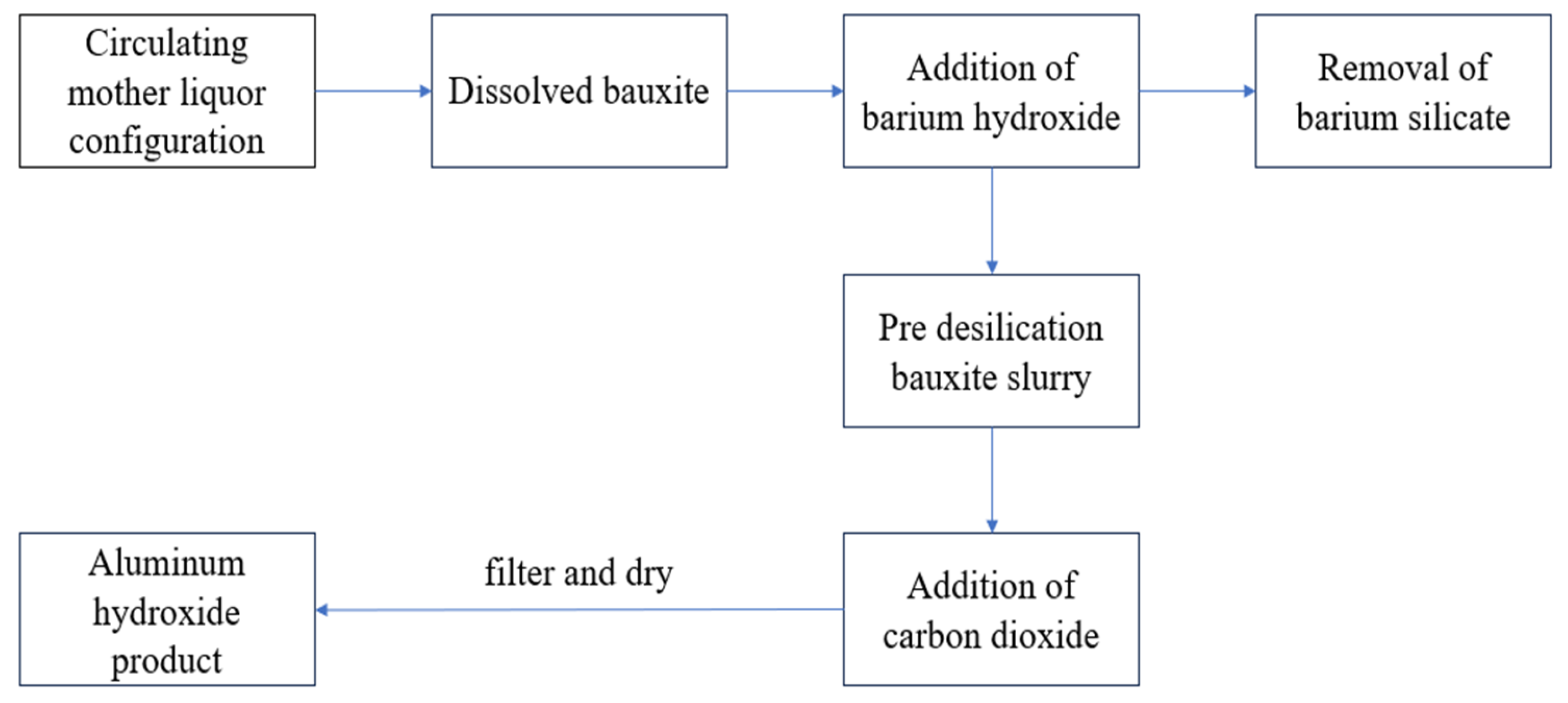
In the bauxite slurry after heat preservation treatment and solid–liquid separation in the previous stage, carbon dioxide gas is continuously injected for 30 min. When the predetermined time is reached, the solution is filtered using a vacuum filter to obtain a white precipitate and a filtrate. The resulting white precipitate is dried in a vacuum drying oven, and the final product is aluminum hydroxide. The experimental process is shown in Figure 1.
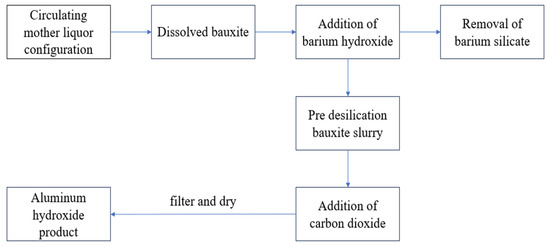
Figure 1.
Experimental process.
3. Results
3.1. Pre-Desiliconization of Bauxite Slurry by Barium Hydroxide Powder
During the experiment, different conditions such as the amount of barium hydroxide added, temperature gradient, and insulation time gradient were set to systematically investigate the effect of barium hydroxide on the pre-desilication rate of the original slurry. The corresponding pre-desilication rate data under different conditions are shown in Table 3. As shown in Table 3, under the condition of a constant insulation temperature of 25 °C, increasing the amount of barium hydroxide added from 2 g to 3 g resulted in a certain improvement in the desilication rate of the samples at different insulation times. At the same time, under the conditions of 2 g and 3 g of barium hydroxide addition, extending the insulation time resulted in an increase in the desilication rate of the samples, and the desilication effect was significantly improved. However, when the amount of barium hydroxide added remains constant and the insulation temperature is increased, the desilication rate decreases to a certain extent, and the desilication effect deteriorates, showing a negative correlation trend, as shown in Table 3. Taking into account the desilication effect, under the experimental conditions of group 6, namely, with a Ba(OH)2 addition of 3 g, a holding temperature of 25 °C, and a holding time of 50 min, the desilication effect is optimal.

Table 3.
Pre-desilication rates corresponding to different conditions.
From the principle of chemical reaction, it is known that barium hydroxide is continuously consumed during the desilication process. Only by ensuring sufficient barium hydroxide can the silicon element be fully reacted and completely removed. In addition, extending the insulation time appropriately makes the reaction more complete, helps the crystallization of barium silicate to be more complete, and makes the reaction more thorough, thereby improving the precipitation efficiency of barium silicate, enhancing the desilication effect, and improving the pre-desilication rate.
3.2. Influence of Insulation Time on Leaching Performance of Aluminum Hydroxide
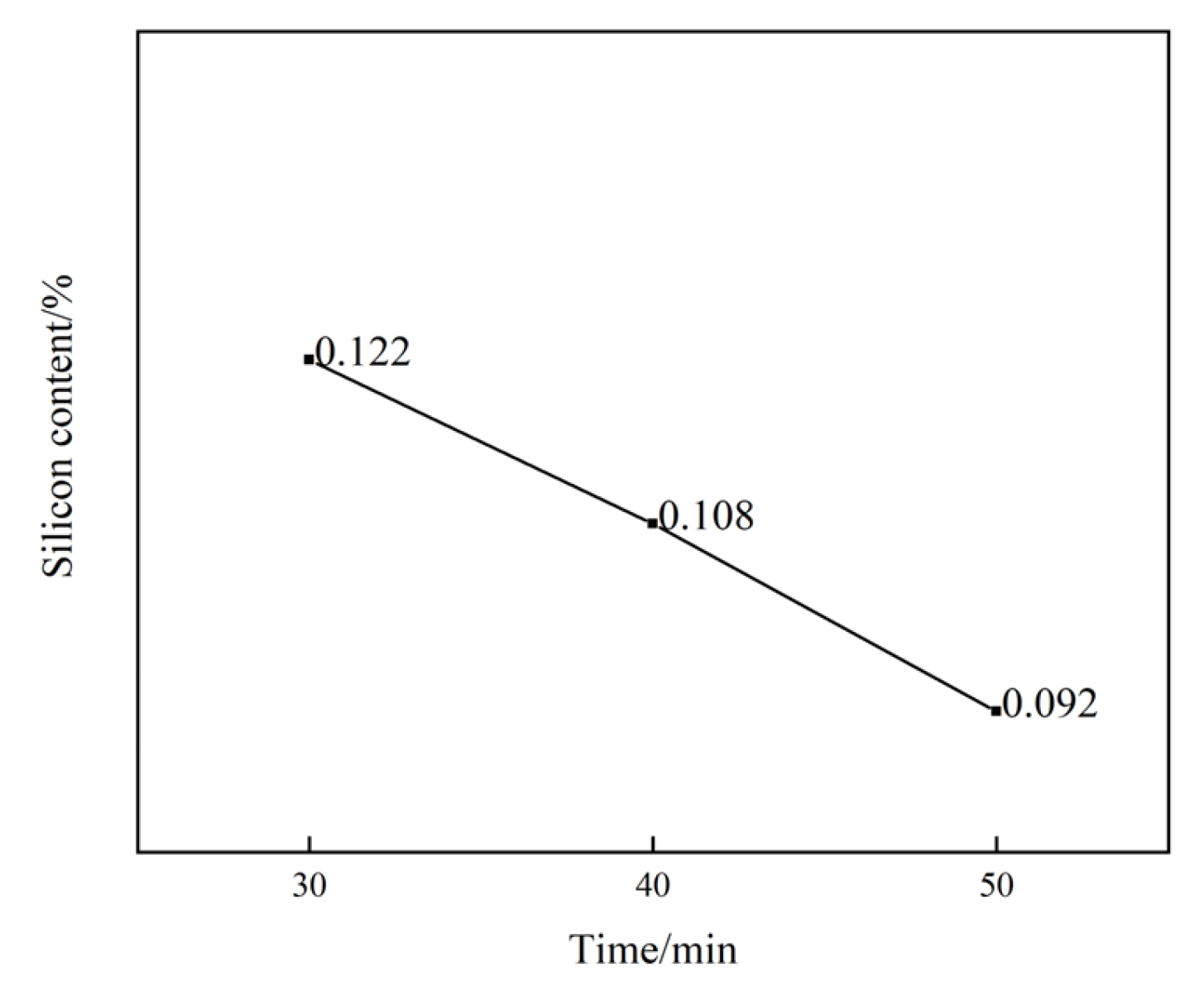
To investigate the effect of holding time on the leaching performance of aluminum hydroxide, experiments were conducted under conditions where the amount of barium hydroxide added remained constant while the holding time was varied. When 3 g of barium hydroxide was added, three time intervals were set, i.e., 30 min, 40 min, and 50 min. Based on the data, the curve depicting the silicon content percentage over time was plotted, as shown in Figure 2. It was clearly observed that, for the same amount of barium hydroxide added, the silicon content percentage gradually decreased as the holding time increased from 30 min to 50 min. This indicated that the holding time significantly impacted the leaching performance of barium hydroxide on aluminum hydroxide. During longer holding times, longer contact and reaction times between barium hydroxide and silicates were allowed. This facilitated the continuous progression of the desilication reaction, promoted a more complete crystallization of barium silicate, improved the precipitation efficiency, and thereby enabled a more effective removal of silicon. Consequently, the purity was enhanced, and the desilication rate was increased. Therefore, in practical production, appropriately extending the holding time to facilitate the desilication reaction is recommended to improve the desilication rate.
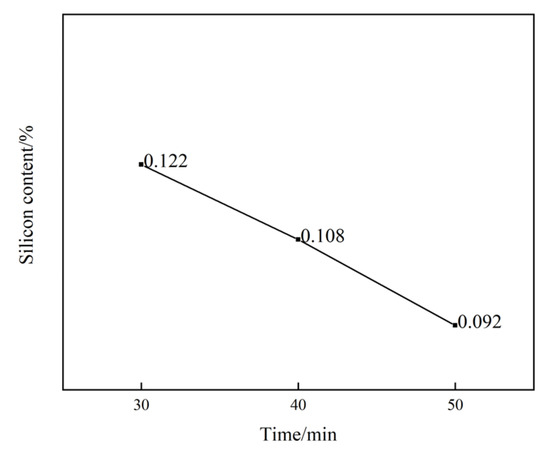
Figure 2.
The proportion of silicon content changes with time under the addition of 3 g of Ba(OH)2.
3.3. XRD and SEM Analyses of Dissolved Aluminum Hydroxide
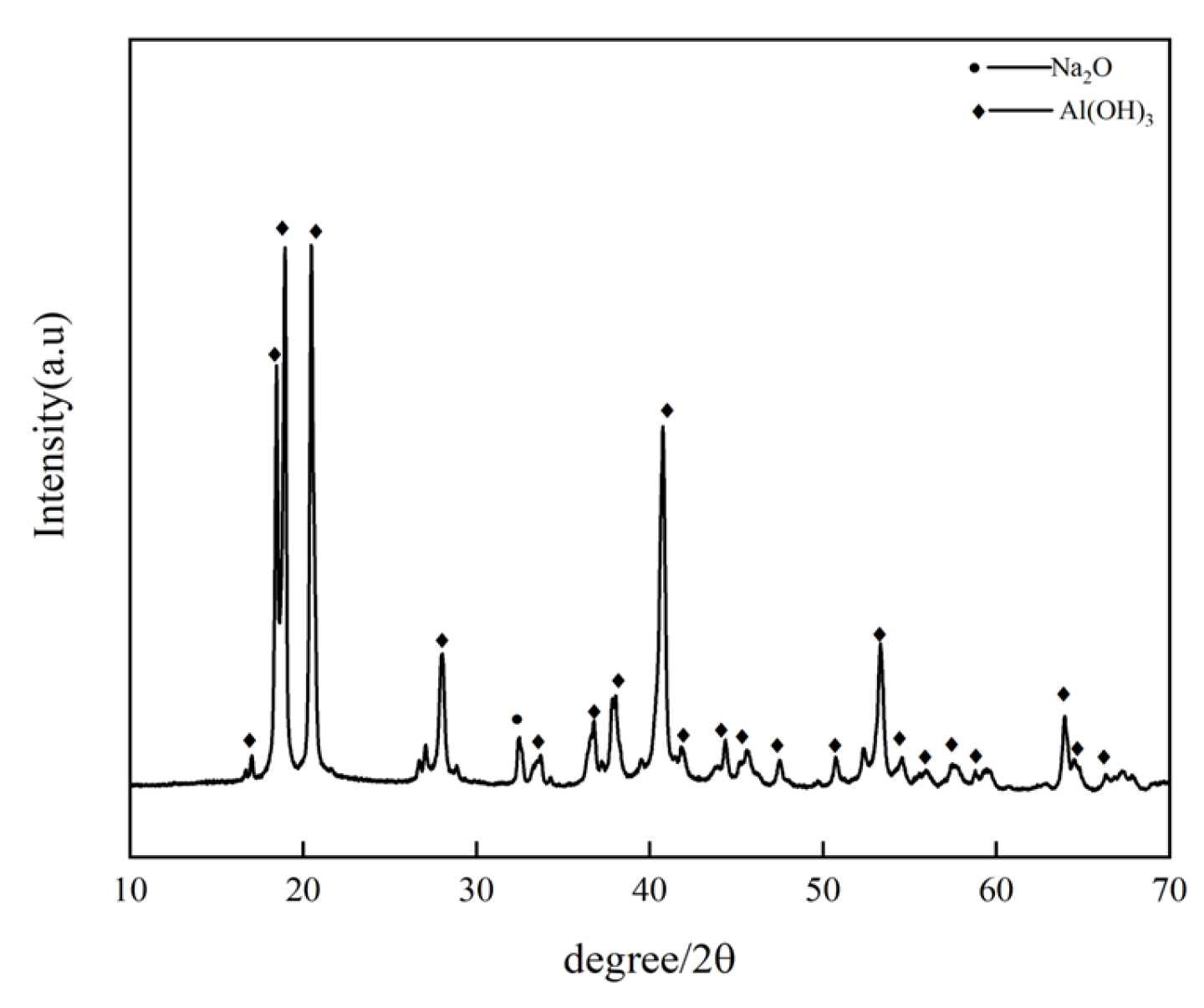
The dissolved aluminum hydroxide precipitate was subjected to XRD phase analysis, as shown in Figure 3. From the figure, it can be seen that the leached precipitate is indeed identified to be aluminum hydroxide, with only a small amount of the impurity phase. This is attributed to the inclusion of sodium during the precipitation of aluminum hydroxide, resulting in impurities [20].
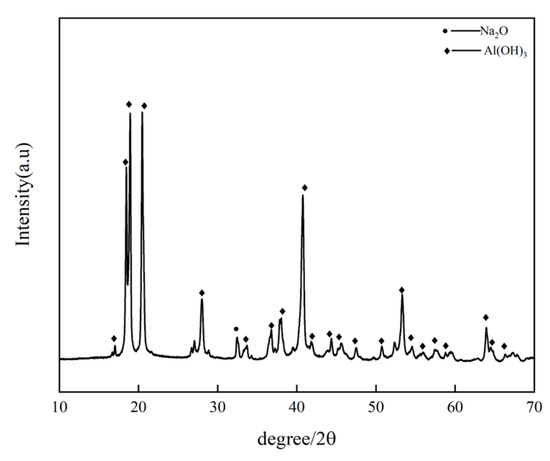
Figure 3.
Dissolved aluminum hydroxide.
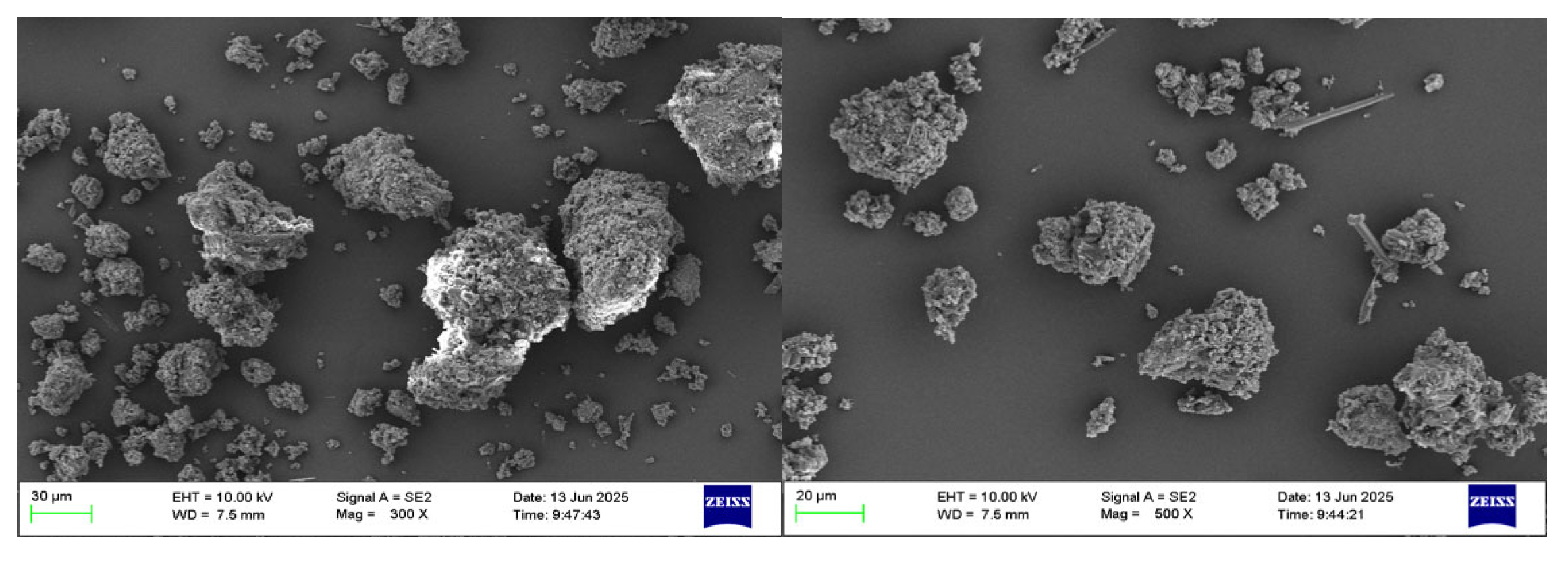
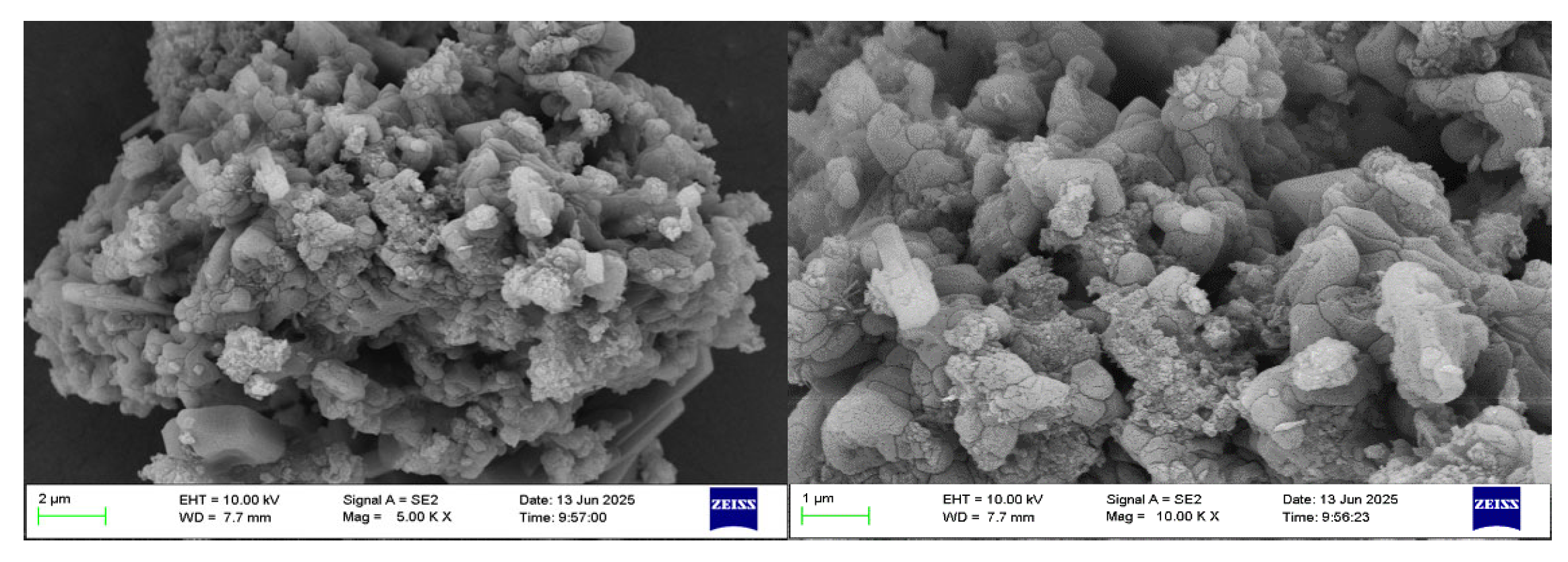
The scanning electron microscope (SEM) characterization was performed on the acquired aluminum hydroxide powder. Figure 4 presents the morphologies observed for the resulting aluminum hydroxide powder. The analysis reveals that the aluminum hydroxide particles exhibit a small particle size and homogeneous distribution.
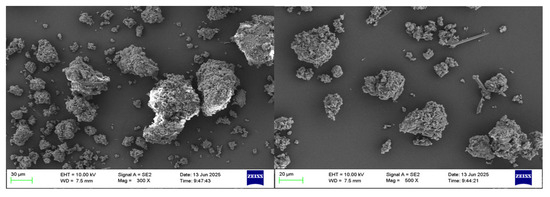
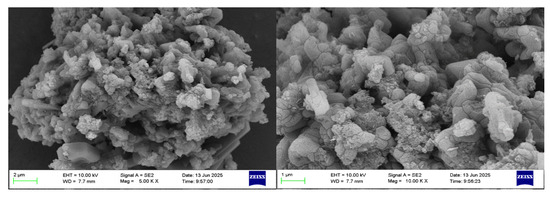
Figure 4.
SEM of aluminum hydroxide powder at different magnifications.
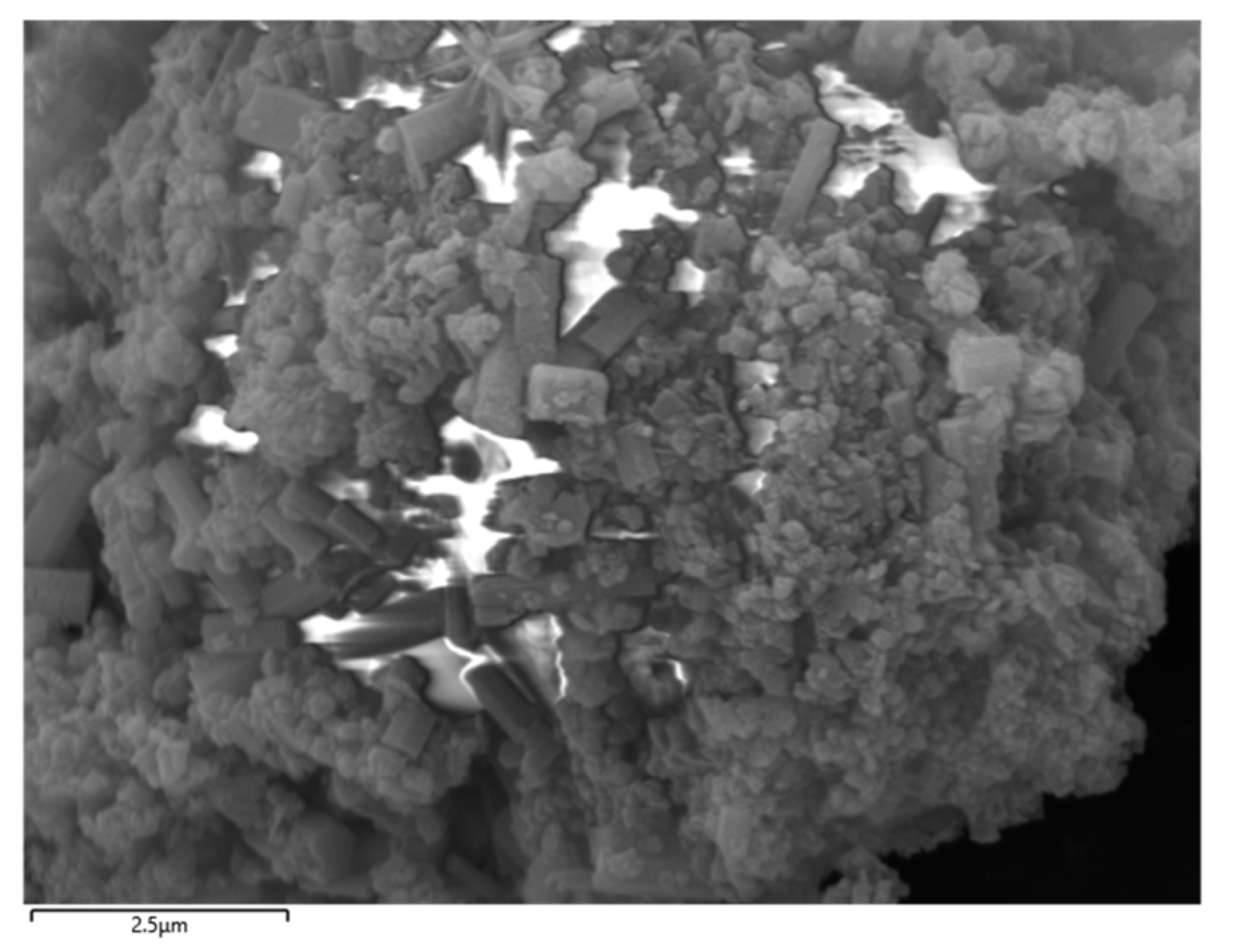
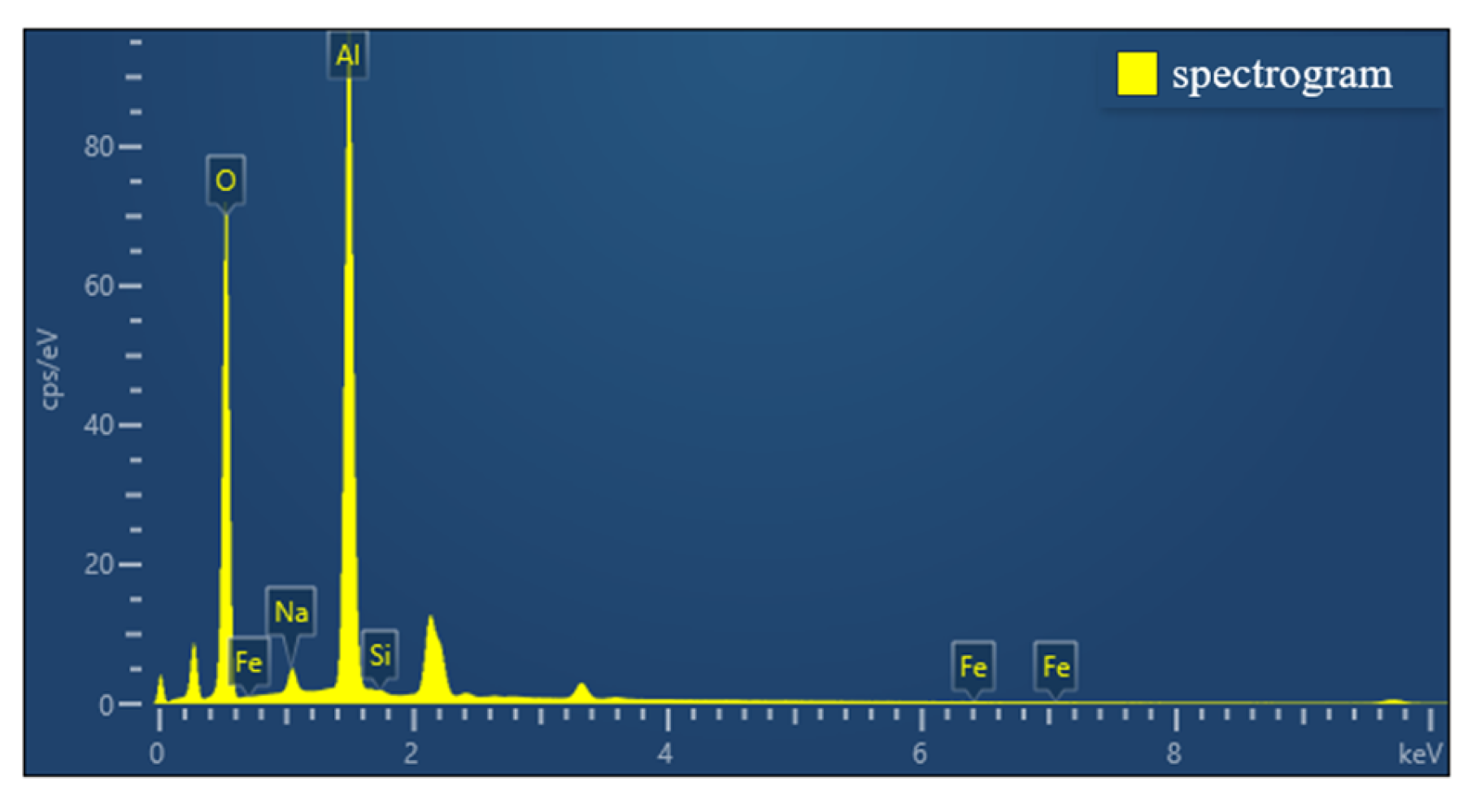
The selected aluminum hydroxide powder underwent localized SEM and EDS investigations, with the results illustrated in Figure 5 and Figure 6.
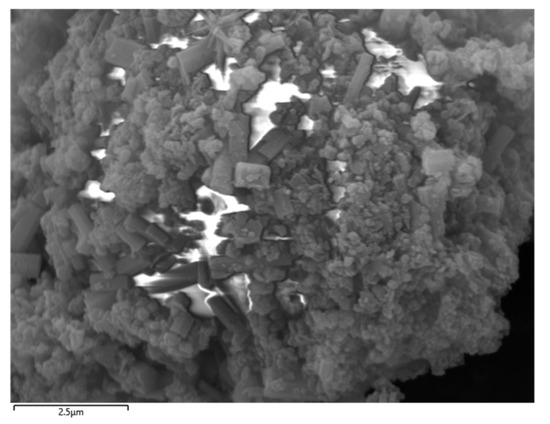
Figure 5.
SEM of localized aluminum hydroxide powder.
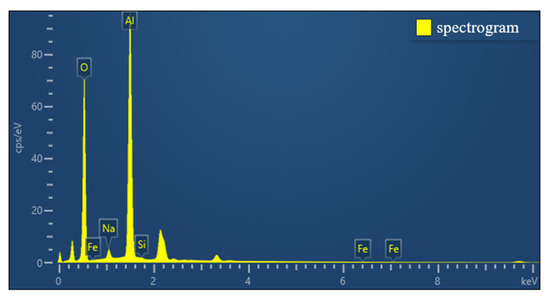
Figure 6.
EDS analysis of aluminum hydroxide powder.
According to the SEM and EDS data analyses, the sample contains a very low concentration of silicon, which exists as fine particles dispersed in the solid gaps of aluminum hydroxide and is classified as a trace impurity. The slightly elevated sodium content can be attributed to its introduction in the previous process. Meanwhile, the iron content is extremely low and has almost no influence.
Compared with the traditional lime-based desilication, barium hydroxide desilication has significant advantages in multiple aspects.
In terms of reaction rate, the advantages of the barium hydroxide method are particularly prominent. The lime method for desilication mainly utilizes the reaction of calcium hydroxide (Ca(OH)2) with the silicon element to generate hydrated calcium silicate precipitate for desilication [21]. However, the solubility of calcium hydroxide in water is relatively low, and the concentration of Ca2+ ions it can provide in aqueous solutions is limited. Chemical reactions are limited by the concentration of Ca2+ ions, resulting in a slower reaction rate. On the contrary, barium hydroxide (Ba(OH)2) has a high solubility in water and can quickly produce a large amount of Ba2+ ions when dissolved in water, increasing the concentration of Ba2+ ions. These Ba2+ ions can quickly react with the silicon element in the solution to form a barium silicate precipitate, greatly improving the rate of desilication reaction.
Barium hydroxide desilication also has significant advantages in aluminum loss. During the process of desilication by the lime method, the generated hydrated calcium silicate precipitate has a large specific surface area, which makes it easier to adsorb aluminum ions, resulting in the chemical loss of aluminum. The precipitation properties of barium silicate generated by the desilication of barium hydroxide are relatively stable, and its adsorption effect on aluminum ions is weak, which can effectively reduce the chemical loss of aluminum.
From the point of view of energy consumption, barium hydroxide desiliconization also has certain advantages. Because the barium hydroxide method has a fast reaction rate, it can achieve a better desiliconization effect in a short time, so the time and energy consumption required for the reaction can be reduced. In contrast, due to the slow reaction rate of lime desiliconization, in order to achieve the ideal desiliconization effect, it often needs a longer reaction time and a higher reaction temperature, which undoubtedly increases the energy consumption [22].
In addition, compared with acid-based desilication, barium hydroxide desilication also has environmental advantages. Acid-based desilication usually uses strong acids such as sulfuric acid and hydrochloric acid, which are costly. During the desilication process, a large amount of acidic wastewater is generated, which is highly corrosive and makes it difficult to separate aluminum later, causing serious pollution to the environment [23]. In the process of desilication by the barium hydroxide method, no acidic wastewater is generated, and the precipitation of barium silicate produced by the desilication reaction can also be reused as building materials, fully meeting the requirements of green environmental protection.
4. Conclusions
- (1)
- This study systematically explored the pre-desilication process of barium hydroxide based on the characteristics of high silicon content and a low aluminum/silicon ratio in a low-grade bauxite mine in Guangxi, aiming to improve the aluminum/silicon ratio and optimize the subsequent alumina production process. The research results indicate that, to a certain extent, the desilication effect is positively correlated with the amount of barium hydroxide added and the holding time, and negatively correlated with the reaction temperature. The research results provide the possibility of achieving efficient desilication technology for low-grade bauxite and improving the utilization rate of mineral resources, thereby better meeting the demand for alumina production in Guangxi and promoting economic development.
- (2)
- In this paper, adding 3 g of barium hydroxide powder to 100 mL bauxite slurry and keeping it at a temperature of 25 °C for 50 min can achieve the best desilication effect. The experimental results showed that, under this condition, the desilication rate reached 97.53%, and the aluminum/silicon ratio was significantly improved, exceeding the control line required for the production of alumina by the Bayer process. At the same time, the properties of the barium silicate precipitate generated by the barium hydroxide method for desilication are relatively stable, and it has a weak adsorption effect on aluminum ions, which can effectively reduce the loss of aluminum during desilication, reduce aluminum loss, and effectively solve the problems of high aluminum loss (5–10%) in the traditional lime method and serious environmental pollution in the acid method.
- (3)
- The research results have demonstrated certain value and advantages in terms of economy and resource utilization. In terms of economy, after desilication treatment with barium hydroxide, the aluminum/silicon ratio of the slurry significantly increases, exceeding the control line required by the Bayer process for producing alumina. This advantage greatly reduces the burden of desilication in the subsequent process of producing alumina using the Bayer process, and correspondingly reduces the equipment investment, chemical reagent consumption, and energy consumption required in the subsequent desilication process, thereby effectively reducing the cost of alumina production and improving the economic efficiency of the enterprise.
- (4)
- Future research can further focus on the development of barium salt cycle process, the collaborative removal mechanism of fine silicon particles, and the optimal design of industrial continuous reaction devices, in order to deepen the application potential of technology and help the efficient development and industrial upgrading of bauxite resources in China.
Author Contributions
Conceptualization, G.H. and A.L.; methodology, A.X.; formal analysis, G.H.; investigation, D.Z.; resources, A.L.; data curation, D.Z.; writing—original draft preparation, G.H. and A.X.; writing—review and editing, A.L. and X.P.; supervision, L.P. and X.Z.; funding acquisition, A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the 2022 Guangxi Key Research and Development Program, grant number GuiKeAB22080015, the Special Fund for Science and Technology Development of Guangxi, grant number AD25069078, 2022 Specific Research Project of Guangxi for Research Bases and Talents, grant number GuiKeAD21238010 and 2021 Special funds for central guidance of local scientific and technological development funds, grant number GuiKeZY21195030.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, H.; Hu, P.; Jiang, J.; Cheng, X.; Wang, J.; Liu, J.; Xiang, P. Distribution, genetic types and current situation of exploration and development of bauxite resources. Geol. China 2021, 48, 68–81. [Google Scholar]
- Chen, Y.; Long, F.; Cao, X.; Li, Y.; Zhang, W.; Zhang, T.; Lv, G. Exploration of Large-Scale Application of Efficient and Clean Utilization of Low-Grade Bauxite. Separations 2023, 10, 336. [Google Scholar] [CrossRef]
- Rao, D.S.; Das, B. Characterization and beneficiation studies of a low grade bauxite ore. J. Inst. Eng. Ser. D 2014, 95, 81–93. [Google Scholar] [CrossRef]
- Modi, M.; Dewangan, P. Beneficiation of Bauxite Ore Characterized by Low-Grade and High Silica Content Using Crushing and Scrubbing Technique. Int. J. Eng. Trends Technol. 2024, 72, 207–213. [Google Scholar] [CrossRef]
- Papadopoulos, N.; Karayianni, H.-S.; Hristoforou, E.; Metaxa, E. Valuable Products Obtained from Red Mud. GR2010000003. 15 July 2010. [Google Scholar]
- Gibson, B.; Wonyen, D.G.; Chelgani, S.C. A review of pretreatment of diasporic bauxite ores by flotation separation. Miner. Eng. 2017, 114, 64–73. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y.; Wang, L.; Hua, Y.; Cheng, T.; Zhang, T.; Zhao, Q. Co-Extraction of Aluminum and Silicon and Kinetics Analysis in Carbochlorination Process of Low-Grade Bauxite. Materials 2024, 17, 3613. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.S.; Palmieri, M.C.; Sponchiado, S.R.P.; Bevilaqua, D. Environmentally sustainable and cost-effective bioleaching of aluminum from low-grade bauxite ore using marine-derived Aspergillus niger. Hydrometallurgy 2020, 195, 105368. [Google Scholar] [CrossRef]
- Ren, S.P.; Xiong, T.; Zhou, Y.C. Study and application of desilication process in mineral processing. Min. Metall. 2013, 10, 195–197. [Google Scholar]
- Massola, C.P.; Lima, J.R.B.; Andrade, C.F. Separation of silica from bauxite via froth flotation. Miner. Eng. 2009, 22, 315–318. [Google Scholar] [CrossRef]
- Abroon, A.; Rahimdad, A. Desilication and flotation techniques for separating them from the bauxite. Adv. Environ. Biol. 2014, 8, 1280–1288. [Google Scholar]
- Xu, Y.; Chen, C.; Li, J. A Novel Sustainable and Facile Method in Alumina Industry: Pre-Treatment of High Silica Bauxite by Cyclic Alkaline Leaching. J. Sustain. Metall. 2025, 11, 645–656. [Google Scholar] [CrossRef]
- Jiang, Z.; Xia, F.; Zhang, S.; Zhang, Q.; Zhang, M. Study on efficient desulfurization and desilication process of low-grade bauxite. Nonferrous Met. Sci. Eng. 2022, 13, 26–34. [Google Scholar]
- Sun, Y.; Pan, A.; Ma, Y.; Chang, J. Extraction of alumina and silica from high-silica bauxite by sintering with sodium carbonate followed by two-step leaching with water and sulfuric acid. RSC Adv. 2023, 13, 23254–23266. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.S.; Palmieri, M.C.; Sponchiado, S.R.P.; Bevilaqua, D. A sustainable approach on biomining of low-grade bauxite by P. simplicissimum using molasses medium. Braz. J. Microbiol. 2022, 53, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sheng, X.-F.; He, L.-Y.; Shan, Y. Improving bio-desilication of a high silica bauxite by two highly effective silica-solubilizing bacteria. Miner. Eng. 2018, 128, 179–186. [Google Scholar] [CrossRef]
- Paz, S.P.A.; Angélica, R.S.; Kahn, H. Optimization of the reactive silica quantification method applied to Paragominas-type gibbsitic bauxites. Int. J. Miner. Process. 2017, 162, 48–57. [Google Scholar] [CrossRef]
- Wu, Y.; Pan, X.; Han, Y.; Yu, H. Novel Process and Mechanism for Chemical Desilication from Kaolinite-Rich Diasporic Bauxite. J. Northeast. Univ. (Nat. Sci.) 2019, 40, 1424–1429. [Google Scholar]
- Yin, Z.; Gu, S. Influence of lime adding method on scaling process in Bayer preheating process of diasporic bauxite slurry. Chin. J. Nonferrous Met. 2001, 11, 910–914. [Google Scholar]
- Dobra, G.; Garcia-Granda, S.; Iliev, S.; Cotet, L.; Negrea, P.; Duteanu, N.; Boiangiu, A. Aluminum hydroxide impurities occlusions and contamination sources. Rev. Chim. 2020, 71, 65–76. [Google Scholar] [CrossRef]
- Zhang, Z.Y. Experimental study on digestion of gibbsite and boehmite mixture. Light Met. 2019, 1, 10–13. [Google Scholar]
- Smith, P. The processing of high silica bauxites—Review of existing and potential processes. Hydrometallurgy 2009, 98, 162–176. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, W.; Liu, W.; Chen, M.; Hu, L. Two-step acid leaching extraction of Fe&Al from karst bauxite and optimization experimental study. Chem. Eng. Process.-Process Intensif. 2025, 208, 110131. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).